Abstract
The E-cadherin/catenin complex is a prime mediator of cell–cell adhesion. APC mutations can result in loss of β-catenin downregulation and an accumulation of β-catenin in the cell. β-CATENIN mutations can have a similar effect. The aim of this study was to investigate the effect of β-CATENIN and APC mutations on the expression and assembly of the E-cadherin/catenin complex. Five colorectal carcinoma cell lines with different APC and β-CATENIN gene status were selected and mutations were confirmed. The expression of members of the E-cadherin/catenin complex was studied by immunohistochemistry and Western blotting. Complex assembly was investigated by immunoprecipitation. It is shown that E-cadherin and catenins are expressed in colorectal carcinoma cell lines with the predominant complex assembly being E-cadherin/β-catenin/α-catenin. The subcellular distribution of the proteins is influenced by cell–cell contact, resulting in membranous localization. The expression and assembly of the E-cadherin/catenin complex does not appear to be affected by the presence of APC and or β-CATENIN mutations.
Keywords: catenins, colorectal carcinoma, E-cadherin
The E-cadherin/catenin complex is a prime mediator of cell–cell adhesion and influences the formation of cell–cell junctions. E-Cadherin facilitates the assembly of specialized intercellular junctions (desmosomes and gap and tight junctions) necessary to link epithelial cells into functional monolayers (Gumbiner et al. 1988; Wheelock & Jensen 1992). Conversely, blockage of cadherin activity with anti-cadherin antibodies induces the dispersion of cell layers (Takeichi 1988; Takeichi 1990). Cadherin-based adhesive contacts also participate in establishing apical–basal polarity (Nelson et al. 1990; Yap et al. 1995), possibly through a role in the targeted delivery of membrane components to the basolateral cell surface (Grindstaff et al. 1998). Cellular studies have also suggested that E-cadherin may influence cell locomotion (Hermiston et al. 1996) and population dynamics (Watabe et al. 1994), properties that could contribute to its morphogenetic influence.
Assembly of endogenous cadherin/catenin complexes appears to be a dynamic process. Large pools of cadherin-bound and cadherin-independent catenins are present in cultured cells (Hinck et al. 1994b; Nathke et al. 1994). E-Cadherin binds to β-catenin or γ-catenin. α-Catenin also binds either to β-catenin or to γ-catenin with the result that complexes of either E-cadherin/β-catenin/α-catenin or E-cadherin/γ-catenin/α-catenin are formed (Hinck et al. 1994b). β-Catenin and γ-catenin competitively bind to the same region of α-catenin. α-Catenin associates with E-cadherin via β-catenin or γ-catenin (Herrenknecht et al. 1991; Nagafuchi et al. 1991) and is the central protein linking the actin cytoskeleton to cadherin/catenin complexes at the cell membrane.
The gene encoding human β-catenin is located on chromosome 3p21 (Kraus et al. 1994). The arm-repeat domain mediates the binding of β-catenin to APC (Hulsken et al. 1994), Axin (Behrens et al. 1998; Ikeda et al. 1998) and Tcf family of transcription factors (van de Wetering et al. 1997). Phosphorylation of both APC and axin by GSK-3β enhances β-catenin binding to the APC–axin complex and targets the protein for ubiquitination and proteasomal degradation (Aberle et al. 1997; Behrens et al. 1998; Ikeda et al. 1998, 2000).
The APC gene is localized to human chromosome 5q21-22 (Bodmer et al. 1987; Leppert et al. 1987) and consists of 16 exons, one non-coding and 15 coding (Groden et al. 1991). The product of the APC gene is a 300 kDa protein composed of 2843 amino acids (Smith et al. 1993). Inherited mutations of APC cause Familial Adenomatous Polyposis (FAP), and acquired APC mutations are present in approximately 80% of sporadic cancers (Miyoshi et al. 1992). Studies of the adenoma–carcinoma sequence in both sporadic cancers and tumours from FAP patients suggest that APC mutation is an early event in the progression of colorectal cancer, occurring before RAS or p53 mutation (Powell et al. 1992; Smith et al. 1993; Kinzler & Vogelstein 1996). These observations have led to the designation of APC as the gatekeeper for colorectal carcinogenesis.
Both β-catenin and γ-catenin bind directly to E-cadherin and APC, whereas α-catenin binds only through its association with either β-catenin or γ-catenin. Thus, cadherin and APC appear to represent parallel systems, both utilizing the same set of associated proteins (Rubinfeld et al. 1995).
