Abstract
This article reviews the evidence that adhesion molecules are critical in leukocyte recirculation and pathogenesis of diseases affecting the closely related tissues of the liver and gut, which offer novel opportunities for treatment.
Keywords: addressin, adhesion molecule, leukocyte recirculation, inflammatory bowel disease, integrin, liver disease, MAdCAM-1
Leukocyte trafficking
Leukocytes continuously recirculate between the blood and tissues. They traffic to organs and subsequently leave the circulation via lymph nodes. Some are naïve, others have been programmed to recognize antigens and interact with endothelium and thus disseminate preferentially (i.e. ‘home’) to the site of original antigen exposure.
In the absence of inflammation, circulating virgin leukocyte traffic preferentially in a physiological circulation to secondary lymphoid tissue (lymph nodes, spleen and gut-associated lymphoid tissue) where requirements for effective antigen presentation and differentiation are fulfilled. The exit from the blood mainly takes place in unique postcapillary venules — high endothelial venules (HEV) (Girard & Springer 1995). These are near-cuboidal cells displaying functional modifications to facilitate leukocyte extravasation. Migration through HEVs appears to be very specific. Leukocytes circulating in the blood are able to discriminate between the HEV endothelium and endothelium lining non-lymphoid tissues. Approximately 1.4 × 104 lymphocytes extravasate from blood into a single lymph node via HEVs every second and 25% of lymphocytes circulating in HEVs will bind and emigrate (Butcher et al. 1979). If lymphocytes are presented with antigens, they proliferate by clonal expansion. Lymphocytes can exit from lymphoid tissues via efferent lymphatics to re-enter the systemic circulation.
When tissues become inflamed, leukocytes including lymphocytes are recruited to those sites. The mechanisms of recruitment involve combinations of molecules expressed on the leukocytes and on the endothelium. Addressins are key partners in these processes, being tissue molecules that provide a unique molecular address, allowing leukocytes expressing the corresponding molecular ligand to target particular organs (Vidal-Vanaclocha et al. 1993 and Salmi et al. 1998). Granulocytes and monocytes also emigrate from the blood stream in response to molecular changes on the surface of blood vessels that signal injury or infection; they cannot recirculate. The processes governing emigration of cells from the circulation into tissues were first defined for neutrophils, but similar processes pertain for lymphocyte homing as well, although the latter process is more complex.
Adhesion molecules, cell trafficking and recruitment
Adhesion molecule is a general term for the molecules involved in the recruitment process, which are surface bound glycoprotein molecules expressed on leukocytes and/or endothelial cells. They share common characteristics, acting as a molecular link between the external and internal milieu of the cell. They are all trans-membrane proteins with different domains, the largest of which is extracellular, attached to an intra-membraneous segment linked to a cytoplasmic functional domain, through which they can influence cell function, e.g. modulating the cytoskeleton of the cell and activating secondary messenger systems.
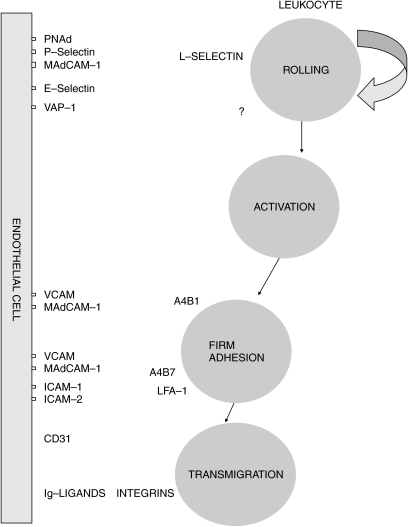
The molecular mechanisms of leukocyte extravasation are well characterized in a ‘multistep paradigm’ (Figures 1 and 2). This describes the overall process of extravasation as a three-step mechanism consisting of (i) tethering and rolling of leukocytes on the endothelium, (ii) activation of integrins and (iii) firm adhesion and transmigration.
Figure 1.
Transmigration of leukocytes through vascular endothelium. In the normal event there is a random contact between leukocytes and vascular endothelium. However, if vascular endothelium becomes activated, leukocytes roll on to it, adhere and transmigrate between the endothelial cells to reach the areas of inflammation.
Figure 2.
Adhesion molecules involved in leukocyte emigration.
Tethering and rolling
The blood-borne leukocytes are displaced from the central flow of the vessel, largely in regions of the microvasculature, i.e. postcapillary venules. These tethering contacts lead to cells rolling at a velocity of 10–50 µm/s – slower than erythrocytes typically 4000 µm/s (Springer 1994). Adhesion molecules called selectins mediate these readily reversible contacts, controlling both tethering and rolling (Pachynski et al. 1998). These molecules are constitutively active and presented on the tip of microvillous projections, considerably higher than the planar surface, making them suitable candidates for initial contacts. The patterns of leukocyte recirculation depend upon the combinations of molecules expressed on the leukocyte and the combinations of addressins and other molecules providing individual tissues with a unique molecular address.
