Abstract
Scarce information exists about the role of lung antigen-presenting cells (APCs) in vivo during pulmonary tuberculosis. As APCs activate cellular immunity, following intratracheal inoculation with virulent Mycobacterium tuberculosis, we assessed in situ lung APC recruitment, distribution, granuloma involvement, morphology and mycobacterial burden by using MHC-CII, CD14, scavenger receptor class A (SRA), the murine dendritic cell (DC)-restricted marker CD11c and Ziehl–Neelsen staining. CD11c+ DC and CD14+ cell recruitment into lungs appeared by day 14, continuing until day 60. MHC-CII+ cells increased since day 7, persisting until day 60. Thus, virulent mycobacteria delays (14–21 days) lung APC recruitment compared to model antigens and nonvirulent bacilli (24–48 h).
Regarding granuloma constitution, highly bacillary CD14+ and SRA+ cells were centrally located. MHC-CII+ cells were more peripheral, with less mycobacteria. CD11c+ cells were heterogeneously distributed within granulomas, with scarce bacilli.
When labelling lung suspensions for MHC-CII and classifying cells as macrophages or DC, then staining for Ziehl–Neelsen, a remarkable segregation was found regarding bacillary burden. Most macrophage-like cells contained numerous bacilli, while DC had no or scarce mycobacteria. This implies differential APC contributions in situ during pulmonary tuberculosis regarding mycobacterial uptake, granuloma involvement and perhaps bacillary growth.
Keywords: APC, DCs and macrophages, lung, Mycobacterium tuberculosis, pulmonary tuberculosis
Pulmonary tuberculosis, an infection caused by Mycobacterium tuberculosis has been declared a global health emergency by the World Health Organization (WHO). Tuberculosis is a re-emergent expanding disease, responsible for a great mortality and morbidity throughout the world. It has been estimated that one-third of the world's population is currently infected with M. tuberculosis, with 8 million new cases per year. Nevertheless, only 5–10% will develop the chronic pulmonary disease (Dye et al. 1999; WHO 2000). Most people will be able to control the infection through efficient immune responses, especially the cellular arm of immunity. However, the bacilli might not be eliminated completely and may persist dormant for years in a latent state, a phenomenon not well understood yet (Flynn & Ernest 2000; Hernández-Pando et al. 2000; Manabe & Bishai 2000). M. tuberculosis spreads from a person with pulmonary tuberculosis through aerosols, entering the body via the respiratory tract, reaching the lungs where phagocytic cells become infected, especially the alveolar macrophages (AMs). Apparently, M. tuberculosis can survive within AM for long-lasting periods, causing either a latent infection or subsequently developing the pulmonary disease (Wiegeshaus et al. 1989; Andersen 1997). This bacillus has evolved several mechanisms to evade the host immune responses, including the inhibition of antimicrobial activity of macrophages (Schluger & Rom 1998).
It is known that the first line of defence against M. tuberculosis is the AM, which interacts with the bacilli through different surface molecules like complement, mannose and Toll-like receptors (Arthur & Dannenberg 1991; Schluger & Rom 1998). Despite the fact that dendritic cells (DCs) are present throughout the respiratory tract, from the upper nasal mucosa to parenchymal lung tissue, little is known about lung DCs in vivo during tuberculosis, either in the naturally occurring disease or in experimental models of infection. Lung DCs are found within pleura and the alveolar septal walls; additionally they can be found, although in very small numbers, in the alveolar spaces (Holt & Schon–Hegrad 1987; McWilliam et al. 1995; Holt 2000).
In vitro assays have shown that monocyte-derived DCs and macrophages internalize the mycobacteria, resulting in DC maturation and activation with a differential production of cytokines. Interleukin-12 (IL-12) was almost exclusively produced by DCs, while macrophages produced IL-10 and the pro-inflammatory cytokines tumour necrosis factor-α (TNF-α), IL-1 and IL-6 (Mohagheghpour et al. 2000; Giacomini et al. 2001). M. tuberculosis grew equally well within nonactivated DCs and macrophages; however, activation with IFN-γ and LPS inhibited the growth of this intracellular microorganism. While macrophages can kill the intracellular bacteria, DCs seem unable to do so and, apparently, only restrict the growth of this bacillus (Bodnar et al. 2001). Experiments conducted in vivo with the M. bovis (BCG) showed that, following intravenous administration, bacilli were detected in similar percentage in splenic macrophages and in splenic DCs. After 2 weeks, BCG bacilli were still viable without apparent growth within DCs (Jiao et al. 2001).