The association of APC with catenins led to the proposal that APC has a role in regulating the activity of the cadherin/catenin complex and, thus, intercellular adhesion (Rubinfeld et al. 1993; Su et al. 1993; Hinck et al. 1994a). Wild-type but not mutant APC has been shown to associate and promote the assembly of microtubules which influence the distribution of actin filaments and the cytoskeletal organization in general (Smith et al. 1994), with possible implications for colon carcinogenesis.
Many APC mutations result in a truncated protein with loss of the β-catenin regulatory activity and cellular accumulation of β-catenin (Inomata et al. 1996). β-CATENIN mutations targeting the phosphorylation sites in exon 3 have a similar result. β-Catenin lacking the amino-terminal structure is known to be stabilized in mammalian cells (Munemitsu et al. 1996), and such mutants have been implicated in cell transformation (Oyama et al. 1994; Kawanishi et al. 1995).
This report describes a set of experiments designed to explore the expression and distribution of members of the E-cadherin/catenin complex in a panel of colorectal carcinoma cell lines. Five human colorectal adenocarcinoma cell lines of different APC and β-CATENIN gene status and of variable grades of differentiation were chosen. The cell lines were chosen to determine whether there was any correlation between the presence of APC and β-CATENIN mutations and the growth pattern and/or the assembly and distribution of the E-cadherin/catenin complex. The expression and assembly of the E-cadherin/catenin complex does not appear to be affected by the presence of APC and or β-CATENIN mutations but is influenced by the presence or absence of cell–cell contact.
Materials and methods
Cell lines
Five human colorectal adenocarcinoma cell lines showing different grades of differentiation and known to express various APC and β-CATENIN mutations were used. The cell lines were SW480, SW620, HT29, HCT116 and Caco-2. SW620 is derived from lymph node metastasis of the primary tumour from which SW480 is derived. All cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA).
Genomic DNA isolation and polymerase chain reaction
DNA was isolated using a high-salt method. The cells were grown until 80% confluent, then harvested and resuspended in SE buffer [75 mm sodium chloride (NaCl), 25 mm ethylenediaminetetraacetic acid (EDTA) (pH 8.0) and 1% sodium dodecyl sulphate (SDS)]. For every 1 × 107 cells, 2 ml of SE buffer was used. Proteinase K (BDH, Poole, UK) was added to a final concentration of 200 mg/ml, and the cells were incubated at 55 °C overnight. Prewarmed (37 °C) NaCl was added to a final concentration of 1.5 m. An equal volume of chloroform (BDH) was added, and the tubes were put on a roller for mixing for 1 h at room temperature and then centrifuged at 2000 × g for 10 min. The top layer was transferred to a clean Falcon, and an equal volume of isopropanol (BDH) was added. The DNA was spooled, washed in alcohol and then dissolved in TE buffer [10 mm Tris (pH 8.0) and 1 mm EDTA (pH 8.0)]. Ribonuclease (20 ml of 10 mg/ml, Advanced Biotechnologies, Columbia, MD, USA) was added to each sample and incubated for 1 h at 37 °C.
A polymerase chain reaction (PCR) was set up to amplify 4 regions of exon 15 of APC and exons 3 and 5 of β-CATENIN. Six pairs of PCR primers were used. [For APC: regions (15C) atttgaatactacagtgttaccc-5′, cttgtattctaatttggcataagg-3′; (15G) aagaaacaatacagacttattgtg-5′, atgagtggggtctcctgaac-3′; (15H) atct ccctccaaaagtggtgc-5′, tccatctggagtactttctgtg-3′; (15I) agtaaatgct gcagttcagagg-5′, ccgtggcatatcatccccc-3′. For β-CATENIN: (exon 3) atttgatggagttggacatggc-5′, ccagctacttgttcttgagtgaagg-3′; (exon 5) ggtggttaataaggctgcagtt-5′, attttcaccagggcaggaat-3′.]
The PCR product (sequencing template) was purified using the QIAquick PCR purification kit (Qiagen, Crawley, UK), to remove dNTPs, enzyme and primers, producing a clean template which was then amplified for sequencing. Each template was amplified once using the forward primer and another using the reverse primer to sequence the region in both directions. The PCR product was then purified using the QIAquick PCR purification kit and then sequenced. Fluorescence-based cycle sequencing was performed using the ABI Prism BigDye Terminator cycle sequencing ready reaction kits (PE Biosystems, Warrington, UK) and the ABI Prism 3700 DNA Analyser.
