Abstract
Aplastic anaemia (AA) is characterized by hypocellular marrow, pancytopenia, and risk of severe anaemia, haemorrhage and infection. AA is often idiopathic, but frequently occurs after exposure to drugs/chemicals. However, the pathogenesis of AA is not clearly understood, and there are no convenient animal models of drug-induced AA. We have evaluated regimens of busulphan (BU) administration in the mouse to produce a model of chronic bone marrow aplasia showing features of human AA. Mice were given 8 doses of BU at 0, 5.25 and 10.50 mg/kg over 23 days; marrow and blood samples were examined at 1, 19, 49, 91 and 112 days after dosing. At day 1 post dosing, in mice treated at 10.50 mg/kg, nucleated marrow cells, CFU-GM and Erythroid-CFU were reduced. Similarly, peripheral blood erythrocytes, leucocytes, platelets and reticulocytes were reduced. At day 19 and 49 post dosing, there was a trend for parameters to return towards normal. However, at day 91 and 112 post dosing, values remained significantly depressed, with a stabilized chronic bone marrow aplasia. At day 91 and 112 post dosing, marrow cell counts, CFU-GM and Erythroid-CFU were decreased; marrow nucleated cell apoptosis and c-kit+ cell apoptosis were increased; peripheral blood erythrocyte, leucocyte, and platelet counts were reduced. We conclude that this is a model of chronic bone marrow aplasia which has many interesting features of AA. The model is convenient to use and has potential in several areas, particularly for investigations on mechanisms of AA pathogenesis in man.
Keywords: busulphan, bone marrow aplasia, mouse
Introduction
AA is characterized by hypocellular bone marrow resulting from damage to haemopoietic stem cells. This produces pancytopenia with risk of severe anaemia, major haemorrhage and life-threatening infections. Once the patient has been stabilized with blood, platelet transfusions and antibiotics, specific treatment comprises either allogeneic stem cell transplantation, or immunosuppressive therapy with antithymocyte globulin (ATG) and cyclosporin (Young 1995). Although AA is a relatively rare disorder, the incidence being 1–3 per million population per year (Gordon-Smith & Issaragrissil 1992), it is associated with significant mortality as well as later complications of relapse and clonal evolution to myelodysplasia, acute myeloid leukaemia and paroxysmal nocturnal haemoglobinuria (Tooze et al. 1999).
In many cases, AA is idiopathic. It may occur following recovery from viral hepatitis, most often non-A, non-B, non-C. Furthermore, AA may occur after exposure to drugs or chemicals (Young 1995). Apart from the predictable dose dependent bone marrow suppression that occurs after antineoplastic chemotherapy, acquired AA occurs unpredictably in a small proportion of the population exposed to a particular drug, chemical or other agent. The unpredictable nature of acquired AA may reflect an underlying genetic predisposition (Marsh et al. 1999), or a pre-existing bone marrow susceptibility resulting from a previous marrow defect.
The fundamental pathophysiology of AA is not well understood. The clinical response of AA to ATG implies an involvement of the immune system. Indeed, there is an extensive literature documenting numerous, though nonspecific, changes in the immune system. However, AA is not a classical autoimmune disease. Autoantibodies have not been identified (Cline & Golde 1978), and while auto-reactive T-cells can occasionally be cloned from aplastic patients, similar clones can also be obtained from normal individuals. Considering the work of our laboratory and others showing intrinsic abnormalities in the stem cells of AA patients, it is not yet clear whether the involvement of the immune system is primary, or whether it is secondary to a pre-existing stem cell defect.
Recently we have shown an increase in the proportion of apoptotic cells within the CD34+ population of AA bone marrow, compared with normal marrow, using 7-amino-actinomycin D (7-AAD) staining (Philpott et al. 1995a,b). This increase was greatest where the absolute number of CD34+ cells is most reduced and appeared to correlate with transfusion dependency as a marker of disease severity. Therefore, accelerated apoptosis appears to be a major contributor to the stem cell deficiency in AA. It has also been shown that aplastic CD34+ cells have increased expression of Fas-receptor, which correlates with increased sensitivity of cells to anti-Fas antibody-mediated inhibition of colony formation (Maciejewski et al. 1995; Killick et al. 2000). In addition, we have shown that the increased apoptosis and increased expression of Fas-receptor in AA CD34+ cells is reduced after ATG therapy (Killick et al. 2000).
We and others have demonstrated a reduction in progenitor cell production in AA in long-term bone marrow culture (LTBMC) (Gibson & Gordon-Smith 1990; Marsh et al. 1990; Novitsky & Jacobs 1991), and a deficiency in the proportion of CD34+ cells (Maciejewski et al. 1994) and earlier CD34+/CD33– cells (Scopes et al. 1994), indicating a defect at the level of the haemopoietic stem cell. In addition, long-term culture initiating cells (LTCIC) are reduced (Maciejewski et al. 1996).
There is a general view that there are no convenient animal models of drug-induced AA which have contributed significantly to understanding the pathogenesis of the human disease (Benestad 1979; Appelbaum & Fefer 1981; Vincent 1986; Young & Maciejewski 1997). However, models of chronic marrow failure in animals induced by benzene, BU, strontium-89 and irradiation have been described (Haak 1980; Camitta et al. 1982; den Ottolander et al. 1982; Vincent 1986; Young & Maciejewski 1997), and studies in mice with genetic anaemias have been carried out (Appelbaum & Fefer 1981). Similarly, mouse models of marrow failure induced by immunological methods have been developed (Barnes & Mole 1967; Kubota et al. 1978, 1979; Wolk et al. 1998), and rat models of marrow aplasia induced by irradiation have been investigated (Knospe et al. 1966, 1968). However, none of these rodent models have become widely used. Nevertheless, in a series of reports, Morley and his coworkers described a mouse model of BU-induced chronic hypoplastic marrow failure (Morley & Blake 1974a,b; Morley et al. 1975). In this model, BU was administered on four occasions, at 14 day intervals, over a six week period, at 20, 20, 20 and 10 mg/kg, respectively, to female Swiss or BALB/c mice, and the animals were studied over the following 300–400 days. We have investigated these findings (Andrews et al. 1993, 1997, 1998), but used the female B6C3F1 mouse, and gave four fortnightly doses of BU at dose levels from 10 to 40 mg/kg. However, in our experiments, lasting up to 497 days, we have been unable to obtain results which closely parallel those of Morley. Accordingly, we have now re-examined various regimens of BU administration in an attempt to produce an easily used mouse model of chronic bone marrow aplasia which shows features of the human disease, and which develops within a relatively short period (about 100 days). We now describe, in female BALB/c mice treated with frequent low doses of BU, the induction of chronic (late stage) bone marrow aplasia in a study lasting 112 days. Animals show statistically significant reductions in peripheral blood WBC, neutrophils, lymphocytes, monocytes, RBC, and platelets, and even more severe reductions in humeral marrow cells, and marrow committed progenitor cells; MCV is increased (Diamanti et al. 1999; Macharia et al. 1999a,b). Interestingly, apoptosis of marrow cells and specifically stem cell apoptosis is increased, which is a key feature of human AA (Maciejewski et al. 1995; Philpott et al. 1995b; Killick et al. 2000).
We believe that this is a mouse model of mild chronic bone marrow aplasia. We consider this new animal model is convenient to use and has the potential for further investigation and studies in four particular areas. Firstly, to prove or refute evidence that AA is caused by specific drugs/chemicals; secondly, to evaluate the potential of new drugs to induce AA; thirdly, to evaluate the usefulness of therapeutic interventions; and fourthly, as a basis for investigating mechanisms of the pathophysiology of the human disease.
Materials and methods
(1) Animals
Weanling female BALB/c mice (A. Tuck and Son Ltd, Beeches Road, Battlesbridge, Essex) were caged in groups of 10–12 on wood shavings with diet (Rat and Mouse No.1, SDS Ltd, Witham, Essex), and mains drinking water ad libitum. A temperature of 19–22 °C was maintained, with a relative humidity of 45–65%, and a light : dark cycle of 12 : 12 h (lights on at 07.00 hours). Animals were acclimatized for at least seven days before the start of each experiment; they were observed daily for signs of ill health during the period of BU treatment, and two or three times each week in the postdosing period. Body weights were determined daily, or two or three times each week, or at appropriate times. All animal procedures were carried out under local Ethical Committee guidelines and approval for Project and Personal Licences, and followed the UK Home Office (1989) “Code of Practice for the Housing and Care of Animals used in Scientific Procedures.”
(2) Administration of busulphan
BU (Sigma Chemical Co Ltd, Poole, Dorset) was dissolved in acetone at concentrations of 5–10 mg/mL. Immediately before administration, deionized water was added to the BU-acetone solution at a volume of 3–10 (water) : 1 (acetone), and the solution given by intraperitoneal (ip) injection at a dose volume of 0.1–0.2 mL per mouse (a maximum of 10 mL/kg body weight); control mice were given acetone : water (vehicle) at the same dose volume.
