Abstract
It has previously been shown that dietary copper can modulate the extent of atherosclerosis in the thoracic aorta of cholesterol-fed rabbits. The metabolism of copper and zinc are closely related, and it has been hypothesized that the balance of dietary copper to zinc may be important in determining coronary risk. Hence, we have investigated the interaction between dietary copper and zinc in atherogenesis in the New Zealand White rabbit. Juvenile male rabbits were randomly allocated to eight groups. Four groups were fed a normal chow diet with zinc (0.5%, w/w), copper (0.2%, w/w), copper plus zinc or neither in their drinking water for 12 weeks. Four other groups were fed a diet containing 0.25–1% (w/w) cholesterol plus zinc, copper, both or neither. Serum cholesterol of individual animals was maintained at approximately 20 mmol/l.
Integrated plasma cholesterol levels were similar for all groups receiving cholesterol and significantly higher than those in the chow-fed groups (P < 0.001). Aortic copper concentrations were higher in the animals receiving cholesterol diets with copper compared to rabbits receiving normal chow and copper (P < 0.001). Aortic zinc content was significantly higher in cholesterol-fed rabbits supplemented with zinc alone or with copper than in those fed cholesterol alone (P < 0.001). Plasma ceruloplasmin concentrations were significantly higher in groups receiving cholesterol, irrespective of their trace element supplementation (P < 0.001). However, trace element supplementation increased the level significantly (P < 0.05). Trace element supplements did not appear to affect erythrocyte superoxide dismutase in the cholesterol-fed animals; however, zinc supplementation was associated with a significant increase in the enzyme in chow-fed animals (P < 0.05). The activity of the enzyme per mg of protein in aortic tissue was higher in animals receiving copper in the presence of cholesterol (P < 0.05) but not significantly so in its absence. Dietary trace element supplementation in cholesterol-fed animals was associated with a significant reduction in aortic lesion area. Plasma thiobarbituric acid-reactive substances and FOX concentrations were both significantly higher in the cholesterol-fed rabbits compared with the animals that fed on a chow diet (P < 0.001), and these were reduced significantly by dietary copper or zinc supplementation (P < 0.001). Hence, dietary supplements of copper or zinc at the doses used both inhibited aortic atherogenesis in the cholesterol-fed rabbits, although there was no significant additional effect when given in combination.
Keywords: antioxidants, atherosclerosis, cholesterol-fed rabbit, dietary copper and zinc, lipid peroxides
Copper and zinc are essential dietary nutrients (Linder 1991). Copper and zinc ions are involved in numerous metabolic reactions, forming part of the functional groups of several key enzymes (Ferns et al. 1997). Among these are enzymes that may be protective against atherosclerosis, including copper-zinc superoxide dismutase (Cu-Zn SOD), and endothelial nitric oxide synthase (eNOS).
Conversely, copper ions have been shown to accelerate the oxidation of low-density lipoprotein (LDL) in vitro, leading to the formation of oxidized LDL and other pro-atherogenic byproducts; and zinc may affect the bioavailability and metabolism of copper (Abdallah & Samman 1993), as well as the cellular oxidation of LDL (Wilkins & Leake 1994). Hence, the ratio of dietary copper to zinc may be an important determinant of coronary risk, a hypothesis first proposed by Klevay (1975). Although zinc may itself affect lipoprotein metabolism (Allen & Klevay 1978; Klevay 1980), this is contested for diets with a zinc content approaching that within the physiological range (Ficher et al. 1980; Frimpong & Magee 1987), and the association between serum copper concentration and serum total cholesterol is inconsistent (Hess et al. 1977; Sandstead et al. 1980; Manthey et al. 1981; Kromhout et al. 1985; Salonen et al. 1991; Iskra et al. 1993). It has also been reported that zinc status may affect LDL oxidisability (Gatto & Samman 1995) or superoxide dismutase activity (Abdallah & Samman 1993).
The interpretation of the association between blood trace element status and coronary risk is further complicated by the fact that caeruloplasmin, the major copper-containing plasma protein, is an acute phase reactant and is elevated in the presence of chronic inflammatory disease that may include atherosclerosis. It is therefore unclear whether these epidemiological data reflect a positive association between coronary heart disease and copper status per se or whether raised serum copper levels are an indication of an underlying inflammatory process.
We aimed to assess the effects of zinc and copper singly and together on atherogenesis in the cholesterol-fed rabbit model.
Materials and methods
Material
All reagents were of at least analytical grade and supplied by Sigma-Aldrich chemicals (Sigma-Aldrich Ltd, Ontario, Canada) unless indicated otherwise. All digestion tubes and any other glass or plastic ware used for trace element determination were cleaned by soaking overnight in 10% (v/v) hydrochloric acid, followed by thorough rinsing with deionized distilled water and drying. Aqueous solutions were made up in deionized distilled water.
Rabbit colonies
Weanling male New Zealand White rabbits (6–10 weeks old) were obtained from and housed within the animal house of The King Fahad Medical Research Center, King Abdul-Aziz University, Jeddah, Saudi Arabia. They were randomly allocated to one of eight dietary groups: chow, or cholesterol-fed with either plain water, or water containing 0.2% copper sulfate (w/w), 0.5% zinc sulfate (w/w) or both. Water and food were allowed ad libitum. Food intake was calculated on a daily basis, and weight was recorded at the start of the experiment and at fortnightly intervals.
Dietary groups
The chow diet was supplied by The Grain Silos and Flourmills Organization, Saudi Arabia. Rabbits were fed either a normal chow diet or a diet containing 0.25–1% (w/w) cholesterol. Cholesterol crystals were dissolved in peroxide-free diethyl ether, mixed with the diet and allowed to dry before feeding to the animals, as previously described. The cholesterol-containing diet was prepared in three concentrations: 0.25, 0.5 and 1%. This enabled the modification of the cholesterol content of the diets to maintain the serum cholesterol at approximately 20 mmol/l. The different cholesterol diets were prepared by mixing the 1% cholesterol diets with the corresponding chow diet to produce individually tailored diets with different cholesterol content. In the cholesterol-fed groups, serum cholesterol levels increased to approximately 20 mmol/l after an average of 4 weeks of commencing the diet containing 1% cholesterol. The serum cholesterol levels were measured at the start of the experiment and at fortnightly intervals.
