Abstract
A study has been carried out in the apolipoprotein (apo) E-deficient mouse to investigate the activity of lacidipine (a calcium antagonist with antioxidant properties) in inhibiting the development of atherosclerotic lesions; of particular interest were changes in the susceptibility of low-density lipoproteins (LDL) to oxidation. Mice receiving a Western-type diet to accelerate the development of atherosclerosis were treated orally with vehicle or lacidipine at 3 or 10 mg/kg/day for 8 weeks. Lacidipine treatment (at 3 or 10 mg/kg) had no effect on the plasma lipid profile. However, a significant (P < 0.01) dose-related reduction of 43 and 50% of the aortic lesion area in respect to vehicle-treated mice was observed. Moreover, the resistance of mouse plasma LDL to undergo lipid peroxidation was significantly (P < 0.01) increased in apo E-deficient mice treated with lacidipine. The native LDL-like particle, derived from apo E-deficient mice treated with lacidipine, contained significantly lower concentrations of malonyldialdehyde than the vehicle-treated control group (P < 0.01). After exposure to human umbilical vein endothelial cells, LDL-like particle vitamin E levels (expressed as area under the curve; AUC), were significantly higher (P < 0.01) in both the 3 and 10 mg/kg lacidipine-treated groups, in comparison with the vehicle-treated control animals. We conclude that lacidipine reduced the extent of the atherosclerotic area in hypercholesterolaemic apo E-deficient mice, and that this reduction may be associated with the capacity of the drug to decrease the susceptibility of LDL to oxidation.
Keywords: antioxidant, apo E-deficient mice, atherosclerosis, dihydropiridine, high-fat diet, lacidipine, oxidized LDL
Several lines of evidence from both in vitro and in vivo studies suggest that the oxidation of low-density lipoprotein (LDL) plays an important role in the pathogenesis of atherosclerosis, although the reasons for this are not fully understood (Avogaro et al. 1988; Boyd et al. 1989; Steinberg et al. 1989; Rosenfeld 1991; Wiklund et al. 1991). Oxidatively modified LDL is taken up by macrophages at an increased rate, in comparison to native LDL, and thus promotes cellular cholesterol accumulation and foam cell formation, which are the hallmarks of early atherosclerotic lesions (Steinberg 1997). Moreover, the oxidation process can induce the expression of adhesion molecules and facilitate transcription factor expression, mechanisms which play an important role in the further development of atherosclerosis (Cominacini et al. 1997a). LDL oxidation, occurring inside the artery wall, can be inhibited by many defence systems such as antioxidants in plasma, if these molecules are preserved, and able to carry out such activity (Steinberg et al. 1989). Because oxidized LDL cannot be detected in the circulation, considerable research has recently involved the measurement of the susceptibility of isolated LDL particles to in vitro oxidation (Esterbauer et al. 1987; Cominacini et al. 1991a). Antioxidant supplementation has been shown to reduce the progression of atherosclerotic lesions in Watanabe hyperlipidaemic rabbits (Mao et al. 1991) and to increase the resistance of LDL to oxidation in both healthy and diabetic subjects (Cominacini et al. 1991b; Dieber-Rotheneder et al. 1991; Babiy et al. 1992).
1,4-dihydropyridine (1,4-DHP) calcium channel blockers (CCBs), although markedly varied in their chemical structures and antihypertensive effects, contain aromatic rings capable of stabilizing oxygen radicals, and a hydrogen-donating reaction may also contribute to their antioxidant activity (Napoli et al. 1999). Of the several DHP CCBs, lacidipine has been demonstrated to have antioxidant properties in models of biological membranes by illustrating an activity comparable to the reference antioxidant compound vitamin E (van Amsterdam et al. 1992). In addition, lacidipine has been shown to reduce the extent of atherosclerotic lesions in cholesterol-fed hamsters (Cristofori et al. 2000a), in apolipoprotein (apo) E-deficient mice that fed on a conventional rodent diet (Cristofori et al. 2000b) and in man (Zanchetti et al. 2002). Apo E-deficient mice are characterized by a spontaneous and very pronounced hypercholesterolaemia and by several atherosclerotic features which are typical of lesions found in other animal models of atherosclerosis (Nakashima et al. 1994; Reddick et al. 1994). Furthermore, the LDL of apo E-deficient mice has been demonstrated to be highly susceptible to oxidation (Hayek et al. 1994). The apo E-deficient mouse model could therefore prove to be invaluable in assessing the atherogenic relevance of factors involved in the oxidative modification of lipoprotein (Breslow 1993).
The aim of the present study was to further investigate the effect of lacidipine on the development of atherosclerotic lesions in the apo E-deficient mouse challenged with a high fat (Western-type) diet and to evaluate the associated susceptibility of LDL to oxidation under conditions of oxidative stress.
Materials and methods
Animals and animal husbandry
Homozygous, female apo E-deficient mice (GlaxoSmithKline Research and Development, Ware, UK), approximately 6 weeks old at the beginning of the experiments, were used. This colony was established from animals purchased from the Jackson Laboratory, which originated from apo E-deficient mice first engineered at the University of North Carolina in the laboratory of Dr Nobuyo Maeda (Chapel Hill, NC, USA) (Piedrahita et al. 1992; Zhang et al. 1992). The mice were randomly allocated to three groups of 20 animals each (one vehicle-treated control and two lacidipine-treated groups).
