Abstract
Abdominal aortic aneurysm is accompanied by the impairment of collagen metabolism in arterial wall. Metalloproteinases and collagen-stimulating factors play an important role in the maintenance of balance between collagen biosynthesis and degradation in tissues. Insulin-like growth factor-I (IGF-I) plays a major role in the stimulation of collagen biosynthesis. Its activity and bioavailability to target cells are modulated by IGF binding proteins (IGFBPs). The potential role of these factors in the mechanism of collagen metabolism deregulation in aortic aneurysm is the purpose of this study. Therefore, we have compared the content of collagen, gelatinolytic activity, IGF-I, IGFBP-1 and IGFBP-3 in normal human aorta and aortic aneurysm. The content of hydroxyproline (representing collagen content) in the proteins of aortic aneurysm was found to be similar to that found in normal aorta. Taking into account that some of the hydroxyproline may be derived from collagen degradation products (CDPs), they were separated and hydroxyproline was determined. It has been found that CDP-derived hydroxyproline content in aortic aneurysm was increased as compared with normal aorta, suggesting an increased collagen degradation. In contrast, zymography showed a decrease of collagenolytic activity in aortic aneurysm tissue, but an increase in mural thrombus, compared to respective controls. IGF-I concentration in aortic aneurysm was decreased, while the concentrations of BP-1 and BP-3 were both increased compared to control. The data suggest that increased collagen degradation in aortic aneurysm is due to the increase in collagenolytic activity in mural thrombus accompanying aneurysm tissue. It suggests that the mural thrombus may play a critical role in the pathogenesis of abdominal aortic aneurysm.
Keywords: abdominal aortic aneurysm, collagen, IGFBP-1, IGFBP-3, IGF-I, metalloproteinases, thrombus
Abdominal aortic aneurysm is defined as an abnormal enlargement in the diameter of the aorta, with at least a 50% increase over the unchanged, proximal section of the vessel (Gloviczki et al. 1990; Adolph et al. 1997). Normal size of the abdominal aorta measured below the origin of renal arteries in adults aged 65 years or more is 2.01 ± 0.5 cm in diameter (the diameter of the aorta gradually increases in size with advancing age). Abdominal aortic aneurysms pose a significant medical problem, due to their incidence rate, reported to be as high as 2–4% of the general population, and serious clinical course. Ruptured aneurysms account for 0.5–0.8% of all deaths (Henney et al. 1993; Dadgar et al. 1997).
Up to date, the factors contributing to the development of the aneurysms still remain unknown. Atherosclerosis has been considered as a main pathogenic cause of the abdominal aortic aneurysm (Zatina et al. 1984). Currently, biochemical changes within aortal wall lining the aneurysm wall with a thrombus as well as atheromatosis are being considered as a major causative mechanism (Wills et al. 1996).
Collagen and elastin are the two most significant structural proteins of the aortal wall. Together with proteoglycans and glycosaminoglycans, they form elastic and collagen fibres, maintaining the ability of reversible deformation and mechanical load-bearing properties of the aorta. The characteristics of elastin and collagen are mutually complementary. Elastic fibres provide elasticity whereas collagen fibres account for strength and durability. Certainly, a deregulation of metabolism of these proteins may play a fundamental role in the pathogenesis of the aortic aneurysm (Rasmussen & Hallet 1997; Campa et al. 1987).
Collagen, which accounts for about one third of total body proteins, is essential for the maintenance of connective tissue architecture in blood vessels and arteries (Rizzo et al. 1989). The interaction between cells and extracellular matrix (ECM) proteins, e.g. collagen can regulate cellular gene expression, differentiation and growth (Bissel 1981; Carey 1991). Therefore, any changes in quantity, structure and distribution of collagens (and other ECM proteins) would be more likely to alter metabolism in arteries and their function. Impaired synthesis and degradation of collagen could potentially play a major role in the regulation of collagen mass in arterial diseases. The way in which the processes are regulated is still poorly understood. It has been postulated that deregulation of collagen synthesis and degradation may be an underlying mechanism for aortic aneurysm (Brophy et al. 1991; Sobolewski et al. 1995). Whether the deregulation takes place in the tissue of aortic aneurysm or it is induced by thrombus lining the aneurysm wall remains to by clarified.