The regular chow diet was analysed for its zinc and copper content in order to calculate the amount required to be added to the drinking water as a supplement. It was found to contain 21.05 µg of copper and 90.09 µg of zinc per gram of diet, respectively. Unsupplemented drinking water provided <4 µg of copper and <33 µg of zinc per day. This was supplemented with zinc as its sulfate at 0.5% (w/w) or with copper as its sulfate at 0.2% (w/w) or with both.
The dietary supplementation of copper was chosen on the basis of previous work that indicated that 0.2% copper supplementation in the drinking water would inhibit lesion formation (Lamb et al. 1999). The 0.5% zinc supplementation of drinking water was chosen because similar concentrations have been used previously in vivo in studies in rabbits, and it was hypothesized that the excess zinc would have a substantial effect on copper metabolism.
Total dietary copper and zinc in the chow-fed rabbits was approximately 3.7 mg/day of copper and approximately 15.8 mg/day of zinc. In the animals on copper supplementation, dietary copper was approximately 350 mg/day, and in the animals on zinc supplementation, dietary zinc was approximately 875 mg/day.
Blood sampling
Special precautions were taken while collecting fasting blood samples to avoid contamination with copper and zinc. Fasting blood was drawn from the marginal ear vein using a siliconized 24-gauge ‘Minicath’ intravenous catheter (Becton-Dickinson and Co. Rutherford, NJ, USA) into heparinized tubes. Samples were centrifuged within 2 h of collection at 1500 × g for 10 min at room temperature. Plasma was collected, the white buffy layer (leucocytes) was discarded and the erythrocytes were washed three times in 10 times its volume of cold saline. The erythrocytes were centrifuged at 10,000 × g for 15 min at 4 °C. The cells were collected after removal of the supernatant, lysed in four times their volume of ice-cold deionized water and stored at −80 °C until analysis.
Cholesterol measurement
Plasma cholesterol levels were determined using a cholesterol oxidase colourimetric kit (Crescent Diagnostics, Jeddah, Kingdom of Saudi Arabia). Readings were carried out on NovaSpec II, spectrophotometer of adjustable wavelength, model 80-2088-71 (Amersham Pharmacia Biotech, Athens, Greece).
Animal killing
After 12 weeks on the formulated diets, the fasting animals were anaesthetized with xylazine (3.5 mg/kg intramuscularly) and ketamine (18 mg/kg intramuscularly) (Alfasan, Woerden, Holland) and heparinized (300 IU/kg intravenously) (Sigma-Aldrich Ltd). An abdominal incision was made to access the abdominal aorta for insertion of a cannula connected to a perfusion apparatus. Rabbits were then killed with an overdose of pentobarbitone (Biochemie GmbH, Vienna, Austria) intravenously and the jugular veins transected for perfusion run-off. Rabbits were perfused with isotonic saline at a rate of 100–120 ml/min/kg body weight. When the run-off was clear, the saline was replaced with 4% paraformaldehyde in phosphate-buffered saline (PBS) at the same flow rate in half the animals from each group. Perfusion was continued for 15 min. The other half, whose tissues were used for tissue enzyme determinations, was perfused with isotonic saline. Following perfusion, the entire thoracic aortae were isolated and carefully cleaned of fascia to avoid stretching and endothelial damage. The aorta was divided longitudinally into halves, pinned, lumen side up and divided into segments.
Tissue processing and histological staining
Five-micron sections of aortic arch were taken at the level of the first and seventh intercostals and fixed in 4% paraformaldehyde overnight prior to paraffin embedding.
Segments from the ascending aorta, the descending aorta and at the eighth intercostal artery branch point were frozen at −70 °C for the determination of trace element content (copper and zinc) and enzyme activity (superoxide dismutase).
Segments from the ascending aorta and at the second intercostal artery branch point from perfusion-fixed aortae were placed at room temperature in 2% glutaraldehyde (Pelco, Redding, CA, USA). Tissue segments for scanning electron microscopy (SEM) were postfixed in 2% osmium tetroxide (Pelco). After dehydration in a graded ethanol series, the segments were critical point dried with carbon dioxide in a critical point drier (Pelco CPD-2, Ted Pella, Inc.) and sputter coated with gold using a Polaron sputter coater (SEM coating E5100, Polaron Equipment limited, Watford, UK). They were then examined using a SEM (XL20, Philips, Holland) at 10 kV. Segments taken from the third upto the sixth intercostal artery branch point were placed in 4% paraformaldehyde, and the extent of atherosclerosis (quantification of lesional area) were determined by staining with oil red O (Sigma-Aldrich Ltd) (Rutherford et al. 1997). The sections were pinned out onto a corkboard and photographed.
Quantification of lesional area
Images were acquired using a JVC CCD camera. The area staining positively with oil red O was quantified using Qwin 550C image analysis software (Leica Microsystems, Cambridge, Cambridgeshire, UK) and expressed as a percentage of the total area analysed as previously described (Lamb et al. 1999).
Plasma ferroxidase activity
The ferroxidase activity of ceruloplasmin was determined by using o-dianisidine as a substrate (Schosinsky et al. 1974). Duplicate plasma samples, each of 0.05 ml, were added to 0.75 ml of 0.1 m acetate buffer pH 5.0. The substrate, 0.2 ml of 7.88 m o-dianisidine 2HCL (Sigma chemical, St Louis, MO, USA), was added and the reaction terminated in the first tube after 5 min and after 15 min in the second tube with 2 ml of 9 m sulfuric acid. The absorbance of both solutions was measured at 540 nm vs. water as a blank and then subtracted. Readings were carried out on NovaSpec II, spectrophotometer of adjustable wavelength, model 80-2088-71 (Amersham Pharmacia Biotech). The activity was calculated using the molar absorption coefficient of the oxidized substrate (9.6 ml/µmol/cm) and expressed as IU/l.