Animals were housed three per cage on wood shavings and fed a Western-type diet (Adjusted Calories Diet, Harlan Tekland TD88137, Madison, WI, USA, containing 42% fat from milk fat and 0.15% cholesterol). Diet and drinking water were available ad libitum. Animals were observed daily, and body weights and food intake were determined weekly. The research complied with national legislation and was performed under a Project Licence obtained according to Italian law (art.7, Legislative Decree number 116, 27 January 1992) which acknowledges European Directive 86/609/EEC. The studies are also in accord with the GlaxoSmithKline policy on the Care and Use of Animals, and also complied with related codes of practice.
Administration of lacidipine
Mice were orally dosed, by gavage, once daily for 8 weeks, with lacidipine (GlaxoSmithKline) at 3 or 10 mg/kg body weight, or vehicle (0.5% methylcellulose; methocel, Sigma Chemical Co., Milan, Italy) at a standard dose volume of 10 ml/kg body weight. In apo E-deficient mice, the 3 mg/kg lacidipine dose level induced plasma drug levels comparable to that achieved with the human therapeutic dose of 4 mg/kg (Zanchetti et al. 2002). The 10 mg/kg dose was chosen in this study to maximize any effect of the treatment.
Biochemical measurements
After the 8-week lacidipine treatment period, mice were fasted overnight and sacrificed under pentobarbital anaesthesia (60 mg/kg, i.p.) by withdrawal of blood from the vena cava. Blood was anti-coagulated with 1 mm tripotassium ethylenediaminetetraacetic acid (EDTA) and centrifuged at 1900 g for 10 min at 4 °C. Total plasma cholesterol and triglyceride levels were determined on fresh plasma samples using an automated enzymatic technique (Boehringer Mannheim GmBH, Monza, Milan, Italy). Separation of total lipoprotein was performed by sequential density ultracentrifugation with a TLA-100.2 rotor (Optima TL Ultracentrifuge, Beckman Instruments, Palo Alto, CA, USA) in NaBr solution at a final density of 1.019, 1.063 and 1.210 for very low-density lipoprotein (VLDL), LDL and high-density lipoprotein (HDL), respectively, with a total centrifugation time of 5 h at 16 °C (Cristofori et al. 2000b).
LDL-like particles (d = 1.006–1.063) were isolated from pooled plasma by sequential-density ultracentrifugation, as previously described (Hayek et al. 1994). In particular, for each dose-level group, six pooled samples of 2.5 ml each were used. Each pooled sample was the result of mixing the plasma from three or four mice. To minimize LDL oxidation during the isolation process, all solutions were deoxygenated by bubbling with argon during the isolation. LDL was stored in the dark under nitrogen at 4 °C, under sterile conditions, and used within 3 days. Immediately before the oxidation incubations, LDL was separated from EDTA and from diffusible low molecular mass compounds by gel filtration on PD-10 Sephadex G-25 m gel (Pharmacia, Upsala, Sweden) in 0.01 mol/l phosphate-buffered saline at pH 7.4.
LDLs derived from apo E-deficient mice were oxidized either with copper ions or with human umbilical vein endothelial cells (HUVECs). The method for LDL oxidation with Cu2+ and for the evaluation of LDL susceptibility to oxidation (i.e. evaluation of the length of time of the lag phase) was based on development of fluorescence during copper-catalysed LDL oxidative modification, as previously described (Cominacini et al. 1991a). The hydroperoxide content of native LDL was determined by evaluating the level of malonyldialdehyde (MDA), as reported by Carbonneau et al. (1991). The extent of LDL oxidation was determined as the thiobarbituric acid adduct, using the high-performance liquid chromatography (HPLC) method of Carbonneau et al. (1991). Analytical separations were performed with a Hewlett-Packard 1050 HPLC connected to a reverse phase C18, 15 × 0.46 cm column (Bio-Rad, Hercules, California, USA); the system included a guard column (Microguard System, Bio-Rad) with a Hewlett-Packard spectrophotometric UV-VIS detector at 532 nm. An MDA standard was prepared by dissolving 220.3 mg of 1,1,3,3-tetraethoxypropane (Aldrich-Chemie, Steinheim, Germany) in 100 ml of deionized water to give a 10 mmol/l stock solution. Measurement of protein was carried out using the Pierce BCA protein assay reagent (Smith et al. 1985).
HUVECs were isolated from human umbilical veins as described by Cominacini et al. (1996) and used at passage 2–4. LDL oxidation was carried out by adding 1.5 ml of serum-free F-12 medium (Gibco, Life Technology, Rockville, MD, USA) containing 200 µg/ml protein to each well of HUVECs and incubating for 24 h at 37 °C as previously described (Cominacini et al. 1996). Vitamin E was measured by HPLC with fluorescence detection as previously described (Cominacini et al. 1991a).
Tissue preparation and quantification of atherosclerotic lesions
At the end of the 8-week experimental period, blood was collected and the animals were perfused with 10% buffered formalin for 10 min. The hearts were rapidly dissected and sectioned according to the method of Tangirala et al. (1995a). Using this technique, the heart was sectioned transversely just below the level of the atria, postfixed in 10% buffered formalin overnight and embedded in paraffin. Sequential 7-µm sections were cut from the apex towards the base of the heart until the aortic valve leaflets appeared. From this point, 17 sections of the aortic root for each animal, representing every second serial section, over a distance of about 240 µm, were taken and stained with haematoxylin and eosin (H&E). The extent of the atherosclerotic lesions were measured using a computerized image analysis system made up of the image analysis software and board, as described previously (Cristofori et al. 2000b), and a black and white video camera (Jai; 711.00-CV) mounted on a Leitz Diaplan microscope (original magnification, ×40). Results are expressed as the mean (in µm2) of the data (±SEM) from the 17 sections analysed for each animal. All assessments were carried out by the same operator in a ‘blind’ fashion.
