Abstract
Transforming growth factor-β (TGF-β) plays a central role in fibrosis, contributing to the influx and activation of inflammatory cells, the epithelial to mesenchymal transdifferentiation (EMT) of cells and the influx of fibroblasts and their subsequent elaboration of extracellular matrix. TGF-β signals through transmembrane receptor serine/threonine kinases to activate novel signalling intermediates called Smad proteins, which modulate the transcription of target genes. The use of mice with a targeted deletion of Smad3, one of the two homologous proteins which signals from TGF-β/activin, shows that most of the pro-fibrotic activities of TGF-β are mediated by Smad3. Smad3 null inflammatory cells and fibroblasts do not respond to the chemotactic effects of TGF-β and do not autoinduce TGF-β. The loss of Smad3 also interferes with TGF-β-mediated induction of EMT and genes for collagens, plasminogen activator inhibitor-1 and the tissue inhibitor of metalloprotease-1. Smad3 null mice are resistant to radiation-induced cutaneous fibrosis, bleomycin-induced pulmonary fibrosis, carbon tetrachloride-induced hepatic fibrosis as well as glomerular fibrosis induced by induction of type 1 diabetes with streptozotocin. In fibrotic conditions that are induced by EMT, such as proliferative vitreoretinopathy, ocular capsule injury and glomerulosclerosis resulting from unilateral ureteral obstruction, Smad3 null mice also show an abrogated fibrotic response. Animal models of scleroderma, cystic fibrosis and cirrhosis implicate involvement of Smad3 in the observed fibrosis. Additionally, inhibition of Smad3 by overexpression of the inhibitory Smad7 protein or by treatment with the small molecule, halofuginone, dramatically reduces responses in animal models of kidney, lung, liver and radiation-induced fibrosis. Small moleucule inhibitors of Smad3 may have tremendous clinical potential in the treatment of pathological fibrotic diseases.
Keywords: fibrosis, halofuginone, Smad3, Smad7, TGF-β, wound healing
Transforming growth factor-β (TGF-β), a 112 amino acid homodimeric protein, was first identified as a factor inducing anchorage-independent growth of normal rat kidney cells. It is now considered to be the quintessential multifunctional cytokine regulating a variety of important biological responses including cell growth and differentiation, apoptosis, cell migration, immune cell function and extracellular matrix (ECM) production (Roberts & Sporn 1990; Massague 1998). The multiple biological actions of TGF-β contribute to the central role that it plays in many fibrotic diseases including cirrhosis, chronic hepatitis, glomerulonephritis, scleroderma and pulmonary fibrosis (Border & Noble 1994). Many fibrotic pathologies are associated with increased levels of TGF-β which initially recruit inflammatory cells and fibroblasts into an area of injury and then stimulate these cells to produce cytokines and extracellular matrix, respectively. As TGF-β not only increases synthesis of matrix proteins but also enhances secretion of protease inhibitors while reducing secretion of proteases, it is a potent stimulator of matrix accumulation (Roberts & Sporn 1996). While the responses elicited by TGF-β play a role in the normal physiology of tissue repair following injury, too often this process does not properly resolve and chronic pathological conditions result.
Mammals express three highly homologous TGF-β isoforms (TGF-βs 1, 2 and 3) which often have similar biological activities in vitro, while eliciting distinct biological responses in vivo. Mice in which the gene for each of the TGF-β ligands has been knockedout show different phenotypes (Letterio & Roberts 1996). Additionally, as platelets are an abundant source of TGF-β1, this isoform is released by degranulating platelets at the site of an injury; hence, TGF-β1 is the isoform thought to play the most significant role in wound healing and possible subsequent fibrosis (Roberts & Sporn 1996). TGF-β also is the founding member of what is now a large superfamily consisting of over 40 ligands including activins, inhibins, bone morphogenetic proteins (BMPs) and the growth and differentiation factors (GDFs) (Kingsley 1994). Many TGF-β family members signal through cell-surface serine/threonine kinase receptors. A family of proteins designated as Smads transduces the ligand signal from the cell surface to the nucleus. This review will focus on the role of Smad3, a mediator of TGF-β signalling, in regulating the fibrotic response, including discussions of Smad3-dependent gene regulation, its contribution to fibrosis in a number of organs and possible therapeutic strategies for blocking unwanted fibrosis.
Signalling through Smads
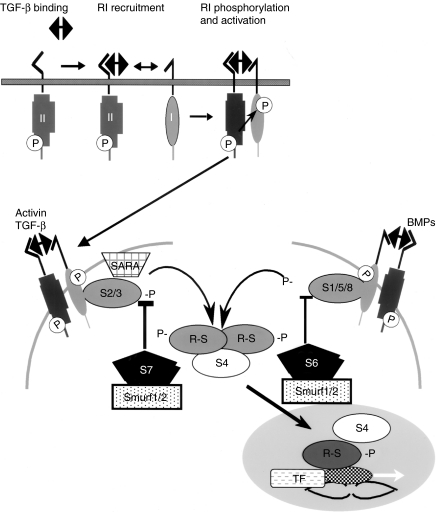
The name Smad is derived from a contraction of the names of TGF-β-like ligand-signalling intermediates first identified in Drosophila (Mad) and Caenorhabditis elegans (Sma). The eight mammalian Smads are grouped into three subfamilies, the five receptor-activated Smads (R-Smads), the one common mediator Smad (Co-Smad) and the two inhibitory Smads (I-Smads) (Moustakas et al. 2001; Derynck & Zhang 2003; Shi & Massague 2003). Of the R-Smads, Smads 2 and 3 signal for TGF-β and activin, while Smads 1, 5 and 8 transduce signals from BMP ligands (Figure 1). For TGF-β signalling, ligand binding to the constitutively active ser/thr kinase Type II receptor recruits the Type I receptor into the complex where it is phosphorylated by the Type II receptor resulting in its activation. Smads 2 and 3 are recruited to the activated Type I receptor by SARA (Smad anchor for receptor activation) and are directly phosphorylated by the Type I TGF-β receptor kinase on the last two serines of a conserved SSXS motif located at the extreme carboxyl terminus of the R-Smads. The phosphorylated R-Smad is released from the receptor complex to form a heteromeric complex of two R-Smads and the co-Smad (Smad4), and this complex translocates to the nucleus where it can interact with various transcription factors and affect transcriptional responses. The I-Smads (Smad 6 for the BMP pathway and Smad7 for the TGF-β/activin pathway) function by binding to the Type I receptor and preventing recruitment and phosphorylation of R-Smads. The I-Smads also bring the E3 ubiquitin ligases Smurfs 1 and 2 (Smad ubiquitination regulatory factors 1 and 2) to the Type I receptor which subsequently ubiquitinate and degrade the receptor.
Figure 1.
Overview of the transforming growth factor-β (TGF-β)/Smad-signalling pathway. At the cell surface, binding of TGF-β ligand to the constitutively active Type II receptor recruits the Type I receptor into the complex where it is phosphorylated. The activated Type I receptor then phosphorylates Smad 2 or 3 which are recruited there by SARA (Smad anchor for receptor activation) at the C-terminal serines. Activin also phosphorylates Smads 2/3, while BMPs phosphorylate Smads 1/5/8. The receptor-activated Smads then complex with the common mediator Smad4 and this complex translocates to the nucleus where it regulates transcription of target genes and binds to a variety of transcription factors (TFs). Activation of R-Smads by Type I receptor kinases is inhibited by Smad6 for the BMP pathway and Smad7 for the TGF-β/activin pathway. The E3 ubiquitin ligases Smurfs 1 and 2 which degrade the R-Smads also interact with Smads 6/7 and ubiquitinate the Type I receptors.
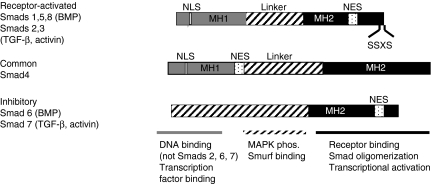
The structural domains of the three Smad subfamilies are shown in Figure 2. R-Smads and the co-Smad contain conserved amino- and carboxyl-terminal MH (mad homology) 1 and 2 domains, respectively, which flank a more divergent proline-rich middle linker region. In I-Smads, the MH1 domain is replaced by a more divergent amino-terminus which does not bind to DNA. The MH1 domain mediates autoinhibition by its interaction with the MH2 domain preventing its phosphorylation in the absence of ligand. The MH1 domain can bind directly to DNA except in the case of the normal splice variant of Smad2 where a 30 amino acid insertion in this domain prevents DNA binding. The minimal Smad-binding element (SBE) contains only four base pairs, 5′-AGAC-3′, but there are reports of binding to other G/C-rich sequences. Protein kinase C phosphorylation of Smads 2 or 3 in this domain abrogates its DNA-binding activity. A nuclear localization signal which is exposed following phosphorylation is also present in the MH1 domain and a variety of transcription factors including c-Jun, SP1, ATF-2 and TFE3 bind here.
Figure 2.
Structural organization and domain function of the three classes of Smad proteins. The overall structure of the receptor-activated and common Smad proteins comprises the conserved MH1 (grey) and MH2 (black) domains and the intervening linker (striped) region. The inhibitory Smads lack the MH1 domain. Receptor-activated Smads are phosphorylated by Type I receptor kinases on the C-terminal SSXS site. The location of proposed nuclear localization and nuclear export signals (NLS and NES, respectively) is shown. The major functions of each domain are listed on the bottom of the figure.