Tissue and plasma trace element content
All reagents were treated with Chelex-100 prior to use. Serum samples were diluted 1 : 10 with deionized distilled water. Tissue samples (0.5 g) from liver, kidney, muscle and (5–15 mg) aorta were dissolved in 7 ml of 3.5 m nitric acid by heating to 150 °C for 30 min in a Techator digestor (Perstop Analytical Ltd, Bristol, UK). When cool, 1 ml of 11.6 m perichloric acid was added and all tubes heated to 150 °C for 30 min, 200 °C for 15 min and 250 °C for 15 min and then allowed to cool. The volume was made to 10 ml with 1% nitric acid. The copper and zinc content was measured by flame atomic absorption spectrophotometer on a Perkin-Elmer model 5000–flame (Perkin-Elmer Corp., Norwalk, CT, USA) equipped with an air–acetylene flame burner. Copper and zinc hollow cathode lamps (Perkin-Elmer Corp.) were operated at 20 mA for copper and at 15 mA for zinc. Atomic absorption measurements were made at a wavelength of 324.8 nm for copper and at 213.9 nm for zinc. Stock atomic absorption standard solutions of copper or zinc (Sigma chemical) containing from 0.05 to 1 mg/l were diluted with 10% (v/v) glycerol (Puchades et al. 1989) to obtain a standard curve. A 10% (v/v) glycerol solution was used as a blank solution as instructed by the manufacturer.
Tissue and erythrocytes lysate antioxidant enzyme activity
Erythrocyte lysates were prepared as described above. For preparation of the aortic supernatant, small frozen sections of aorta (5–15 mg) or liver were rinsed in 50 mm phosphate buffer saline, pH 7.4, containing 0.3 m potassium bromide and 3% (v/v) proteases cocktail inhibitor (Sigma, Poole, Dorset, UK). Tissues were then homogenized in 4–8 volume (v/w) of 50 mm Tris-HCL (pH 7.5, containing 5 mm EDTA and 1 mm 2-mercaptoethanol), using a microhomogenizer. The homogenate was then sonicated for 5 min at full power in an Ultrawave sonicating water bath (Philip Harris Scientific, London, UK) and the supernatant retained following centrifugation at 10,000 × g for 20 min at 4 °C. The enzyme activity was expressed as U/mg protein in tissue samples. Protein content of the tissue homogenates was measured by the method of Lowry et al. (1951).
The enzyme activity was expressed as U/g haemoglobin in the erythrocyte haemolysates. Haemoglobin concentration was determined in a 1 : 20 dilution of erythrocyte haemolysates prepared as previously described, using a 96-well plate reader (Molecular devices-VMAX, kinetic microplate reader, Sunnyvale, CA, USA). The haemoglobin assay kit (Crescent Diagnostics) is based on the colourimetric cyanmethemoglobin method.
Superoxide dismutase activity
SOD activity was measured in erythrocyte lysates and tissue samples using a Randox kit (Randox Laboratories Ltd, Crumlin, County Antrim, Northern Ireland). This is specific for the Cu/Zn SOD at the pH employed for the assay (pH 10.2). The enzyme activity was assessed by its ability to inhibit the reduction of 2-(4-iodophenyl)-3-(4-nitophenol)-5-phenyltetrazolium chloride (INT) as previously described (Marklund 1976). The activity was expressed in U/g of haemoglobin in erythrocytes and in U/mg of protein in aortic and liver tissues. One unit of enzyme activity was defined as the activity of SOD required for 50% inhibition of the reaction.
Lipid peroxides
Plasma lipid peroxides were measured by the FOX assay (Nourooz-Zadeh et al. 1995) or thiobarbituric acid reactive substances (TBARSs) (Ohkawa et al. 1979). All readings were carried out on a NovaSpec II spectrophotometer of adjustable wavelength (model 80-2088-71).
One hundred microlitre of plasma was added to 900 µl of FOX reagent (100 µm xylenol orange, 250 µm Fe2+, 25 mm sulfuric acid and 4 mm butylated hydroxytoluene in 90% (v/v) methanol), and subsequently, the absorbance was read at 560 nm (ɛ = 4.3 × 104/m/cm) (Nourooz-Zadeh et al. 1995).
Aldehyde products of lipid peroxidation were measured by the TBARS method (Ohkawa et al. 1979). Plasma samples of 0.5 ml were incubated with 0.25 ml of 1% (w/v) 2-thiobarbituric acid (Sigma chemical) in 50 mm NaOH and 0.25 ml of 30% (w/v) trichloroacetic acid in a boiling water bath for 15 min to prevent lipid peroxidation; 25 µl of 0.88% of butylated hydroxytoluene in ethanol was added to the samples. The solutions were then cooled in ice for 5 min, after which the absorbance at 535 nm was measured. The molar extinction coefficient, as determined from the standard samples, was 1.56 × 105/m/cm. This was used to calculate the concentrations in samples.
Precision of enzyme assays
Control samples were analysed with each assay to assess analytical day-to-day precision. Within-batch imprecision of all assays were below 8%, whereas between-batch imprecision coefficient of variation (CVs) of all assays was below 10%.
Statistical analysis
The results obtained were either expressed as mean ± SD for normally distributed parameters or as a median and range for non-normal distributed parameters. Statistical analyses were performed using Wilcoxon signed rank test for nonparametric data when comparing within the same group or Mann–Whitney U-test when the comparing is between two different groups for nonparametric data. A Kruskal–Wallis test was performed to compare mean values of repeated measures of non-normally distributed parameters. A Bonferroni correction was made for multiple comparisons. Statistical significance was assumed with a P value <0.05. All analyses were performed using spss (version 10) software.