Statistical analysis
Results are expressed as the mean (±SEM.) anova one-way analysis following Dunnett's test was used to compare the differences between the lacidipine groups and the vehicle-treated (control) group, and P < 0.05 was considered significant.
Results
During the 8-week period of lacidipine treatment at 3 and 10 mg/kg, the drug was well tolerated at both dose levels; there were no clinical signs in the animals attributable to the compound. Body weight increase in apo E-deficient vehicle control mice was the same as in the lacidipine-treated groups. The administration of lacidipine did not affect either the plasma total cholesterol triglyceride levels, or the cholesterol distribution in the different lipoprotein fractions (Table 1). Mean levels of total cholesterol were 36.88 ± 0.28 mmol/l in the apo E-deficient control group, and 35.20 ± 0.31 mmol/l and 35.20 ± 0.22 mmol/l in apo E-deficient mice treated with lacidipine at 3 and 10.0 mg/kg/day, respectively.
Table 1.
Plasma lipid profile in control (vehicle-treated) apo E-deficient mice and in mice treated with lacidipine at 3 and 10 mg/kg for 8 weeks
| Dose level of lacidipine (mg/kg) | Total cholesterol* (mmol/l) | Triglyceride* (mmol/l) | VLDL† cholesterol (mmol/l) | LDL† cholesterol (mmol/l) | HDL† cholesterol (mmol/l) |
|---|---|---|---|---|---|
| Control | 36.88 ± 0.28 | 0.87 ± 0.03 | 10.00 ± 0.07 | 1.52 ± 0.02 | 0.22 ± 0.02 |
| 3 | 35.20 ± 0.31 | 1.23 ± 0.07 | 11.30 ± 0.05 | 1.72 ± 0.01 | 0.25 ± 0.02 |
| 10 | 35.20 ± 0.22 | 1.18 ± 0.04 | 12.00 ± 0.03 | 1.43 ± 0.03 | 0.33 ± 0.01 |
Values are expressed as means (±SEM); n = 20 mice in each group.
VLDL, very low-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein. The level of total cholesterol in the three main lipoprotein classes (VLDL, LDL and HDL) was quantified from six pooled plasma samples taken from mice in each dose-level group. Each pooled sample was the result of taking plasma from three or four mice.
Quantification of atherosclerotic lesions
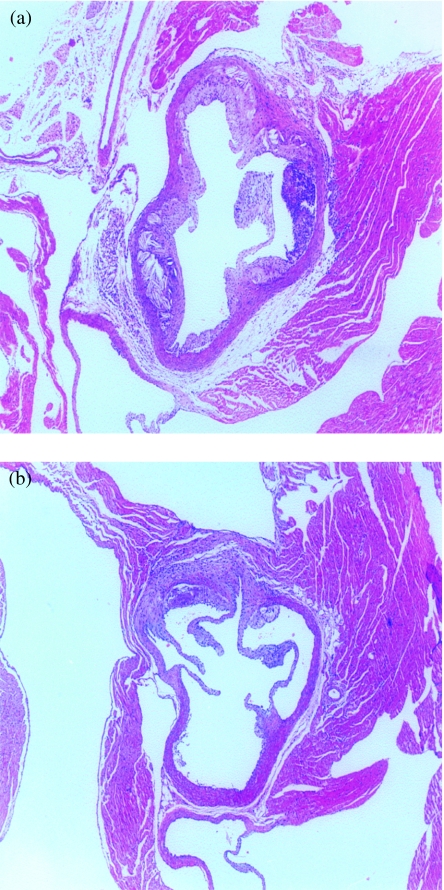
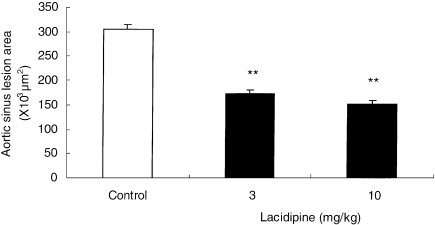
In control apo E-deficient mice, severe atherosclerosis developed during the 8-week period of the study and covered almost the entire aortic surface at the level of the aortic sinus (Figure 1). When the extent of the atherosclerosis was quantified at the aortic root of the heart, a significant dose-related reduction in aortic lesion development was found in mice that were given lacidipine at 3 and 10 mg/kg (P < 0.01 at both dose levels). The average lesion area was 305.5 ± 8.8 × 103µm2 in the apo E-deficient control group (Figure 2), 173.7 ± 6.7 × 103µm2 in animals treated at 3 mg/kg lacidipine and 152.6 ± 7.6 × 103µm2 in the 10 mg/kg lacidipine group (reductions of 43.1% and 50.1% of the control lesion area, respectively). All the results were expressed as mean ± SEM. Histological assessment of the aortic arch demonstrated advanced atherosclerotic disease with plaques showing foamy macrophages, calcification and fibrous caps, a morphology which closely resembles human atherosclerosis. No significant differences in the histological appearance were noted.
Figure 1.