Although the carboxyl-terminal MH2 domain does not bind DNA directly, it is involved in a variety of protein–protein interactions. This domain in R-Smads interacts with the Type I receptor, contains the SSXS phosphorylation site and mediates formation of R-Smad/Smad4 oligomers. Additionally, the MH2 domain contains a nuclear export signal and thus plays a role in Smad nucleocytoplasmic shuttling. The MH2 domain of I-Smads lacks the SSXS motif but does stably bind to activated Type I receptor kinase to prevent phosphorylation of R-Smads. The MH2 domain of both R-Smads and Smad4 interacts with a variety of transcriptional coactivators including p300, CBP and SMIF as well as transcriptional corepressors, such as TGIF, c-ski and SnoN. These negative regulators can interfere with the binding of the MH2 domain to the transcriptional coactivator CBP/p300, with recruitment of histone deacetylases, and can also compete with R-Smad for binding to Smad4 (Shi & Massague 2003).
Although the middle linker regions between the MH1 and MH2 domains are divergent among Smads, this region contains a number of phosphorylation sites which are now appreciated to mediate the observed crosstalk between the Smad pathway and a variety of other signalling mechanisms (Lutz & Knaus 2002; Derynck & Zhang 2003). For example, the Erk subfamily of MAP kinases, which become activated by Ras or by binding of hepatocyte growth factor or epidermal growth factor to tyrosine kinase receptors, can phosphorylate Erk consensus sites (PXSP) in the linker regions of Smads 1, 2 or 3 and inhibit nuclear translocation of the R-Smads. Similarly, Ca2+-calmodulin-dependent protein kinase II phosphorylates Smad2 in the linker region, inhibiting nuclear translocation and signalling. The c-Jun N-terminal kinase can phosphorylate Smad3 at sites outside the C-terminal SSXS motif and enhance nuclear translocation and transcriptional activation. It is important to remember that, in many cases, the effects of phosphorylation are cell-type dependent. For example, ERK inhibition decreases phosphorylation of Smad2/3 in human mesangial cells but does not alter phosphorylation in mouse mammary epithelial NmuMG cells (Hayashida et al. 2003).
The linker region also contains a conserved PY motif that interacts with the WW domains of the Smad-interacting proteins Smurf1 and Smurf2 (Attisano & Tuen Lee Hoeflich 2001) that catalyse ubiqutin-mediated degradation of Smads, some Smad-associated proteins, such as SnoN, and TGF-β receptor complexes themselves. However, the bulk of nuclear Smad2/3 is not targeted for degradation but is dephosphorylated by as yet unidentified phosphatases and then relocated to the cytoplasm.
Smad2 vs. Smad3
While both Smads 2 and 3 mediate signals from TGF-β and activin, these two Smads clearly have nonredundant functions. While Smad2 knockout (KO) mice fail to gastrulate, form mesoderm and establish an anterior–posterior axis leading to death between E7.5 and E8.5 (Nomura & Li 1998; Waldrip et al. 1998; Weinstein et al. 1998), Smad3 KO mice are viable and usually die from defects in mucosal immunity at 1–6 months of age (Datto et al. 1999; Yang et al. 1999). Smad3 KO mice also show diminished T-cell responsiveness to TGF-β and skeletal abnormalities due to defects in differentiation of hypertrophic chondrocytes, resulting in a progressive degenerative cartilage disease (Yang et al. 2001).
As Smad3 binds DNA directly through its MH1 domain and Smad2 instead activates transcription indirectly by binding to transcription factors, one would expect these two Smads to have distinct effects on regulation of target genes. This has been confirmed by showing that mouse embryo fibroblasts (MEFs) derived from either Smad 2 or 3 KO mice show different patterns of gene induction by TGF-β. TGF-β-mediated induction of matrix metalloprotease-2 (MMP-2) was Smad2 dependent, induction of c-fos, Smad7 and TGF-β1 autoinduction were Smad3 dependent and fibronectin induction was independent of either Smad 2 or 3 (Piek et al. 2001). Microarray analyses using these cells and a 9000 gene chip suggests that Smad3 is the essential mediator of TGF-β signalling, because it directly activated genes encoding transcriptional regulators and signal transducers through Smad3/4 DNA-binding motif characteristic of immediate early target genes of TGF-β. Smad2 instead predominantly transmodulated regulation of both immediate early and intermediate genes by TGF-β/Smad3 (Yang et al. 2003). Using antisense oligonucleotides to knockdown expression of Smads 2 or 3 in HaCaT keratinocytes, Kretschmer et al. (2003) showed that TGF-β-induced growth arrest was mediated by Smad3 which modulated the expression of c-Myc, p21Cip1 and the phosphorylation status of Rb. Smad2 was not required for any of these immediate events linked to growth inhibition but was required for TGF-β-induced heme oxygenase-1 expression.
The mechanisms which determine whether TGF-β receptor binding will activate signalling through Smad2 or Smad3 in a particular cell type are not known. Based on the embryonic lethality of Smad2 KO mice, it seems that Smad2 may be more involved in mediating signals during development than Smad3. Also, using Smad2- and Smad3-deficient MEFs, activation of the SBE4-Lux reporter by TGF-β was found to require Smad3, but not Smad2, whereas activation of the activin-response element Lux reporter required Smad2, suggesting specific roles for Smads 2 and 3 in signalling (Piek et al. 2001). Additionally, recent data suggests that phosphorylation of Smads 2 and 3 does not occur on receptors localized to the plasma membrane but rather in endosomes following clatharin-dependent endocytosis (Hayes et al. 2002; Penheiter et al. 2002). Data demonstrating independent phosphorylation of Smads 2 and 3 suggest that they may be phosphorylated by distinct receptor pools in different subdomains of endosomes, as has been shown for the Smad2/3-binding protein SARA (Hu et al. 2002). A recently discovered protein, TLP (TRAP-1-like protein) (Felici et al. 2003) blocks Smad3-dependent transcription through selective inhibition of Smad3/4 complex formation, while activating Smad2-dependent responses. TLP might be involved in localizing Smad4 to specific receptor/Smad complexes in different endosomal subcompartments, such that it is available to activated Smad2 but not activated Smad3. These results suggest that there may be other accessory proteins involved in regulating the balance of Smad2 vs. Smad3 signalling.
Regulation of extracellular matrix gene expression by Smad3
Accumulation of ECM in fibrotic diseases usually results from elevated mRNA levels of fibrillar collagens due to increased transcriptional activation. TGF-β plays an essential role in modulating ECM gene expression, and a growing body of evidence suggests that this is a Smad3-dependent process. Using differential hybridization of a cDNA expression array containing 265 known ECM-related genes, Verrecchia et al. (2001) identified a number of collagen gene promoters in human dermal fibroblasts which were induced by TGF-β1 and dependent upon Smad3. These included COL1A1, COL1A2, COL3A1, COL5A2, COL6A1 and COL6A3. In all cases, activation of the promoter by TGF-β was blocked by both dominant-negative Smad3 and inhibitory Smad7 expression vectors and promoter transactivation by TGF-β did not occur in Smad3 null MEFs.
The interaction of Smad3 with the human COL1A2 promoter has been the most extensively characterized. A proximal region between −353 and −148 bp contains a SBE CAGA motif and is required for stimulation by TGF-β in fibroblasts (Chen et al. 2000). The involvement of the transcriptional coactivator CBP/p300 is required for maximal activation (Ghosh et al. 2000). Additionally, Sp1 binding to the COL1A2 promoter in the region of −313 to −250 bp cooperates synergistically in transactivating the promoter (Zhang et al. 2000). Similarly, in human glomerular mesangial cells, Sp1 binding is required for Smad3 stimulation of COL1A2 promoter activity in response to TGF-β (Poncelet & Schnaper 2001), and in these cells, TGF-β1-stimulated activation of PKCδ positively regulated Smad transcriptional activity of the COL1A2 promoter (Runyan et al. 2003). The antagonistic effects of interferon-γ (IFN-γ) (Ghosh et al. 2001; Higashi et al. 2003) and tumour necrosis factor-α (TNF-α) (Verrecchia et al. 2003) on TGF-β activation of the COL1A2 promoter also occur through effects on Smad3. The IFN-γ-signalling pathway competes with Smad3 for limited amounts of CBP/p300, while JNK activation by TNF-α somehow blocks Smad3-dependent upregulation of COL1A2 induced by TGF-β. In contrast, TGF-β and TNF-α synergistically enhance expression of COL7A1 in human dermal fibroblasts through binding of RelA and Smad3 to different enhancer elements in the promoter (Kon et al. 1999). There is also evidence that sphingolipid mediators play a role in regulating TGF-β signalling, as cotransfection of Smad3 and sphingosine 1-phosphate phosphatase in fibroblasts caused a dramatic increase in COL1A2 promoter activity (Sato et al. 2003a).
TGF-β contributes to fibrosis not only by enhancing ECM synthesis but also by inhibiting ECM degradation by downregulating expression of matrix-degrading enzymes and increasing expression of MMP inhibitors. Several studies suggest that this too may be a Smad3-dependent process. In dermal fibroblasts, negative regulation of the MMP-1 promoter is mediated by Smad3 (it does not occur in Smad3 null MEFs) (Yuan & Varga 2001) with involvement of an AP-1 site (Hall et al. 2003). In contrast, TGF-β upregulates activity of the plasminogen activator inhibitor-1 (PAI-1) promoter through binding of Smad3 to a CAGA motif (Dennler et al. 1998) with association between Smad3 and Sp1 being required for maximal promoter activation in several cell lines (Datta et al. 2000). The induction of the tissue inhibitor of metalloprotease-1 (TIMP-1) by TGF-β also appears to be Smad3 dependent (Verrecchia et al. 2001).