(3) Tissue sampling
Mice were killed by ip injection of pentobarbitone sodium (Sagatal, Rhône Mérieux Ltd, Harlow, Essex) and blood removed from the right ventricle following a thoracotomy incision. Blood (0.5 mL) was anticoagulated with 1.5 mg/mL dipotassium EDTA (Teklab, Sacriston, Durham). The contents of the right humerus or femur were aspirated into 1.0 mL phosphate buffered saline (PBS) to prepare a marrow cell suspension; a marrow smear was prepared from the contents of the left humerus or femur. The right and left femora were removed with surrounding muscle and placed in 5 mL sterile PBS; under sterile conditions, the muscle and epiphyses were removed from each femur and the marrow flushed into 3.5 mL sterile Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Paisley, UK) supplemented with 100 IU/mL penicillin/streptomycin. The spleen and sternum were removed and placed in 10.5% phosphate buffered formalin fixative.
(4) Analysis of blood and marrow suspensions
Blood samples and bone marrow suspensions were analysed with a Technicon H*1 hamatology analyser (Bayer Diagnostics UK Ltd, Newbury, Berks) with mouse-specific software (Technicon, Swords, County Dublin, Eire), as described previously (Turton et al. 1999, 2000). Reticulocyte analysis was carried out with a Sysmex R-1000 (Toa Medical Electronics, Milton Keynes, Bucks), with voltage gain adjusted optimally for mouse blood; three equal divisions of the total number of reticulocytes gave the percentage low (L), mid (M) and high fluorescence ratio reticulocytes (HFR). For the humeral or femoral marrow cell suspension in PBS, the total nucleated cell count (humeral or femoral nucleated cell count; HNCC, FNCC), was obtained from the basophil chanel of the H*1. Humeral or femoral marrow smears were stained with May-Grünwald-Geimsa and differential counts performed by eye on 200 cells to give the myeloid : erythroid (M : E) ratio. Sternal marrow sections were assessed for cellularity, myelopoiesis, erythropoiesis, megakaryopoiesis and the presence of fat. After fixation, the spleen was weighed and weight expressed as absolute and relative weight. Sections of spleen and sternum were prepared and stained with haematoxylin and eosin for histological examination.
(5) Bone marrow clonogenic assay
Femoral bone marrow was diluted 1 : 1 in IMDM, supplemented with 100 IU/mL penicillin-streptomycin and 10 U/mL preservative-free heparin (Leo Laboratories Ltd, Princes Risbrough, Bucks). Diluted marrow was centrifuged on Ficoll-Hypaque (Amersham Pharmacia Biotech, St Albans, Herts) at 400 g for 25 min at room temperature to obtain the mononuclear cells (BMMC) which were washed twice in the above supplemented medium. Cells were cultured at 5 × 104 in 1 mL IMDM supplemented with 15% preselected FCS, 1% deionized bovine serum albumin (BSA; Sigma), 10−4 M mercaptoethanol (Sigma), and 0.8% methylcellulose (Stem Cell Technologies Inc, Vancouver, Canada), with mouse stem cell factor (mSCF, 10 ng/mL; R & D Systems, Abingdon, Berks), mouse interleukin-3 (mIL-3, 10 ng/mL; R & D Systems), human interleukin-6 (hIL-6, 10 ng/mL; Novartis Pharmaceuticals UK Ltd, Camberley, Surrey) and human erythropoietin (EPREX, 2 U/mL; Janssen-Cilag Ltd, High Wycombe, Bucks) as growth factor stimulus. Cultures were set up in duplicate in 35 mm dishes and incubated at 37 °C. On day 14, granulocyte-macrophage colony-forming units (CFU-GM), erythroid burst-forming units (BFU-E) and colonies containing both granulocyte-macrophage and erythroid elements (CFU-GEM) were counted. Results were expressed as CFU-GM or total erythroid colonies (BFU-E + CFU-GEM) as percentages of the mean colony numbers of the untreated control animals.
(6) Dual staining for c-kit/CD117 and 7-amino actinomycin (7-AAD)
BMMC (0.5–1.0 × 106) were centrifuged, medium discarded and the pellet resuspended in residual volume of approximately 10μL. Two microlitres of undiluted fluorescein isothiocyanate (FITC)-conjugated rat antimouse c-kit/CD117 antibody (Clone 2B8; BD Pharmingen, Oxford) was added and incubated for 30 min at 4 °C. Negative controls consisted of isotype-matched irrelevant rat IgG2b-FITC antibody (Pharmingen). Cells were then washed twice in PBS supplemented with 1% FCS/0.05% azide, and stained for 7-AAD, as described by Philpott (Philpott et al. 1995a,b). Samples were analysed on a FACScan flow cytometer (Becton Dickinson BioSciences, Oxford) within 30 min of fixation with 200μL 2% paraformaldehyde. Data on 20 000 cells was acquired and processed using Lysys II software (Becton Dickinson). Regions were drawn around clear-cut populations having negative (R2), dim (R3) and bright (R3) 7-AAD fluorescence, corresponding to live, apoptotic and dead cells, respectively (Philpott et al. 1995a). The proportion of cells within each region was calculated. A second scattergram was created by combining right-angle light scatter with c-kit fluorescence and a region drawn around cells with low right-angle light scatter and high c-kit fluorescence (c-kit+) (R1). A logical gate was then defined to quantify cells satisfying both c-kit+ and 7-AAD-negative, -dim, and -bright regions. Results are expressed as percentages of the controls for the proportion of c-kit+ cells, and the proportion of apoptotic cells in the total mononuclear population and in the c-kit+ subpopulation.
(7) Statistical analysis
Treated and control (vehicle-treated) groups were compared using Student's t-tests with Fig. P for Windows (Biosoft) and GraphPad Prism (GraphPad Software, San Diego, CA).
(8) Experimental design
Experiment 1: Preliminary study
Female BALB/c mice, mean body weight 16.0 g, were dosed with BU on 8 occasions over a period of 25 days (day 1, 5, 8, 12, 16, 19, 23, 25) with vehicle, or with BU at 5, 10, 15, 20 and 25 mg/kg (n = 16–24). Animals were observed daily for clinical signs of toxicity and weighed at frequent intervals for 165 days post dosing; any animals showing evidence of BU toxicity, from which it was considered they would not recover, were immediately killed. At 1, 90 and 165 days after the final BU dose, 5–7 mice from the vehicle-dosed control group and from the groups dosed at 5 and 10 mg/kg BU, were sampled for blood and marrow investigations.
Experiment 2: Main study
201 BALB/c mice, mean body weight 15.6 g were divided into 3 groups: group 1 (vehicle dosed), n = 71; group 2 (BU, 5.25 mg/kg), n = 63; group 3 (BU, 10.50 mg/kg), n = 67. Animals were dosed with vehicle or BU on 8 occasions over a period of 23 days (day 1, 3, 8, 11, 15, 18, 21, 23) and observed as in Experiment 1. At 1, 19, 49, 91 and 112 days after the final BU dose, mice from each group (n = 6–12) were sampled for blood and marrow examination, and bone marrow cell culture and apoptosis measurement. A group of 7 untreated (control) mice were also sampled on the first day of vehicle/BU dosing.
Results
Experiment 1. Preliminary Study
Clinical signs and mortality
In groups dosed at 25 mg/kg BU (n = 24), 20 mg/kg (n = 21) and 15 mg/kg (n = 21), some mice showed signs of BU toxicity, with loss of condition after the fourth BU dose (day 12 of the dosing period). The fur became dull and dry, there was a reduction in activity and responses, and an abnormal gait and hunched posture developed. After the fifth BU dose (day 16), two mice died in the 25 mg/kg BU group, and one in each of the 20 and 15 mg/kg BU groups; the administration of BU to these three groups was therefore discontinued. From day 17 to 23, in the 25 mg/kg BU group, the remaining 22 mice lost condition and were either killed, or in a small number of cases, were found dead, at the morning inspection; all such mice are referred to as intercurrent death (ICD) animals. The paws, tail and ears of mice which became ill often lost their pink colour (the BALB/c is a white mouse), and became white. These changes were also seen in the 20 mg/kg BU group mice, with 20 ICD mice between day 17 and 23, and in the 15 mg/kg group, with 20 ICD animals from day 19 to 40.
In control (vehicle-dosed) mice (n = 17), and mice treated with BU at 5 mg/kg (n = 16) and 10 mg/kg (n = 20), there was no evidence of BU toxicity during or after drug administration. In the 10 mg/kg BU group, there were 3 ICD mice, on days 11, 154 and 155 post dosing; there were no ICD animals in the 5 mg/kg BU or control groups.
B. Hematology findings
Blood and marrow samples were taken from 5 mice treated with BU at 15 and 20 mg/kg, and from 4 mice at 25 mg/kg, on day 19 or 23 after the first BU dose; control mice, and mice treated at 5 and 10 mg/kg were sampled at day 1, 90 and 165 post dosing (n = 5–7). In mice treated with 5 BU doses at 15, 20 and 25 mg/kg, sampled on day 19/23 after the first BU dose, there was pronounced marrow depression as shown by significant decreases in erythrocyte values, all leucocytes, and in platelets, reticulocytes, and FNCC; the changes were not dose related.