Several growth factors are implicated in the regulation of collagen metabolism. One of them is insulin-like growth factor-I (IGF-I), a multifunctional growth factor with potent growth, collagen- and proteoglycan-stimulating activity (Oyamada et al. 1990). The effects of IGF-I are regulated by a family of IGF-I binding proteins, designated IGF-I BP-1 through BP-6 (Clemmons 1997; Kim et al. 1997). The different IGFBPs have been shown to restrict IGF tissue availability, regulate IGF transport to cells and modulate IGF binding to membrane receptors (Le Roith et al. 1995).
Under normal conditions, most of the IGF-I circulates in adult human plasma in a form bound to BP-3. It has been postulated that it prolongs the half-life of IGF-I and increase cell responsiveness to IGF-I stimulation (Clemmons 1998). The other BPs (BPs: 1 and 2 as well as BPs: 4, 5 and 6) are present in plasma and tissues in concentrations sufficient to modify IGF-I action (Sara & Hali 1990; Lamson et al. 1991; Levitt et al. 1991; Le Roith et al. 1995; Clemmons 1997).
Because collagen metabolism is disturbed in aortic aneurysm, we assumed that the changes might be due to the deregulation of IGF-I activity or collagenolitic activity in this tissue. Therefore, the current study was undertaken to determine collagen, IGF-I, IGFBPs contents in control aorta and aortic aneurysm and collagenolitic activity in these tissues as well in parietal thrombus accompanying aneurysm wall.
Materials and methods
Hydroxyproline, gelatin, bovine serum albumin (BSA) were purchased from Sigma Chemicals (Poznan, Poland) as were most other chemicals used.
IGF-I radioimmunoassay (RIA) kit was purchased from Incstar Corporation, Stillwater, MN, USA.
[125I]-IGF-I, Amerlex-M-Separation reagent was obtained from Amersham (Little Chalfont, UK). IGFBP-1 immunoradiometric assay (IRMA) and IGFBP-3 (RIA) kits were purchased from Diagnostic Systems Laboratories, Inc., Webster, TX, USA. Bio-gel P-60, Coomassie Brilliant Blue and Tween-20 were obtained from Bio-Rad Laboratories (Warsaw, Poland).
Material
Aneurysms were obtained from 10 male patients at age of 56–72 years with the following lipid parameters: cholesterol (118–239 mg/dl), triglycerides (73–307 mg/dl), high-density lipoprotein (22–60 mg/dl) and low-density lipoprotein (110–202 mg/dl). Comparative material consisted of normal aorta obtained from 10 male (46–51 years old) donors of kidney for transplantation. The lipid parameters were within the normal range for this group of patients. Mural thrombus was easily separated from aortic aneurysm after longitudinal incision of this tissue. Aortic aneurysm was washed in PBS, and the fragment of tissue (that was in contact with thrombus) was used for preparation of aortic aneurysm tissue extract.
Preparation of tissue extracts
Tissue homogenates (20% w/v) were prepared in 0.05 mol/l Tris–HCl, pH 7.6 with the use of knife homogenizer (Polytron, Bad Wildbad, Germany) and subsequently were sonicated at 0 °C. Homogenates were centrifuged at 16 000 × g for 10 min at 4 °C. Supernatant (tissue extract) was used for assays.
Hydroxyproline determination
Hydroxyproline was determined according to the method of Prockop and Udenfriend 1960.