Role of Smad3 in wound healing
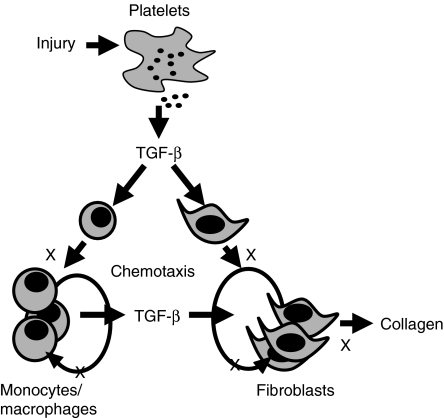
Wound healing has been used to study the effects of loss of Smad3 in vivo, as this model involves the interaction of a variety of cell types within a specific tissue architecture in a defined temporal sequence. TGF-β is produced by all of the cell types involved in the wound healing process, and these cells also can respond to TGF-β (Roberts & Sporn 1996). At the site of a wound, platelet degranulation releases a bolus of TGF-β1 which recruits inflammatory cells and fibroblasts into the area and stimulates these cells to produce TGF-β (Figure 3). In fact, topical application of TGF-β improves wound healing in a variety of animal models of impaired healing (Roberts & Sporn 1996). These observations suggested that deletion of a downstream mediator of TGF-β signalling, such as Smad3, might impair healing. Unexpectedly, cutaneous incisional wounds made in Smad3 KO mice healed more rapidly than those wounds made in Smad3 wildtype (WT) littermate controls (Ashcroft et al. 1999). The incisions made in the Smad3 KO skin re-epithelialized faster than that in the WT skin, as Smad3 null keratinocytes proliferate more rapidly, having reduced sensitivity to growth inhibition by TGF-β. There was reduced influx of inflammatory cells into the wound bed in Smad3 KO mice, as Smad3 null monocytes are impaired in their chemotactic response to TGF-β as well as in their ability to autoinduce TGF-β. There was also decreased accumulation of ECM in the granulation tissue in Smad3 KO mice. The responses of Smad3 heterozygous (HT) mice were approximately midway between those of WT and KO mice, suggesting that the role of Smad3 is limiting in certain responses.
Figure 3.
Proposed role of transforming growth factor-β (TGF-β)/Smad3 in wound healing and fibrosis. Degranulation of platelets releases TGF-β1 which is chemotactic for monocytes and fibroblasts with subsequent autoinduction of TGF-β by these cells and induction of collagen and other matrix proteins by fibroblasts. Processes marked with an X are blocked by loss of Smad3.
Wound contraction, occurring after granulation tissue formation, is necessary to reduce the size of the wound defect, so that eventual closure will occur; however, excessive tissue contraction after injury can lead to debilitating contractile scarring. Myofibroblasts, α-smooth muscle actin (α-SMA)-expressing fibroblasts, are largely responsible for wound contraction. A floating collagen gel with fibroblasts incorporated in it is often used as an in vitro model of wound contraction, and TGF-β treatment augments contraction in this model. It appears that this aspect of wound healing is also Smad3 dependent. Overexpression of Smad3 in the fibroblasts enhanced collagen gel contraction, while Smad7 overexpression suppressed it (Sumiyoshi et al. 2003). Furthermore, TGF-β augmented contraction of Smad2 KO, Smad2 WT and Smad3 WT but not Smad3 KO MEFs (Liu et al. 2003).
Smad3 is an important mediator of pathological fibrotic conditions
Many aspects of wound healing are shared with those of fibrotic disease, including inflammation, angiogenesis and fibrogenesis. In normal physiologic wound healing when sufficient matrix has accumulated in the wound bed, matrix production is terminated and scarring is prevented. If this response fails to resolve or if a tissue is subjected to chronic injury, pathological progressive fibrosis can result. TGF-β has been implicated as being an important mediator in a number of fibrotic diseases, and increased levels of TGF-β are often present in tissues where an uncontrolled fibrotic response occurs. The most direct evidence in the involvement of TGF-β in fibrosis comes from a variety of animal models of fibrotic disease where agents that block TGF-β function reduce the fibrotic response. For example, administration of TGF-β antibodies or antisense oligonucelotides reduces kidney fibrosis in several experimental models, administration of soluble TβRII is beneficial in hepatic and intestinal fibrosis, and TGF-β antibodies prevent skin and lung fibrosis in murine sclerodermatous graft-vs.-host disease (Flanders & Burmester 2003). Diminishing TGF-β function through loss of Smad3 implies that Smad3 KO mice might also be resistant to fibrosis. This hypothesis is supported by the evidence that loss of Smad3 interferes with chemotaxis of macrophages and TGF-β autoinduction (Ashcroft et al. 1999). Autoinduction of TGF-β in inflammatory cells is essential to keep levels of TGF-β ligand high in the wound bed. This TGF-β can then recruit fibroblasts into the area which also autoinduce TGF-β as well as produce collagen. All of these processes appear to be Smad3 dependent (Figure 3). Indeed, there are now a number of reports of animal models of fibrosis where loss of Smad3 results in a diminished fibrotic response. The results of these studies will be summarized below.
Skin
Radiation therapy used to treat malignancies can induce a pathological fibrotic response which compromises patients' quality of life and complicates later surgical intervention. Our laboratory used Smad3 KO mice to investigate its role in radiation-induced fibrosis in the skin. Six weeks after local exposure to 30 Gy of ionizing irradiation, the skin from Smad3 KO mice showed significantly less epidermal acanthosis (Figure 4a–d); dermal influx of mast cells, neutrophils and macrophages and decreased expression of TGF-β than did the irradiated skin of WT littermates (Flanders et al. 2002). The results in Smad3 HT mice were between those of the Smad3 WT and KO. Additionally, the dermis of Smad3 KO skin contained fewer myofibroblasts than did WT skin. As with other types of Smad3-null cells, we found that Smad3 null dermal fibroblasts did not respond to the chemotactic effects of TGF-β as well as their WT counterparts. However, both genotypes responded equally when treated with TGF-β to induce differentiation to myofibroblasts, as judged by α-SMA expression (Flanders et al. 2003).
Figure 4.
Skin of Smad3 KO mice shows less epidermal acanthosis and fibrosis than skin of WT mice 6 weeks after exposure to 30 Gy of γ-irradiation. H&E staining of irradiated WT (a and b) and KO (c and d) skin. Note the enhanced thickness of the epidermis and density of the dermis in irradiated WT compared to KO skin. Original magnification ×100. In (e–h), skin sections were stained with Picrosirius red and photographed under polarized light. The arrow marks the position of the epidermis. Non-irradiated WT (e) and KO (f) skin show similar dermal architecture. In contrast, irradiated WT (g) skin shows thicker collagen fibres with orange–red birefringence indicative of a scarring phenotype, while the architecture of the irradiated KO (h) skin more resembles that of nonirradiated skin. Original magnification ×200.
As Smad3 KO skin contained fewer myofibroblasts, the cells thought to be responsible for the majority of ECM deposition, we proposed that there would be less scarring in Smad3 KO skin as compared to WT skin. This was confirmed by staining tissue sections with Picrosirius red and viewing them under polarized light. This method provides information about both the thickness and organizational pattern of the collagen fibrils (Flanders et al. 2003). Normal dermal architecture, similar in the skin of Smad3 WT and KO mice, was characterized by thin, weakly birefingent yellow-greenish fibres in a basket-weave pattern (Figure 4e,f). In contrast, 6 week after cutaneous irradiation, the dermis of WT (Figure 4g), but not KO skin (Figure 4h), was characterized by the prominent appearance of thicker collagen fibres with an orange–red birefringence, suggestive of a scarring phenotype.
We also saw differences in gene expression patterns in Smad3 WT and KO dermal fibroblasts when they were treated with a combination of 5 Gy ionizing irradiation followed by 5 ng/ml of TGF-β1. This combination treatment was done to mimic the in vivo situation where irradiation induces production of TGF-β (Barcellos-Hoff et al. 1994; Randall & Coggle 1995; Hauer-Jensen et al. 1998). Smad3 WT, but not KO, dermal fibroblasts showed a synergistic induction of mRNA levels of both TGF-β1 and connective tissue growth factor (CTGF) (Flanders et al. 2003). As it has been proposed that the combination of CTGF and TGF-β is needed for optimal matrix synthesis (Mori et al. 1999), the decreased levels of these two fibrogenic cytokines induced in Smad3 KO dermal fibroblasts may partially explain the decreased scarring observed on Smad3 KO skin after irradiation. Smad3 may also play a role in the excessive matrix accumulation found in keloids, because Chin et al. (2001) have reported that keloid fibroblasts show increased phosphorylation of Smad3 relative to normal human dermal fibroblasts.
Radiation therapy and surgery are often used to treat malignancies, but impaired healing of wounds in irradiated tissues can present clinical complications (Tibbs 1997). We found that, even in previously irradiated skin, incisional wounds were still able to heal more rapidly in Smad3 KO mice than in WT mice (Flanders et al. 2003). Five weeks postirradiation, when skin lesions resulting from irradiation had healed, incisions were made in the irradiated field. Wounds were narrower, had a smaller area and re-epithelialized 2–3 times more quickly in Smad3 KO mice than in WT mice. Wound beds of the Smad3 KO mice showed less inflammation and fewer myofibroblasts than did those of WT mice.