In mice given 8 doses of BU at 10 mg/kg, and sampled at day 1 post dosing, red blood cell count (RBC), haemoglobin (Hb), and haematocrit (HCT) were significantly reduced; neutrophils, lymphocytes, monocytes and eosinophils were decreased, as were platelets and reticulocytes. The FNCC was reduced to 23.5% of the controls. At day 90 post dosing, in 10 mg/kg BU mice, there were significant decreases in RBC, Hb and HCT, lymphocytes and FNCC; at day 165, there were significant reductions in RBC, Hb and HCT, platelets, lymphocytes, monocytes and FNCC. Changes in the 5 mg/kg BU mice compared with animals treated at 10 mg/kg, but the alterations were less pronounced.
Experiment 2. Main Study
A. Clinical signs, mortality, body weight changes
Mice were dosed with BU on eight occasions over a 23-day period at 10.50 mg/kg (n = 67) and 5.25 mg/kg (n = 63); there were 71 vehicle-dosed (control) animals. No mice showed evidence of BU toxicity during the dosing period, either at 10.50 or 5.25 mg/kg BU. However, on day 5 post dosing, there were 3 ICD mice in the 10.50 mg/kg group as a result of BU toxicity, and between day 6 and 76 post dosing, there were a further 30 ICD mice in this group. These deaths were unexpected. The mean day of death for the 33 ICD mice was 23.5 days post dosing. The clinical signs of toxicity shown by these animals paralleled the changes seen in Experiment 1. Marrow and blood samples from the ICD mice were not taken for analysis as the haematological and marrow picture of such animals had been defined in Experiment 1. There were no ICD animals in the 5.25 mg/kg BU group after the dosing period, nor in any control mice during or after the dosing period.
The mean body weights of mice on the first day of dosing were, 16.0 g (control), 15.5 g (5.25 mg/kg), and 16.1 g (10.50 mg/kg); at the end of the dosing period the mean weights were 19.1 g, 18.1 g and 17.2 g, giving body weight increases of 19.2%, 12.4% and 5.7% during the dosing period, respectively.
At day 109 post dosing, the mean body weights were 24.1 g (control), 22.2 g (5.25 mg/kg) and 22.9 g (10.50 mg/kg), giving post dosing increases of 26.2%, 22.1% and 33.3%, respectively. From the end of dosing to day 109, mice were weighed on 14 occasions. The patterns of body weight gain in both BU treatment groups were similar (there was no dose-related effect). However, in both BU groups, the mean body weights, at all time points, were lower than the controls. Over the 14 weighing points, the reductions in body weight averaged 5.56% in the 5.25 mg/kg BU group in comparison with the controls, and in the 10.50 mg/kg group the average reduction was 5.81%.
B. Haematology findings
At day 1 post dosing, the administration of BU at 10.50 mg/kg induced a “predictable” marrow depression (Table 1). There were statistically significant reductions in RBC, Hb and HCT, and increases in MCV and MCH. There were significant decreases in neutrophils, lymphocytes, monocytes and eosinophils. Platelet counts were also reduced, as were total reticulocytes and LFR and MFR. HNCC and relative spleen weight were decreased. This pattern of changes was also generally evident in the 5.25 mg/kg BU-dosed mice, but the effect was not as great as at 10.50 mg/kg BU.
Table 1.
Mean haematological results from female BALB/c mice dosed with BU at 5.25 and 10.50 mg/kg, on 8 occasions over 23 days, and sampled at 1, 19, 49, 91 and 112 days after the final BU dosea
| Day −23b | Day 1 | Day 19 | Day 49 | Day 91 | Day 112 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Control | 5.25 mg/kg | 10.50 mg/kg | Control | 5.25 mg/kg | 10.50 mg/kg | Control | 5.25 mg/kg | 10.50 mg/kg | Control | 5.25 mg/kg | 10.50 mg/kg | Control | 5.25 mg/kg | 10.50 mg/kg | |
| n | 7 | 7 | 7 | 7 | 7 | 7 | 6 | 7 | 6 | 6 | 11 | 9 | 6 | 12 | 12 | 7 |
| RBC | 10.44 | 10.29 | 10.06* | 7.27** | 10.27 | 9.36*** | 5.49** | 10.46 | 10.46 | 9.29*** | 10.64 | 10.20* | 9.69 | 10.70 | 10.45 | 9.92** |
| HCT | 0.520 | 0.501 | 0.517* | 0.377** | 0.493 | 0.491 | 0.338 | 0.511 | 0.535** | 0.493 | 0.508 | 0.500 | 0.490 | 0.506 | 0.511 | 0.489 |
| Hb | 15.5 | 15.3 | 15.6* | 11.5** | 15.4 | 15.1 | 10.0* | 15.7 | 16.3* | 15.0 | 15.8 | 15.4 | 15.1 | 15.9 | 15.9 | 15.3 |
| MCV | 50.0 | 48.7 | 51.4*** | 51.9* | 48.0 | 52.5*** | 61.4*** | 48.8 | 51.2*** | 53.1*** | 47.8 | 49.0** | 50.8* | 47.3 | 48.9** | 49.3* |
| MCH | 14.8 | 14.9 | 15.6*** | 15.8** | 15.0 | 16.2* | 18.1*** | 15.0 | 15.6** | 16.2*** | 14.9 | 15.1* | 15.7 | 14.8 | 15.2* | 15.4 |
| Retic | 400.0 | 291.5 | 263.1 | 109.6*** | 294.5 | 325.0 | 526.4 | 241.4 | 297.2* | 279.0 | 282.9 | 247.2 | 272.8 | 254.4 | 226.4 | 271.0 |
| LFR | 217.1 | 154.9 | 148.8 | 58.6** | 160.1 | 156.0 | 215.6 | 136.8 | 159.0 | 141.2 | 158.2 | 143.0 | 137.5 | 138.8 | 126.3 | 145.0 |
| MFR | 128.1 | 96.5 | 79.2* | 29.1*** | 102.0 | 110.1 | 179.3 | 76.3 | 98.7 | 87.2 | 91.1 | 74.1* | 86.5 | 84.3 | 74.1 | 91.4 |
| HFR | 54.9 | 40.1 | 35.1 | 21.9 | 32.4 | 58.9** | 131.4** | 28.2 | 39.5 | 50.5 | 33.6 | 30.1 | 48.8 | 31.3 | 26.1 | 34.6 |
| Plt | 780 | 845 | 435*** | 61*** | 801 | 358*** | 216** | 881 | 578** | 302** | 886 | 599* | 412* | 992 | 726*** | 694** |
| WBC | 2.6 | 1.9 | 1.0* | 0.4** | 1.4 | 1.2 | 0.6*** | 1.1 | 1.3 | 0.8 | 1.5 | 1.5 | 0.4** | 1.4 | 2.1 | 0.7*** |
| Neut | 0.76 | 0.41 | 0.16* | 0.02** | 0.26 | 0.21 | 0.13 | 0.21 | 0.37 | 0.26 | 0.35 | 0.33 | 0.14** | 0.32 | 0.46 | 0.23 |
| Lymph | 1.67 | 1.39 | 0.82 | 0.41** | 0.99 | 0.96 | 0.37*** | 0.75 | 0.80 | 0.42* | 1.04 | 0.99 | 0.26*** | 0.97 | 1.43 | 0.40*** |
| Mono | 0.07 | 0.04 | 0.01** | 0.00** | 0.04 | 0.02* | 0.01** | 0.04 | 0.03 | 0.01** | 0.05 | 0.04 | 0.01** | 0.05 | 0.08 | 0.02** |
| Eo | 0.04 | 0.06 | 0.02** | 0.00*** | 0.05 | 0.02** | 0.01** | 0.08 | 0.05 | 0.04 | 0.08 | 0.08 | 0.03* | 0.08 | 0.05* | 0.02** |
| HNCC | 0.64 | 0.53 | 0.43 | 0.08*** | 0.61 | 0.32*** | 0.12*** | 0.56 | 0.51 | 0.28** | 0.68 | 0.53* | 0.32*** | 0.64 | 0.30*** | 0.26*** |
| Spleen | 6507 | 5381 | 4751 | 3494** | 5560 | 4998 | 6903 | 4690 | 5147 | 5327 | 4650 | 4145* | 3936* | 4716 | 3853*** | 3845*** |
Abbreviations and units: n, number of mice per group; RBC, red blood cells, ×1012/l; HCT, haematocrit, l/l; H, haemoglobin, g/dl; MCV, mean cell volume, fl; MCH, mean cell haemoglobin, pg; Retic, absolute reticulocyte count, ×109/l; LFR, MFR, HFR, low, mid and high fluorescence reticulocytes, ×109/l; Plt, platelets, ×109/l; WBC, white blood cells, ×109/l; Neut, neutrophils, ×109/l; Lymph, lymphocytes, ×109/l; Mono, monocytes, ×109/l; Eo, eosinophils, ×109/l; HNCC, humeral nucleated cell count, ×107; Spleen, relative spleen weight, mg/kg body weight.