Atherosclerotic lesions in sections of the aortic root of the heart in a control apo E-deficient mouse (a), and in an apo E-deficient mouse receiving 3 mg/kg lacidipine (b) (haematoxylin and eosin-stained; original magnification, ×40). In the control mouse (a) extensive, advanced lesions with a well-defined fibrous cap and a necrotic core with nodular deposits of calcification are present. The volume of the lesions in apo E-deficient mouse treated with lacidipine at 3 mg/kg (b) appears markedly reduced, and the lesion is less complex.
Figure 2.
Effect of lacidipine at 0 (control), 3 and 10 mg/kg on atherosclerotic lesions measured in the aortic root of apo E-deficient mice after 8-week treatment. Results are expressed as mean ± SEM of the lesion area (×103µm2) measured in 17 cross-sections through the aortic root of each mouse, over a distance of 240 µm n = 20 in each group; **P < 0.01, in lacidipine-treated animals compared with the controls.
Susceptibility of mouse LDL to undergo lipid peroxidation
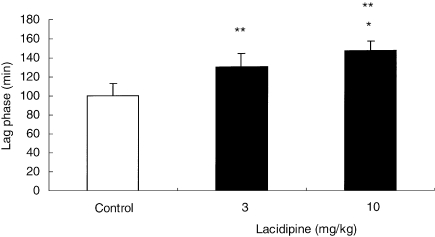
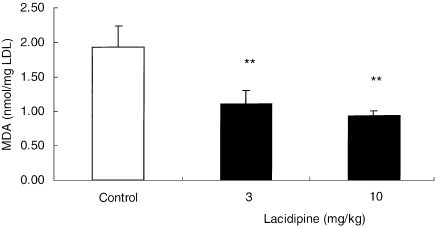
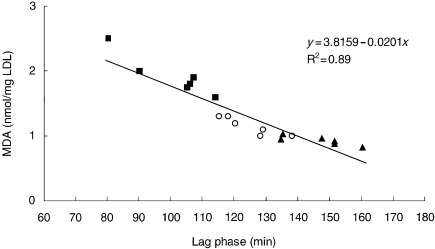
After 8-week treatment with lacidipine at 3 and 10 mg/kg, the susceptibility of mouse LDL to undergo lipid peroxidation following incubation with CuSO4 was significantly reduced (P < 0.01) in comparison with the control group. In the vehicle-treated control group, the lag time required for the initiation of CuSO4-induced LDL oxidation was 100.3 ± 12.7 min (Figure 3). In contrast, in the two lacidipine-treated groups, the time required for LDL oxidation was increased, being 125.8 ± 15.2 min with lacidipine at 3 mg/kg and 146.8 ± 10.0 min at 10 mg/kg. This result paralleled the significantly (P < 0.01) lower concentrations of hydroperoxide content (MDA), detected in the LDL-like particles, derived from both lacidipine-treated groups, compared with the vehicle-treated controls (Figure 4). The mean MDA values, measured in vehicle and lacidipine-treated groups at 3 and 10 mg/kg, were 1.93 ± 0.31, 1.21 ± 0.30 and 0.93 ± 0.07 nmol/mg LDL, respectively. By plotting the lag-phase values detected in individual pooled samples from apo E-deficient mice treated with lacidipine or vehicle with the corresponding MDA values (Figure 5), a significant relationship was identified (R2 = 0.89; P < 0.01).
Figure 3.
The susceptibility of the mouse low-density lipoprotein (LDL) to undergo lipid peroxidation following incubation with CuSO4. LDL-like particles were derived from plasma of apo E-deficient mice after 8-week treatment with vehicle (control) or lacidipine at 3 or 10 mg/kg. Results are expressed as mean ± SEM of the lag phase (min); n = 6 pooled samples in each dose-level group; *P < 0.05 in comparison with the 3 mg/kg lacidipine group; **P < 0.01 in comparison with the control group.
Figure 4.
Effect of lacidipine on the hydroperoxide content of native low-density lipoprotein (LDL). Malondialdheyde (MDA) content in LDL-like particles derived from apoE-deficient mice after 8-week treatment with vehicle (control) or lacidipine at 3 or 10 mg/kg. The results are expressed as mean ± SEM; n = 6 pooled samples in each dose-level group; **P < 0.01 in comparison with the control group.
Figure 5.
Relationship between the hydroperoxide content of native low-density lipoprotein (LDL) [measured as malonyldialdehyde (MDA) in LDL-like particles] and the resistance to oxidation of LDL-like particles (expressed as lag phase in minutes) from the plasma of individual apoE-deficient mice treated with vehicle (control; solid squares) or lacidipine at 3 mg/kg (open circles) or 10 mg/kg (solid triangles). n = 6 pooled samples in each dose-level group.
HUVEC-mediated oxidation of LDL-like particles
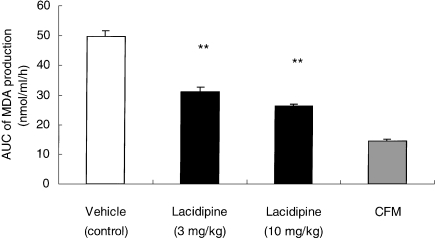
Changes in MDA production and vitamin E content of LDL-like particles derived from apo E-deficient mice were also monitored and quantified during a 24-h incubation period with HUVECs. The amount of MDA formation in the cell-free medium (CFM) over the 24-h period was less than 1 nmol/ml. However, with HUVECs incubated with LDL-like particles from apo E-deficient mice treated with vehicle, a significant increase in MDA production was detected (P < 0.01). The formation of MDA was significantly (P < 0.01) reduced by lacidipine treatment, because lower quantities of MDA were detected from the fourth to the 24th hour of the incubation period. Expressing these results as area under the curve (AUC), calculated by using the mean values of MDA production at various time intervals, during the 24-h incubation, a significant (P < 0.01) dose-related reduction in the lacidipine-treated mice was detected (Figure 6).