In this study, using four different markers in situ, we describe the main lung antigen-presenting cells (APCs) in the susceptible BALB/c mice during experimental pulmonary tuberculosis, which is induced by intratracheal inoculation of the virulent strain of M. tuberculosis H37Rv. Pulmonary infection with M. tuberculosis induced an increase of parenchymal lung APC; these cells displayed differential tissue distribution and morphological features during the infection and, apparently, also a differential capacity to interact with bacilli in vivo.
Materials and methods
Experimental model of pulmonary tuberculosis in mice
The experimental model for murine pulmonary tuberculosis has been previously described in detail (Hernández-Pando et al. 1995; 1996). Briefly, male BALB/c mice from 6 to 8 weeks of age were used. M. tuberculosis strain H37Rv was cultured in Proskauer and Beck medium modified by Youmans. After 1 month of culture, bacilli were harvested in sterile endotoxin-free isotonic saline solution (EFISS), aliquoted and maintained at −70 °C until use. Before use, bacilli were adjusted at 10 × 106/ml and their viability was checked using fluorescein diacetate (Jarnagin & Luchsinger 1980). To induce the experimental pulmonary tuberculosis, mice were anaesthetized with intraperitoneal Pentothal (56 mg/kg), the trachea was exposed by a small midline incision and 100 µl of EFISS with 1 × 106 suspended viable bacilli was injected. The incision was then closed with sterile silk, and the mice were maintained in a vertical position until the effect of anaesthesia passed. Control mice were subjected to the same surgical procedure but were inoculated only with EFISS. Experiments were performed in a P3 biosecurity facilities in accordance to the institutional guidelines for animal care and experimentation. Three separate experiments were performed, and at least three to five mice were killed in each selected time point along the infection.
Lung immunohistochemistry
The lungs were perfused intratracheally with absolute ethanol and fixed for 24 h and were then sectioned through the hilus and embedded in paraffin. Lung sections of 5 µm thickness were mounted on silane (Sigma, St Louis, MO, USA)-treated slides and were then deparaffinized. Antigen retrieval was achieved with citrate buffer (Dako, CA, USA) in a pressure cooker at high pressure for 8 min. Endogenous peroxidase activity was blocked with 6% of H2O2 in PBS for 15 min. After that, lung sections were incubated with primary antibodies overnight at room temperature, at optimal dilutions, which were determined previously. The primary antibodies used were against MHC-CII I-A/I-E (Rat IgG2bk, clone M5/114.15.2, Pharmingen, San Diego, CA, USA), CD14 (Rat IgG1k, clone rmC5-3, BD Pharmingen) and scavenger receptor class A (SRA), type I and II, also known as CD204 (Rat IgG2b, clone 2F8, Serotec, Oxford, UK). Secondary, biotinylated antibody was used to detect the primary bound antibody, followed by HPR-conjugated streptavidin. Enzyme-linked antibody was revealed by reacting with 3,3′- diaminobenzidine (DAB)/H2O2 for 10–15 min at room temperature. Finally, tissue sections were stained with Ziehl–Neelsen or counterstained with haematoxylin. In order to evaluate the CD11c+ subset of cells, frozen sections were used instead of paraffin sections and the antibody used was biotinylated anti-CD11c (Armenian hamster IgG, clone HL3, BD Pharmingen).
Lung cell suspensions
Mice were killed, and the respiratory tract was surgically exposed. Lungs were perfused via the right ventricular cavity of the heart with 10 ml of PBS to remove the peripheral blood cells. Lungs were carefully removed to avoid bronchial tissue and then were cut in small pieces for enzymatic digestion in RPMI 1640, 5% FCS (Gibco, BRL, Carlsbad, CA, USA), 1 mg/ml of collagenase type 2 (Worthington Biochemical Corporation, Lakewood, NJ, USA) and 2 U/ml of DNase I (Gibco, BRL). Lung pieces were digested for 1.5 h at 37 °C. After this period, lungs were homogenized by pipetting with a Pasteur pipette and lung cell suspensions finally filtered through cell strainers (Falcon, BD). Lung cells were subjected to red blood cell lysis and washed and viability was determined with Trypan Blue exclusion. Bronchoalveolar lavage (BAL) was performed with 5 × 1 ml of sterile PBS.
Immunolabel of cells in cytospin preparations
Lung cell suspensions from M. tuberculosis-infected mice were subjected to metrizamide gradients in order to enrich for APC, specifically DCs. Enriched cells were fixed with paraformaldehyde and spun onto slides. Cytospin preparations were then dried and used for immunolabelling of MHC-CII molecules with the anti-I-A/I-E. Ziehl–Neelsen staining was subsequently performed to detect the bacilli.