Separation of tissue intact collagen from collagen degradation products
The method of Kang et al. (1967) was employed for separation of collagen degradation products (CDPs) from intact tissue collagen. Tissue homogenate (20% w/v) was incubated with equal volume of 1 mol/l of acetic acid for 2 h at room temperature. Intact collagen was precipitated by the addition of sodium chloride at final concentration of 2.5 mol/l and centrifuged at 16 000 × g for 15 min at 4 °C. The supernatant containing low molecular weight CDPs was concentrated to 0.5 ml, submitted to hydrolysis in 6 mol/l of hydrochloric acid at 124 C for 16 h in nitrogen atmosphere and hydroxyproline was determined. Similar procedure was employed for the determination of hydroxyproline in the pellet.
Zymography
Gelatinolytic activity was determined according to the method of Unemori and Werb (1986). Tissue extract was mixed with Laemmli sample buffer, Laemmli (1970) containing 2.5% SDS (without reducing agent). Equal amounts (about 20 µg) of protein were electrophoresed under non-reducing conditions on 10% polyacrylamide gels impregnated with 1 mg/ml of gelatin. After electrophoresis, the gels were incubated in 2% Triton X-100 for 30 min at 37 °C to remove SDS and incubated for 18 h at 37 °C in substrate buffer (50 mm Tris–HCl buffer, pH 8, containing 5 mm CaCl2). After staining with Coomassie Brilliant Blue R250, gelatin-degrading enzymes present in tissue extract were identified as clear zones in a blue background.
Separation of IGF-I from BPs
At acidic pH, the IGFBPs complex dissociates releasing free IGF-I which may be determined by RIA. This procedure has been described in detail in previous papers (Palka et al. 1989; Palka & Peterkofsky 1988). Briefly, 250 µl of tissue extract or 25 µl of serum in 1 m acetic acid were submitted to gel filtration on Bio-gel P-60 (100–200 mesh) column (1 × 40 cm) and eluted with 1 m acetic acid. Fractions of 1 ml were collected. To determine the position of IGF-I in eluate, the 125I-labelled IGF-I was added to 250 µl of tissue extract or 25 µl of serum and chromatographed as described above.
Fractions of eluate, containing IGF-I were pooled, evaporated to dryness, redissolved in 0.5 ml of assay buffer, consisted of 0.03 m sodium phosphate, 0.2 mg/ml protamine sulphate, 0.02% sodium azide, 0.01 m EDTA and 0.25% BSA. Aliquots were submitted to RIA for IGF-I, as described below.
Fractions of eluate containing BPs were pooled, evaporated, redissolved in 0.5 ml of 0.1 m Tris–HCl buffer, pH 7.6 and submitted to specific BP assays.
RIA for IGF-I
The assay was performed according to slightly modified protocol provided by Amersham with Somatomedin C reagent pack for RIA (code IM 1721). The reaction mixture contained 100 µl of assay buffer, supplemented with either unlabelled IGF-I (0.05–3.2 ng) or the pooled low molecular weight fractions from the Bio-gel P-60 column (20–40 µl diluted with assay buffer 1 : 10) and 100 µl of antibody diluted 1 : 4000 with assay buffer. Samples were incubated for 30 min at room temperature and then 100 µl of [125I]-IGF-I (about 15 000 cpm, specific activity about 74 TBq/mmol = 2000 Ci/mmol) was added. Incubation was continued for 48 h, at 4 °C after which 500 µl of Amerlex-M second antibody reagent was added. The mixture was incubated for 10 min at room temperature with occasional mixing, centrifuged at 25 000 × g for 10 min and radioactivity of the sediment was determined. A standard competition curve was established using 0.05–3.2 ng of unlabelled IGF-I per tube. The radioactivity of control samples (containing 100 ng of unlabelled IGF-I) was subtracted from the radioactivity of the test samples to correct for non-specific binding.
IRMA for IGFBP-1
The assay was performed according to protocol provided by Diagnostic Systems Laboratories Inc. with Active™ Total IGFBP-1 Coated-Tube Immunoradiometric Assay Kit (code DSL-7800). The assay is based on the procedure of Miles et al. (1974). The reaction mixtures contained 10 or 25 µl of samples (1 : 10 diluted pooled fraction of BPs from serum or tissue extracts).