Control mice were also sampled on day −23, the first day of BU dosing.
significantly different from control animals, P < 0.05
P < 0.01
P < 0.001.
At day 19 post dosing, in mice treated at 10.50 mg/kg, the effect on some erythrocyte parameters was greater than at day 1 post dosing (RBC, Hb, HCT, MVC, MCH); however, total reticulocyte, LFR, MFR and HFR counts were higher than the controls (a rebound reticulocytosis), as was relative spleen weight (see Table 1 for statistically significant changes). Other blood parameters, in 10.50 mg/kg mice, although reduced, appeared to be returning to normal (lymphocytes, monocytes, platelets, HNCC).
On day 49 post dosing, at 10.50 mg/kg BU, although many parameters were still reduced, there was general evidence of a trend of a return towards control values; this applied to erythrocyte values (RBC, Hb, HCT), leucocytes (lymphocytes, monocytes), and platelets and HNCC; MCV and MCH were still raised. However, reticulocytes, neutrophils and relative spleen weight were normal.
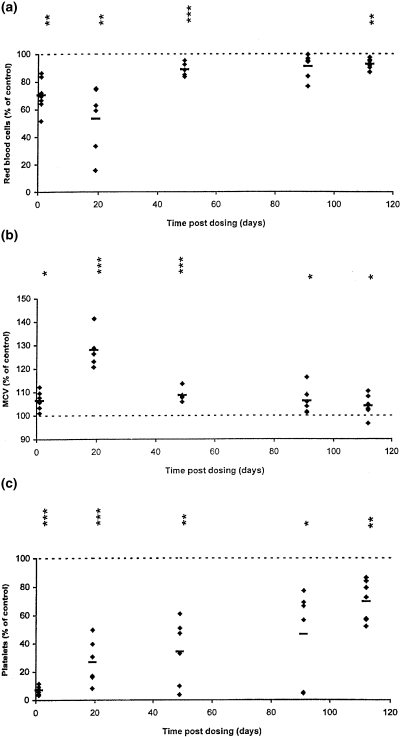
On day 91 and 112 post dosing, there was clear evidence that the trend of a general return towards normal, seen at day 49 post dosing, had not been completed; many blood parameters were still significantly affected at these late-stage time points. This was particularly evident in the 10.50 mg/kg BU group, but parameters in mice treated at 5.25 mg/kg also showed some effects. At day 91 and 112 post dosing, at 10.50 mg/kg, there was evidence of stastically significant late-stage reductions in RBC, neutrophils, lymphocytes, monocytes, eosinophils, platelets, HNCC and spleen relative weight; MCV and MCH showed significant increases. Figure 1 illustrates the stabilized late stage reductions in RBC, platelets and lymphocytes, and the increase in MCV, in mice treated with 10.50 mg/kg BU; results are expressed as percentage increase/decrease, in relation to the mean control value, at each time point.
Figure 1.
Hematological parameters from individual mice, expressed as a percentage of the mean control value at each time point, at 1, 19, 49, 91 and 112 days after BU dosing at 10.50 mg/kg (a), red blood cells; (b), mean cell volume; (c), platelets; (d), lymphocytes. Horizontal bars indicate group means; *P < 0.05, **P < 0.01, ***P < 0.001 are presented vertically above the data points (see Table 1).
C. Bone marrow and spleen cellularity and morphology
Humeral marrow smears and sternal marrow sections from 5 randomly selected control and BU-treated (10.50 mg/kg) mice were examined at day 1, 19, 49, 91 and 112 post dosing; spleen sections from 1 control and 2 BU-treated (10.50 mg/kg) mice at day 1, 49 and 112 post dosing were also studied.
Humeral marrow myeloid cells at day 1 post dosing in BU-treated mice were reduced to 0.4% of the control value (Table 2). The count then showed some recovery towards normal at day 19 and 49 post dosing. However, on day 91 and 112 post dosing, the counts remained reduced, at 41.2% and 35.5% of the control, respectively. Humeral erythroid cells were reduced to 1.2% of control at day 1 post dosing. There was a recovery towards control values at day 19 and 49, but on days 91 and 112 after dosing the counts remained reduced at 50.6% and 60.1% of controls, respectively. Humeral lymphoid cells were relatively spared at day 1 and 19 (31.4% and 27.2% of control values, respectively). At day 49 and 91, there was evidence of a recovery, with counts of 64.5% and 71.0% of the control mean; however, on day 112 post dosing the count was only 29.4% of the control mean.
Table 2.
Mean numbers (n = 5) of humeral marrow smear myeloid, erythroid and lymphoid cells, and total humeral nucleated cell counts, in female BALB/c mice treated with BU at 10.50 mg/kg on 8 occasions over 23 days, and sampled at 1, 19, 49, 91 and 112 days after the final BU dosea
| Myeloid | Erythroid | Lymphoid | Other | HNCC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Control (×106) | BU (×106) | BU (% Control) | Control (×106) | BU (×106) | BU (% Control) | Control (×106) | BU (×106) | BU (% Control) | Control (×106) | BU (×106) | BU (% Control) | Control (×106) | BU (×106) | BU (% Control) |
| 1 | 1.89 | 0.01*** | 0.4 | 1.56 | 0.02*** | 1.2 | 1.83 | 0.57*** | 31.4 | 0.12 | 0.02 | 17.3 | 5.40 | 0.62*** | 11.5 |
| 19 | 2.27 | 0.23*** | 10.3 | 1.90 | 0.47*** | 24.5 | 2.05 | 0.56*** | 27.2 | 0.22 | 0.10 | 46.4 | 6.44 | 1.36*** | 21.1 |
| 49 | 2.05 | 0.84*** | 41.0 | 1.45 | 0.61** | 42.3 | 1.32 | 0.85 | 64.5 | 0.11 | 0.10 | 93.0 | 4.92 | 2.40** | 48.8 |
| 91 | 2.53 | 1.04* | 41.2 | 1.97 | 0.99** | 50.6 | 1.18 | 0.84 | 71.0 | 0.04 | 0.27 | 640.7 | 6.72 | 3.16** | 47.0 |
| 112 | 2.76 | 0.98*** | 35.5 | 1.55 | 0.93* | 60.1 | 1.82 | 0.53*** | 29.4 | 0.12 | 0.03*** | 27.4 | 6.24 | 2.48*** | 39.7 |
Results are expressed as mean absolute numbers of cells from control and BU-treated mice, and from BU-treated mice as a percentage of the mean control value. 5 mice were selected at random from the control and BU-treated animals at each time point, and a differential cell count (200 cells) carried out by eye on the humeral marrow smear. The total humeral nucleated cell count was obtained from the opposing humeral marrow sample, and the absolute number of myeloid, erythroid and lymphoid cells calculated from the differential cell count. HNCC = humeral nucleated cell count.
significantly different from control animals, P < 0.05
P < 0.01
P < 0.001.
Microscopic examination of sternal marrow sections from 5 control and 5 BU-treated mice, was carried out at each time point. At day 1 post dosing, BU-treated mice showed marked marrow hypoplasia with fatty replacement (Table 3); there was an almost complete absence of haematopoietic elements, but where these were present, they consisted of small foci of active cells (Fig. 2A,2B,2C). At day 19 post dosing, very slight myeloid activity was present, but this was still a localized response (Fig. 2D); erythropoiesis and megakaryopoiesis were generally absent. At day 49 after dosing, myelopoiesis was comparable to the controls (i.e. approximately grade 3; Table 3) in 3 of 5 mice; however, very slight erythropoiesis was only evident in 1 of the 5 animals; very slight megakaryopoiesis was present in 4 mice, and slight megakaryopoiesis in 1 animal. On day 91 after BU administration, erythropoietic activity was generally slight/very slight. At day 112 post dosing erythropoiesis was slight in 4 mice, or very slight in 1 mouse (Fig. 2E,2F); myelopoiesis had returned to normal (moderate) levels, and megakaryopoiesis remained as very slight in 3 animals and slight/moderate in 2 animals. One BU mouse at day 112 post BU administration had a lymphoreticular tumour which had infiltrated into tissue adjacent to the sternum.
Table 3.