Flow cytometry analysis
Lung cell suspensions were used to evaluate lung APC by FACS. The antibodies used were anti-CD11c-APC (Armenian hamster IgG, clone HL3, BD Pharmingen), anti-I-A/I-E FITC (Rat IgG2ak, clone 2G9, BD Pharmingen) and anti-SRA (Rat IgG2b, clone 2F8, Serotec). For analysis, 1 × 106 lung cells per well were used in 96-well, U-bottomed plates. Lung cell suspensions were incubated with the primary antibody for 30 min at 4 °C in the dark; cells were then washed three times and, in the case of nonlabelled antibodies, followed by incubation with antirat FITC antibody for 15 min at 4 °C. Finally, cells were washed and fixed with 4% paraformaldehyde to be analysed in a FACScalibur (Becton Dickinson, San Jose, CA, USA).
Results
Distribution of CD11c+ cells in lung tissue
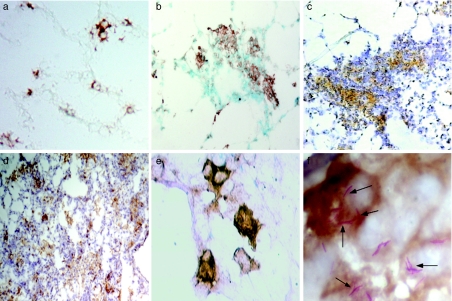
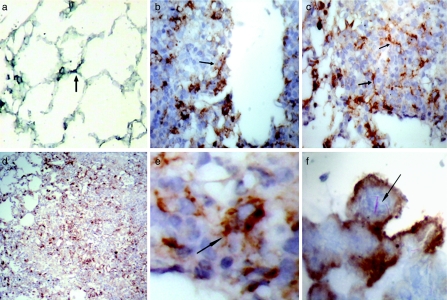
In order to evaluate the presence of DCs in lung tissue, we used antibodies to CD11c molecules, as mouse DCs are reported to specifically express this molecule (Lambrecht et al. 2001; Julia et al. 2002; Shortman & Liu 2002). Lung frozen sections from saline-inoculated control and M. tuberculosis-infected mice were used. In normal noninfected lung tissue, the CD11c+ cells are mainly localized within the interstitial alveolar walls; some of these cells have fine prolongations in the membrane (Figure 1a); a very small proportion of CD11c+ cells are found in the alveolar spaces (data not shown). In the M. tuberculosis-infected lung tissue, the CD11c+ cells were present in the tuberculous lesions, both in granulomas (Figure 1b, c) and in pneumonic areas (Figure 1d). The interstitial cells had a dendritic-like morphology, while the alveolar cells have a rather rounded shape (Figure 1e, f). Additionally, the number of CD11c+ cells in the alveolar spaces increased during the infection (Figure 1b–f), in comparison with the control noninfected tissue (Figure 1a). Red-coloured M. tuberculosis bacilli were detected by Ziehl–Neelsen, and the staining was preferentially observed in the alveolar CD11c+ cells (Figure 1f).
Figure 1.
Distribution of lung CD11c+ cells during experimental tuberculosis. Immunohistochemistry was performed to visualize the distribution and morphology of CD11c+ cells (brown label) in frozen sections from lungs of control, noninfected mice (a) and from Mycobacterium tuberculosis-infected mice, at different time points (b–f). In M. tuberculosis-infected lungs, CD11c+ cells were readily evident within the primary lesions at day 21 postinfection (b) and in granulomas at day 28 (c). CD11c+ cells were also present in the pneumonic areas at day 60 (d). Lung interstitial CD11c+ cells had a more evident dendritic morphology during infection (e). Frequently, alveolar CD11c+ cells might be seen containing M. tuberculosis bacilli in vivo (arrows,f)Magnifications: a–c ×200; d ×100; e ×400; f ×1000. Bacilli (red colour) were stained with Ziehl–Neelsen.
Lung MHC-CII+ cells during tuberculosis
Lung paraffin sections from healthy controls as well as from M. tuberculosis-infected mice were used to characterize the distribution of lung APC-expressing MHC-CII molecules. An antigen retrieval process was implemented aiming for a good detection of MHC-CII molecules. For this reason, most of the positive cells detected are MHC-CIIhigh. However, the antigen retrieval process affected the detection of bacilli by Zielh–Neelsen staining. Thus, for the colocalization of MHC-CII+ cells and the bacilli, the immunohistochemistry was performed in frozen sections.