Results
Changes in weight and blood cholesterol
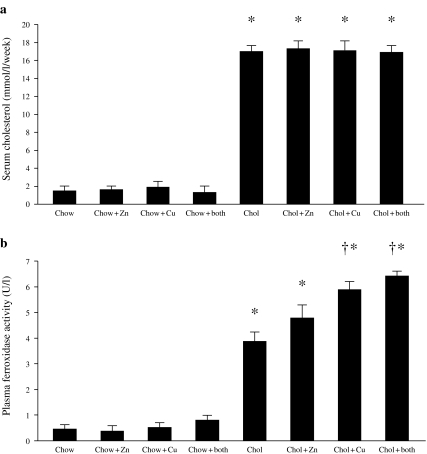
All diets were well tolerated; however, animals receiving the control chow gained significantly more weight over the duration of the experiment compared with the supplemented diets (P < 0.05). Weight gain did not differ significantly in animals receiving cholesterol with or without trace elements. Integrated blood cholesterol values were similar for all the cholesterol-fed animal groups and were also comparable for all the groups of animals receiving normal chow (Figure 1a). As would be expected, integrated serum cholesterol levels were significantly higher in all animals receiving the cholesterol-enriched diet compared with all groups of animals on normal chow (P < 0.001 in all cases). The integrated cholestero value was derived by calculating the mean serum cholesterol value for each animal over the period of the measurement.
Figure 1.
Integrated plasma cholesterol (a) and caeruloplasmin (ferroxidase activity) (b) in rabbits that were fed with chow or cholesterol diets and receiving either copper, zinc, copper and zinc or no additions to their drinking water. *P < 0.001 (all cholesterol vs. all chow diets); †P < 0.05 (cholesterol + copper; cholesterol + Cu + Zn vs. cholesterol alone).
Blood chemistry
Effects of diets on plasma trace element concentrations
The mean plasma copper concentration was significantly higher in the copper-supplemented animals, whether cholesterol was added (P = 0.001) or not (P < 0.05) compared to the nonsupplemented animals, and the presence of zinc did not have any significant effect on plasma copper concentration (P > 0.05) (Table 1). Similarly, the mean plasma zinc concentration increased significantly in supplemented animals in the presence or absence of cholesterol (P = 0.001), and the presence of copper did not have any effect on plasma zinc concentration (P > 0.05).
Table 1.
Serum parameters in rabbits that were fed with chow or cholesterol diets and receiving either copper, zinc, copper and zinc or no additions to their drinking water
| Chow | Chow + Zn | Chow + Cu | Chow + Zn + Cu | Cholesterol | Cholesterol + Zn | Cholesterol + Cu | Cholesterol + Zn + Cu | |
|---|---|---|---|---|---|---|---|---|
| n | 8 | 7 | 8 | 7 | 8 | 8 | 8 | 8 |
| Copper (µmol/l) | 4.11 ± 1.6 | 5.5 ± 2.9 | 7.04 ± 2.5* | 7.58 ± 1.2* | 6.32 ± 1.4* | 7.64 ± 1.9 | 11.3 ± 1.9§ | 10.3 ± 3.0‡ |
| Zinc (µmol/l) | 12.9 ± 1.2§ | 24.9 ± 2.8† | 12.7 ± 5.9 | 24.4 ± 5.3† | 8.39 ± 1.4 | 22.1 ± 4.3§ | 10.9 ± 3.8 | 23.0 ± 3.4§ |
| TBARS (µM) | 0.24 ± 0.01 | 0.28 ± 0.01 | 0.28 ± 0.01 | 0.23 ± 0.01 | 0.61 ± 0.02† | 0.36 ± 0.04†§ | 0.33 ± 0.04†§ | 0.42 ± 0.02†§ |
TBARS, thiobarbituric acid reactive substances.
P < 0.05 (vs. chow).
P < 0.001 (vs. chow).
P < 0.05 (vs. cholesterol alone).
P < 0.001 (vs. cholesterol alone).
Plasma caeruloplasmin: Plasma caeruloplasmin levels (measured as ferroxidase activity) were significantly higher in animals receiving a cholesterol-enriched diet, irrespective of their trace element supplementation (P < 0.001). Among the cholesterol-fed animals, plasma ferroxidase activity was significantly higher in animals receiving copper in the presence or absence of zinc compared to those receiving cholesterol alone (P < 0.05) (Figure 1b).
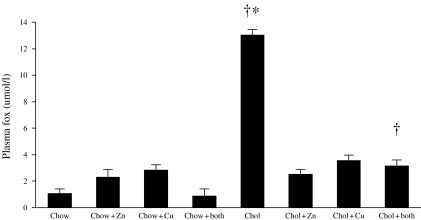
Plasma lipid peroxides: Plasma lipid peroxides, estimated by the FOX assay, were significantly higher in the animals on the cholesterol diet compared to animals on chow diets with or without zinc or copper supplements (P = 0.001) (Figure 2). Levels were significantly lower in the cholesterol-fed animals receiving copper or zinc supplements compared to those on no supplements (P < 0.001) (Figure 2). Similar results were obtained for plasma TBARS (Table 1).
Figure 2.
Plasma levels of lipid peroxides estimated by the FOX assay in rabbits that were fed with chow or cholesterol diets and receiving either copper, zinc, copper and zinc or no additions to their drinking water. *P < 0.001 (cholesterol alone vs. cholesterol + copper; cholesterol + zinc and cholesterol + copper and zinc); †P = 0.001 (all cholesterol vs. all chow diets).
Effects of diets on blood antioxidant enzymes
Erythrocyte superoxide dismutase: The mean erythrocyte superoxide dismutase was significantly higher in the cholesterol-fed rabbits compared with those on normal chow diet (P < 0.01). Supplements with copper or zinc individually or together did not appear to affect erythrocyte superoxide dismutase in the cholesterol-fed animals (Table 2); however, zinc supplementation was associated with a significant increase in erythrocyte superoxide dismutase in the chow-fed animals (P < 0.05). A nonsignificant increase was also noted due to supplementation with copper alone or together with zinc in chow-fed animals.
Table 2.