Histological examination of sternal marrow sections from female BALB/c mice dosed with BU at 10.50 mg/kg on 8 occasions over 23 days, and sampled at 1, 19, 49, 91 and 112 days after the final BU dosea
| Cellularity | Myelopolesis | Erythropoiesis | Megakaryopoiesis | Fat | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | Control | BU | Control | BU | Control | BU | Control | BU | Control | BU |
| 1 | 3.0 | 0.6 | 3.0 | 0.2 | 3.0 | 0.0 | 3.0 | 0.0 | 0.0 | 4.8 |
| 19 | 3.0 | 1.2 | 3.0 | 1.2 | 3.0 | 0.0 | 3.0 | 0.4 | 0.2 | 4.6 |
| 49 | 3.0 | 2.4 | 3.0 | 2.6 | 3.0 | 0.2 | 3.0 | 1.2 | 0.2 | 1.8 |
| 91 | 3.0 | 2.0 | 3.0 | 2.2 | 3.0 | 1.6 | 3.0 | 1.4 | 0.2 | 2.2 |
| 112 | 3.0 | 2.8 | 3.0 | 3.0 | 3.0 | 1.8 | 3.0 | 1.6 | 0.4 | 2.4 |
Values are means; n = 5 for control and BU-treated mice at each point. Sections were assessed for cellularity, myelopoiesis, erythropoiesis, megakaryopoiesis and the presence of fat and graded on a 6 point scale: 0 = absent; 1 = very slight; 2 = slight; 3 = moderate; 4 = marked; 5 = very marked. Results were not analysed stastically.
Figure 2.
H&E stained sections of mouse sterna from control (vehicle-treated) and BU-treated (10.50 mg/kg) animals. (a) and (b), control mouse at day 1 after vehicle dosing showing normal marrow cellularity. (A, original magnification [OM] × 100; B, OM × 400). (c), BU-treated mouse at day 1 after dosing, showing marked hypocellularity, fatty replacement (arrow heads), small megakaryocytic foci (arrows), and a haemorrhagic area (H). (OM × 100). (d), mouse treated with BU at 19 days after dosing; there is marked hypocellularity with fatty replacement, a large cystic space, and an occasional myeloid focus (arrow) (OM × 100). (d) and (f), sternal marrow from a BU-treated mouse at 112 days after dosing; there is some hypocellularity, but with only slight erythroid activity (E, OM × 100; F, OM × 400).
Examination of marrow smears from 5 BU-treated mice at each time point showed considerable variability in comparison with the controls. At day 1 post dosing (Table 2), BU mice showed marked hypocellularity, and this was also evident at day 19. Furthermore, at day 49, 91 and 112 post dosing, 1 or 2 of the 5 animals examined at each time point continued to show marked hypocellularity (Fig. 3a,3b,3c). The clastogenic effects of BU were evident at day 1 post dosing, with increased Howell-Jolly bodies (micronuclei, Fig. 3d). At day 19 post dosing, when erythroid regeneration was taking place, diserythropoietic morphology, such as multinucleation (Fig. 3e) and megaloblastosis (Fig. 3f) was seen.
Figure 3.
May-GrünwaldBGiemsa stained mouse humeral marrow smears (a-f.) from control (vehicle-treated) and BU-treated (10.50mg/kg) animals. (a), control mouse at day 1 after vehicle dosing illustrating the normal distribution of myeloid (arrow), erythroid (open arrow) and lymphoid (arrow head) elements (OM × 1000). (b), BU-treated mouse at day 91 after dosing, showing hypocellularity, with fat cells and mast cells predominating; normal haemopoietic elements are absent (OM × 1000). (c), BU-treated mouse at day 112 post dosing; the marrow shows significant depletion of erythroid precursors (OM × 1000). (D), BU-treated mouse at day 19 post dosing; the marrow is hypocellular, with few myeloid cells, and many Howell-Jolly bodies (arrows) are present (OM × 1000). (e), day 19 after BU dosing: dyserythropoiesis; many late normoblasts (arrows) are multinucleated (OM × 1000). (f), day 19 after BU dosing: dyserythropoiesis; megaloblastic development (arrows) of intermediate and late normoblasts (OM × 1000).
Spleens from 1 control, and 2 BU mice (10.50 mg/kg), were examined at day 1, 49 and 112 after the administration of BU. At day 1 post dosing, in BU mice, haematopoietic activity was reduced in the red pulp; myelopoiesis and erythropoiesis was very slight in 1 animal, but in the other mouse examined there were foci of haematopoiesis of primitive cells of both lineages. Megakaryopoiesis was reduced or absent. On day 49 post dosing, there was very marked haematopoiesis in both erythroid and myeloid lineages; megakaryopoiesis was moderate. At 112 days post dosing, haematopoietic activity was very pronounced; myelopoiesis, erythropoiesis and megakaryopoiesis were all marked/very marked.
D. Bone marrow clonogenic assay
Figure 4a and 4b show the effect of BU on CFU-GM and erythroid colony numbers, respectively. 5.25 mg/kg BU reduced the CFU-GM counts at day 1 post dosing to 67% of the control, was comparable to the control group at days 19 and 49 post dosing (102% and 137% of the control, respectively), before decreasing at days 91 and 112 post dosing to 87% and 89% of the control, respectively. 10.50 mg/kg BU had a greater effect, with significant decreases at days 1 and 19 post dosing (to 3% and 16% of the control, respectively), returned to control levels at day 49 (79% of the control) and becoming statistically significantly decreased again at days 91 and 112 post dosing to 40% and 47% of the control, respectively (Fig. 4a).
Figure 4.
Committed progenitor cell content of femoral marrow from mice treated with BU at 1, 19, 49, 91 and 112 days after BU dosing. CFU-GM (A) and erythroid colony numbers (B) in BU-treated mice (▴ BU, 5.25 mg/kg; • BU, 10.50 mg/kg) as percentages (mean ± SEM) of the control mice. *P < 0.05, **P < 0.01, ***P < 0.001.
Similar effects were observed for the erythroid colony counts (Fig. 4b). 5.25 mg/kg BU significantly reduced the counts at days 1 and 19 post dosing (18% and 22% of the control, respectively), was comparable to the control group at day 49 (131% of the control), decreased at day 91 to 71% of the control and returned to normal levels at day 112 post dosing (105% of the control). The effect of 10.50 mg/kg BU on the erythroid colony counts was highly significant. At all time points post dosing, there was a significant reduction in the counts compared to the controls. On day 1, 19, 49, 91 and 112, erythroid colony counts were reduced in comparison to control levels, to 0%, 2%, 47%, 13% and 15%, respectively.
E. Bone marrow apoptosis
Figure 5(a) shows the proportion of apoptotic cells in the BU-treated mice as a percentage of the control groups at each time point. Apoptosis after a 5.25-mg/kg dose was reduced to 86% of the control on day 1 after BU dosing, significantly reduced to 26% of the control on day 19, returned to normal levels on day 49 (105% of the control), and significantly increased to 129% and 131% of the control on days 91 and 112 post dosing, respectively. Apoptosis after 10.50 mg/kg treatment was reduced to 69% of the control on day 1 post dosing, significantly reduced to 31% of the control on day 19, returned to normal levels on day 49 (79% of the control), and significantly increased to 143% and 132% of the control on days 91 and 112 post dosing, respectively.
Figure 5.
Apoptosis of femoral marrow from mice treated with BU at 1, 19, 49, 91 and 112 days post dosing. Marrow nucleated cell apoptosis (A), proportion of c-kit+ cells (B), and apoptosis in c-kit+ cells (C) in BU-treated mice (▴ BU, 5.25 mg/kg; • BU, 10.50 mg/kg) as percentages (mean ± SEM)) of the control mice. Asterisks indicating degree of significance are as for Figure 4.
Figure 5(b) shows the proportion of c-kit+ cells in the BU-treated mice as a percentage of the control mice. On day 1 after BU dosing, c-kit+ cells were reduced by 70% compared to the control after 5.25 mg/kg treatment and to 15% of the control after 10.50 mg/kg treatment. On day 19 post dosing, c-kit+ cells decreased to similar levels (30% of the control) at both 5.25 and 10.50 mg/kg, while on day 49 increased to 80% of the control at both BU levels. On day 91 and 112 post dosing, counts at 5.25 and 10.50 mg/kg increased to levels comparable to the controls. Figure 5(c) shows the proportion of c-kit+ cells that were apoptotic as a percentage of the control groups. The percentage of apoptotic cells in both the BU-treated groups was low compared to controls from day 1 to day 49 post dosing, but on day 91 apoptosis increased by 30% compared to the controls at both BU dose levels. On day 112, apoptosis was significantly increased for both 5.25 and 10.50 mg/kg dose groups, by 55% and 89% compared to the control, respectively.
Discussion
Experiment 1 (Preliminary study) followed on from a series of investigations (Andrews et al. 1993, 1997, 1998) where BU was administered to female B6C3F1 mice, and the experimental design was based on Morley & Blake (1974a,b) involving BU dosing on four fortnightly occasions. However, Experiment 1, a dose-ranging study, demonstrated that BU could be administered repeatedly (on 8 occasions), over a relatively short period (25 days), and indicated dose levels of BU that could be used. The clinical features of BU toxicity were also defined and the blood and marrow parameters of ICD animals with significant marrow depression characterized. In Experiment 2, the dosing regimen was modified further. BU was again given on 8 occasions, at the level of 10.50 (and 5.25) mg/kg, and over a reduced period of 23 days. At 10.50 mg/kg, an initial “predictable” marrow depression was induced, characterized by decreased RBC, platelets, neutrophils and lymphocytes, and a reduced HNCC and relative spleen weight (Table 1). At day 19 and 49 post dosing there was a return of parameters towards normal, however, at a late stage (day 91 and 112 post dosing), evidence of a stable, chronic, mild bone marrow aplasia was present.