Immunocytochemistry
Cells were grown on tissue-culture multispot glass microscope slides (Hendley, Loughton, UK), in a 37 °C and 10% CO2 incubator to different levels of confluence. The cells were then fixed in acetone : methanol (1 : 1) for 10 min at −20 °C. Endogenous peroxidase was blocked by incubating the slides in 0.3% hydrogen peroxide (H2O2, BDH) in phosphate-buffered saline (PBS) for 15 min. The slides were incubated in normal goat serum (1/20, DAKO, Cambridge, UK) for 15 min to block non-specific binding. Primary antibody was then added to the cells (E-cadherin, 1 : 500; α-catenin, 1 : 250; β-catenin, 1 : 500; γ-catenin, 1 : 500), and the slides were incubated overnight at 4 °C. Primary antibody was added to three wells, and to the fourth well, used as a negative control, only PBS was added. The cells were washed in PBS, and then secondary antibody [biotinylated goat antimouse immunoglobulin (Ig), DAKO] was added to each well. The slides were incubated for 30 min at room temperature, washed in PBS and then incubated with streptavidin–peroxidase conjugate (1 : 250, Amersham Pharmacia Biotech, Bucks, UK) for 30 min at room temperature. Peroxidase activity was demonstrated by activated 3,3′-diaminobenzidine tetrahydrochloride solution and 0.1% H2O2. The slides were counterstained in Cole's haematoxylin (Pioneer Research Chemicals, Colchester, UK), dehydrated and mounted using Pertex mountant (CellPath, Hemel Hempstead, UK). The slides were examined by a conventional light microscope, and the level of expression and cellular distribution of the proteins between membrane, cytoplasm and nucleus were assessed.
Western blotting and immunoprecipitation
Cells were grown to approximately 80% confluence and then harvested. The cells were solubilized by cytoskeleton buffer (Pasdar & Nelson 1988). Eight hundred millilitres of soluble fraction lysis buffer (0.5% Triton X-100 (TX-100), 5 mm NaCl, 10 mm Pipes, 3 m MgCl2, 300 mm sucrose and protease inhibitor cocktail tablets (Boehringer Mannheim, Lewes, UK) (one tablet/50 ml lysis buffer)] was added to the Petri dish, and the cells were scraped with a rubber policeman and pipetted with the lysis buffer into a prechilled Eppendorf. Tubes were kept on ice for 20 min with repeated vortexing and then were centrifuged at 13 000 × g for 10 min at 4 °C. The resulting supernatant (with the TX-100-soluble fraction of the cell protein) was collected. The remaining pellet was incubated in 100 ml of the lysis buffer for the insoluble fraction [15 mm Tris–HCl (hydrochloric acid), 5 mm EDTA, 2.5 mm EGTA [ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′-tetraacetic acid] and 1% SDS] at 100 °C for 10 min and then centrifuged at 13 000 × g for 10 min at 4 °C. The supernatant (with the TX-100-insoluble fraction of the cell protein) was collected.
Protein concentration was quantified using the Bradford protein assay kit (Bio-Rad, Hemel Hempstead, UK). Equal protein loading of corresponding lanes was observed. The calculated volumes of lysates were mixed with sample buffer and resolved by SDS-PAGE, and the resolved proteins were transferred by electroblotting onto nitrocellulose membranes (Millipore, Hesle, UK). The membranes were probed by antibodies against α-catenin (1 : 250), E-cadherin and β- and γ-catenin (1 : 500) (Transduction Laboratories, Lexington, KY, USA) followed by the secondary antibody [Horse radish peroxidase (HRP)-conjugated rabbit antimouse Ig, DAKO]. Detection was by enhanced chemiluminescence reagent (Amersham Pharmacia Biotech), followed by exposure to hyperfilm (Amersham Pharmacia Biotech).