In normal noninfected lung tissue, these cells were observed in the interstitial walls, similar to CD11c+ cells, displaying an elongated shape when they were located in the septal alveolar wall (arrow in Figure 2a). In the infected tissue, MHC-CII+ cells were both within granulomas (located rather in the periphery) (Figure 2b, c) and in the pneumonic areas (Figure 2d). These cells have a dendritic-like morphology (Figure 2e) and were observed surrounding the centre of the lesions, where big MHC-CII-negative cells with a large cytoplasm were arranged (Figure 2b, c). Regarding the colocalization of MHC-CII+ cells and red Ziehl–Neelsen+ bacilli, MHC-CII+ cells had a few or no bacilli inside (Figure 2f).
Figure 2.
MHC-CII+ cells in Mycobacterium tuberculosis-infected lungs. Paraffin sections of M. tuberculosis-infected lungs were subjected to antigen retrieval procedures to increase the label (brown colour) of positive cells. (a) MHC-CII+ cells in normal noninfected lung. (b–f) MHC-CII+ cells in M. tuberculosis-infected lungs at day 60. Frequently, MHC-CII+ cells in granulomatous lesions displayed a dendritic-like morphology (arrows in b, c and e) and were localized around the centre of these lesions and in pneumonic areas (d). (f) MHC-CII+ cells plus Ziehl–Neelsen staining in frozen sections, to show red stained bacilli (arrow) (×1000). Magnifications: a–c ×200; d ×100; E ×600.
The distribution of CD11c+ and MHC-CII+ was very similar in normal lungs, but in infected tissue, they had a differential localization, suggesting the presence of different subsets of cells inside the tuberculous lesions. One of these subsets (CD11c+/MHC-CII+) localized preferentially in the periphery of lesions, while the other subset (CD11c+/MHC-CII–) mainly in the centre of granulomas.
Lung macrophages display a concentric localization in the tuberculous lesions
CD14+ macrophages in normal lung tissue were mainly detected in the alveolar spaces and less frequently in the alveolar walls (Figure 3a). Expression of SRA in control noninfected tissue was practically negative (data not shown). The expression of CD14, as well as the number of positive cells, increased during infection with M. tuberculosis, with the distribution of these cells being rather concentric in lung lesions. Macrophages (CD14+ cells) were mainly in the centre of granulomas (Figure 3b, c). Also, the number of cells expressing SRA increased notably with the infection and had a similar distribution to the CD14+ cells (Figure 3d). Their appearance corresponded well to phagocytic cells and, like the CD14+ cells, had a great number of intracellular bacilli (Figure 3e, f).
Figure 3.
CD14+ and scavenger receptor class A+ (SRA+) macrophages accumulate at the centre of the lesions in Mycobacterium tuberculosis-infected lungs. CD14 and SRA were ascertained by immunohistochemistry in paraffin lung sections from noninfected mice(a)and M. tuberculosis-infected mice (b–f) at day 60 postinfection. CD14+ cells are located mainly in alveolar spaces in normal uninfected lung tissue (arrows in A) and increase during infection (b–c). CD14+ (b, c and e) and SRA+ cells (d and f) were localized mostly in the centre of granulomas. Both the CD14+ and the SRA+ cells contain a large number of bacilli, red staining in(e)and(f), respectively. Magnifications: a, b, c and d ×200; e and f ×400.
Cell influx to M. tuberculosis-infected lung tissue
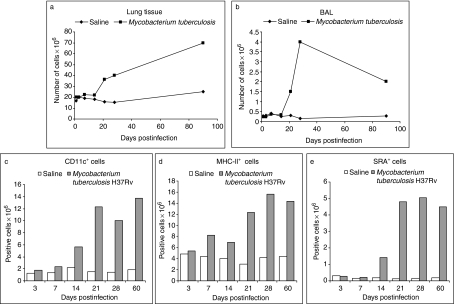
During the experimental pulmonary tuberculosis, an influx of cells seems to occur, resulting in a substantial increment of total cells within the lung tissue. Apparently, these cells arrive in interstitial and alveolar spaces. To quantify the cells in each compartment, first we obtained the cells from the alveolar spaces by bronchoalveolar lavage, and the remaining parenchymal lung tissue was subjected to digestion in order to obtain cell suspensions. Viable cells were then counted by Trypan Blue exclusion and reported at different times postintratracheal inoculation of either M. tuberculosis or endotoxin-free isotonic saline solution (control group). The total number of cells in lung parenchyma increased notably by day 21 in the M. tuberculosis-infected mice; this increase was still observed by day 90 postinfection (Figure 4a). In contrast, the number of cells in BAL fluid increased at the same time as in lung parenchyma, reached a peak at day 28, but then decreased after that, and by day 90, there were similar values to those at day 21 (Figure 4b). However, these cells were still sevenfold above the basal levels in the alveolar spaces, indicative of the reactions still ongoing within lung tissue.