RIA for IGFBP-3
The assay was performed according to protocol provided by Diagnostic Systems Laboratories Inc. with IGFBP-3 Radioimmunoassay Kit (code DSL-6700). The reaction mixtures contained 100 µl of samples (1 : 100 diluted pooled fraction of BPs from serum or tissue extracts).
Radioactivity assay
Radioactivity was measured with the use of a Mini-gamma 1275 counter (LKB Wallac, Boston, MA, USA).
Statistical analysis
In all experiments, the mean values with standard deviations for 10 assays were calculated. The results were submitted to statistical analysis using the Student's t-test, accepting P < 0.05 as significant.
Results
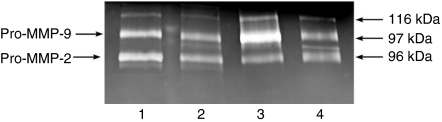
The characteristic feature of collagen is the presence of hydroxyproline (1/8 of collagen mass), which very rarely occurs in other proteins (Birkedal-Hansen 1987). Therefore, the amount of this amino acid in tissue proteins multiplied by 8 represent approximately the amount of tissue collagen. Total amount of hydroxyproline was determined in normal aorta and aneurysm.The content of this amino acid in the proteins of aortic aneurysm (about 400 µg/mg of dry tissue) was found to be similar to that found in normal aorta (about 360 µg/mg of dry tissue), suggesting similar content of collagen in both tissues. Taking into account that some of the tissue hydroxyproline may be derived from CDPs, they were separated as described in Materials and methods, hydrolysed and released hydroxyproline was determined. As summarized in Table 1, CDP-derived hydroxyproline content in aortic aneurysm was significantly increased as compared with normal aorta. It suggests that the increase in CDP content in aneurysm may result from an increase in gelatinolytic activity in this tissue. Figure 1 shows that this is not the case. Gelatinolytic activity determined by zymography was found to be higher in control aorta than in aortic aneurysm. Both tissues contain the following gelatinases; first, represented by about 92 kDa gelatinase [presumably latent metalloproteinase-9 (MMP-9)], and second, represented by about 62 kDa gelatinase (presumably an active form of MMP-2). Both of them are well-defined tissue gelatinases (Brown et al. 1993). Interestingly, the activity of both gelatinases, particularly 92 kDa gelatinase, is increased in mural thrombus accompanying aneurysm tissue as compared with normal aorta and aneurysm tissue as well serum. Although the data present average gelatinolytic activity in aortic tissues from 10 patients, the individual patient's samples showed similar results, implying the mechanism for increased CDP in aortic aneurysm.
Table 1.
Hydroxyproline (Hyp) content of total collagen and collagen degradation product (CDP) preparations from normal aorta and aneurysm tissue
| Aorta | Total Hyp (µg/mg of protein) | CDP-derived Hyp (µg/mg of protein) | CDP (% of total) |
|---|---|---|---|
| Normal | 19.8 ± 3.6 | 3.3 ± 0.8 | 16.6 |
| Aneurysm | 24.7 ± 5.3 | 11.1 ± 5.9* | 44.9 |
The assays were performed on 10 individual samples and mean values with standard deviations are presented.
P < 0.05 compared to control values
Figure 1.
Zymography of tissue homogenates from control aorta (lane 1), aortic aneurysm (lane 2), mural thrombus (lane 3) and serum (lane 4). The assay was performed with 20 µg of protein of 10 pooled tissue homogenates or 2 µl of serum of 10 pooled patient's sera. The following molecular weight standards were used: galactosidase (116 kDa), phosphorylase b (97 kDa) and bovine serum albumin (66 kDa). MMP, metalloproteinase.