Figure 6.
HUVEC-mediated oxidation of low-density lipoprotein (LDL)-like particles, expressed as the area under the curve (AUC, in nmol/ml/h; mean ± SEM) of malonyldialdehyde (MDA) production during a 24-h incubation period. LDL-like particles were derived from the plasma of apo E-deficient mice after 8-week treatment with vehicle, or lacidipine at 3 and 10 mg/kg. A dose-related reduction in AUC values was observed at both dose levels of lacidipine. **P < 0.01 in comparison with the cell-free medium (CFM) and the vehicle control; n = 6 for vehicle control; n = 11 for lacidipine at 3 mg/kg; n = 6 for lacidipine at 10 mg/kg and n = 8 for CFM.
Vitamin E content of LDL-like particles
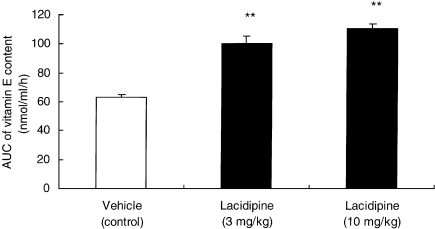
No difference was detected in the basal LDL-like particle concentrations of vitamin E between apo E-deficient mice treated with vehicle or lacidipine (5.43 ± 0.09 µg/mg LDL in control mice vs. 5.85 ± 0.29 and 5.86 ± 0.16 µg/mg LDL protein in the 3 and 10 mg/kg lacidipine-treated groups, respectively). All the results were expressed as mean ± SEM. However, after 24-h exposure to HUVECs, a significant decrease in the LDL-like particle concentration of vitamin E in the vehicle (control) group was observed as, at the end of the incubation period, a value below 15 nmol/ml/h was measured. Nevertheless, in apo E-deficient animals treated with lacidipine at 3 and 10 mg/kg, levels of vitamin E were maintained with concentrations significantly (P < 0.01) higher in both lacidipine-treated groups in comparison with the vehicle-treated control mice. Expressing the data as AUC, calculated as the mean values of vitamin E content, at specific time points during the 24-h incubation period, a significant (P < 0.01) and dose-related preservation of vitamin E levels in the lacidipine-treated mice was demonstrated (Figure 7).
Figure 7.
Vitamin E content, expressed as the area under the curve (AUC, in nmol/ml/h; mean ± SEM) during a 24-h incubation period with human umbilical vein endothelial cells (HUVECs). Low-density lipoprotein (LDL)-like particles were derived from the plasma of apo E-deficient mice after 8-week treatment with vehicle or lacidipine at 3 or 10 mg/kg. Dose-related increases in the AUC values were observed at both dose levels of lacidipine in comparison with the vehicle-treated control group; **P < 0.01; n = 6 for vehicle control; n = 11 for lacidipine at 3 mg/kg and n = 6 for lacidipine at 10 mg/kg.
Discussion
The present study demonstrates that lacidipine treatment significantly reduced the progression of aortic lesions in apo E-deficient mice that fed on a high-fat diet and that this effect was paralleled by a marked decrease in the propensity for the oxidation of plasma LDL, induced by different forms of oxidative stress. However, the anti-atherosclerotic activity of lacidipine in apo E-deficient mice was not caused by a reduction in plasma cholesterol levels. Therefore, the present results tend to reinforce the concept that mode of action of lacidipine on the atherosclerotic processes may be related to the known antioxidant properties of the compound (van Amsterdam et al. 1992). There is now growing evidence of a relationship between the susceptibility of LDL to oxidation and atherosclerotic risk (Regnstrom et al. 1992; Cominacini et al. 1993,1994a,1994b; Sasahara et al. 1994; Cominacini et al. 1997b; Sobal et al. 2001). Oxidatively modified LDL is taken up by macrophages at an increased rate, in comparison to native LDL, and this process can therefore promote cellular cholesterol accumulation and foam cell formation, which are the hallmarks of early atherosclerotic lesions (Steinberg 1997).
Several studies have demonstrated the important role of the inhibition of LDL oxidation in reducing the atherosclerosic process. Angiotensin-converting enzyme (ACE) inhibiting drugs such as captopril (Hayek et al. 1998) and fosinopril (Hayek et al. 1999) have been reported to inhibit LDL oxidation and the atherosclerotic process in apo E-deficient mice. Other potent antioxidative agents, such as vitamin E, are also reported to attenuate atherosclerosis in this animal model (Maor et al. 1997). Similarly, Tangirala et al. (1995b) also demonstrated anti-atherogenic effects in apo E-deficient mice using the antioxidant N,N′-diphenyl-1,4-phenylenediamine (DPPD).
Lacidipine belongs to the class of 1,4-DHP drugs, and an analysis of structural–functional relationships on the oxidative modification of LDL suggests an important role for the 2-substitution of the phenyl ring (Napoli et al. 1999). In addition, calcium antagonists of the DHP type are highly lipophilic and therefore their antioxidative effects could also be explained by hypothesizing a direct interaction with plasma lipoproteins (Rojstaczer & Triggle 1996).