For immunoprecipitation, equivalent aliquots of each of the fractions were precleared by incubation with 50 ml/lane protein G-Sepharose (Pharmacia, Bucks, UK) for 1 h at 4 °C. The clarified supernatants were incubated with 5 mg/ml of anti-E-cadherin antibody for 1 h at 4 °C with rotation. Purified mouse Ig (Sigma, Poole, UK) was used with negative control samples. Fifty millilitres of G-Sepharose (Amersham Pharmacia Biotech) was then added and incubated overnight at 4 °C. The beads were washed sequentially with high stringency buffer [15 mm Tris–HCL (pH 7.5), 5 mm EDTA, 2.5 mm EGTA, 1% TX-100, 1% sodium deoxycholate, 0.1% SDS, 120 mm NaCl and 25 mm KCl], high-salt buffer [15 mm Tris–HCl (pH 7.5), 5 mm EDTA, 2.5 mm EGTA, 1% TX-100, 1% sodium deoxycholate, 0.1% SDS and 1 m NaCl] and low-salt buffer [15 mm Tris–HCl (pH 7.5) and 5 mm EDTA]. The beads were then resuspended in an equal volume of sample buffer, boiled for 10 min and centrifuged at 13 000 × g for 5 min, and the supernatant was resolved by SDS-PAGE on an 8% polyacrylamide gel and transferred by electroblotting onto nitrocellulose membrane and, subsequently, probed with anti-E-cadherin, anti-α-catenin, anti-β-catenin and anti-γ-catenin antibodies (Transduction Laboratories).
Results
Mutation analysis
Sequencing of the amplified regions of the APC and β-CATENIN genes showed that SW480, SW620 and HT29 had mutant APC. SW480 and SW620 had one mutant allele with loss of the other allele, whereas in HT29, both alleles were mutant. HCT116 expressed wild-type APC and mutant β-catenin. Caco-2 had both APC and β-CATENIN gene mutations (Ilyas et al. 1997). In Caco-2, there was one mutant APC allele with loss of the other allele. Table 1 summarizes the details of the mutations in the cell lines.
Table 1.
APC and β-CATENIN gene status in five colorectal adenocarcinoma cell lines
| Cell line | APC mutation | β-CATENIN mutation |
|---|---|---|
| SW480 | CAG–TAG (stop) (codon 1338) | Wild-type |
| SW620 | CAG–TAG (stop) (codon 1338) | Wild-type |
| HCT116 | Wild-type | Three-base deletion |
| Exon 3 (codon 45) | ||
| HT29 | 1 bp insertion (codon 1555) | Wild-type |
| Caco-2 | CAG–TAG (stop) (codon 1367) | GGC–GCC (missense) |
| Exon 5 (codon 245) |
Immunodetection of E-cadherin and catenins
SW480 and SW620
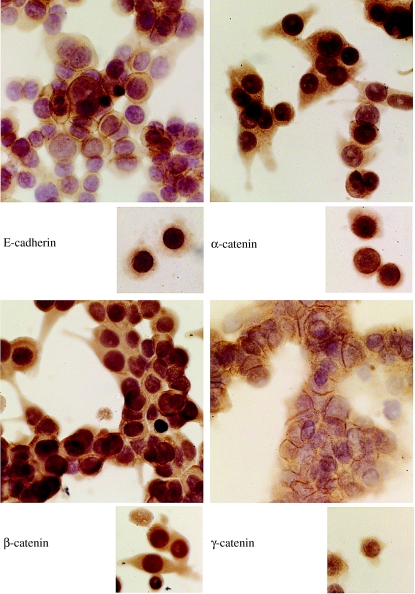
Scattered cells of both cell lines showed cytoplasmic immunoreactivity for E-cadherin and the three catenins, with no membranous localization. All catenins also showed nuclear localization. When confluent, these cells grew as discohesive cell aggregates where there was some membranous localization of E-cadherin and catenins (Figure 1). Prominent nuclear localization of β- and γ-catenin was also observed in clustered cells.
Figure 1.
Expression of E-cadherin and catenins in the cell line SW480, when cells were dispersed (subsets) and when they were clustered. When clustered, cells grew as discohesive aggregates with little membranous localization of the proteins.