Our present studies in the BALB/c mouse derive from the earlier work of Morley & Blake (1974a,b) and Morley et al. (1975, 1976, 1978). However, there are relevant new features in our model. The mouse model of Morley & Blake (1974a) involves four fortnightly doses of BU at 20, 20, 20 and 10 mg/kg (total 70 mg/kg), administered over 42 days. With such a regimen, BU dosing at 14 day intervals allows the depressed marrow to return towards normal by the time of the next dose. In our present model, a lower dose level of BU (10.50 mg/kg) was used, but administered repeatedly (every 2–5 days). Previous studies (Andrews et al. 1993) have shown that the nadir of marrow depression after a single BU dose, occurs at day 2–7. Thus with our present dosing regimen, doses of BU are given during the nadir.
We have also attempted to reduce from 42 to 23 days the time period over which BU is given. However, the total dose of BU administered (84 mg/kg) is higher than the 70 mg/kg used by Morley. The time period of the experiment has also been significantly reduced; Morley & Blake (1974a,b) studied animals for 300–400 days, and other workers who have based their techniques on Morley's regimen, have continued their experiments for 200–350 days (Robin et al. 1981; Bhoopalam et al. 1986), or 12–18 months (Hays et al. 1982), or 40 weeks (McManus & Weiss 1984).
In the initial paper of Morley & Blake (1974a), high mortality was reported during and after BU dosing. After day 60, mice were described as being in a “latent” phase, showing only minor haematological abnormalities, but at some time during the period 60–313 days, individual latent animals began to show a developing marrow aplasia, with body weight loss, pallor and illness, and a marked depression of all circulating blood cells and tibial marrow cell count. This condition developed rapidly and the affected animals sickened and died; these animals were referred to as “aplastic” mice. In this way therefore, Morley's BU-treated animals consisted of two changing populations of mice, an initially larger group of latent mice, and at various times, individuals developing or showing clear evidence of frank aplasia.
In comparing the above observations of Morley & Blake (1974a) with our present findings, mice sampled at day 91 and 112 post BU dosing may equate with Morley's latent animals. Morley & Blake (1974a,b) and Morley et al. (1975, 1976, 1978) give information on peripheral blood and marrow cell counts of latent animals: platelet counts were normal, HCT was minimally depressed, reticulocyte counts were normal, neutrophil/lymphocyte/monocyte counts were moderately depressed, as were tibial marrow cell counts. Our present findings (day 91 and 112 post dosing) would therefore probably compare with these data in respect to RBC (HCT), neutrophil/lymphocyte/monocyte counts, and marrow cell and reticulocyte counts. However, in contrast, platelet counts were significantly depressed in the present study (Table 1), to 58% of control values. Also, interestingly, at all time points studied in mice treated with both 5.25 and 10.50 mg/kg, MCV was significantly increased which is a characteristic feature of patients with AA.
However, Morley & Blake (1974b) reported that during the latent period, CFU-S and CFC were severely depressed. This is in agreement with our demonstration of reduced bone marrow CFU-GM and erythroid colonies (BFU-E + CFU-GEM) (Fig. 4). In addition, in Experiment 2, the marrow of BU-treated mice contained a greater proportion of apoptotic cells than the controls, and although the proportion of stem cells (c-kit+ cells) was normal or slightly above normal on days 91 and 112 post dosing, a greater proportion of such cells were apoptotic (Fig. 5). The mechanism by which BU-treated marrow is able to maintain a relatively mild cytopenia in peripheral blood, in spite of significantly reduced marrow cell counts and marrow progenitor cells, and increases in the proportion of apoptotic stem cells, is unclear but does parallel the human condition in patients with AA even after recovery after immunosuppressive ATG therapy (Marsh et al. 1990, 1991; Maciejewski et al. 1994; Philpott et al. 1995b). Podesta et al. (1998), who followed patients after ATG for up to 20 years, observed that although all achieved normal peripheral blood counts, the marrow cell counts and numbers of committed progenitor cells remained subnormal, and there was an even more significant reduction in LTCIC. This suggests that even a severely reduced stem cell population is able to maintain steady-state haemopoiesis. Proliferation potential of LTCIC was also reduced in these patients and this may suggest that more stem cells in AA are unable to enter the cell cycle to proliferate normally (Podesta et al. 1998). Increased division by more mature progenitor cells may compensate for this. In addition, we have demonstrated reduced regeneration of progenitors from 5-fluorouracil-treated AA marrow cells (Gibson et al. 1996). These reports suggest dysfunctional and deficient primitive noncycling stem cells in this disease, reflecting abnormal proliferation and differentiation kinetics, with increased apoptosis as a major contributor to this stem cell defect.
Botnick et al. (1976) showed by serial transplantation that the proliferation capacity of stem cells from BU-treated mice was permanently damaged which may explain why the animals progress from this latent phase of normal/near normal peripheral blood counts and markedly reduced stem cell number to a period of severe aplasia (Morley & Blake 1974a,b, 1198).
Normal haemopoiesis is sustained by interactions between stem cells and marrow stroma, which consists of a wide variety of cell types and extracellular matrix (Toogood et al. 1980; Strobel et al. 1986; Wilkins & Jones 1995). Marrow stroma in vivo as seen in trephine biopsies and in vitro in LTBMC consists of macrophages, fat cells, T cells, endothelial cells and fibroblasts. Several studies have investigated stromal function in BU-treated mice in addition to stem cells and propose that although the principal defect is one of stem cells, a contributing factor may be abnormal stroma (Morley et al. 1975; Hays et al. 1982; McManus & Weiss 1984). Interestingly, defective stromal function has also been reported by a number of groups in a few cases of AA (Hotta et al. 1985; Juneja & Gardner 1985; Marsh et al. 1990).
We feel justified in using the term “chronic” to describe the late-stage bone marrow aplasia of the present studies. The mean life-span of the BALB/c mouse is about 595 days (Festing 1979). In Experiment 2, mice were treated with BU for 23 days and studied for 112 days post dosing (135 days in total), that is, about 23% of the life-span. If human life expectancy is taken as 75 years, 23% of a human life span would equate to the presence of aplasia at 17 years after first exposure to the drug.
We appreciate that further studies are required on the present mouse model, to define its characteristics more fully, in comparison with those of human AA. However, we believe that, the present model could be adapted to evaluate the potential of drugs to cause AA. Studies might commence with compounds which have been associated with the induction of AA in man (e.g. gold salts, phenylbutazone, penicillamine, phenytoin) (Baumelou et al. 1993; Young & Alter 1994; Issaragrisil et al. 1997). The usefulness of the mouse model should also be defined by investigating the potential of the three drugs recently associated with AA in clinical use, remoxipride (Comitttee on Safety of Medicine/Medicines Control Agency 1993; Philpott et al. 1993), felbamate (Brodie & Pellock 1995), and ticlopidine (Mataix et al. 1992; Troussard et al. 1992; Lesesve et al. 1994; Rodriguez et al. 1994), to induce marrow aplasia in the mouse. Future studies should also involve using this model to investigate the pathogenesis of the disease, and in therapeutic interventions to inhibit or prevent the development of AA (e.g. ATG, cyclosporin A and antiapoptotic drugs, such as pentoxifylline and ciprofloxacin) (Shetty et al. 1996). In addition, it is interesting that full recovery was not seen in this model, and investigations into whether chronic bone marrow failure leads to secondary myelodysplasia, involving clonal events and karyotypic abnormalities, should be performed.
We consider the present model of chronic bone marrow aplasia (consisting of a predictable marrow depression immediately post dosing, and developing into an apparently stable, long-lasting mild marrow aplasia), is convenient to use and has several advantages. First, the model is easy to generate and use; second, the BALB/c mouse has advantages, being a robust, easily available, commonly used, docile, genetically defined, inbred strain; third, the marrow aplasia develops rapidly; fourth, many features of AA in man are present (Marsh et al. 1990, 1991; Maciejewski et al. 1994; Philpott et al. 1995b; Podesta et al. 1998). However, there are drawbacks: the unexpectedly high mortality; the lack of a pronounced anaemia, reticulocytopenia and neutropenia; the generally mild nature of the aplasia. Experiments are in progress to address these points, particularly the first. Preliminary studies indicate that further modification of the 10.50 mg/kg BU dosing regimen, with slightly reduced dose levels, will produce mice with a chronic marrow aplasia but without high mortality. We are also attempting to define the patterns of the various cellular responses, to identify more clearly the time periods of the “predictable” marrow depression and the late stage aplasia.