Figure 4.
Kinetics of lung cells during Mycobacterium tuberculosis infection. The total number of cells increased within lung tissue in relation to the time postinfection(a), whereas in bronchoalveolar lavage (BAL), total cells apparently decreased after day 28(b)From day 21 postinfection, the changes in the number of cells are very obvious in M. tuberculosis-infected tissue compared to the control, noninfected group. The total number of CD11c+, MHC-CII+ and scavenger receptor class A+ cells in M. tuberculosis-infected lungs was measured by FACS (c–e).
Lung APC during M. tuberculosis infection
By means of flow cytometry, we quantified the lung APC using antibodies to CD11c, MHC-CII, SRA and CD14. CD11c+ cells increased slowly during M. tuberculosis infection, showing a significant increment at day 14 (Figure 4c), and continued to accumulate during the chronic phase of the infection. These cells reached their maximum values at day 21 postinfection, maintaining their high levels along the period of study, up to 60 days (Figure 4c). Lung MHC-CII+ cells also showed a minor elevation at early days, but the important increment was seen at day 21 in M. tuberculosis-infected mice. These cells increased over four times the basal numbers of the control group (Figure 4d). MHC-CII+ cells reached a peak at day 28 and were also maintained elevated by day 60 postinfection. To evaluate lung macrophages, we initially used anti-CD14 antibody, but in our hands, this marker was not very well detected by FACS. We then used the SRA to evaluate these cells in lung cell suspensions. SRA+ cells did not change until 2 weeks postinfection, when an elevation was already noticed, but the important increase was seen by day 21, approximately 16-fold over the control group (Figure 4e). Like the other two markers, SRA+ cells were also elevated during the chronic phase of the infection (Figure 4e).
Differential bacillary burden in lung APC
Lung sections from M. tuberculosis-infected mice, previously immunolabelled with antibodies against CD11c, MHC-CII, CD14 and SRA molecules, were stained with Ziehl–Neelsen, and the number of mycobacteria were counted in each positive cell (Table 1). CD14+ and SRA+ cells showed the higher percentage of cells bearing bacilli, 64 and 57%, respectively, in comparison with 36% for CD11c+ cells and 39% for MHC-CII+ cells. There were not statistically significant differences between CD11c+ and MHC-CII+ and between CD14+ and SRA+ cells regarding the bacillary load. However, there was a significant difference between CD14+ and CD11c+ (P < 0.001) or MHC-CII+ (P < 0.01) cells. In addition to the highest proportion of cells with bacilli, CD14+ APCs were also the group of cells with the largest number of intracellular bacilli per individual cell (Table 1), with a statistically significant difference vs. CD11c+ (P < 0.001), and MHC-CII+ (P < 0.005) cells.
Table 1.
Bacillary burden in lung antigen-presenting cells*
| Lung cells | % of positive cells bearing intracellular bacilli | No. of bacilli in positive cells for each marker |
|---|---|---|
| CD11c | 35.5 ± 7.5 | 2.9 ± 1.8 |
| MHC-CII | 39.1 ± 9.6 | 3.8 ± 3.7 |
| CD14 | 64.4 ± 8.1 | 5.9 ± 3.2 |
| SRA | 56.9 ± 10.6 | 5.1 ± 3.8 |
Only cells positive for the markers indicated were included.
Lung macrophages and lung DCs apparently displayed a differential bacillary burden in vivo. To asses the interaction between M. tuberculosis and these lung APCs, lung cell suspensions from H37Rv-infected mice were enriched by metrizamide gradients and then used for cytospin preparations. Cytospins were double stained for MHC-CII and then for Zielh–Neelsen. The morphological appearance was used as a basic criterion to discriminate between lung DCs and macrophages. Once cells were primarily classified as either macrophages or DCs, we then ascertained the association of each one to red, Ziehl–Neelsen+ bacilli. In these preparations, lung macrophages had a rounded shape, lacked long and fine cytoplasmic extensions and had intermediate positivity for MHC-CII staining (Figure 5a, b). Distinctively, these cells had also a great number of bacilli. The size of lung macrophages was variable, usually small macrophages had less bacilli than big macrophages. Frequently, big macrophages were observed as highly vacuolated cells (Figure 5b). In contrast, lung cells classified as DCs had a rather characteristic appearance with very delicate and extended cytoplasmic projections (Figure 5c–f). Unlike lung macrophages, only a very low proportion of lung DCs was found bearing Ziehl–Neelsen+ bacilli (arrows in Figure 5e, f). Moreover, this low percentage of bacilli+ cells had each very few intracellular bacilli, and their MHC-CII expression was much more intense than that observed in lung macrophages (Figure 5).