Vitamin E is the major lipid-soluble antioxidant present in blood. It acts synergistically with other circulating and cellular antioxidants to protect cells from damage and lysis induced by oxidative stress. Most of the vitamin E in blood plasma is present in the LDL fraction, and hence vitamin E is optimally placed to prevent free-radical-mediated modification of this lipoprotein. In the present study, the amount of vitamin E in apo E-deficient mice treated with lacidipine, and in vehicle-treated control groups, was measured. Significant differences in the basal LDL-like particle concentrations of vitamin E were found between the apo E-deficient mice treated with lacidipine and the vehicle-treated control group. The levels of vitamin E were maintained in animals treated with lacidipine at both the 3 and 10 mg/kg dose levels, because the vitamin E concentrations were significantly (P < 0.01) higher in both lacidipine-treated groups in comparison with the controls. This could mean that lacidipine may have reduced the progression of aortic atherosclerosis by lengthening the LDL-lag phase and working as a radical scavenger. This concept is consistent with the classic kinetic model proposed by Niki for lipid peroxidation (Niki 1987), where the length of the lag phase is directly related to the amount of antioxidant contained in the lipoprotein. Another possibility in explaining the effect of lacidipine on LDL-lag phases in apo E-deficient mice derives from the scheme proposed by Esterbauer et al. (1989) and by Yoshida & Niki (1992). According to these authors, the role of the copper in the oxidative process of LDL is to catalyse the conversion of trace amounts of preformed lipid hydroperoxides to alkoxy and peroxy radicals, which in turn start another lipid peroxidation reaction. In our study, hydroperoxides were reduced in the LDL-like particles of apo E-deficient mice treated with lacidipine, in comparison with the vehicle-treated control group, and there was a direct correlation between the hydroperoxide concentrations in LDL and the length of lag phase. Therefore, lacidipine may have lengthened the LDL-lag phase also by reducing the LDL content of the lipid hydroperoxides.
In the present study, the antioxidative properties of lacidipine were also assessed in the in vitro model of LDL oxidation obtained by incubation with HUVECs, and interesting results were obtained. It is well known that the process of LDL modification induced by such endothelial cells in vitro closely resembles the corresponding process in vivo. Although cell-mediated oxidative modification of LDL has been the subject of several studies in recent years, the mechanism by which cells initiate LDL oxidation still remains unclear. Both extracellular superoxide radicals (Cathcart et al. 1989) and lipoxygenase activity (Cathcart et al. 1991; Chamulitrat et al. 1991) have been proposed. Attention has also focused on the reaction of superoxide with nitric oxide (Bruckdorfer 1993). Nitric oxide can react with superoxide to form the peroxynitrite anion. Decomposition of the peroxynitrite anion generates a strong oxidant with reactivity similar to the hydroxyl radical, which has been shown to initiate lipid peroxidation (Bruckdorfer 1993). Whichever mechanism of oxidation may be involved, this led to the initial hypothesis that the action of modifying cells is to accelerate the formation of lipid peroxides within the LDL particle. In the present study, the reduction in the content of hydroperoxides found in LDL-like particles of apo E-deficient mice treated with lacidipine may therefore also be the result of the action of the drug on endothelial cells. This conclusion is consistent with the demonstrated inhibitory effect of lacidipine on the generation of reactive oxygen species induced by pro-oxidant stimuli in endothelial cells (Cominacini et al. 1998). The results of the present study also show that LDL vitamin E consumption during HUVEC oxidation was reduced in apo E-deficient mice treated with lacidipine, in comparison with the vehicle-treated control group. Although the mechanism underlying this effect of lacidipine on vitamin E is not clear, one hypothesis was proposed by Thomas et al. (1995) which was that lacidipine acts as co-antioxidant.
In conclusion, in the present study, it has been demonstrated that lacidipine reduces the extent of the atherosclerotic lesion in the hypercholesterolaemic apo E-deficient mouse with a decrease in the susceptibility of LDL to oxidation. These effects of lacidipine may be related to the reducing property of the molecule, as well as the capacity of the drug to interfere in the cellular production of peroxidative products.
Acknowledgments
We thank Miss Sara Munaro and Mrs Monica Bonato, Daniela Spagnolo and Valentina Zantedeschi for their excellent technical assistance.