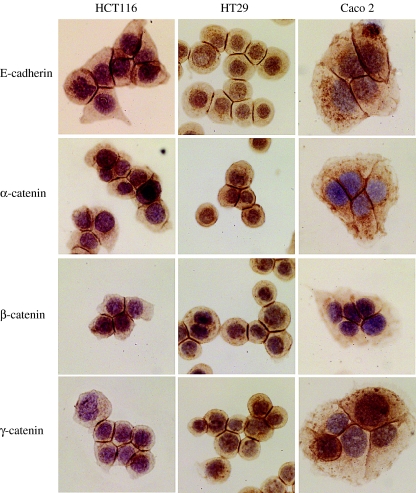
HCT116, HT29 and Caco-2
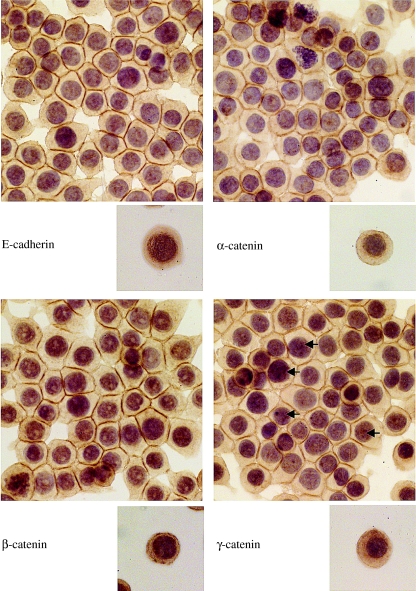
When the immunoprofile of E-cadherin and the catenins was studied in preconfluent cells of HCT116, HT29 and Caco-2, strong membranous localization of the proteins was only observed at sites of cell–cell contact, whereas the free borders of the cells did not show any membranous staining (Figure 2). As the cells grew into confluent monolayers, there was a shift of the proteins from the cytoplasm to the cell membrane. Much of the cytoplasmic immunoreactivity and the perinuclear accentuation seen in scattered cells was lost (see Figure 3 for an example of the immunoprofile of HT29 as representative of this group of cell lines).
Figure 2.
Expression of E-cadherin and catenins in the cell lines HCT116, HT29 and Caco-2. Membranous localization of the proteins is seen only at points of cell–cell contact.
Figure 3.
Expression of E-cadherin and catenins in the cell line HT29, when cells were dispersed (subsets) and when confluent. Confluent cells grew as cohesive sheets with strong membranous expression at points of cell–cell contact. Arrows indicate nuclei showing punctate immunoreactivity.
The presence of β-catenin and APC mutations did not seem to influence cell morphology, growth pattern or the expression of members of the E-cadherin/catenin complex. However, these parameters seemed to be influenced by the presence of cell–cell contact.
Western blotting and immunoprecipitation
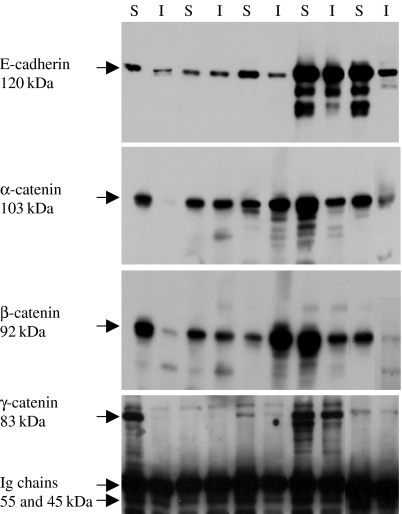
Western blotting showed expression of E-cadherin (120 kDa), α-catenin (103 kDa), β-catenin (92 kDa) and γ-catenin (83 kDa) in both the TX-100-soluble (cytoplasmic) and the TX-100-insoluble (cytoskeleton bound) fractions (data not shown).
The E-cadherin immunoprecipitates of the cell lysates showed the presence of E-cadherin and the three catenins in the TX-100-soluble and the TX-100-insoluble cell protein fractions of all cell lines, indicating the integrity of the complex. The presence of both β-catenin and γ-catenin in the immunoprecipitates in both protein fractions indicates that the two complex assemblies, E-cadherin/β-catenin/α- catenin and E-cadherin/γ-catenin/α-catenin, exist in all cell lines. However, the γ-catenin bands were weak in comparison with those of E-cadherin, β-catenin and α-catenin and only appeared on prolonged exposure (Figure 4), suggesting the predominance of the E-cadherin/β-catenin/α-catenin complex assembly over that of the E-cadherin/γ-catenin/α-catenin.
Figure 4.
Immunoprecipitation of the E-cadherin/catenin complex in the TX-100-soluble (S) and TX-100-insoluble (I) cell protein fractions, using the anti-E-cadherin antibody. Bands of α-, β- and γ-catenin are seen in both fractions of all cell lines; SW480 (lanes 1 and 2), SW620 (lanes 3 and 4), HCT116 (lanes 5 and 6), HT29 (lanes 7 and 8) and Caco-2 (lanes 9 and 10).
Discussion
In the present study, three of the cell lines, SW480, SW620 and HT29, were chosen as they express only mutant APC. A fourth cell line, HCT116, expresses wild-type APC, yet expresses mutant β-catenin. The fifth cell line that was chosen, Caco-2, has both APC and β-CATENIN gene mutations (Ilyas et al. 1997). This study aimed to determine whether such mutations influence the expression and distribution of E-cadherin and the catenins in the cell and the protein–protein interactions between the members of the complex.