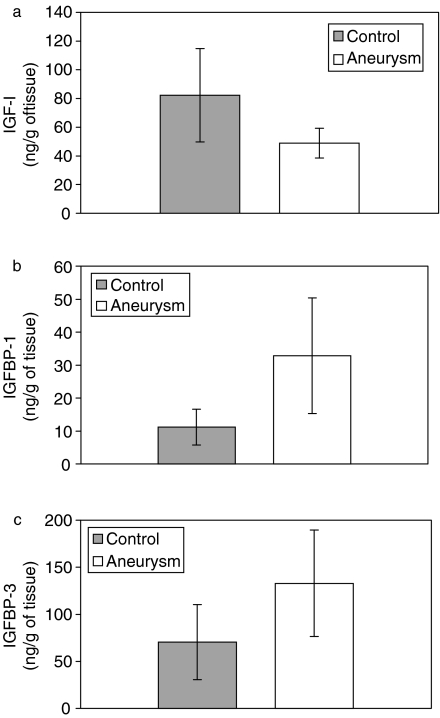
The concentration of IGF-I was measured in normal human aorta and aortic aneurysm. The amount of IGF-I in aortic aneurysm was significantly decreased compared to that in control tissue (Figure 2a). BP-1 (Figure 2b) and BP-3 (Figure 2c) were both increased compared to controls.
Figure 2.
The contents of insulin-like growth factor-I (IGF-I) (a) and its binding proteins (BPs): IGFBP-1 (b) and IGFBP-3 (c) in control and aneurysm tissues. The assays were performed on 10 individual samples and mean values with standard deviations are presented. P < 0.05 compared to control values.
Discussion
Some studies have previously found that aortic aneurysm is accompanied by the increased activity of various proteases, including enzymes degrading elastin, collagen as well as non-specific lysosomal cathepsins (Gacko & Glowinski 1998). Our results show an increased amount of CDPs in aortic aneurysm, compared to that in normal aorta. It may be expected that increased gelatinolytic activity should be found in aortic aneurysm tissue. Our results, however, provide evidence that gelatinolytic activity in aneurysm tissue is not different from that in normal aorta. Instead, increased gelatinolytic activity was found in thrombus accompanying aneurysm. All aortic aneurysm contained mural thrombus. Thrombus and aortic aneurysm tissues were derived from individual patients, paired and measured individually. Although the zymography present data on pooled tissues, in all individual cases, an increase in gelatinolytic activity in thrombus compared to aneurysm tissue was observed. It can be suggested that increased CDPs in aortic aneurysm result from the action of gelatinases originated in mural thrombus accompanying the aneurysm tissue. The cellular composition of parietal thrombus is not constant. During the time of thrombus deposition, neutrophils, macrophages, lymphocytes and endothelial cells infiltrate the thrombus. The cells play an important role in the reorganization of thrombus and underlying aortic tissue. Sometimes, neo-vascularization of internal and medial wall of aneurysm may be observed (Holmes et al. 1995). It seems possible that cells present in thrombus may participate in forming aneurysm. This hypothesis is supported by the observation that most of proteases are synthesized in macrophages and granulocytes infiltrating the external and middle layers of the aneurysm wall. Increased levels of non-specific proteases in aneurysm serve probably for rapid utilization of CDP. It cannot be excluded that the non-specific enzymes may play an important role in gelatinase activation. Among MMPs found in the thrombus lining aneurysm wall, MMP-9 was found as a latent enzyme and only MMP-2 was found as an active form. However, the expression of MMP-2 was not different between thrombus, aneurysm and normal aorta, suggesting minor contribution of this MMP to CDP production in aneurysm tissue. In contrast, the expression of latent pro-MMP-9 was increased in thrombus, compared to other studied tissues. It can be speculated that the pro-MMP-9 from the thrombus is transported into the surrounding tissues, where it is activated. This mechanism may explain the increased CDP in aortic aneurysm that may reflect local increase in extracellular collagen degradation during the disease.