Copper and zinc levels in liver and aortae in rabbits that were fed with chow or cholesterol diets and receiving either copper, zinc, copper and zinc or no additions to their drinking water
| Chow | Chow + Zn | Chow + Cu | Chow + Zn + Cu | Cholesterol | Cholesterol + Zn | Cholesterol + Cu | Cholesterol + Zn + Cu | |
|---|---|---|---|---|---|---|---|---|
| n | 8 | 7 | 8 | 7 | 8 | 8 | 8 | 8 |
| Erythrocyte SOD (U/g Hb) | 164 ± 42.4 | 232.3 ± 29.3† | 196.9 ± 43.3 | 197.9 ± 60.1 | 245 ± 51.1* | 260.9 ± 33.8 | 238.9 ± 13.5 | 217 ± 55.6 |
| n | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| Aorta SOD (U/mg protein) | 2.08 ± 0.11 | 2.11 ± 0.04 | 3.88 ± 1.17 | 2.69 ± 0.77 | 1.82 ± 0.41 | 2.16 ± 0.4 | 3.5 ± 1.43‡ | 2.29 ± 0.2 |
| Liver SOD (U/mg protein) | 0.77 ± 0.1 | 1.22 ± 0.04* | 1.94 ± 0.62* | 2.06 ± 0.39* | 1.37 ± 0.97 | 1.47 ± 0.78 | 1.58 ± 0.8 | 2.65 ± 1.5 |
P < 0.01 (vs. chow).
P < 0.001 (vs. chow).
P < 0.05 (vs. cholesterol alone).
Tissue chemistry
Effects of the diets on tissue copper and zinc concentrations
Supplementation with copper or zinc or both caused a significant increase (P < 0.05 and P < 0.001, respectively) in liver copper content in chow-fed rabbits (Table 3). The addition of cholesterol to the diet caused a significant increase in hepatic copper as well (P < 0.05). Moreover, in these animals, supplementation with copper alone or with zinc caused a nonsignificant increase in hepatic copper content. The mean copper content of the aorta was significantly higher in cholesterol-fed rabbits supplemented with copper alone (P = 0.001) or with copper and zinc (P < 0.05) than those that were fed with cholesterol alone. No significant effects of either copper or zinc were observed in the chow-fed rabbits (Table 3).
Table 3.
Superoxide dismutase activity in erythrocytes, aortae and liver in rabbits that were fed with chow or cholesterol diets and receiving either copper, zinc, copper and zinc or no additions to their drinking water
| Chow | Chow + Zn | Chow + Cu | Chow + Zn + Cu | Cholesterol | Cholesterol + Zn | Cholesterol + Cu | Cholesterol + Zn + Cu | ||
|---|---|---|---|---|---|---|---|---|---|
| n | 8 | 7 | 8 | 7 | 8 | 8 | 8 | 8 | |
| Cu | Liver (µg/g wet tissue) | 2.24 ± 0.83 | 3.63 ± 0.53* | 7.84 ± 3.58* | 5.18 ± 1.12† | 3.4 ± 1.33* | 3.5 ± 1.45 | 4.08 ± 1.48 | 4.65 ± 1.36 |
| Aorta (µg/g wet tissue) | 1.95 ± 0.82 | 1.9 ± 0.68 | 2.4 ± 0.59 | 2.24 ± 1.18 | 1.49 ± 0.48 | 1.46 ± 0.32 | 4.66 ± 1.25§ | 3.79 ± 1.39‡ | |
| Zn | Liver (µg/g wet tissue) | 25 ± 6.83 | 41.8 ± 8.98* | 28.3 ± 3.72 | 41.3 ± 7.83* | 25.5 ± 5 | 29.3 ± 1.72 | 27.4 ± 3.57 | 31.1 ± 2.86‡ |
| Aorta (µg/g wet tissue) | 1.5 ± 0.15‡ | 2.84 ± 1.27* | 1.04 ± 0.65* | 4 ± 0.97† | 0.51 ± 0.36 | 3.71 ± 0.59§ | 1.01 ± 0.67 | 2.92 ± 1.41‡ |
P < 0.01 (vs. chow).
P < 0.05 (vs. chow).
P < 0.05 (vs. cholesterol alone).
P < 0.001 (vs. cholesterol alone).
The hepatic zinc content was significantly higher in cholesterol-fed rabbits supplemented with zinc plus copper than in those that were fed with cholesterol alone. The mean of hepatic zinc of those fed with regular chow and supplemented with zinc alone or plus copper was significantly higher than the mean of the nonsupplemented group (P < 0.05) (Table 3). The mean zinc content of the aorta was significantly higher in cholesterol-fed rabbits supplemented with zinc alone or with both zinc and copper (P < 0.001) than in those that were fed with cholesterol alone (Table 3). Similarly, supplementation with zinc or copper or both caused a significant increase in aortic zinc content in the chow-fed rabbits.
Effects of diets on tissue antioxidant enzyme concentrations
The activity of superoxide dismutase per mg of protein in aortic tissue was higher in animals receiving copper supplements in the presence of cholesterol (P < 0.05) but not significantly so in its absence (Table 2). Supplementation with copper or zinc or both caused a significant increase in hepatic enzyme activity in the absence of cholesterol (P < 0.05). However, no such effect was noted in the cholesterol-fed animals. Although supplementation with both copper and zinc seemed to increase the level but due to small number of animals per group, no significance was found (Table 2). Furthermore, hepatic superoxide dismutase per mg of protein was significantly higher in cholesterol-fed animals receiving zinc supplement compared to rabbits receiving normal chow and zinc (P = 0.05) (Table 2).
Effects of copper and zinc supplementation on atherosclerosis in the thoracic aorta of cholesterol-fed rabbits
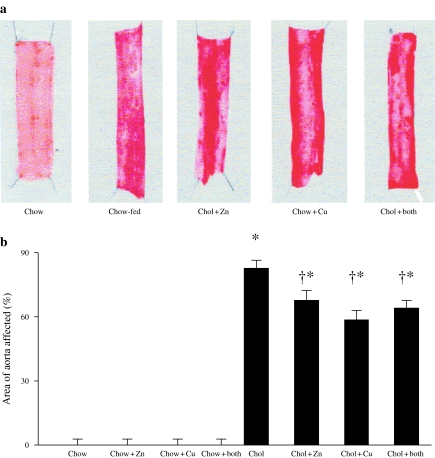
Oil Red O staining
Longitudinal segments of thoracic aorta were stained with oil red O for quantification of regions of aortae containing macroscopic lesions. Figure 3a shows typical aortae from a rabbit fed with control chow and from rabbits in the cholesterol-fed groups with or without dietary copper and/or zinc supplements. The percentage of the aortae staining positively for lipid using oil red O in all the chow-fed groups was essentially nil. In the rabbits receiving cholesterol alone, the area affected was 82.8 ± 4.8% compared to 58.6 ± 7.0% in animals receiving concomitant copper (P = 0.01) and 67.7 ± 5.6% in those receiving zinc (P < 0.05). The animals receiving both copper and zinc had a mean value of 64 ± 7.3, and this did not differ significantly from those animals receiving a single supplement (P > 0.05) (Figure 3b).