Systemic sclerosis (SSc) is an autoimmune disease of unknown aetiology characterized by vascular abnormalities and excessive ECM accumulation leading to progressive cutaneous and visceral fibrosis. Increasing evidence implicates Smad3 as at least a partial mediator of the fibrosis. Smad3 expression was upregulated in SSc skin lesion in humans in vivo and in cultured SSc fibroblasts in vitro with accompanying decreases of Smad7 expression (Dong et al. 2002). Smad3 phosphorylation and PAI-1 induction were augmented in SSc fibroblasts as compared to normal fibroblasts and adenovirus-mediated overexpression of Smad7 restored normal PAI-1 production in SSc fibroblasts (Dong et al. 2002). Moreover, in a murine model of SSc induced by injection of bleomycin into the skin, the localization of Smad 2/3 in fibroblasts was predominantly nuclear with no induction of Smad7 to counteract Smad3 activation (Takagawa et al. 2003). There is also evidence that CTGF which is highly expressed in SSc lesions plays a role in the maintenance of the fibrotic phenotype in SSc fibroblasts (Denton & Abraham 2001). TGF-β induction of CTGF was Smad3 dependent, but mutation of the SBE did not reduce the high level of CTGF promoter activity observed in dermal fibroblasts cultured from SSc patients (Holmes et al. 2001), suggesting that the maintenance of the fibrotic phenotype in SSc fibroblasts may be independent of Smad3.
Liver
High levels of TGF-β are often found in hepatic fibrosis and it has been implicated as a mediator of fibrosis in many liver diseases (Gressner et al. 2002). It is believed that release of TGF-β by necrotic hepatocytes may be one of the first signals to activate adjacent quiescent hepatic stellate cells (HSC) resulting in their transdifferentiation into proliferative, fibrogenic and contractile myofibroblasts. Primary cultures of HSC are spontaneously activated by contact with tissue culture plastic, and this is used as an in vitro model of fibrogenesis. In this system, interactions of Smad3 and Sp1 mediated TGF-β stimulation of the COL1A2 promoter in HSC. In parenchymal hepatocytes, however, Sp3 rather than Sp1 bound to this regulatory element and did not activate the promoter, suggesting cell lineage-specific COL1A2 transcription (Inagaki et al. 2001). Using antibodies specific to either the phosphorylated C-terminus or middle linker region of Smad3, Furukawa et al. (2003) showed that, in HSC, TGF-β-dependent Smad3 phosphorylation at the C-terminus decreased, but phosphorylation of the middle linker region by the p38 MAPK pathway increased during transdifferentiation. They propose that, as HSCs become fully differentiated to myofibroblasts, TGF-β RI-mediated Smad3 signal decreases and p38 MAPK-mediated Smad3 signal predominates, stimulating ECM production leading to liver fibrosis.
Smad3 seems to be activated in chronic fibrotic hepatic disease, as HSCs derived from cirrhotic rat liver showed constitutive phosphorylation and nuclear localization of Smad3 as well as increased transcription of COL1A2 and PAI-1 genes (Inagaki et al. 2001). When acute liver injury was induced by administration of CCl4, Smad3 KO mice showed approximately one-half of the induction of hepatic collagen type I mRNA as did Smad3 WT mice, while α-SMA expression was similar (Schnabl et al. 2001). Thus, Smad3 is not necessary for HSC activation as assessed by α-SMA expression, as has been reported in Smad3 KO dermal fibroblasts (Flanders et al. 2003), but does seem to be required for maximal collagen Type I expression. Different expression patterns of Smad7 in HSCs and myofibroblasts following acute and chronic CCl4 administration, respectively, may contribute to the fibrosis seen in chronic conditions. While Smad7 was induced in HSCs in acute liver injury, levels of Smad7 remained low in myofiboblasts in the chronic condition, perhaps explaining why the fibrotic signals are not inhibited (Tahashi et al. 2002).
Kidney
Renal interstitial fibrosis, in which normal glomerular tissue is replaced by ECM, is caused by a number of clinical entities including urinary tract obstruction, glomerulonephritis and diabetes. The condition is progressive and potentially lethal. Stressors such as hypertension or hyperfiltration can injure visceral epithelial cells or podocytes initiating a cascade of signalling events which results in mesangial cell expansion and ECM accumulation (Schnaper et al. 2003). TGF-β is believed to play a role in glomerulosclerosis, as its expression is associated with increased mesangial matrix in several glomerular diseases, and intrarenal infusion of the TGF-β gene causes sclerosis in rats, while infusion of TGF-β antisense oligonucleotides decrease sclerosis in experimental nephropathy (Schnaper et al. 2003). In response to TGF-β, Smad3 stimulated COL1A2 promoter activity in human glomerular mesangial cells (Poncelet & Schnaper 2001). In this system, TGF-β also activated the PI3K-PDK1-Akt pathway which phosphorylated Smad3 in the middle linker region and enhanced Smad3 transcriptional activity, leading to increased collagen I expression (Runyan et al. 2004). Overexpression of Smad7 in mouse mesangial cells abrogated TGF-β-dependent stimulation of COL1A2 promoter activity (Chen et al. 2002).
Activation of Smad3 is a feature of mouse models of both type 1 and 2 diabetes. In db/db mice, a genetic model of type 2 diabetes, there was increased nuclear localization of Smad3 in glomerular and tubular cells and increased binding of nuclear proteins to the SBE compared to control mice (Hong et al. 2001). Similar increases in nuclear localization of Smad3 and activation of SBE were observed in kidneys of streptozotocin-induced diabetic mice (type 1 diabetes), and these increases were prevented by insulin treatment (Isono et al. 2002). When Smad3 KO mice were treated with streptozotocin, diabetes-induced thickening of glomerular basement membrane, albuminuria and upregulation of fibronectin and COL4A3 expression were attenuated, suggesting that local inhibition of Smd3 may be beneficial in the prevention or treatment of diabetic glomerulonephropathy (Fujimoto et al. 2003). The high circulating level of glucose found in diabetes may actually activate Smad3, as culturing renal cells in high glucose induced phosphorylation and nuclear translocation of Smad3 (Li et al. 2003) as well as activation of collagen I synthesis (Li et al. 2003) and fibronectin promoter activity (Isono et al. 2002). Both of these matrix-inducing effects were blocked in vitro by overexpression of Smad7.
Smad3 also is involved in renal interstitial fibrosis induced by other aetiologies. For example, in human focal segmental glomerulosclerosis in which injury to podocytes is the first event to occur in the disease, significant increases in TGF-β1 and phosphoSmad3 were observed (Kim et al. 2003). In unilateral ureteral obstruction (UUO), upregulation of TGF-β is thought to induce epithelial–mesenchymal transition (EMT) of renal tubules that then go on to deposit ECM. Following UUO, Smad3 KO mice were protected from tubulointersitial fibrosis as evidenced by the blocking of EMT and decreased monocyte influx and collagen accumulation (Sato et al. 2003b). Kidneys from Smad3 WT mice following UUO showed a threefold to sixfold increase in TGF-β1 expression compared to kidneys from Smad3 KO mice or sham-operated animals. Primary cultures of renal tubular epithelial cells from Smad3 WT mice underwent EMT following TGF-β treatment, as evidenced by loss of E-cadherin expression and induction of α-SMA and Snail expression, which did not occur in cells prepared from Smad3 KO mice. Moreover, mechanical stretch of cultured epithelial cells, mimicking renal tubular distention due to accumulation of urine after UUO, induced EMT following Smad3-mediated upregulation of TGF-β1.
Eye
Healing of ocular wounds is often initiated by EMT. Lens epithelium is known to undergo pathologic EMT following traumatic injury, such as in cataract surgery and implantation of an artificial lens, which can lead to production of ECM resulting in contraction of the capsular tissue and opacification of the capsule containing the artificial lens. Similarly, in proliferative vitreoretinopathy (PVR), a common cause of failure in retinal reattachment surgery, EMT of retinal pigment epithelial (RPE) cells can lead to fibrosis and to traction detachment of the retina. Saika et al. (2001) observed that, 8–24 h following puncture of the anterior capsule of the mouse lens, Smads 3 and 4 translocate from the cytoplasm to the nucleus in cells next to the capsular break. Additionally, nuclear Smads 3 and 4 were also seen in lens epithelial cells adjacent to regenerating lens fibres in humans during capsular healing and exogenous TGF-β-induced nuclear translocation of Smad3 in lens epithelial cells of anterior capsule specimens in explant culture (Saika et al. 2002). The importance of Smad3 signalling in EMT in the eye has been demonstrated in two studies using Smad3 KO mice. Following injury to the lens capsule (Saika et al. 2004) or retinal detachment in Smad3 WT mice, lens epithelial cells and RPE cells, respectively, undergo EMT as evidenced by induction of snail, α-SMA and collagen I. These responses were completely blocked in Smad3 KO mice. In WT mice, the lens defect closed in 5 days with fibroblast-like cells, while in KO mice, the defect was closed by more epithelial-like cells, but this was not complete until 8 week postinjury. Furthermore, primary cultures of lens epithelial cells isolated from Smad3 KO mice did not undergo EMT upon TGF-β treatment, as did cells from WT mice. These studies suggest that inhibition of the Smad3-signalling pathway might be desired clinically to prevent secondary cataracts and PVR.