References
- van Amsterdam FTM, Roveri A, Maiorino M, Ratti E, Ursini F. Lacidipine: a dihydropyridine calcium antagonist with antioxidant activity. Free Radic. Biol. Med. 1992;12:183–187. doi: 10.1016/0891-5849(92)90025-c. [DOI] [PubMed] [Google Scholar]
- Avogaro P, Bittolo Bon G, Cazzolato G. Presence of a modified low density lipoprotein in humans. Atherosclerosis. 1988;8,:79–87. [PubMed] [Google Scholar]
- Babiy AV, Gebicky JM, Sullivan DR. Increased oxidazibility of plasma lipoproteins in diabetic patients can be decreased by probucol therapy and is not due to glycation. Biochem. Pharmacol. 1992;43:995–1000. doi: 10.1016/0006-2952(92)90604-h. [DOI] [PubMed] [Google Scholar]
- Boyd HC, Gown AM, Wolfbauer G, Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am. J. Pathol. 1989;135:815–824. [PMC free article] [PubMed] [Google Scholar]
- Breslow J. Transgenic mouse models of lipoprotein metabolism and atherosclerosis. Proc. Natl. Acad. Sci. USA. 1993;90:8314–8318. doi: 10.1073/pnas.90.18.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckdorfer KR. Non-enzymatic oxidation of lipids lipoproteins: the role of metals and nitric oxide. Curr. Opin. Lipidol. 1993;4:238–243. [Google Scholar]
- Carbonneau MA, Peuchant E, Sess D, Canioni P, Clerc M. Free and bound malonyldialdehyde measured as thiobarbituric acid adduct by HPLC. Clin. Chem. 1991;37:1423–1429. [PubMed] [Google Scholar]
- Cathcart MA, McNally AK, Chisholm GM. Lipoxygenase-mediated transformation of human low density lipoprotein to an oxidized cytotoxic complex. J. Lipid Res. 1991;32:63–70. [PubMed] [Google Scholar]
- Cathcart MA, McNally AK, Morel DW, Chisholm GM. Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein to a cytotoxin. J. Immunol. 1989;142:1963–1969. [PubMed] [Google Scholar]
- Chamulitrat W, Hughes MF, Eling TE, Mason RP. Superoxide and peroxy radical generation from the reduction of polynsaturated fatty acid hydroperoxides by sobean lipoxygenase. Arch. Biochem. Biophys. 1991;290:153–159. doi: 10.1016/0003-9861(91)90601-e. [DOI] [PubMed] [Google Scholar]
- Cominacini L, Garbin U, Cenci B, et al. Predisposition to LDL oxidation during copper-catalyzed oxidative modification and its relation to alpha-tocopherol content in humans. Clin. Chim. Acta. 1991b;204:57–68. doi: 10.1016/0009-8981(91)90217-z. [DOI] [PubMed] [Google Scholar]
- Cominacini L, Garbin U, Davoli A, et al. A simple test for predisposition to LDL oxidation based on the fluorescence development during copper-catalyzed oxidative modification. J. Lipid Res. 1991a;32:349–358. [PubMed] [Google Scholar]
- Cominacini L, Garbin U, De Santis A, Campagnola M, Davoli A, Fratta Pasini A. Mechanisms involved in the in vitro modification of low density lipoprotein by human umbilical vein endothelial cells and copper ions. J. Lipid Med. 1996;13:19–33. doi: 10.1016/0929-7855(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Cominacini L, Garbin U, Fratta Pasini A, et al. Antioxidants inhibit the expression of intracellular cell adhesion molecole-1 and vascular cell adhesion molecole-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic. Biol. 1997a;22:117–127. doi: 10.1016/s0891-5849(96)00271-7. [DOI] [PubMed] [Google Scholar]
- Cominacini L, Garbin U, Fratta Pasini A, et al. Oxidized low-density lipoprotein increases the production of intracellular reactive oxygen species in endothelial cells: inhibitory effect of lacidipine. J. Hypertens. 1998;16:1913–1919. doi: 10.1097/00004872-199816121-00010. [DOI] [PubMed] [Google Scholar]
- Cominacini L, Garbin U, Pastorino AM, et al. Effects of troglitazone on in vitro oxidation of LDL and HDL induced by copper ions and endothelial cells. Diabetologia. 1997b;40:165–172. doi: 10.1007/s001250050658. [DOI] [PubMed] [Google Scholar]
- Cominacini L, Garbin U, Pastorino AM, et al. Predisposition to LDL oxidation in patients with and without angiographically established coronary artery disease. Atherosclerosis. 1993;99:63–70. doi: 10.1016/0021-9150(93)90051-u. [DOI] [PubMed] [Google Scholar]
- Cominacini L, Garbin U, Pastorino AM, et al. Increased susceptibility of LDL to in vitro oxidation in patients with insulin-dependent and non-insulin-dependent diabetes mellitus. Diabetes Res. 1994a;26:173–184. [PubMed] [Google Scholar]
- Cominacini L, Pastorino AM, Garbin U, et al. The susceptibility of LDL to in vitro oxidation is increased in hypercholesterolemic patients. Nutrition. 1994b;10:527–531. [PubMed] [Google Scholar]
- Cristofori P, Lanzoni A, Gaviraghi G, Turton J, Sbarbati A. Anti-atherosclerotic activity of the calcium antagonist lacidipine in cholesterol-fed hamsters. Biomed. Pharmacother. 2000a;54:93–99. doi: 10.1016/S0753-3322(00)88858-7. [DOI] [PubMed] [Google Scholar]
- Cristofori P, Lanzoni A, Quartaroli M, et al. The calcium-channel blocker lacidipine reduces the development of atherosclerotic lesions in the apoE-deficient mouse. J. Hypertens. 2000b;18:1429–1436. doi: 10.1097/00004872-200018100-00010. [DOI] [PubMed] [Google Scholar]
- Dieber-Rotheneder M, Puhl H, Waeg G, Striegl G, Esterbauer H. Effect of oral supplementation with alpha-tocopherol on the vitamin E content of human low density lipoproteins and resistance to o"xidation. J. Lipid Res. 1991;32:1325–1332. [PubMed] [Google Scholar]