Figure 3.
Representative section of aortae stained with oil red O (a) and the percentage en face aortic area covered by oil red O staining (b) in rabbits that were fed with chow or cholesterol diets and receiving either copper, zinc, copper and zinc or no additions to their drinking water. *P < 0.001 (all cholesterol vs. all chow diets); †P < 0.05 (cholesterol + copper; cholesterol + zinc vs. cholesterol alone).
Electron microscopy
The cholesterol-fed group of rabbits developed the typical morphological features of atherosclerosis, with leucocyte adherence at sites of predeliction, and fatty-streak formation, particularly around and between intercostal branch points. There were no gross differences in morphology between the groups (data not shown).
Discussion
We have previously described a biphasic relationship between dietary copper intake and atherosclerosis in the cholesterol-fed rabbit (Lamb et al. 2001). It has also been suggested that the balance between dietary copper and zinc modulates plasma cholesterol concentrations (Klevay 1975). In Klevay's original Copper/Zinc hypothesis, the ratio between the two was proposed as a risk factor of coronary disease in man, this being mediated in part by its effects on lipoprotein metabolism (Klevay 1975; Mielcarz et al. 1997). Recent data from Abiaka et al. (2003) have challenged the hypothesis.
There have been previous studies on the individual effects of copper, or zinc status on the vasculature in rodents; however, most of these have focused on trace element deficiency (Shields et al. 1962; Coulson & Carnes 1965; Hunt & Carlton 1965; Petering et al. 1986; Vlad et al. 1993; Pucheu et al. 1995; Disilvestro & Blostein-Fujii 1997; Hamilton et al. 2000).
Although the original hypothesis of Klevay (1975) suggested that it was the effect of the balance of dietary copper and zinc on blood cholesterol levels that contributed to atherogenesis, other putative mechanisms for the effects of these trace elements on atherogenesis have been proposed (Hennig et al. 1992; Wilkins & Leake 1994; Disilvestro & Blosteinfujii 1997; Ferns et al. 1997; Wu et al. 1998). Because of the possibility of interactions between dietary copper and zinc, we investigated the effects of the balance between dietary copper and zinc on atherogenesis in dietary sufficiency, or excess, using a well-established model of atherogenesis.
Dietary supplements of zinc or copper reduced the extent of atherosclerosis
In rabbits matched for integrated levels of plasma cholesterol, we found that dietary supplementation of either zinc or copper was associated with a reduction in extent of atherosclerosis. The effects of copper supplementation were consistent with our previous results (Lamb et al. 1999). The reduction in the extent of atherosclerosis associated with zinc supplementation was of a similar magnitude as that seen in the copper-supplemented rabbits. However, no synergy was observed in the group of animals receiving both supplements. This may indicate that the protective effects are being mediated by similar or related mechanisms.
Effects of experimental diets on tissue and plasma trace elements
Dietary copper or zinc supplementation was associated with increased plasma copper and zinc levels, respectively, in both chow- and cholesterol-fed rabbits. When both trace elements were concomitantly administered, no significant reciprocal effect on plasma levels was observed; similar plasma levels of zinc or copper were attained as for animals receiving a single supplement. Hence, the levels of dietary copper and zinc supplementation used in this study were not associated with significant reciprocal interference in absorption as previously suggested (Lamb et al. 1997). Hepatic tissue copper levels were significantly increased in the chow-fed rabbits receiving copper supplements in the presence or absence of zinc. This is expected, as the liver is the main organ involved in whole body copper and zinc metabolism. Nevertheless, dietary copper supplementation was associated with a significant increase in aortic copper in the cholesterol-fed rabbits. This was associated with an elevated level of plasma caeruloplasmin, which might be related to increased inflammatory response in this group. Increased endothelial cell permeability reported in inflammation would allow caeruloplasmin-associated copper to accumulate in the vascular wall. Caeruloplasmin may contribute to LDL oxidation (Ehrenwald & Fox 1996), and the higher levels of caeruloplasmin in the copper-supplemented cholesterol-fed rabbits may have been expected to exacerbate the atherosclerotic process. This was not found to be so in our study and may be related to effects on aortic superoxide dismutase level (discussed further below).
Levels of hepatic zinc were higher in association with zinc supplementation in chow-fed rabbits but not significantly so in cholesterol-fed rabbits. However, dietary supplementation with both elements was associated with a modest increase in hepatic zinc in the presence or absence of cholesterol. Whether this was due to modified tissue kinetics remains to be clarified; however, the interaction between dietary copper, zinc and cholesterol in relation to both copper and zinc absorption, transport and tissue distribution appears complex.
In aortic tissue, zinc supplementation increased zinc accumulation in the presence or absence of cholesterol, even though there was no concomitant increase in aortic superoxide dismutase activity. This might suggest a different mechanism for the inhibitory effect of zinc supplementation on the atherogenesis process, as zinc has been reported to have a direct antioxidant effect in vitro (Wilkins & Leake 1994).