Lung
Pulmonary fibrosis is characterized by mesenchymal cell proliferation and transdifferentiation of some of these cells to myofibroblasts, leading to excessive collagen accumulation in the alveolar and interstitial compartments of the lung. An inflammatory response as a result of injury sometimes initiates these fibroproliferative events. In a mouse model of cystic fibrosis, a disease characterized by an aggressive inflammatory response, bacterial binding and altered cell-surface glycosylation, there was dramatic reduction in Smad3 expression on the nasal epithelium (Kelley et al. 2001). Cystic fibrosis phenotype epithelial cells, as well as in A549 cells expressing dominant-negative Smad3, were unable to support TGF-β1-mediated inhibition of either the interleukin-8 (IL-8) or nitric oxide synthase-2 promoter. The inability of TGF-β to inhibit the activation of these potent inflammatory mediators because of decreased Smad3 expression may account for the severe inflammation in cystic fibrosis.
In a bleomycin-induced model of pulmonary fibrosis, Smad3 KO mice showed fewer fibrotic lesions and decreased expression of collagen I and fibronectin mRNA and protein than did Smad3 WT mice (Zhao et al. 2002). There were similar increases in inflammatory cell influx and TGF-β1 levels in the lungs of each genotype. In another model of pulmonary fibrosis in which the condition is induced by inhalation of an adenovirus expressing active TGF-β1, Smad3 KO mice showed virtually no fibrotic lesions, while Smad3 WT mice accumulated ECM (P. Bonniaud and J. Gauldie, personal communication). Four days after the administration of the adenovirus, lungs from WT mice demonstrated increased expression of mRNAs for CTGF, COL3A1, TIMP1 and PAI-1, whereas there was no increased expression in Smad3 KO lungs. Additionally, these researchers noted that, at 4 months of age, there was a dramatic enlargement in the peripheral air space of Smad3 KO mice, a condition characteristic of emphysema. In emphysema, there is a progressive destruction of ECM thought to occur through an imbalance in protease–antiprotease activity. Indeed, lungs from untreated Smad3 KO mice showed higher levels of MMP-9 and MMP-12 than did lungs from Smad3 WT mice, while other genes implicated in ECM enhancement (CTGF, PAI-1 and TIMP-1) were expressed at similar levels. In gene reporter assays in RAW264.7 cells (a mouse macrophage cell line), Smad3 was able to inhibit expression of MMP-12 following lipopolysaccharide treatment (Werner et al. 2000), and expression of dominant-negative Smad3 blocked the inhibitory effect of TGF-β on the MMP-12 promoter (Feinberg et al. 2000). The loss of Smad3 signalling appears to abrogate the ability of TGF-β to negatively regulate the expression of these proteases, resulting in the observed ECM loss in the lungs of Smad3 KO mice.
Cardiovascular system
Myocardial infarction of the rat heart induced expression of TGF-β1, collagen type I and Smads 2, 3 and 4 in the infarct scar as well as in the border zone which contains remnant myocytes (Hao et al. 1999). Additionally, there was decreased expression of the inhibitory Smad7 protein in the infarct (Wang et al. 2002). Ongoing remodelling of the infarct scar by cardiomyofibroblasts inappropriately expressing these molecules is a possible mechanism for the progressive loss of cardiac function after a large myocardial infarction.
Early atherosclerotic lesions are characterized by influx of inflammatory cells, intimal smooth muscle cell (SMC) proliferation and migration and ECM deposition. Activation of peroxisome proliferator-activated receptor γ (PPARγ) after balloon injury inhibits vascular SMC proliferation and neointima formation. Fu et al. (2001) have shown that ligand stimulation of PPAR γ inhibited TGF-β-induced CTGF expression in human aortic SMC and that overexpression of Smad3 rescued this inhibition. PPARγ physically associated with Smad3 in in vitro glutathione-S-transferase pull-down experiments, suggesting that PPARγ inhibited TGF-β-induced CTGF expression in human aortic SMC by directly interfering with the Smad3-signalling pathway. Furthermore, in bovine aortic endothelial cells, Smad3 regulated TGF-β induction of the PDGF-B chain promoter (Taylor & Khachigian 2000). PDGF-B has been implicated in the pathogenesis of atherosclerosis due to its ability to stimulate endothelial cell proliferation and contribute to neointima formation.
TGF-β-signalling cascades in endothelial cells have recently been shown to be mediated through two Smad pathways (Goumans et al. 2003). Stimulation of endothelial cells with TGF-β activates the two type I receptors, ALK1 and ALK5, with different kinetics and threshold levels. ALK5 signals through the Smad2/3 pathway, while ALK1 activates the Smad1/5 pathway. ALK1 and ALK5 show clear differences in transcriptional regulation patterns in endothelial cells with ALK5 inducing ECM-, PAI-1- and smooth muscle-related genes, while ALK1 induced c-myc (Goumans et al. 2002; Ota et al. 2002). These results indicate that ALK1 signalling stimulates endothelial cell proliferation and migration, while ALK5 signalling promotes vascular maturation. This adds an additional level of complexity to TGF-β-induced signalling with TGF-β activating both arms of the Smad pathway.
Inhibition of Smad3 signalling as a potential antifibrotic therapy
Due to the pleiotropic biological actions of TGF-β mediated by multiple signalling pathways, therapies that target TGF-β expression/activation or binding of TGF-β to its receptor may potentially induce a number of unwanted side effects. Agents that target specific signalling pathways downstream of the TGF-β receptor are more likely to have the desired effects while avoiding complications. As Smad3 plays such a critical role in mediating the pathobiology of fibrotic disease, as evidenced by the previously cited studies, inhibition of Smad3 signalling could be a prime target for intervention in fibrotic conditions. Figure 5 shows that multiple steps in the fibrotic process are Smad3 dependent and could be targets for an inhibitor whether the disease process is initiated by inflammation, such as radiation-induced fibrosis, or by EMT, such as in PVR. Several different mechanisms to inhibit Smad3 in vivo have been reported and are discussed below.
Figure 5.
Many processes which contribute to fibrotic disease are mediated by Smad3. These include: (1) epithelial to mesenchymal transdifferentiation (EMT) which is important in the induction of renal interstitial fibrosis and proliferative vitreoretinopathy (PVR); (2) recruitment of inflammatory cells and fibroblasts and autoinduction of transforming growth factor-β (TGF-β) in these cells which is involved in induction of radiation-induced fibrosis and pulmonary fibrosis and (3) induction of collagen synthesis by TGF-β. Inhibitors of Smad3 could act at multiple sites to inhibit fibrosis.
Cytokines
Several antifibrotic cytokines can modulate the Smad pathway. The antifibrotic effects of IFN-α which is used for the treatment of chronic hepatitis C are mediated by actions on Smad3. Inagaki et al. (2003) have shown that IFN-α induced phosphorylation of Stat1 which competes with Smad3 for limited amounts of CBP/p300, thereby inhibiting basal and TGF-β/Smad3-stimulated COL1A2 transcription in activated HSC, providing a molecular basis for the antifibrotic effects of IFN-α. BMP-7 counteracted TGF-β-induced EMT of murine distal tubular epithelial cells by inhibiting the suppressive effects of TGF-β on the E-cadherin promoter (Zeisberg et al. 2003). In the presence of TGF-β, treatment with BMP-7 restored E-cadherin expression to normal levels in association with nuclear localization of phosphorylated Smads 1, 2 and 3, suggesting that both Smad pathways were operating simultaneously. Additionally, BMP-7 treatment of mice with nephritic serum nephritis, a model of progressive chronic renal injury associated with EMT, prevented tubular atrophy and accumulation of interstitial fibrosis. In a mouse model of bleomycin-induced pulmonary fibrosis, intraperitoneal injections of IL-7 decreased both the TGF-β and collagen content of the lung (Huang et al. 2002). In human pulmonary fibroblasts isolated from patients with idiopathic pulmonary fibrosis, IL-7 inhibited TGF-β-induced collagen production by inducing Smad7; in the presence of IL-7, dominant-negative Smad7 fibroblasts restored TGF-β-induced collagen synthesis (Huang et al. 2002).
Smad7 vectors
While some cytokines may decrease fibrosis by inducing Smad7 which ultimately inhibits activity of Smad3, several studies have directly introduced vectors expressing Smad7 into cells or animals. Achieving sufficient levels of Smad7 in vivo through efficient transfection/infection of these expression vectors is challenging, but some encouraging results have been reported in these types of gene therapy studies. Lan et al. (2003) have transferred a doxycyline-regulated Smad7 gene or control empty vector into rat kidneys via the renal artery using an ultrasound-microbubble (Optison)-mediated system immediately after ligation of the ureter. By adjusting the dose of doxycyline given in the drinking water, Smad7 transgene expression was seen in 70–90% of the cells in the kidney. Following UUO, induction of Smad7 resulted in complete inhibition of Smad2/3 nuclear translocation and tubulointerstitial fibrosis, as evidenced by decreased expression of α-SMA and collagens I and III. Decreased fibrosis using this model was also seen when Smad 7 was introduced one day after ureter ligation by injection of adenoviral CMV-Smad7 into the pelvic space followed by electroporation (Terada et al. 2002). In a murine bleomycin-induced model of lung fibrosis, a single intratracheal injection of a recombinant adenovirus carrying Smad7 cDNA near the start of bleomycin administration resulted in suppression of collagen expression and no morphological fibrotic response when compared with mice administered adenoviral Smad6 (Nakao et al. 1999). Interestingly, the administration of adenoviral Smad7 did not alter pulmonary inflammation or TGF-β production when compared to controls. When fibrosis was induced in rat liver by ligating the common bile duct, a 50% reduction in the expression of collagen and α-SMA was achieved 3 weeks after ligation when adenoviral Smad7 was injected into the portal vein during surgery and into the tail vein at later times (Dooley et al. 2003). A smaller, but significant, 30% reduction was observed in animals with established fibrosis when Smad7 was not administered until 1 week after ligation. Adenoviral expression of Smad7 also arrested transdifferentiaiton of primary cultures of HSC and while it did not decrease expression of α-SMA, overexpression of Smad7 seemed to destroy the fibrillar organization of the actin cytoskeleton.