Acknowledgments
This study was supported by grants from GlaxoSmithKline Research and Development, and The Aplastic Anaemia Trust (formerly The Marrow Environment Fund). We wish to acknowledge with thanks the assistance of the technical staff at the School of Pharmacy for their husbandry of the animals, David McCarthy for preparation of photographs, Vicky Welsh and Malika Chibane for preparation of the manuscript, and Peter Buckley for advice on the presentation of the results. We also gratefully acknowledge the assistance of staff in the Clinical Pathology Unit and the Histology Laboratory at GlaxoSmithKline for analysis of blood and marrow samples and preparation of tissue sections.
References
- Andrews CM, Dash LM, Williams TC, Craig Gray J, Turton JA. Long-term effects of busulphan on lymphocyte subpopulations in female B6C3F1 mice. Comp. Haematol. Int. 1997;7:230–237. [Google Scholar]
- Andrews CM, Spurling NW, Turton JA. Characterisation of busulphan-induced myelotoxicity in B6C3F1 mice using flow cytometry. Comp. Haematol. Int. 1993;3:538–546. [Google Scholar]
- Andrews CM, Williams TC, Turton JA. Long-term haematological alterations in female B6C3F1 mice treated with busulphan. Comp. Haematol. Int. 1998;8:125–138. [Google Scholar]
- Appelbaum FR, Fefer A. The pathogenesis of aplastic anaemia. Sem. Haematol. 1981;18:241–257. [PubMed] [Google Scholar]
- Barnes DWH, Mole RH. Aplastic anaemia in sublethally irradiated mice given allogeneic lymph node cells. Br. J. Haematol. 1967;13:482–491. doi: 10.1111/j.1365-2141.1967.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Baumelou E, Guiguet M, Mary JY. Epidemiology of aplastic anemia in France: a case-control study. I. Medical history and medication use. The French Cooperative Group for Epidemiological Study of Aplastic Anaemia. Blood. 1993;81:1471–1478. [PubMed] [Google Scholar]
- Benestad H. Drug mechanisms in marrow aplasia. In: Geary CG, editor. Aplastic Anaemia. London: Ballière-Tindall; 1979. pp. 26–42. [Google Scholar]
- Bhoopalam N, Price K, Norgello H, Barone-Varelas J, Fried W. Busulphan and chloramphenicol induced T cell lymphoma: cell surface characteristics and functional properties. Clin. Exp. Immunol. 1986;64:646–655. [PMC free article] [PubMed] [Google Scholar]
- Botnick LE, Hannon EC, Hellman S. Limited proliferation of stem cells surviving alkylating agents. Nature. 1976;262:68–70. doi: 10.1038/262068a0. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Pellock JM. Taming the brain storm: felbamate updated. Lancet. 1995;346:918–919. doi: 10.1016/s0140-6736(95)91550-8. [DOI] [PubMed] [Google Scholar]
- Camitta BM, Storb R, Thomas ED. Aplastic anaemia. Pathogenesis, diagnosis, treatment and prognosis. N. Engl. J. Med. 1982;306:645–652. doi: 10.1056/NEJM198203183061105. [DOI] [PubMed] [Google Scholar]
- Cline MJ, Golde DW. Immune suppression of hematopoiesis. Am. J. Med. 1978;64:301–310. doi: 10.1016/0002-9343(78)90060-8. [DOI] [PubMed] [Google Scholar]
- Comitttee on Safety of Medicine/medicines Control Agency. Remoxipride (Roxiam) – aplastic anaemia. Curr. Probl. Pharmacovigil. 1993;19:9. [Google Scholar]
- Diamanti V, Turton JA, Sharpe S, Macharia G, Andrews M, Williams T, Marsh JCW, Gibson FM. Induction of chronic bone marrow aplasia with busulphan: studies on marrow cell counts and colony forming assays in a new experimental model in the BALB/c mouse. Human Exp. Toxicol. 1999;18:763. [Google Scholar]
- Festing MFW. London: MacMillan; 1979. Inbred Strains in Biomedical Research. [Google Scholar]
- Gibson FM, Gordon-Smith EC. Long term culture of aplastic anaemia bone marrow. Br. J. Haematol. 1990;75:421–427. doi: 10.1111/j.1365-2141.1990.tb04358.x. [DOI] [PubMed] [Google Scholar]
- Gibson FM, Scopes J, Gordon-Smith EC. Regeneration of aplastic anemia progenitor cells from 5-fluorouracil treated bone marrow in long-term culture. Exp. Haematol. 1996;24:209. [Google Scholar]
- Gordon-Smith EC, Issaragrissil S. Epidemiology of aplastic anaemia. Bailliere's Clin. Haematol. 1992;5:475–491. doi: 10.1016/s0950-3536(11)80028-4. [DOI] [PubMed] [Google Scholar]
- Haak HL. Experimental drug-induced aplastic anaemia. Clin. Haematol. 1980;9:621–639. [PubMed] [Google Scholar]
- Hays EF, Hale L, Villarreal B, Fitchen JH. Exp. Haematol. Vol. 10. 1982. “Stromal” and haemopoietic stem cell abnormalities in long-term cultures of marrow from busulphan-treated mice; pp. 383–392. [PubMed] [Google Scholar]
- Home Office. London: Her Majesty's. Stationary Office; 1989. Code of Practice for the Housing and Care of Animals used in Scientific Procedures. [Google Scholar]
- Hotta T, Kato T, Maeda H, Yamao H, Yamada H, Saito H. Functional changes in marrow stromal cells in aplastic anemia. Acta Hematologica. 1985;74:65–69. doi: 10.1159/000206171. [DOI] [PubMed] [Google Scholar]
- Issaragrisil S, Kaufman DW, Anderson T, Chansung K, Thamprasit T, Sirijirachai J, Piankijagum A, Porapakkham Y, Vannasaeng S, Leaverton PE, Shapiro S, Young NS. Low drug attributability of aplastic anaemia in Thailand. Blood. 1997;89:4034–4039. [PubMed] [Google Scholar]
- Juneja HS, Gardner FH. Functionally abnormal marrow stromal cells in aplastic anemia. Exp. Hematol. 1985;13:194–199. [PubMed] [Google Scholar]
- Killick S, Cox C, Marsh JCW, Gordon-Smith EC, Gibson FM. Mechanisms of bone marrow progenitor cell apoptosis in aplastic anaemia and the effect of antithymocyte globulin: Examination of the role of the Fas–Fas L interaction. Br. J. Haematol. 2000;111:1164–1169. doi: 10.1046/j.1365-2141.2000.02485.x. [DOI] [PubMed] [Google Scholar]
- Knospe WH, Blom J, Crosby WH. Regeneration of locally irradiated bone marrow. I. Dose-dependent, long-term changes in the rat, with particular emphasis on vascular and stromal reaction. Blood. 1966;28:398–415. [PubMed] [Google Scholar]
- Knospe WH, Blom J, Crosby WH. Regeneration of locally irradiated bone marrow. II. Induction of regeneration in permanently aplastic medullary cavities. Blood. 1968;31:400–405. [PubMed] [Google Scholar]
- Kubota K, Mizoguchi H, Miura Y, Kano S, Takaku F. Experimental hypoplastic marrow failure in the mouse. Exp. Haematol. 1978;6:791–800. [PubMed] [Google Scholar]
- Kubota K, Mizoguchi H, Miura Y, Kano S, Takaku F. Hemopoietic stem cells in experimental hyoplastic marrow failure of the mouse. In: Baum SJ, Ledney GD, editors. Experimental Haematology Today. New York: Springer-Verlag; 1979. pp. 239–245. [Google Scholar]
- Lesesve JF, Callat MP, Lenormand B, Monconduit M, Noblet C, Moore N, Caron F, Humbert G, Stamatoullas A, Tilly H. Haematological toxicity of ticlopidine. Am. J. Haematol. 1994;47:149–150. doi: 10.1002/ajh.2830470226. [DOI] [PubMed] [Google Scholar]
- Macharia G, Andrews CM, Williams TC, Gibson F, Marsh J, Diamanti V, Sharpe S, Turton JA. Peripheral blood changes in a new model of busulphan-induced chronic bone marrow aplasia (aplastic anaemia) in the BALB/c mouse. Human Exp. Toxicol. 1999b;18:764. [Google Scholar]
- Macharia G, Gibson F, Andrews M, Diamanti V, Sharpe S, Williams T, Turton J. Chronic hypoplastic marrow failure in the mouse; a possible new model for the assessment of drugs with potential to induce aplastic anaemia (AA) J. Pharm. Pharmacol. 1999a;51(S) [Google Scholar]
- Maciejewski JP, Anderson S, Katevas P, Young NS. Phenotypic and functional analysis of bone marrow progenitor cell compartment in bone marrow failure. Br. J. Haematol. 1994;87:227–234. doi: 10.1111/j.1365-2141.1994.tb04903.x. [DOI] [PubMed] [Google Scholar]
- Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br. J. Haematol. 1995;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. A severe and consistent deficit in marrow and circulating primitive hematopoietic cells (long-term culture-initiating cells) in acquired aplastic anemia. Blood. 1996;88:1983–1991. [PubMed] [Google Scholar]
- Marsh JCW, Chang H, Testa NG, Hows JM, Dexter TM. The haemopoietic defect in aplastic anaemia assessed by long term marrow culture. Blood. 1990;76:1748–1757. [PubMed] [Google Scholar]
- Marsh JCW, Chang J, Testa NG, Hows JM, Dexter TM. In vitro assessment of marrow “stem cell” and stromal cell function in aplastic anaemia. Br. J. Haematol. 1991;78:258–267. doi: 10.1111/j.1365-2141.1991.tb04426.x. [DOI] [PubMed] [Google Scholar]
- Marsh JCW, Choudry J, Parry-Jones N, Ellis SW, Muir KR, Gordon-Smith EC, Tucker GT. Study of the association between cytochromes P450 2D6 and 2E1 genotypes and the risk of drug and chemical induced idiosyncratic aplastic anaemia. Br. J. Haematol. 1999;104:266–270. doi: 10.1046/j.1365-2141.1999.01190.x. [DOI] [PubMed] [Google Scholar]
- Mataix R, Ojeda E, Del Carmen Perez M, Jimenez S. Ticlopidine and severe aplastic anaemia. Br. J. Haematol. 1992;80:125–126. doi: 10.1111/j.1365-2141.1992.tb06412.x. [DOI] [PubMed] [Google Scholar]
- McManus PM, Weiss L. Busulphan-induced chronic bone marrow failure: changes in cortical bone, marrow stromal cells, and adherent cell colonies. Blood. 1984;64:1036–1041. [PubMed] [Google Scholar]
- Morley A, Blake J. An animal model of chronic aplastic marrow failure. I. Late marrow failure after busulphan. Blood. 1974a;44:49–56. [PubMed] [Google Scholar]
- Morley A, Blake J. Haemopoietic precursor cells in experimental hypoplastic marrow failure. Aust. J. Exp. Biol. Med. Sci. 1974b;52:909–914. doi: 10.1038/icb.1974.90. [DOI] [PubMed] [Google Scholar]
- Morley A, Trainor K, Blake J. A primary stem cell lesion in experimental chronic hypoplastic marrow failure. Blood. 1975;45:681–688. [PubMed] [Google Scholar]
- Morley A, Trainor K, Remes J. Residual marrow damage: Possible explanation for idiosyncrasy to chloramphenicol. Br. J. Haematol. 1976;32:525–531. doi: 10.1111/j.1365-2141.1976.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Morley AA, Trainor KJ, Seshadri RS. Chronic hypoplastic marrow failure and residual injury. Blood Cells. 1978;4:253–265. [PubMed] [Google Scholar]
- Novitsky N, Jacobs P. Marrow stem cell and stromal cell function in aplastic anaemia. Br. J. Haematol. 1991;79:531–533. doi: 10.1111/j.1365-2141.1991.tb08074.x. [DOI] [PubMed] [Google Scholar]
- den Ottolander GJ, te Velde J, Veenhof W, Kleiverda K, Haak HL, Spaander PJ. Busulphan aplasia in rabbits: a model for human aplastic anaemia. Br. J. Haematol. 1982;51:265–276. doi: 10.1111/j.1365-2141.1982.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Philpott NJ, Marsh JCW, Gordon-Smith EC. Aplastic anaemia and remoxipride. Lancet. 1993;342:1244–1245. doi: 10.1016/0140-6736(93)92230-q. [DOI] [PubMed] [Google Scholar]
- Philpott NJ, Scopes J, Marsh JCW, Gordon-Smith EC, Gibson FM. Increased apoptosis in aplastic anaemia progenitor cells: possible pathophysiological significance. Exp. Haematol. 1995b;23:642–1648. [PubMed] [Google Scholar]
- Philpott NJ, Turner AJC, Scopes J, Westby M, Marsh JCW, Gordon-Smith EC, Dalgleish AG, Gibson FM. The use of 7-amino-actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared to other techniques. Blood. 1995a;87:2244–2251. [PubMed] [Google Scholar]
- Podesta M, Piaggio G, Frassoni F, Pitto A, Zikos P, Sessarego M, Abate M, Teresa Van Lint M, Berisso G, Bacigalupo A. The assessment of the hematopoietic reservoir after immunosuppressive therapy or bone marrow transplantation in severe aplastic anemia. Blood. 1998;91:1959–1965. [PubMed] [Google Scholar]
- Robin E, Berman M, Bhoopalam N, Cohen H, Fried W. Induction of lymphomas in mice by busulphan and chloramphenicol. Cancer Res. 1981;41:3478–3482. [PubMed] [Google Scholar]
- Rodriguez JN, Fernandez-Jurado A, Dieguez JC, Amian A, Prados D. Ticlopidine and severe aplastic anaemia. Am. J. Haematol. 1994;47:332. doi: 10.1002/ajh.2830470418. [DOI] [PubMed] [Google Scholar]
- Scopes J, Bagnara M, Gordon-Smith EC, Ball SE, Gibson FM. Haemopoietic progenitor cells are reduced in aplastic anaemia. Br. J. Haematol. 1994;86:427–430. doi: 10.1111/j.1365-2141.1994.tb04761.x. [DOI] [PubMed] [Google Scholar]
- Shetty V, Dar S, Hussaini S, Alvi S, Span L, Mundle S, Gexer S, Gregory S, Venugopal P, Hines C, Chopra H, Borok R, Robin E, Rifkin S, Alston D, Hernandez B, Hsu WT, Preisler H, Raza A. Effect of anti-phosphatidic drugs on the expression of cytokines in myelodysplastic syndromes (MDS) Exp. Hematol. 1996;24:573a. [Google Scholar]
- Strobel E-S, Gay RE, Greenberg PL. Characterisation of the in vitro stromal micro-environment of human bone marrow. Int. J. Cell Cloning. 1986;4:341–356. doi: 10.1002/stem.5530040506. [DOI] [PubMed] [Google Scholar]
- Toogood IRG, Dexter TM, Allen TD, Suda T, Lajtha LG. The development of a liquid culture system for the growth of human bone marrow. Leuk. Res. 1980;4:449–461. doi: 10.1016/0145-2126(80)90027-2. [DOI] [PubMed] [Google Scholar]
- Tooze J, Marsh JCW, Gordon-Smith EC. Clonal evolution of aplastic anaemia to myelodysplastic syndrome/acute myeloid leukaemia and paroxysmal nocturnal haemoglobinuria. Leuk. Lymph. 1999;33:231–241. doi: 10.3109/10428199909058423. [DOI] [PubMed] [Google Scholar]
- Troussard X, Mayo P, Mosquet B, Reman O, Leporrier M. Ticlopidine and severe aplastic anaemia. Br. J. Haematol. 1992;82:779–780. doi: 10.1111/j.1365-2141.1992.tb06964.x. [DOI] [PubMed] [Google Scholar]
- Turton JA, Havard AC, Robinson S, Holt DE, Andrews CM, Fagg R, Williams TC. An assessment of chloramphenicol and thiamphenicol in the induction of aplastic anaemia in the BALB/c mouse. Food Chem. Toxicol. 2000;38:925–938. doi: 10.1016/s0278-6915(00)00087-9. [DOI] [PubMed] [Google Scholar]
- Turton JA, Yallop D, Andrews M, Fagg R, York M, Williams TC. Haemotoxicity of chloramphenicol succinate in the CD-1 mouse and Wistar Hanover rat. Human Exp. Toxicol. 1999;18:566–576. doi: 10.1191/096032799678845098. [DOI] [PubMed] [Google Scholar]
- Vincent PC. Drug-induced aplastic anaemia and agra- nulocytosis. Incidence and mechanisms. Drugs. 1986;31:52–63. doi: 10.2165/00003495-198631010-00004. [DOI] [PubMed] [Google Scholar]
- Wilkins BS, Jones DB. Immunohistochemical characterisation of intact adherent layers from human long-term bone marrow cultures. Br. J. Haematol. 1995;90:757–766. doi: 10.1111/j.1365-2141.1995.tb05193.x. [DOI] [PubMed] [Google Scholar]
- Wolk A, Simon-Stoos K, Nami I, Concannon J, Mawe J, Tanawattanacharoen P, Maung C, Dunn D, Young NS, Bloom M. A mouse model of immune-mediated aplastic anemia. Blood. 1998;92:639a. [Google Scholar]
- Young N. Aplastic anaemia. Lancet. 1995;346:228–232. doi: 10.1016/s0140-6736(95)91273-8. [DOI] [PubMed] [Google Scholar]
- Young NS, Alter B. Acquired and Inherited. Philadelphia: W.B. Saunders Co; 1994. Aplastic Anaemia; pp. 100–132. [Google Scholar]
- Young NS, Maciejewski JP. The pathophysiology of acquired aplastic anaemia. N. Engl. J. Med. 1997;336:1365–1372. doi: 10.1056/NEJM199705083361906. [DOI] [PubMed] [Google Scholar]