Effects of experimental diets on tissue and plasma SOD and lipid peroxides
Atherogenesis, at least in experimental animal models, is associated with oxidative stress. Biochemical markers of LDL oxidation are found in plasma and plaque tissue, and pro-atherogenic genes are induced in vascular cells by oxidative stress (Parthasarathy et al. 1992). Our two measures of lipid peroxidation, TBARS and the FOX assay, showed consistent changes in the different groups of rabbits. These assays showed that single supplementation with either copper or zinc was associated with a reduction in plasma lipid peroxides. We have previously reported a reduction in plasma lipid peroxide levels with copper supplementation (Lamb et al. 1999). Zinc deficiency was associated with an increased susceptibility of LDL oxidation in the rat (Disilvestro & Blosteinfujii 1997). Furthermore, zinc deficiency is associated with impaired endothelial cell barrier function (Hennig et al. 1992) and endothelial cell apoptosis (Meerarani et al. 2000; Szuster-Ciesielska et al. 2000). The former would allow entry of plasma constituents, including caeruloplasmin, which is capable of oxidizing LDL (Ehrenwald & Fox 1996). Zinc supplementation is expected to maintain endothelial cell barrier function, thus reducing the possibility of entry of caeruloplasmin and consequently decreasing the risk of oxidizing LDL.
We have also previously found that copper supplementation increases aortic copper/zinc superoxide dismutase (Cu/Zn SOD) activity (Lamb et al. 2001); this enzyme is involved in the removal of superoxide, a highly toxic reactive oxygen species. In the present study, aortic tissue SOD levels were higher in the copper-supplemented animals. Thus, it is possible that copper supplements induce Cu/Zn SOD activity, thereby reducing oxidative stress, inhibiting pro-atherogenic gene expression, which is in part associated with increased plasma caeruloplasmin level (Lamb et al. 1997), and increasing nitric oxide bioavailability. Indeed, we have reported that copper supplementation increases endothelium-dependent responses in isolated carotid rings from cholesterol-fed rabbits (Lamb et al. 1997). We also observed increased hepatic superoxide dismutase activity in chow-fed rabbits given copper and zinc supplementation, either singly or in combination. These findings suggest that SOD expression might be induced by a response to both copper and zinc supplementation in hepatic tissue.
Conclusions
Dietary supplements of copper or zinc inhibit atherogenesis in the cholesterol-fed rabbit. Although zinc supplementation did not appear to reduce the efficacy of copper supplements, there was no significant additional effect when used in combination. At the doses used, both trace elements reduced plasma lipid peroxides, indicating that they may act by an effect on lipid oxidation.
Acknowledgments
EA was supported by a scholarship from the joint programme of the King Abdul Aziz University. DL was supported by the British Heart Foundation and University of Surrey Bridging fund.
References
- Abdallah S, Samman S. The effect of increasing dietary zinc on the activity of superoxide-dismutase and zinc concentration in erythrocytes of healthy female subjects. Eur. J. Clin. Nutr. 1993;47:327–332. [PubMed] [Google Scholar]
- Abiaka C, Olusis S, Lawadhi A. Serum microminerals and the indices of lipid metabolism in an apparently healthy population. J. Clin. Lab. Anal. 2003;17:61–65. doi: 10.1002/jcla.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KG, Klevay LM. Cholesterolemia and cardiovascular abnormalities in rats caused by copper deficiency. Atherosclerosis. 1978;29:8–93. doi: 10.1016/0021-9150(78)90096-5. [DOI] [PubMed] [Google Scholar]
- Coulson W, Carnes W. Cardiovascular studies on Copper-deficient swine. II. Mechanical properties of the aorta. Lab. Invest. 1965;11:1316–1321. [PubMed] [Google Scholar]
- Disilvestro RA, Blostein-Fujii A. Moderate zinc deficiency in rats enhances lipoprotein oxidation in vitro. Free Radic. Biol. Med. 1997;22:739–742. doi: 10.1016/s0891-5849(96)00344-9. [DOI] [PubMed] [Google Scholar]
- Ehrenwald E, Fox PL. Role of endogenous ceruloplasmin in low density lipoprotein oxidation by human U937 monocytic cells. J. Clin. Invest. 1996;97:884–890. doi: 10.1172/JCI118491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns GAA, Lamb DJ, Taylor A. The possible role of copper ions in atherogenesis: the Blue Janus. Atherosclerosis. 1997;133:139–152. doi: 10.1016/s0021-9150(97)00130-5. [DOI] [PubMed] [Google Scholar]
- Ficher P, Giroux A, Belonje B, et al. The effect of dietary copper and zinc on cholesterol metabolism. Am. J. Clin. Nutr. 1980;33:1019–1025. doi: 10.1093/ajcn/33.5.1019. [DOI] [PubMed] [Google Scholar]
- Frimpong NA, Magee AC. Effects of dietary copper and zinc on serum-lipid parameters of young male-rats. Nutr. Rep. Int. 1987;35:551–559. [Google Scholar]
- Gatto LM, Samman S. The effect of zinc supplementation on plasma-lipids and low-density lipoprotein oxidation in males. Free Radic. Biol. Med. 1995;19:517–521. doi: 10.1016/0891-5849(95)00041-u. [DOI] [PubMed] [Google Scholar]
- Hamilton I, Gilmore WS, Strain J. Marginal copper deficiency and atherosclerosis. Biol. Trace Elem. Res. 2000;78:179–189. doi: 10.1385/BTER:78:1-3:179. [DOI] [PubMed] [Google Scholar]
- Hennig B, Wang Y, Ramasamy S, Mcclain CJ. Zinc-deficiency alters barrier function of cultured porcine endothelial-cells. J. Nutr. 1992;122:1242–1247. doi: 10.1093/jn/122.6.1242. [DOI] [PubMed] [Google Scholar]
- Hess F, King J, Margen S. Effect of low zinc intake and oral contraceptive agents on nitrogen utilization and clinical findings in young women. J. Nutr. 1977;107(12):2219–2227. doi: 10.1093/jn/107.12.2219. [DOI] [PubMed] [Google Scholar]