Small molecule inhibitors
Concern about the safety, efficacy and possible side effects of gene therapy strategies or systemic cytokine administration to inhibit fibrotic disease makes the use of small molecule inhibitors of Smad3-attractive therapeutic agents. Adding 1,25-dihydroxyvitamin D3 to diets of mice or rats for 14 days resulted in decreased levels of Vitamin D receptor, Smad3 and bioactive TGF-β protein in the kidney (Aschenbrenner et al. 2001); this may define a partial mechanism whereby vitamin D3 prolongs allograft survival in a rat renal allograft model of chronic rejection.
Several drugs used to treat patients for other conditions may also have antifibrotic properties based on their abilities to modulate the Smad-signalling pathway. In large clinical trials, angiotensin-converting enzyme inhibitors improve clinical outcome in patients with progressive renal disease. Plasma levels of the haemoregulatory peptide N-acetyl-ser-asp-lys-pro (Ac-SDKP) are increased in treated patients and Ac-SDKP ameliorates cardiac and renal fibrosis in hypertensive animal models. Kanasaki et al. (2003) found that Ac-SDKP treatment of human mesangial cells inhibited TGF-β-induced increases in PAI-1 and COL1A2 mRNA as well as nuclear translocation of Smads 2 and 3 which was accompanied by nuclear export of Smad7. These observations may explain the antifibrotic properties of Ac-SDKP. The chemotherapeutic agent 5-fluorouracil (5-FU) also shows some efficacy in treatment and prevention of hypertrophic scars and keloids. In dermal fibroblasts, 5-FU antagonized TGF-β-driven COL1A2 transcription and inhibited formation of Smad3/4 DNA complexes in a JNK-dependent manner, providing a molecular explanation to the observed clinical benefits of 5-FU as an antifibrotic agent (Wendling et al. 2003).
A new class of water-soluble small molecule inhibitors, related to imidazole inhibitors of p38, have recently been shown to inhibit the kinase activity of TGF-β Type I receptors ALK4 and ALK5. An initial inhibitor in this class, SB-431542, inhibited TGF-β-induced phosphorylation and nuclear translocation of Smad3 as well as TGF-β-induced COL1A1 mRNA levels (Laping et al. 2002). A newly synthesized inhibitor, SB-505124, developed as a competitive inhibitor of the ATP-binding site of ALK5, is three to five times more potent in inhibiting Smad2/3 signalling than SB-431542 (Byfield et al. 2004). SB-505124 also inhibits TGF-β-induced MAPK pathway components but does not alter BMP-induced signalling through Smads 1, 5 and 8. It also abrogates death of FaO cells resulting from treatment with TGF-β, but not from treatment with TNF-α. SB-505124 may have potential as a therapeutic agent and will also be a valuable tool in elucidating the complex pathway and crosstalk of the TGF-β-signalling pathway both in vitro and in animal models in vivo.
Halofuginone, a low molecular weight plant alkaloid used as an anticoccidiostat, inhibits collagen synthesis in several animal models of fibrotic disease (Pines & Nagler 1998). Studies now show that halofuginone can inhibit activation of Smad3. In several cultured cell lines, halofuginone decreased TGF-β-induced phosphorylation of Smads 2 and 3 and also rapidly induced expression of Smad7 mRNA (Xavier et al. 2004). These researchers also found that intraperitoneal injection of halofuginone in mice reduced ionizing radiation-induced hind leg contraction resulting from fibrosis (Figure 6). In this assay, the extent of contraction of the irradiated leg is compared to the nonirradiated contra-lateral leg of the same animal. Irradiated legs of the control group extended 70–80% of nonirradiated legs, while the extension was 90% in the halofuginone-treated mice. This increased extension was maintained even after halofuginone injections were discontinued. Importantly, the effectiveness of radiation treatment of subcutaneous tumours in mice was not affected by halofuginone, suggesting that halofuginone may have potential uses as a therapeutic agent in protecting normal tissue from undergoing unwanted fibrosis in patients receiving radiation therapy to treat malignancies.
Figure 6.
Halofuginone treatment protects against radiation-induced leg contracture. 35 Gy irradiation was given to the right leg and leg extension compared to the nonirradiated contralateral leg of C3H/Hen female mice. Vehicle (white bars) or halofuginone (1 µg/mouse/day) (black bars) was given as a daily i.p. injection. The arrow marks the time when daily injections were stopped. *P < 0.05 compared to control.
Intraperitoneal injection of halofuginone was also effective in inhibiting spontaneous dermal fibrosis and cutaneous hyperplasia in the tight skin mouse model of scleroderma (McGaha et al. 2002). In cultured dermal fibroblasts, halofuginone inhibited TGF-β-induced upregulation of collagen protein and activity of the COL1A2 promoter as well as phosphorylation and activation of Smad3, but not Smad2. In order to begin developing a local therapy modality, dermal application of halofuginone (0.01% in cream) in the tight skin mouse model was found to be almost as effective as systemic administration in the reduction of skin COL1A1 gene expression and skin thickness (Pines et al. 2003).
Several clinical trials have been conducted with halofuginone (Pines et al. 2003). Topical application of 0.1% halofuginone in healthy volunteers showed no skin irritation or systemic absorption. In one patient with chronic graft-vs.-host disease, 6 months of daily topical application of 0.03% halofuginone to the skin of a sclerotic neck improved rotation of the neck and decreased the level of COL1A1 gene expression in skin biopsies taken from treated skin. However, 3 months after cessation of treatment, collagen levels had returned to baseline. The transient effect of halofuginone was probably the result of the chronic stimulation of fibrogenesis. In a phase II trial with systemic sclerosis patients, daily topical application of halofuginone resulted in five of 12 patients responding with reduction in mean total skin score after 3 months. Safety results showed that there were no clinically relevant changes in vital signs and laboratory evaluations. Phase I studies of oral administration of halofuginone have shown it to be well tolerated with some incidences of gastrointestinal adverse effects at higher doses.
Halofuginone has great potential as an antifibrotic therapeutic. In a variety of animal models, systemic administration has been well tolerated and topical administration is well tolerated in humans. Additionally, in animal models of fibrosis, halofuginone has a minimal effect on collagen levels in nonfibrotic animals, while exerting strong inhibitory effects in fibrotic organs. It mainly affects stimulated collagen synthesis without altering the usually low physiological level of collagen expression. In most animal models, halofuginone was used as a preventative agent, being administered before or together with the fibrotic stimulus. Two promising studies suggest that halofuginone may be useful in reversing existing fibrosis. In the tight skin mouse, halofuginone caused a decrease in the preexisting fibrotic condition as measured by changes in collagen level and skin morphology (McGaha et al. 2002). In rats with established thioacetamide-induced liver fibrosis, addition of halofuginone to the diet caused almost complete resolution of the fibrotic condition as measured by hydroxyproline levels in the liver (Bruck et al. 2001).
Conclusions and perspectives
An increasing body of evidence demonstrates that inhibiting TGF-β signalling through blocking Smad3 can decrease fibrotic responses both in vitro and in vivo. As TGF-β can signal through multiple pathways, including Smad2/3, MAP kinases and PI3 kinase, it is amazing that elimination of one specific signalling arm dependent on Smad3 can have such profound effects. Because activin also signals through Smad3, some of the responses induced by blocking Smad3 may be the result of altered activin signalling. Continued studies of the mechanisms of TGF-β signalling in different cell types will contribute to our understanding of exactly what cellular processes are mediated by a given signalling pathway and may contribute to the development of therapeutics with very specific biological actions. Questions that require further investigation include determining the specificity of Smad signalling from TGF-βvs. activin, the effects of phosphorylation of the linker region on Smad activity and the ability of TGF-β to signal through Smads 1/5/8 under certain conditions (Goumans et al. 2003).
While a therapeutic agent that blocks only signalling through Smad3 would be ideal to inhibit fibrosis with minimal side effects, agents that inhibit actions of both Smads 2 and 3 have been successful when used in animal models of fibrosis. Viral vectors expressing Smad7 would be expected to block both Smads 2 and 3, and halofuginone inhibits TGF-β-induced phosphorylation of both Smads 2 and 3 in several cell lines (Xavier et al. 2004). Microarray studies in MEFs suggest that over 95% of the TGF-β-dependent transcriptional effects in these cells are Smad3 dependent (Yang et al. 2003); hence, blocking Smad2 may have only minimal impact. Indeed, systemic administration of halofuginone in animal models and humans has been well tolerated (Pines et al. 2003).