It cannot be excluded that disturbances of collagen metabolism in aortic aneurysm may to some extent result from deregulation of IGF-I homeostasis. IGF-I is considered as a potent stimulator of collagen biosynthesis (Oyamada et al. 1990), and the concentration of IGF-I in aortic aneurysm tissue is significantly decreased compared to that in control aorta. It could suggest a decrease in collagen biosynthesis and deposition in the tissue. However, insignificant changes in collagen content were found in aneurysm compared to control aorta. It is possible that the discrepancy may result from the increase in both IGFBP-1 and IGFBP-3 concentrations in aneurysm tissue. The IGF-I binding proteins may concentrate IGF-I in the tissue maintaining the required level for collagen biosynthesis. Therefore, it seems that disturbances in collagen metabolism in aortic aneurysm are not a result of deregulation of IGF-I homeostasis in this tissue. Thus, in summary, the data suggest that increased collagen degradation in aortic aneurysm is due to the increase in gelatinolytic activity in mural thrombus accompanying aneurysm tissue. It suggests that the mural thrombus may play critical role in the pathogenesis of abdominal aortic aneurysm.
References
- Adolph R, Vorp DA, Stad DL, Webster MW, Kameneva MV, Watkins S. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J. Vasc. Surg. 1997;25:916–926. doi: 10.1016/s0741-5214(97)70223-4. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Catabolism and turnover of collagens: collagenases. Methods Enzymol. 1987;144:140–150. doi: 10.1016/0076-6879(87)44177-3. [DOI] [PubMed] [Google Scholar]
- Bissel M. How does extracellular matrix direct gene expression? J. Theor. Biol. 1981;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Brophy CM, Reilly JM, Smith GJW, Tilson MD. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann. Vasc. Surg. 1991;5:229–233. doi: 10.1007/BF02329378. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kayser M, McLaughlin B, Weiss JB. Collagenase and gelatinase production by calcifying growth plate chondrocytes. Exp. Cell Res. 1993;208:1–9. doi: 10.1006/excr.1993.1216. [DOI] [PubMed] [Google Scholar]
- Campa JS, Greenhalgh RM, Powell JT. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis. 1987;65:13–21. doi: 10.1016/0021-9150(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Carey DJ. Control of growth and differentiation of vascular cells by extracellular matrix. Annu. Rev. Physiol. 1991;53:161–177. doi: 10.1146/annurev.ph.53.030191.001113. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Insulin-like growth factor binding proteins and their role in controlling IGF-actions. Cytokine Growth Factor Rev. 1997;8:45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF-actions. Mol. Cell. Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Dadgar L, Marois Y, Deng X, Guidoin R. Arterial wall mechanical characteristics after treatment in collagenase. Clin. Invest. Med. 1997;22:25–34. [PubMed] [Google Scholar]
- Gacko M, Glowinski S. Activities of proteases in parietal thrombus of aortic aneurysm. Clin. Chim. Acta. 1998;271:171–177. doi: 10.1016/s0009-8981(97)00246-5. [DOI] [PubMed] [Google Scholar]
- Gloviczki P, Pairolero P, Welch T, et al. Multiple aortic aneurysms: the results of surgical management. J. Vasc. Surg. 1990;11:19–27. doi: 10.1067/mva.1990.16620. [DOI] [PubMed] [Google Scholar]
- Henney AM, Adiseshiah M, Poulter N, MacSweeney STR, Greenhalgh RM, Poell JT. Abdominal aortic aneuysm. Lancet. 1993;341:215–220. [Google Scholar]
- Holmes DR, Liao S, Parks WC, Thomson RW. Medial neovascularisation in abdominal aortic aneurysm: a histopathologic marker of aneurysmal degradation with pathophysiologic implication. J. Vasc. Surg. 1995;21:761–772. doi: 10.1016/s0741-5214(05)80007-2. [DOI] [PubMed] [Google Scholar]