- Hunt T, Carlton WW. cardiovascular lesions associated with experimental copper deficiency in the rabbit. J. Nutr. 1965;87:385–393. doi: 10.1093/jn/87.4.385. [DOI] [PubMed] [Google Scholar]
- Iskra M, Patelski J, Majewski W. Concentrations of calcium, magnesium, zinc and copper in relation to free fatty-acids and cholesterol in serum of atherosclerotic men. J. Trace Elem. Electrolytes Health Dis. 1993;7:185–188. [PubMed] [Google Scholar]
- Klevay LM. Coronary heart diseases: the zinc/copper hypothesis. Am. J. Clin. Nutr. 1975;28:764–774. doi: 10.1093/ajcn/28.7.764. [DOI] [PubMed] [Google Scholar]
- Klevay LM. Interactions of copper and zinc in cardiovascular diseases. Ann. N. Y. Acad. Sci. 1980;355:140–151. doi: 10.1111/j.1749-6632.1980.tb21334.x. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Wibowo AA, Herber RF, et al. Trace metals and coronary heart disease risk indicators in 152 elderly men (the Zutphen study) Am. J. Epidemiol. 1985;122:378–385. doi: 10.1093/oxfordjournals.aje.a114118. [DOI] [PubMed] [Google Scholar]
- Lamb D, Avades T, Ferns G. Biphasic modulation of atherosclerosis induced by graded dietary copper supplementation in the cholesterol-fed rabbit. Int. J. Exp. Pathol. 2001;82:287–294. doi: 10.1046/j.1365-2613.2001.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb D, Reeves G, Taylor A, Ferns G. Dietary copper supplementation increases monocyte binding to carotid endothelium in the cholesterol-fed rabbit. Atherosclerosis. 1997;134:330. [Google Scholar]
- Lamb D, Reeves G, Taylor A, Ferns G. Dietary copper supplementation reduces atherosclerosis in the cholesterol-fed rabbit. Atherosclerosis. 1999;146:33–43. doi: 10.1016/s0021-9150(99)00123-9. [DOI] [PubMed] [Google Scholar]
- Linder MC. Nutrition and metabolism of the trace elements. In: Linder MC, editor. Nutrition Biochemistry Metabolism. Amsterdam: Elsevier; 1991. pp. 36–49. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr L, Randall RJ. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:267–275. [PubMed] [Google Scholar]
- Manthey J, Stoeppler M, Morgenstern W, et al. Magnesium and trace metals: risk factors for coronary heart diseases? Circulation. 1981;64:722–729. doi: 10.1161/01.cir.64.4.722. [DOI] [PubMed] [Google Scholar]
- Marklunds S. Spectrophotometric study of spontanious disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J. Biol. Chem. 1976;251(23):7504–7507. [PubMed] [Google Scholar]
- Meerarani P, Ramadass P, Toborek M, et al. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor alpha. Am. J. Clin. Nutr. 2000;71(1):81–87. doi: 10.1093/ajcn/71.1.81. [DOI] [PubMed] [Google Scholar]
- Mielcarz G, Howard A, Williams N, et al. Copper and zinc status as a risk factor for ischemic heart disease: a comparison between Japanese in Brazil and Okinawa. J. Trace Elem. Exp. Med. 1997;10:29–35. [Google Scholar]
- Nourooz-Zadeh J, Tajaddinisarmadi J, Birlouezaragon I, Wolff S. Measurement of hydroperoxides in edible oils using the ferrous oxidation in xylenol orange assay. J. Agric. Food Chem. 1995;43:17–21. [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Steinberg D, Witztum J. The role of oxidized LDL in the pathogenesis of atherosclerosis. Annu. Rev. Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- Petering HG, Murthy L, Stemmer KL, et al. Effects of copper deficiency on the cardiovascular system of the rat: the role of dietary sucrose and excessive zinc. Biol. Trace Elem. Res. 1986;9:271–270. [Google Scholar]
- Puchades R, Maquiera A, Planta M. Rapid digestion procedure for the determination of lead in vegetable tissues by electro thermal atomization atomic absorption spectrophotometer. Analyst. 1989;114:1397–1399. [Google Scholar]
- Pucheu S, Coudray C, Tresallet N, et al. Effect of dietary antioxidant trace element supply on cardiac tolerance to ischemia-reperfusion in the rat. J. Mol. Cell. Cardiol. 1995;27(10):2303–2314. doi: 10.1016/s0022-2828(95)91839-6. [DOI] [PubMed] [Google Scholar]
- Rutherford C, Martin W, Carrier M, et al. Endogenously elicited antibodies to platelet derived growth factor-BB and platelet cytosolic protein inhibit aortic lesion development in the cholesterol-fed rabbit. Int. J. Exp. Pathol. 1997;78:21–32. doi: 10.1046/j.1365-2613.1997.d01-237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JT, Salonen R, Seppanen K, et al. Interactions of serum Copper, Selenium and LDL-C in atherogenesis. BMJ. 1991;302:756–760. doi: 10.1136/bmj.302.6779.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstead H, Klevay I, Mahalko J, et al. Marginal zinc nutriture: effect on lipid metabolism and plasma Zn. Clin. Res. 1980;28:600A. [Google Scholar]
- Schosinsky KH, Lehmann HP, Beeler M. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin. Chem. 1974;20:1556–1563. [PubMed] [Google Scholar]
- Shields G, Coulson W, Kimball D, et al. Studies on copper metabolism. 32. Cardiovascular lesions in copper-deficient swine. Am. J. Pathol. 1962;41:603–621. [PMC free article] [PubMed] [Google Scholar]
- Szuster-Ciesielska A, Stachura A, Slotwinska M, et al. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology. 2000;145(2–3):159–171. doi: 10.1016/s0300-483x(00)00144-x. [DOI] [PubMed] [Google Scholar]
- Vlad M, Bordas E, Tomus R, et al. Effect of copper-sulfate on experimental atherosclerosis. Biol. Trace Elem. Res. 1993;38:47–54. doi: 10.1007/BF02783981. [DOI] [PubMed] [Google Scholar]
- Wilkins G, Leake D. The oxidation of low-density lipoprotein by cells or iron is inhibited by zinc. FEBS Lett. 1994;341:259–262. doi: 10.1016/0014-5793(94)80468-0. [DOI] [PubMed] [Google Scholar]
- Wu J, Reaves S, Wang Y, et al. Zinc deficiency decreases plasma level and hepatic mRNA abundance of apolipoprotein A-I in rats and hamsters. Am. J. Physiol. Cell Physiol. 1998;44:C1516–C1525. doi: 10.1152/ajpcell.1998.275.6.C1516. [DOI] [PubMed] [Google Scholar]