There may be, however, potentially serious adverse effects of long-term partial suppression of TGF-β signalling through inhibition of Smad3. In the initial stages of carcinogenesis, TGF-β is thought to have tumour suppressor functions (Wakefield & Roberts 2002). However, transgenic mice continually expressing the chimeric Fc:TβRII TGF-β antagonistic protein are free of serious disease and have no changes in tumour incidence or progression (Yang et al. 2002). Additionally, TGF-β is a potent immunosuppressive agent and plays a role in the pathogenesis of chronic inflammatory diseases, so that blocking TGF-β may stimulate the development of autoimmune diseases. Monteleone et al. (2001) have reported that in active lesions from patients with inflammatory bowel disease, there was increased expression of Smad7 protein that was not present in areas of endoscopically normal mucosa from the same patients. Abundant phospho-Smad3 was seen in normal mucosa but not in diseased samples; this loss of Smad3 activity presumably blocks the anti-inflammatory effects of TGF-β and contributes to the disease. To avoid these and similar potential complications, fibrotic conditions in which Smad3 inhibitors can be applied locally may be most amenable to treatment. Treatment of fibrotic ocular conditions leading to blindness, improvement of wound healing and protection of normal tissue in patients undergoing radiation therapy are all conditions where Smad3 inhibitors could improve outcome.
References
- Aschenbrenner JK, Sollinger HW, Becker BN, Hullett DA. 1,25-(OH(2)D(3) alters the transforming growth factor beta signaling pathway in renal tissue. J. Surg. Res. 2001;100:171–175. doi: 10.1006/jsre.2001.6221. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Attisano L, Tuen Lee-Hoeflich S. The smads. Genome Biol. 2001;2:3010.1–3010.8. doi: 10.1186/gb-2001-2-8-reviews3010. reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J. Clin. Invest. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Bruck R, Genina O, Aeed H, Alexiev R, Nagler A, Avni Y, Pines M. Halofuginone to prevent and treat thioacetamide-induced liver fibrosis in rats. Hepatology. 2001;33:379–386. doi: 10.1053/jhep.2001.21408. [DOI] [PubMed] [Google Scholar]
- Byfield SAD, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of TGF-β type I receptors ALK4, ALK5 and ALK7. Mol. Pharmacol. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- Chen R, Huang C, Morinelli TA, Trojanowska M, Paul RV. Blockade of the effects of TGF-beta1 on mesangial cells by overexpression of Smad7. J. Am. Soc. Nephrol. 2002;13:887–893. doi: 10.1681/ASN.V134887. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Yuan W, Lo S, Trojanowska M, Varga J. Interaction of smad3 with a proximal smad-binding element of the human alpha2(I) procollagen gene promoter required for transcriptional activation by TGF-beta. J. Cell Physiol. 2000;183:381–392. doi: 10.1002/(SICI)1097-4652(200006)183:3<381::AID-JCP11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Chin GS, Liu W, Peled Z, et al. Differential expression of transforming growth factor-beta receptors I and II and activation of smad 3 in keloid fibroblasts. Plast. Reconstr. Surg. 2001;108:423–429. doi: 10.1097/00006534-200108000-00022. [DOI] [PubMed] [Google Scholar]
- Datta PK, Blake MC, Moses HL. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-beta -induced physical and functional interactions between smads and Sp1. J. Biol. Chem. 2000;275:40014–40019. doi: 10.1074/jbc.C000508200. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol. Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, Ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton CP, Abraham DJ. Transforming growth factor-beta and connective tissue growth factor: key cytokines in scleroderma pathogenesis. Curr. Opin. Rheumatol. 2001;13:505–511. doi: 10.1097/00002281-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dong C, Zhu S, Wang T, et al. Deficient Smad7 expression: a putative molecular defect in scleroderma. Proc. Natl. Acad. Sci. USA. 2002;99:3908–3913. doi: 10.1073/pnas.062010399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dooley S, Hamzavi J, Breitkopf K, et al. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178–191. doi: 10.1016/s0016-5085(03)00666-8. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, Jain MK, Werner F, et al. Transforming growth factor-beta 1 inhibits cytokine-mediated induction of human metalloelastase in macrophages. J. Biol. Chem. 2000;275:25766–25773. doi: 10.1074/jbc.M002664200. [DOI] [PubMed] [Google Scholar]
- Felici A, Wurthner JU, Parks WT, et al. TLP, a novel modulator of TGF-beta signaling, has opposite effects on Smad2- and Smad3-dependent signaling. EMBO J. 2003;22:4465–4477. doi: 10.1093/emboj/cdg428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC, Burmester JK. Medical applications of transforming growth factor-β. Clin. Med. Res. 2003;1:13–20. doi: 10.3121/cmr.1.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC, Major CD, Arabshahi A, et al. Interference with transforming growth factor-beta/ Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am. J. Pathol. 2003;163:2247–2257. doi: 10.1016/s0002-9440(10)63582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC, Sullivan CD, Fujii M, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am. J. Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Zhang J, Zhu X, et al. Peroxisome proliferator-activated receptor gamma inhibits transforming growth factor beta-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. J. Biol. Chem. 2001;276:45888–45894. doi: 10.1074/jbc.M105490200. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Maezawa Y, Yokote K, et al. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem. Biophys. Res. Commun. 2003;305:1002–1007. doi: 10.1016/s0006-291x(03)00885-4. [DOI] [PubMed] [Google Scholar]
- Furukawa F, Matsuzaki K, Mori S, et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Yuan W, Mori Y, Chen S, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth factor-beta. Integration at the level of p300/CBP transcriptional coactivators. J. Biol. Chem. 2001;276:11041–11048. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Yuan W, Mori Y, Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-beta involves functional cooperation with p300/CBP transcriptional coactivators. Clin. Exp. Nephrol. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-β receptor signaling pathways. Trends Cardiovasc. Med. 2003;13:301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, Ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front. Biosci. 2002;7:d793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- Hall MC, Young DA, Waters JG, et al. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-beta 1. J. Biol. Chem. 2003;278:10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- Hao J, Ju H, Zhao S, et al. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J. Mol. Cell Cardiol. 1999;31:667–678. doi: 10.1006/jmcc.1998.0902. [DOI] [PubMed] [Google Scholar]
- Hauer-Jensen M, Richter KK, Wang J, Abe E, Sung CC, Hardin JW. Changes in transforming growth factor beta1 gene expression and immunoreactivity levels during development of chronic radiation enteropathy. Radiat. Res. 1998;150:673–680. [PubMed] [Google Scholar]
- Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- Hayes S, Chawla A, Corvera S. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi K, Inagaki Y, Fujimori K, Nakao A, Kaneko H, Nakatsuka I. Interferon-gamma interferes with transforming growth factor-beta signaling through direct interaction of YB-1 with Smad3. J. Biol. Chem. 2003;278:43470–43479. doi: 10.1074/jbc.M302339200. [DOI] [PubMed] [Google Scholar]
- Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and smads, maintenance of scleroderma phenotype is independent of smad signaling. J. Biol. Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- Hong SW, Isono M, Chen S, Iglesias-De La Cruz MC, Han DC, Ziyadeh FN. Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am. J. Pathol. 2001;158:1653–1663. doi: 10.1016/s0002-9440(10)64121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chuang JZ, Xu K, McGraw TG, Sung CH. SARA, a FYVE domain protein, affects Rab5-mediated endocytosis. J. Cell Sci. 2002;115:4755–4763. doi: 10.1242/jcs.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Sharma S, Zhu LX, et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J. Clin. Invest. 2002;109:931–937. doi: 10.1172/JCI14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Y, Mamura M, Kanamaru Y, et al. Constitutive phosphorylation and nuclear localization of Smad3 are correlated with increased collagen gene transcription in activated hepatic stellate cells. J. Cell Physiol. 2001;187:117–123. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1059>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Nemoto T, Kushida M, et al. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38:890–899. doi: 10.1053/jhep.2003.50408. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Nemoto T, Nakao A, et al. Interaction between GC box binding factors and Smad proteins modulates cell lineage-specific alpha 2(I) collagen gene transcription. J. Biol. Chem. 2001;276:16573–16579. doi: 10.1074/jbc.M010485200. [DOI] [PubMed] [Google Scholar]
- Isono M, Chen S, Won HS, Carmen Iglesias-De La Cruz, Ziyadeh F. Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-beta-induced fibronectin in mesangial cells. Biochem. Biophys. Res. Commun. 2002;296:1356–1365. doi: 10.1016/s0006-291x(02)02084-3. [DOI] [PubMed] [Google Scholar]
- Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-Acetyl-Seryl-Aspartyl-Lysyl-Proline inhibits TGF-beta-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J. Am. Soc. Nephrol. 2003;14:863–872. doi: 10.1097/01.asn.0000057544.95569.ec. [DOI] [PubMed] [Google Scholar]