There did not seem to be any correlation between the presence of β-CATENIN and APC mutations and cellular morphology and/or growth pattern. However, the cell morphology seemed to be influenced by the presence of cell–cell contact, particularly in the cell lines (HCT116, HT29 and Caco-2) that grew as cohesive confluent monolayers. When dispersed, the cells assumed a round/oval shape (HT29 and Caco-2) or a stellate appearance (HCT116) but were polygonal when confluent. However, in the cell lines that grew as clusters of discohesive cells (SW480 and SW620), the cell density and the presence or absence of cell–cell contact did not seem to influence cellular morphology and the cells always grew as a mixture of round and spindle-shaped cells.
Immunocytochemistry demonstrated a link between cell–cell contact and the distribution of E-cadherin and catenins. In cell lines that grew as confluent monolayers, there was strong membranous localization of all members of the complex but only at points of cell–cell contact and not in singly scattered cells or at the free cell borders at the periphery of small-cell clusters. In SW480 and SW620, there was little difference in the distribution of E-cadherin and catenins between dispersed and clustered cells. Taken together, these observations strongly suggest that physical cell–cell contact plays a role in the recruitment of E-cadherin and catenins to the cell membrane and is a prerequisite for the membranous localization of the E-cadherin/catenin complex. This is expected at least in normal cells, though not necessarily in malignant cells, as cell–cell contact is associated with reorganization of the cytoskeleton and the E-cadherin/catenin complex at the adherens junctions is linked to the cytoskeleton. The E-cadherin/catenin complex thus seems to be responsible for the stabilization, rather than the initiation, of cell–cell adhesion, as free cell borders never exhibited membranous localization of the proteins.
These differences in distribution have been observed in other cell lines. In epithelioid breast carcinoma cell lines, E-cadherin is localized at cell–cell borders and is not present at free cell borders. In interleukin-6-treated cultures of either ZR-75-1-TX or ZR-75-1-Ro cells, the free cell borders showed little or no staining for E-cadherin, while remaining cell junctions stained strongly for E-cadherin, particularly in the apical region of the cell (Tamm et al. 1994). NBT-II cells (rat bladder carcinoma cells) also express E-cadherin along cell junctions, with free cell borders being devoid of immunostaining (Boyer et al. 1992).
Quinlan & Hyatt (1999) demonstrated a relationship between actin organization and the localization and function of the components of the cadherin/catenin complex. Depolymerization of the apical cortical actin ring resulted in epithelial cells in which components of the cadherin/catenin complex were found only in intracellular vesicles and never at the surface. Re-establishment of the filamentous actin ring beneath the cell surface restored membranous localization of the cadherin/catenin complex, implicating the actin ring in the transport and delivery of cadherin and catenins to the cell surface.
It has been suggested that the equilibrium between free and bound pools of catenins could play a critical role in regulating cellular responses to extracellular signals for cell–cell adhesion and cell proliferation. It was proposed (Hinck et al. 1994b) that a shift in the equilibrium towards assembly of catenins with growth factor receptors could facilitate the transduction of signals for cell proliferation. Thus, shifts in balance between adhesion and proliferation will determine whether the cell maintains cell contacts or whether it loses cell contacts and divides. Increased levels of cytoplasmic catenins seem to be associated with translocation of proteins to nucleus. In the present study, nuclear localization of catenins was notably observed in dispersed cells and only occasionally in confluent cells. It is possible that established cell–cell adhesion results in feedback signals that control the levels of expression of E-cadherin and catenins. In the absence of cell–cell adhesion and, hence, such feedback signals, there is accumulation of E-cadherin and catenins in the cell, owing to either increased expression or decreased degradation with probable transfer to the nucleus. We have shown that nuclear localization of β- and γ-catenin occurs in both sporadic and familial colorectal carcinomas, which do show increased levels of expression of both protein and RNA, as demonstrated by immunohistochemistry and in situ hybridization (El-Bahrawy et al. 2001, 2002a). There was a higher frequency of nuclear localization of β-catenin at the invasive front of the tumours, especially in cells invading singly or in small groups (El-Bahrawy et al. 2001), which is very consistent with our in vitro results (Figures 1 and 3).