Figure 5.
Macrophages and lung DCs from cell suspensions of Mycobacterium tuberculosis-infected lungs at day 28 postinfection. Metrizamide-enriched APCs obtained from lung of M. tuberculosis-infected mice were used to prepare cytospin and were then stained for MHC-CII (brown) and Ziehl–Neelsen (red) for H37Rv bacilli. Macrophages frequently had large quantities of M. tuberculosis H37Rv Ziehl–Neelsen+ bacilli (a–b). DCs clearly express high levels of MHC-CII molecules, but usually contain only a few bacilli (arrows in e–f) (×1000).
Discussion
During experimental pulmonary tuberculosis in mice infected by intratracheal inoculation with the virulent strain H37Rv, we found an important influx of cells into the lung tissue. The timing of this cell influx is more apparent after 3 weeks of infection. We do not know the mechanism of this timing, but it may be related to the low growth rate of M. tuberculosis or to the presence of sui generis antigenic compounds such as lipids with immunoregulatory effects. In the first phase of pulmonary tuberculosis, bacillary growth is apparently controlled (Arthur & Dannenberg 1991), but 2 weeks after, a dramatic increase in the number of bacilli is noticed (Andersen & Heron 1993; Hernández-Pando et al. 1997). Perhaps more available bacteria infect more macrophages, provoking a strong inflammatory reaction. While the number of cells in lung parenchyma continued to increase during the infection, it decreases in BAL fluid in the chronic phase (Figure 4), but without reaching naïve levels. This reduction is probably both due to the consolidation of tuberculous lesions and to the reduced number of alveolar spaces, as the chronic inflammatory reaction proceeds. Indeed by day 90, cell quantities in BAL are still seven times over the normal levels. Alternative explanations should consider AM, because they are known to participate in the control of inflammatory responses within lung tissue (Holt et al. 1988; Bilyk & Holt 1993); perhaps this reflects a compensatory reaction during the unrestrained bacillary growth in chronic stages.
CD14+ pulmonary macrophages were preferentially localized in the centre of granulomas, a distribution also shared by the SRA+ cells. SRA was also detected by FACS to evaluate the influx of lung macrophages during experimental tuberculosis. SRA+ cells had an important increment until day 14 postinfection and reached a peak on day 28. These cells accumulated in the chronic stages forming an important part of the tuberculous lesions.
A high proportion of cells identified by these two markers, CD14 and SRA, were positive for intracellular bacilli, and each had a good bacillary burden (Figure 3e, f) (Table 1). This fact might be related to the high capacity of these cells for capturing and internalizing the bacilli or to differential competence of these cells to control the bacillary growth. Our group has previously reported (Hernández-Pando et al. 1997) that the bacillary burden in the lungs of infected BALB/c increases in relation to the time of infection. Although the main role of these cells would seem to control the bacteria, in this model of pulmonary tuberculosis, they are obviously unable to do so. It is likely that they were recruited by cytokines and chemokines released during the infection. In previously reported works (Andersen & Heron 1993; Chan et al. 1995; Hernández-Pando et al. 1996; Pinxteren et al. 2000), the production of IFN-γ, TNF-α and other TH1-like cytokines was observed and the activation of macrophages was also seen. In this model, the control of infection only seems to occur in the first stages, while in the chronic phase the mycobacteria seem to overcome this and spread throughout the lung tissue. Conceivably, this might be related to the delayed influx and activation of these cells into the site of infection, compared to the influx of APC reported in other tissues, under the influence of either model or other microbial antigens (McWilliam et al. 1996; Holt 2000; Lambrecht et al. 2001).
In normal noninfected lung tissue, CD11c+ cells were mainly localized in the interstitial walls. In the M. tuberculosis-infected lung tissue, the distribution was different and CD11c+ cells were present in tuberculous lesions and also in the alveolar spaces, in large numbers compared to the normal tissue (Figure 1). The morphology of CD11c+ cells located in the alveolar spaces corresponded to that of macrophages, big and rounded cells with a large cytoplasm. These alveolar CD11c+ cells were more positive for intracellular bacilli than the other CD11c+ cells localized in the interstitial walls. CD11c+ cells present in the interstitial inflammation had a more dendritic shape (Figure 1e). According to these results, we would favour the notion that alveolar CD11c+ cells might correspond to alveolar macrophages expressing CD11c (Gonzalez-Juarrero et al. 2003; Lagranderie et al. 2003), while interstitial CD11c+ cells correspond to lung DCs. This might help to explain why these CD11c+ cells are observed both in the periphery and in the centre of granulomas (Figure 1b, c) and the differential bacillary burden observed in comparison to CD14+ and SRA+ cells (Table 1). In line with this, human DCs have been reported in the periphery of granulomas in patients with tuberculosis (Uehira et al. 2002), a compartment rich in lymphocytes, suggesting potential in situ interactions between T cells and DCs, driving the local response.