- Kelley TJ, Elmer HL, Corey DA. Reduced Smad3 protein expression and altered transforming growth factor-beta1-mediated signaling in cystic fibrosis epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001;25:732–738. doi: 10.1165/ajrcmb.25.6.4574. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim BK, Moon KC, Hong HK, Lee HS. Activation of the TGF-beta/Smad signaling pathway in focal segmental glomerulosclerosis. Kidney Int. 2003;64:1715–1721. doi: 10.1046/j.1523-1755.2003.00288.x. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kon A, Vindevoghel L, Kouba DJ, Fujimura Y, Uitto J, Mauviel A. Cooperation between SMAD and NF-kappaB in growth factor regulated type VII collagen gene expression. Clin. Exp. Nephrol. 1999;18:1837–1844. doi: 10.1038/sj.onc.1202495. [DOI] [PubMed] [Google Scholar]
- Kretschmer A, Moepert K, Dames S, Sternberger M, Kaufmann J, Klippel A. Differential regulation of TGF-beta signaling through Smad2, Smad3 and Smad4. Clin. Exp. Nephrol. 2003;22:6748–6763. doi: 10.1038/sj.onc.1206791. [DOI] [PubMed] [Google Scholar]
- Lan HY, Mu W, Tomita N, et al. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J. Am. Soc. Nephrol. 2003;14:1535–1548. doi: 10.1097/01.asn.0000067632.04658.b8. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Grygielko E, Mathur A, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol. Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Transforming growth factor-beta1-deficient mice: identification of isoform-specific activities in vivo. J. Leukoc. Biol. 1996;59:769–774. doi: 10.1002/jlb.59.6.769. [DOI] [PubMed] [Google Scholar]
- Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63:2010–2019. doi: 10.1046/j.1523-1755.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Wen FQ, Kobayashi T, et al. Smad3 mediates the TGF-beta-induced contraction of type I collagen gels by mouse embryo fibroblasts. Cell Motil. Cytoskeleton. 2003;54:248–253. doi: 10.1002/cm.10098. [DOI] [PubMed] [Google Scholar]
- Lutz M, Knaus P. Integration of the TGF-beta pathway into the cellular signalling network. Cell Signal. 2002;14:977–988. doi: 10.1016/s0898-6568(02)00058-x. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- McGaha TL, Phelps RG, Spiera H, Bona C. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J. Invest. Dermatol. 2002;118:461–470. doi: 10.1046/j.0022-202x.2001.01690.x. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J. Clin. Invest. 2001;108:601–609. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J. Cell. Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J. Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- Nakao A, Fujii M, Matsumura R, et al. Transient gene transfer and expression of Smad7 prevents bleomycin- induced lung fibrosis in mice. J. Clin. Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Ota T, Fujii M, Sugizaki T, et al. Targets of transcriptional regulation by two distinct type I receptors for transforming growth factor-beta in human umbilical vein endothelial cells. J. Cell Physiol. 2002;193:299–318. doi: 10.1002/jcp.10170. [DOI] [PubMed] [Google Scholar]
- Penheiter SG, Mitchell H, Garamszegi N, Edens M, Dore JJ, Leof EB. Internalization-dependent and –independent requirements for transforming growth factor beta receptor signaling via the Smad pathway. Mol. Cell Biol. 2002;22:4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek E, Ju WJ, Heyer J, et al. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem. 2001;276:19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- Pines M, Nagler A. Halofuginone: a novel antifibrotic therapy. Gen. Pharmacol. 1998;30:445–450. doi: 10.1016/s0306-3623(97)00307-8. [DOI] [PubMed] [Google Scholar]
- Pines M, Snyder D, Yarkoni S, Nagler A. Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. BiolBlood Marrow Transplant. 2003;9:417–425. doi: 10.1016/s1083-8791(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. J. Biol. Chem. 2001;276:6983–6992. doi: 10.1074/jbc.M006442200. [DOI] [PubMed] [Google Scholar]
- Randall K, Coggle JE. Expression of transforming growth factor-beta 1 in mouse skin during the acute phase of radiation damage. Int. J. Radiat. Biol. 1995;68:301–309. doi: 10.1080/09553009514551231. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. The transforming growth factors-β. In: Roberts AB, Sporn MB, editors. Handbook of Experimental Pharmacology 95. New York: Springer-Verlag; 1990. pp. 419–472. [Google Scholar]
- Roberts AB, Sporn MB. Transforming growth factor-β. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. pp. 275–308. [Google Scholar]
- Runyan CE, Schnaper HW, Poncelet AC. Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am. J. Physiol. Renal Physiol. 2003;285:F413–F422. doi: 10.1152/ajprenal.00082.2003. [DOI] [PubMed] [Google Scholar]
- Runyan CE, Schnaper HW, Poncelet AC. The PI3K-Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to TGF-beta 1. J. Biol. Chem. 2004;279:2632–2639. doi: 10.1074/jbc.M310412200. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, et al. Smad3 singaling is required for epithelial-mesenchymal transistion of lens epithelium post-injury. Am. J. Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Miyamoto T, Ishida I, et al. TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br. J. Ophthalmol. 2002;86:1428–1433. doi: 10.1136/bjo.86.12.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Okada Y, Miyamoto T, Ohnishi Y, Ooshima A, McAvoy JW. Smad translocation and growth suppression in lens epithelial cells by endogenous TGFbeta2 during wound repair. Exp. Eye Res. 2001;72:679–686. doi: 10.1006/exer.2001.1002. [DOI] [PubMed] [Google Scholar]
- Sato M, Markiewicz M, Yamanaka M, et al. Modulation of transforming growth factor-beta (TGF-beta) signaling by endogenous sphingolipid mediators. J. Biol. Chem. 2003a;278:9276–9282. doi: 10.1074/jbc.M211529200. [DOI] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 2003b;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;34:89–100. doi: 10.1053/jhep.2001.25349. [DOI] [PubMed] [Google Scholar]
- Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-beta signal transduction and mesangial cell fibrogenesis. Am. J. Physiol. Renal Physiol. 2003;284:F243–F252. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi K, Nakao A, Setoguchi Y, Okumura K, Tsuboi R, Ogawa H. Smads regulate collagen gel contraction by human dermal fibroblasts. Br. J. Dermatol. 2003;149:464–470. doi: 10.1046/j.1365-2133.2003.05490.x. [DOI] [PubMed] [Google Scholar]
- Tahashi Y, Matsuzaki K, Date M, et al. Differential regulation of TGF-beta signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology. 2002;35:49–61. doi: 10.1053/jhep.2002.30083. [DOI] [PubMed] [Google Scholar]
- Takagawa S, Lakos G, Mori Y, Yamamoto T, Nishioka K, Varga J. Sustained activation of fibroblast transforming growth factor-beta/Smad signaling in a murine model of scleroderma. J. Invest. Dermatol. 2003;121:41–50. doi: 10.1046/j.1523-1747.2003.12308.x. [DOI] [PubMed] [Google Scholar]
- Taylor LM, Khachigian LM. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-beta involves transactivation by Smads. J. Biol. Chem. 2000;275:16709–16716. doi: 10.1074/jbc.275.22.16709. [DOI] [PubMed] [Google Scholar]
- Terada Y, Hanada S, Nakao A, Kuwahara M, Sasaki S, Marumo F. Gene transfer of Smad7 using electroporation of adenovirus prevents renal fibrosis in post-obstructed kidney. Kidney Int. 2002;61:94–98. doi: 10.1046/j.1523-1755.2002.0610s1094.x. [DOI] [PubMed] [Google Scholar]
- Tibbs MK. Wound healing following radiation therapy: a review. Radiother. Oncol. 1997;42:99–106. doi: 10.1016/s0167-8140(96)01880-4. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Tacheau C, Wagner EF, Mauviel A. A central role for the JNK pathway in mediating the antagonistic activity of pro-inflammatory cytokines against transforming growth factor-beta -driven SMAD3/4-specific gene expression. J. Biol. Chem. 2003;278:1585–1593. doi: 10.1074/jbc.M206927200. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Wang B, Hao J, Jones SC, Yee MS, Roth JC, Dixon IM. Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1685–H1696. doi: 10.1152/ajpheart.00266.2001. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Yang X, Li C, Xu X, Gotay J, Deng CX. Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc. Natl. Acad. Sci. USA. 1998;95:9378–9383. doi: 10.1073/pnas.95.16.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling J, Marchand A, Mauviel A, Verrecchia F. 5-fluorouracil blocks transforming growth factor-beta-induced alpha2 type I collagen gene (COL1A2) expression in human fibroblasts via c-Jun NH2-terminal kinase/activator protein-1 activation. Mol. Pharmacol. 2003;64:707–713. doi: 10.1124/mol.64.3.707. [DOI] [PubMed] [Google Scholar]
- Werner F, Jain MK, Feinberg MW, et al. Transforming growth factor-beta 1 inhibition of macrophage activation is mediated via Smad3. J. Biol. Chem. 2000;275:36653–36658. doi: 10.1074/jbc.M004536200. [DOI] [PubMed] [Google Scholar]
- Xavier S, Piek E, Fujii M, et al. Amelioration of radiation-induced fibrosis: inhibition of TGF-β signaling by halofuginone. J. Biol. Chem. 2004;279:15167–15176. doi: 10.1074/jbc.M309798200. [DOI] [PubMed] [Google Scholar]
- Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J. Cell Biol. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YA, Dukhanina O, Tang B, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J. Clin. Invest. 2002;109:1607–1615. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Piek E, Zavadil J, et al. Hierarchical model of gene regulation by transforming growth factor-beta. Proc. Natl. Acad. Sci. USA. 2003;100:10269–10274. doi: 10.1073/pnas.1834070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Varga J. Transforming growth factor-beta repression of matrix metalloproteinase-1 transcription in dermal fibroblasts involves Smad3. J. Biol. Chem. 2001;276:38502–38510. doi: 10.1074/jbc.M107081200. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription. J. Biol. Chem. 2000;275:39237–39245. doi: 10.1074/jbc.M003339200. [DOI] [PubMed] [Google Scholar]
- Zhao J, Shi W, Wang YL, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L585–L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]