Both β-catenin and γ-catenin are known to translocate to the nucleus where they bind to nuclear transcription factors (Cavallo et al. 1997; Morin et al. 1997; Simcha et al. 1998; Omer et al. 1999; Prieve & Waterman 1999). α-Catenin also translocates to nucleus, as seen in the present study. We had confirmed this using immunofluorescence and confocal microscopy and also demonstrated it in colorectal carcinomas (El-Bahrawy et al. 2002b).
E-Cadherin, α-catenin and β-catenin form a complex in a 1 : 1 : 1 protein ratio (Ozawa & Kemler 1992; Aberle et al. 1994; Hinck et al. 1994b). In this study, immunoprecipitation showed that in these cell lines the predominant complex was E-cadherin/β-catenin/α-catenin rather than E-cadherin/γ-catenin/α-catenin, as shown by the presence of weak γ-catenin bands detected only on prolonged exposure of the blots, in contrast to the strong β-catenin bands that were readily detected.
Adams et al. (1996) showed that E-cadherin/β-catenin complex is preferentially involved in initiation of cell adhesion between Madin–Darby canine kidney cells and that γ-catenin is associated with cell–cell contacts at a later stage, which may reflect interaction of γ-catenin with desmosomal cadherin complexes that form after initial E-cadherin-mediated cell–cell contact (Pasdar & Nelson 1988). These data implied that β-catenin played a role in the formation of new contacts, while γ-catenin is involved in the subsequent stabilization of contacts (Lampugnani et al. 1995). This may explain the weak expression of γ-catenin in the E-cadherin/catenin complex, as detected in this study by immunoprecipitation, despite its strong expression in the cells, as seen by immunocytochemistry and Western blotting.
In conclusion, we show that E-cadherin and the catenins are highly expressed in colorectal carcinoma cell lines, with the predominant complex assembly being that of E-cadherin/β-catenin/α-catenin. The subcellular distribution of the proteins is influenced by cell–cell contact, the latter resulting in membranous localization, showing that the E-cadherin/catenin complex plays a role in the stabilization, rather than the initiation, of cell–cell contact. The complex expression and assembly does not appear to be affected by the presence of the APC and/or β-catenin mutations, suggesting that the role played by such mutations in tumour development is unlikely to be through disturbances in the E-cadherin/catenin complex.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin–catenin complex in vitro with recombinant proteins. J. Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin–catenin–actin reorganization during development of cell–cell adhesion. J. Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Bodmer WF, Bailey CJ, Bodmer J, et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328:614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Boyer B, Dufour S, Thiery JP. E-cadherin expression during the acidic FGF-induced dispersion of a rat bladder carcinoma cell line. Exp. Cell Res. 1992;201:347–357. doi: 10.1016/0014-4827(92)90283-e. [DOI] [PubMed] [Google Scholar]
- Cavallo R, Rubenstein D, Peifer M. Armadillo and dTCF: a marriage made in the nucleus. Curr. Opin. Genet. Dev. 1997;7:459–466. doi: 10.1016/s0959-437x(97)80071-8. [DOI] [PubMed] [Google Scholar]
- El-Bahrawy M, Poulsom R, Jeffery R, Talbot I, Alison MR. The expression of E-cadherin and catenins in sporadic colorectal carcinoma. Hum. Pathol. 2001;32:1216–1224. doi: 10.1053/hupa.2001.28948. [DOI] [PubMed] [Google Scholar]
- El-Bahrawy M, Talbot I, Poulsom R, Alison M. Variable nuclear localization α-catenin in colorectal carcinoma. Lab. Invest. 2002b;82:1167–1174. doi: 10.1097/01.lab.0000028821.41246.6a. [DOI] [PubMed] [Google Scholar]
- El-Bahrawy M, Talbot I, Poulsom R, Jeffery R, Alison M. The expression of E-cadherin and catenins in colorectal tumours from familial adenomatous polyposis patients. J. Pathol. 2002a;198:69–76. doi: 10.1002/path.1168. [DOI] [PubMed] [Google Scholar]
- Grindstaff KK, Bacallao RL, Nelson WJ. Apiconuclear organization of microtubules does not specify protein delivery from the trans-Golgi network to different membrane domains in polarized epithelial cells. Mol. Biol. Cell. 1998;9:685–699. doi: 10.1091/mbc.9.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Wong MH, Gordon JI. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 1996;10:985–996. doi: 10.1101/gad.10.8.985. [DOI] [PubMed] [Google Scholar]
- Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc. Natl. Acad. Sci. USA. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Beta-catenin: a common target for the regulation of cell adhesion by Wnt-1 and Src signaling pathways. Trends Biochem. Sci. 1994a;19:538–542. doi: 10.1016/0968-0004(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J. Cell Biol. 1994b;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J. Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF. Beta-catenin mutations in cell lines established from human colorectal cancers. Proc. Natl. Acad. Sci. USA. 1997;94:10330–10334. doi: 10.1073/pnas.94.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M, Ochiai A, Akimoto S, Kitano S, Hirohashi S. Alteration of beta-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res. 1996;56:2213–2217. [PubMed] [Google Scholar]