- Kang AH, Borstein P, Piez KA. The amino acid sequence of peptides from the cross-linking region of rat skin collagen. Biochemistry. 1967;6:788–795. doi: 10.1021/bi00855a019. [DOI] [PubMed] [Google Scholar]
- Kim HS, Nagalla SR, Oh Y, Wilson E, Roberts CT, Rosenfeid RG. Identification of a family of low-affinity insulin-like growth factor binding proteins (IGFBPs): characterization of connective tissue growth factor as a member of the IGFBP superfamily. Proc. Natl. Acad. Sci. USA. 1997;94:12981–12986. doi: 10.1073/pnas.94.24.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamson G, Giudice LC, Rosenfeid RG. Insulin-like growth factor binding protein: structural and molecular relationships. Growth Factors. 1991;5:19–28. doi: 10.3109/08977199109000268. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Werner H, Beitner-Johnson D, Roberts Jr CT. Molecular and cellular aspects of the insulin-like growth factor-I receptor. Endocr. Rev. 1995;16:143–164. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Levitt MS, Denyer GS, Cooney GJ, Baxter RC. Insulin-like growth factor-binding protein-1 modulates blood glucose levels. Endocrinology. 1991;129:2254–2256. doi: 10.1210/endo-129-4-2254. [DOI] [PubMed] [Google Scholar]
- Miles LEM, Lipschitz DA, Bieber CP, Cook JD. Measurement of serum ferritin by a 2-site immunoradiometric assay. Anal. Biochem. 1974;61:209–224. doi: 10.1016/0003-2697(74)90347-9. [DOI] [PubMed] [Google Scholar]
- Oyamada I, Palka J, Schalk EM, Takeda K, Peterkofsky B. Scorbutic and fasted guinea pig sera contain an insulin-like growth factor I-reversible inhibitor of proteoglycan and collagen synthesis in chick embryo chondrocytes and adult human skin fibroblasts. Arch. Biochem. Biophys. 1990;276:86–93. doi: 10.1016/0003-9861(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Palka J, Bird TA, Oyamada I, Peterkofsky B. Similar hormonal changes in sera from scorbutic and fasted (vitamin C-supplemented) guinea pigs, including decreased IGF-I and appearance of an IGF-I reversible mitogenic inhibitor. Growth Factors. 1989;1:147–156. doi: 10.3109/08977198909029124. [DOI] [PubMed] [Google Scholar]
- Palka J, Peterkofsky B. Salt stimulation of serum insulin-like growth factor binding protein activity. Anal. Biochem. 1988;175:442–449. doi: 10.1016/0003-2697(88)90568-4. [DOI] [PubMed] [Google Scholar]
- Prockop DW, Udenfriend S. A specific method for analysis of hydroxyproline in tissues and urine. Anal. Biochem. 1960;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen TE, Hallet JW. Inflammatory aortic aneurysms. Ann. Surg. 1997;225:155–164. doi: 10.1097/00000658-199702000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo RJ, McCarthy WJ, Dixit SN, et al. Collagen types and matrix protein content in human abdominal aortic aneurysms. J. Vasc. Surg. 1989;10:365–373. doi: 10.1067/mva.1989.13151. [DOI] [PubMed] [Google Scholar]
- Sara VR, Hali K. Insulin-like growth factors and their binding proteins. Physiol. Rev. 1990;70:591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- Sobolewski K, Wolańska M, Bańkowski E, Gacko M, Glowińnski S. Collagen, elastin and glycosaminoglycans in aortic aneurysms. Acta Biochim. Pol. 1995;42:301–308. [PubMed] [Google Scholar]
- Unemori EN, Werb Z. Reorganization of polymerized actin: A possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J. Cell Biol. 1986;103:1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A, Thompson MM, Crowther M, Sayers RD, Bell PRF. Pathogenesis of abdominal aortic aneurysms. Cellular and biochemical mechanisms. Eur. J. Vasc. Endovasc. Surg. 1996;12:391–400. doi: 10.1016/s1078-5884(96)80002-5. [DOI] [PubMed] [Google Scholar]
- Zatina MA, Zarins CK, Gewertz BL, Glasgow S. Role of median lamellar architecture in pathogenesis of aortic aneurysm. J. Vasc. Surg. 1984;1:442–448. [PubMed] [Google Scholar]