CD11c+ cells increased during the M. tuberculosis infection. This would imply that DCs and macrophages are being attracted to the infected lung tissue during the infection, with the exception of the first 2 weeks, when only a minor influx of these cells occurred (Figure 4c). The total number of CD11c+ cells would correspond well to the addition of alveolar macrophages plus DCs, and it is conceivable that they might share a common monocytic precursor. We do not have a precise explanation for this delayed APC recruitment, but it might be related to the presence of M. tuberculosis in AM (putatively suppressors). Altered expression of chemokines and their receptors in lung tissue (Peters et al. 2001) might be another possibility affecting the flow of cells during the early phase.
MHC-CII+ cells, already present in normal noninfected lung, started to increase from day 7 postinfection and continued increasing during the chronic stage. This indicates that either APCs arrive in the affected tissue and are maintained there for a long time or local precursors are induced to mature locally. These cells were distributed around the centre of the tuberculous lesions, had a very characteristic morphology, most of them displaying long cytoplasmic prolongations (Figure 2e), like typical DCs. Interestingly, the cells in the centre of granulomas were usually negative for MHC-CII and exhibited a morphology more like macrophages. MHC-CIIhigh cells had only a few intracellular bacilli, and not all these cells were associated to M. tuberculosis (Figure 2f). These results suggest that DCs, which express high amounts of MHC-CII molecules, had a much lower proportion of bacilli than the cells with a typical macrophage morphology. This is also in accordance with reports indicating that DCs are less permissive to mycobacterial growth (Mohagheghpour et al. 2000; Tailleux et al. 2003). Trying to test this, cells in cytospin preparations from lung cell suspensions enriched for APC were classified according to their basic morphological appearance as lung macrophages or as lung DCs. Both expressed MHC-CII, but comparatively, DCs had a stronger expression than macrophages. Lung DCs were very difficult to find bearing intracellular bacilli, and the number of bacilli per individual cell was very low. In contrast, lung macrophages clearly had a great number of bacilli (Figure 5). Frequently, lung macrophages containing more bacilli were of bigger size. Likewise, in these circumstances, they were often multivacuolated cells.
These basic histopathological findings suggest to us a differential in situ role for lung APCs during the course of pulmonary tuberculosis. For instance, those lung macrophages seem more engaged in trying to control the bacillary growth, as they were mainly present in the core of the tuberculous granulomatous lesions, precisely where the bacillary burden is concentrated within lungs. Additionally, lung macrophages seem more capable of capturing a great number of bacilli, although ultimately they may not be able to control their growth. Conceivably, this could be due to a delayed arrival of other inflammatory or immune cells in the lung tissue. In contrast, DCs seem to be involved mostly in sampling the bacteria, as they had only a reduced number of intracellular bacilli. Another obvious possibility is that lung DCs are much less permissive to mycobacterial growth than lung macrophages are. The high expression of MHC-CII in DCs is likely related to presentation of antigenic determinants to lymphocytes. Certainly, more in vivo studies are required about the APC functions during this chronic pulmonary infectious disease.
Acknowledgments
We thank Victor Hugo Rosales-García (Cytometry Unit) and Selene Meza-Pérez for their excellent help. This work was supported in part by grants from CONACYT 30757-M (LF-R) and Conacyt G36923 (RH-P). Alexander Pedroza-González and Gina Stella García-Romo are fellow holders from CONACYT. Leopoldo Flores-Romo, Rogelio Hernández-Pando and Iris Estrada-García are SNI members.