- Kawanishi J, Kato J, Sasaki K, Fujii S, Watanabe N, Niitsu Y. Loss of E-cadherin-dependent cell–cell adhesion due to mutation of the beta-catenin gene in a human cancer cell line, HSC-39. Mol. Cell. Biol. 1995;15:1175–1181. doi: 10.1128/mcb.15.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kraus C, Liehr T, Hulsken J, et al. Localization of the human beta-catenin gene (CTNNB1) to 3p21: a region implicated in tumor development. Genomics. 1994;23:272–274. doi: 10.1006/geno.1994.1493. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, et al. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J. Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert M, Dobbs M, Scambler P, et al. The gene for familial polyposis coli maps to the long arm of chromosome 5. Science. 1987;238:1411–1413. doi: 10.1126/science.3479843. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Nagase H, Ando H, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum. Mol. Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence beta-catenin in vivo and promotes hyperphosphorylation of the adenomatous polyposis coli tumor suppressor protein. Mol. Cell. Biol. 1996;16:4088–4094. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S. The 102 kDa cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nathke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J. Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Shore EM, Wang AZ, Hammerton RW. Identification of a membrane–cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin–Darby canine kidney epithelial cells. J. Cell Biol. 1990;110:349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer CA, Miller PJ, Diehl RE, Kral AM. Identification of Tcf4 residues involved in high-affinity beta-catenin binding. Biochem. Biophys. Res. Commun. 1999;256:584–590. doi: 10.1006/bbrc.1999.0379. [DOI] [PubMed] [Google Scholar]
- Oyama T, Kanai Y, Ochiai A, et al. A truncated beta-catenin disrupts the interaction between E-cadherin and alpha-catenin: a cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res. 1994;54:6282–6287. [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Molecular organization of the uvomorulin–catenin complex. J. Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar M, Nelson WJ. Kinetics of desmosome assembly in Madin–Darby canine kidney epithelial cells: temporal and spatial regulation of desmoplakin organization and stabilization upon cell–cell contact. I. Biochemical analysis. J. Cell Biol. 1988;106:677–685. doi: 10.1083/jcb.106.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SM, Zilz N, Beazer Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Prieve MG, Waterman ML. Nuclear localization and formation of beta-catenin–lymphoid enhancer factor 1 complexes are not sufficient for activation of gene expression. Mol. Cell. Biol. 1999;19:4503–4515. doi: 10.1128/mcb.19.6.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MP, Hyatt JL. Establishment of the circumferential actin filament network is a prerequisite for localization of the cadherin–catenin complex in epithelial cells. Cell Growth Differ. 1999;10:839–854. [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, et al. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P. The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin. J. Biol. Chem. 1995;270:5549–5555. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- Simcha I, Shtutman M, Salomon D, et al. Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin. J. Cell Biol. 1998;141:1433–1448. doi: 10.1083/jcb.141.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Johnson KA, Bryan TM, et al. The APC gene product in normal and tumor cells. Proc. Natl. Acad. Sci. USA. 1993;90:2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Levy DB, Maupin P, Pollard TD, Vogelstein B, Kinzler KW. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–3675. [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell–cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell–cell adhesion. Annu. Rev. Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tamm I, Cardinale I, Kikuchi T, Krueger JG. E-cadherin distribution in interleukin 6-induced cell–cell separation of ductal breast carcinoma cells. Proc. Natl. Acad. Sci. USA. 1994;91:4338–4342. doi: 10.1073/pnas.91.10.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell–cell association and retardation of growth by activation of the E-cadherin–catenin adhesion system in a dispersed carcinoma line. J. Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Jensen PJ. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E-cadherin. J. Cell Biol. 1992;117:415–425. doi: 10.1083/jcb.117.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Stevenson BR, Keast JR, Manley SW. Cadherin-mediated adhesion and apical membrane assembly define distinct steps during thyroid epithelial polarization and lumen formation. Endocrinology. 1995;136:4672–4680. doi: 10.1210/endo.136.10.7664688. [DOI] [PubMed] [Google Scholar]