- Esterbauer H, Jugens G, Quehenberger O, Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J. Lipid Res. 1987;28:495–509. [PubMed] [Google Scholar]
- Esterbauer H, Rotheneder M, Striegl G, et al. Vitamin E and other lipophilic antioxidants protect LDL against oxidation. Fat. Sci. Technol. 1989;91:16–324. [Google Scholar]
- Hayek T, Attias J, Coleman R, et al. The angiotensin-converting enzyme inhibitor, fosinopril, and the angiotensin II receptor antagonist, lasortan inhibit LDL oxidation and attenuate atherosclerosis independent of lowering blood pressure in apolipoprotein E deficient mice. Cardiovasc. Res. 1999;44:579–587. doi: 10.1016/s0008-6363(99)00239-4. [DOI] [PubMed] [Google Scholar]
- Hayek T, Attias J, Smith J, Breslow JL, Keidar S. Antiatherosclerotic and antioxidative effects of captopril in apolipoprotein E-deficient mice. J. Cardiovasc. Pharmacol. 1998;31:540–544. doi: 10.1097/00005344-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Hayek T, Oiknine J, Brook JG, Aviram M. Increased plasma and lipoprotein lipid peroxidation in apo E deficient mice. Biochem. Biophys. Res. Commun. 1994;201:1567–1574. doi: 10.1006/bbrc.1994.1883. [DOI] [PubMed] [Google Scholar]
- Mao SJ, Yates MT, Rechtin AE, Jackson RI, Van Sicule WA. Antioxidant activity of probucol and its analogues in hypercholesterolemic Watanabe rabbits. J. Med. Chem. 1991;34:298–302. doi: 10.1021/jm00105a046. [DOI] [PubMed] [Google Scholar]
- Maor I, Hayek T, Coleman R, Aviram M. Plasma LDL oxidation leads to its aggregation in the atherosclerotic apo E deficient mouse. Arterioscler. Thromb. Vasc. Biol. 1997;17:2995–3005. doi: 10.1161/01.atv.17.11.2995. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raine EW, Brelow J, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the aortic tree. Arterioscler. Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Napoli C, Salomone S, Godfraind T, et al. 1,4-dihydropyridine calcium channel blockers inhibit plasma and LDL oxidation and formation of oxidation-specific epitopes in the arterial wall and prolong survival in stroke-prone spontaneously hypertensive rats. Stroke. 1999;30:1907–1915. doi: 10.1161/01.str.30.9.1907. [DOI] [PubMed] [Google Scholar]
- Niki E. Antioxidants in relation to lipid peroxidation. Chem. Phys. Lipids. 1987;44:227–253. doi: 10.1016/0009-3084(87)90052-1. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick RI, Zhang SH, Maeda N. Atherosclerosis in mice lacking apoE: evaluation of lesional development and progression. J. Atheroscler. Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Regnstrom JN, Nilsson J, Tornavall P, Landou C, Hamsten A. Susceptibility to low density lipoprotein oxidation and coronary atherosclerosis in man. Lancet. 1992;339:1183–1186. doi: 10.1016/0140-6736(92)91129-v. [DOI] [PubMed] [Google Scholar]
- Rojstaczer N, Triggle DJ. Structure-function relationships of calcium antagonists. Effect on oxidative modification of low density lipoprotein. Biochem. Pharmacol. 1996;51:141–150. doi: 10.1016/0006-2952(95)02162-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME. Oxidized LDL affects multiple atherogenic cellular responses. Circulation. 1991;83:2137–2140. doi: 10.1161/01.cir.83.6.2137. [DOI] [PubMed] [Google Scholar]
- Sasahara M, Raines EW, Chait A, et al. Inhibition of hypercolesterolemia-induced atherosclerosis in the nonhuman primate by probucol. Is the extent of atherosclerosis related to resistance of LDL to oxidation? J. Clin. Invest. 1994;94:155–164. doi: 10.1172/JCI117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia A, Gartner FH, Provenzano AC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;105:293–299. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sobal G, Menzel EJ, Sinzinger H. Calcium antagonists as inhibitors of in vitro low density lipoprotein oxidation and glycation. Biochem. Pharmacol. 2001;61:373–379. doi: 10.1016/s0006-2952(00)00548-7. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modification of low-density lipoprotein that increases its atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Casanada F, Miller E, Witztum J, Steinberg D, Palinski W. Effect of the antioxidant N,N'-diphenylenediamine (DPPD) on atherosclerosis in apo E deficent mice. Arterioscler. Thromb. Vasc. Biol. 1995b;15:1625–1630. doi: 10.1161/01.atv.15.10.1625. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid Res. 1995a;36:2320–2328. [PubMed] [Google Scholar]
- Thomas ST, Neuzil J, Mohr D, Stocker R. Coantioxidants make alpha-tocopherol and efficient antioxidant for low density lipoprotein. Am. J. Clin. Nutr. 1995;62:1357–1364. doi: 10.1093/ajcn/62.6.1357S. [DOI] [PubMed] [Google Scholar]
- Wiklund O, Mattson L, Bjornheden T, Camejo G, Bondjers G. Uptake and degradation of low density lipoproteins in atherosclerotic rabbit aorta: role of local LDL modification. J. Lipid Res. 1991;32:55–62. [PubMed] [Google Scholar]
- Yoshida Y, Niki E. Oxidation of methyl linoleate in acqueous dispersions induced by copper and iron. Arch. Biochem. Biophys. 1992;295:107–114. doi: 10.1016/0003-9861(92)90494-h. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Gene Bond M, Hennig M, et al. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis. Circulation. 2002;106:2422–2427. doi: 10.1161/01.cir.0000039288.86470.dd. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercolesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]