References
- Andersen P. Host response and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand. J. Immunol. 1997;45:115–131. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- Andersen P, Heron I. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect. Immun. 1993;61:844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M, Dannenberg J. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol. Today. 1991;12(7):228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- Bilyk N, Holt PG. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulation factor. J. Exp. Med. 1993;177:1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar KA, Serbina NV, Flynn JL. Fate of Mycobacterium tuberculosis within dendritic cells. Infect. Immun. 2001;69:800–809. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Tanaka K, Carroll D, et al. Effect of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C, Scheele S, Dolin P, et al. Global burden of tuberculosis. Estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- Flynn J, Ernest JD. Immune responses in tuberculosis. Curr. Opin. Immunol. 2000;12:432–436. doi: 10.1016/s0952-7915(00)00116-3. [DOI] [PubMed] [Google Scholar]
- Giacomini E, Iona E, Ferroni L, et al. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 2001;166:7033–7041. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Shim TS, Kipnis A, et al. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J. Immunol. 2003;171:3128–3135. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- Hernández-Pando R, Jeyanathan M, Mengistu G, et al. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356:2133–2138. doi: 10.1016/s0140-6736(00)03493-0. [DOI] [PubMed] [Google Scholar]
- Hernández-Pando R, Orozco H, Honour J, et al. Adrenal changes in murine pulmonary tuberculosis; a clue to pathogenesis. FEMS Immunol. Med. Microbiol. 1995;12:63–72. doi: 10.1111/j.1574-695X.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Pando R, Orozco H, Sampiere A. Correlation between the kinetics of Th1-/Th-2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- Hernández-Pando R, Orozco H, Arriaga K, et al. Analysis of the local kinetics and localization of interleukin-1α, tumor necrosis factor-α and transforming growth factor-β, during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–617. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG. Antigen presentation in the lung. Am. J. Respir. Crit. Care Med. 2000;162:s151–s156. doi: 10.1164/ajrccm.162.supplement_3.15tac2. [DOI] [PubMed] [Google Scholar]
- Holt PG, Schon-Hegrad MA. Localization of T cells, macrophages and dendritic cells in rat respiratory tract tissue: implications for immune function studies. Immunology. 1987;62:349–356. [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Schon-Hegrad MA, Oliver J. MHC clas II antigen-bearing dendritic cells in pulmonary tissues of the rat: regulation of antigen presentation activity by endogenous macrophage populations. J. Exp. Med. 1988;167:262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin JL, Luchsinger DW. The use of fluorescein diacetate and ethidium bromide as stain for evaluating viability of mycobacteria. Stain Technol. 1980;55(4):253–258. doi: 10.3109/10520298009067249. [DOI] [PubMed] [Google Scholar]
- Jiao X, Lo-Man R, Guermonprez P, et al. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 2001;168:1294–1301. doi: 10.4049/jimmunol.168.3.1294. [DOI] [PubMed] [Google Scholar]
- Julia V, Hessel EM, Malherbe L, et al. A restricted subset of dendritic cells captures airbone antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- Lagranderie M, Nahori MA, Balazuc AM, et al. Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology. 2003;108(3):352–364. doi: 10.1046/j.1365-2567.2003.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Prins J-B, Hoogsteden HC. Lung dendritic cells and host immunity to infection. Eur. Respir. J. 2001;18:692–704. [PubMed] [Google Scholar]
- Manabe YC, Bishai WR. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat. Med. 2000;6(12):1327–1329. doi: 10.1038/82139. [DOI] [PubMed] [Google Scholar]
- McWilliam AS, Napoli S, Marsh AM, et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J. Exp. Med. 1996;184:2429–2432. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam AS, Nelson DJ, Holt PG. The biology of airway dendritic cells. Immunol. Cell Biol. 1995;73:405–413. doi: 10.1038/icb.1995.63. [DOI] [PubMed] [Google Scholar]
- Mohagheghpour N, Vollenhoven AV, Goodman J, et al. Interaction of Mycobacterium avium with human monocyte-derived dendritic cells. Infect. Immun. 2000;68:5824–5829. doi: 10.1128/iai.68.10.5824-5829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W, Scott HM, Chambers HF, et al. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2001;98(14):7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinxteren LAHV, Cassidy JP, Smedegaard BHC, et al. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 2000;30:3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Schluger NW, Rom WN. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 1998;157:679–691. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- Shortman K, Liu Y-J. Mouse and human dendritic cells subtypes. Nat. Rev. Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Tailleux L, Neyrolles O, Honore-Bouakline S, et al. Constrained intracellular survival of Mycobacterium tuberculosis in human dendritic cells. J. Immunol. 2003;170(4):1938–1948. doi: 10.4049/jimmunol.170.4.1939. [DOI] [PubMed] [Google Scholar]
- Uehira K, Amakawa R, Ito T, et al. Dendritic cells are decreased in blood and accumulated in granuloma in tuberculosis. Clin. Immunol. 2002;105(3):296–303. doi: 10.1006/clim.2002.5287. [DOI] [PubMed] [Google Scholar]
- WHO. Tuberculosis. Fact Sheet(104)
- Wiegeshaus E, Balasubramanian V, Smith DW. Immunity to tuberculosis from the perspective of pathogenesis. Infect. Immun. 1989;57(12):3671–3676. doi: 10.1128/iai.57.12.3671-3676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]