Activation of integrins
The integrin activation step is thought to involve binding of chemotactic cytokines presented by the endothelium to the trans-membrane receptors (Gunn et al. 1998). The leukocyte is ‘activated’, and is able to bind to the endothelium, resist the high shearing forces imposed on it and stop within the vessel (Berlin et al. 1993).
Firm adhesion and migration
The ‘stable’ cell then seeks inter-endothelial junctions through which it can migrate within tissues, and adhesion molecules expressed on the surface of endothelial cells ensure an orderly sequence of cell–cell interactions.
The main classes of adhesion molecules are intercellular adhesion molecules, integrins, selectins, and cadherins. As already mentioned, endothelial adhesion molecules with a dominant role in tissue-specific migration are often called ‘vascular addressins’; their counter-receptors on the leukocyte are called ‘homing receptors’. Cells can express adhesion molecules constitutively (e.g. endothelial cells of the HEV in lymph nodes), or up-regulate them on exposure to cytokines, chemokines, or other proinflammatory molecules such as complement activation products and microbial metabolites. There are several families of adhesion molecules, which participate variously in immune and inflammatory processes, based on structure, function, and location.
Leukocyte migration into the liver and gut
The gut has a specialized immune system appropriate to its exposure to the major antigen challenge from the lumen, consisting of food products and bacteria. Antigen enters intestinal mucosa via ‘M’ (microfolded) cells, the specialized epithelium above the lymphoid follicles. Peyer's patches represent organized lymphoid structures, appearing different from lymphoid follicles because they lack afferent lymphatics. Within Peyer's patches the mucosal immune response is initiated by the uptake and processing of antigenic material by macrophages and follicular dendritic cells and its presentation to T and B cells. Here, lymphocytes bind to the specialized HEVs. If the naïve lymphocyte is not exposed to antigen it leaves Peyer's patches and returns into the systemic circulation via efferent lymphatics. However, if the lymphocyte makes contact with its antigen it divides and differentiates into effector/memory cells. These immunoblasts are transported via the lymphatics into mesenteric lymph nodes and eventually into the blood, with wide dispersion throughout the body.
The liver shares a common embryological origin from the endoderm with the gut, and it too has a distinct endothelial phenotype characterized by expression of several adhesion molecules. Furthermore, the liver also like the gut epithelium is an important site of exposure to foreign antigens, particularly through the portal vein, and thus needs to be able to respond efficiently to pathogens. Mechanisms by which the liver may modify gut-derived antigens range from conceptually simple filter functions of the reticulo-endothelial system to complex processes such as induction of systemic tolerance.
The resident leukocytes within normal liver include natural killer lymphocytes and a large number of functional memory/effector T cells which are removed from the circulation by the liver but which continue to provide ongoing immune surveillance. Common mechanisms for leukocyte homing to both the gut and liver would make good sense in evolutionary terms, as the first port of call for the blood from the gut carrying the nutrients (and potentially hazardous compounds), is the liver. However, unlike the gut, lymphocyte recirculation in the liver is less well defined and the exact route of entry for lymphocytes into portal tracts remains unclear. There are three vascular routes by which T cells may enter the liver: via portal vessels in the portal tracts; or directly into the parenchyma from the hepatic sinusoids; or via the central vein of the lobule. The sinusoids comprise a unique vascular bed which has a low velocity blood flow and which unlike other vascular beds does not require capture mediated by selectins. Lymphocytes are seen in portal tracts in the normal liver and heavy infiltration is observed in inflammatory liver diseases, e.g. Hepatitis C. The portal vessels empty into sinusoids but do not normally appear to have morphological evidence of postcapillary venules (high endothelial venules). However, the presence of new vessels at sites of lymphocytic infiltration is intriguing. These vessels appear to have phenotypic similarities to high endothelial venules; they have been described within portal tracts in chronic viral hepatitis (Garcia-Monzon et al. 1995), where they could facilitate the recruitment of lymphocytes and may be important in regulating T-cell recirculation to the liver.
Molecular classes of cell adhesion molecule in liver and gut
(A) Selectins
The selectin family shares a common mosaic structure consisting of a lectin, carbohydrate and single epidermal growth factor-like domains, including a series of 2–9 short repeats similar to those found in complement regulatory proteins. The lectin domain is the most central structure of the molecule in ligand binding, because it interacts efficiently with molecules containing fucosylated, sialylated and sulphated carbohydrate structures (Adams et al. 1994). Selectin function is uniquely restricted to the vascular system and expression of individual selectins varies in disease states (Table 1).
Table 1.
The selectin family
| Selectin | Location | Expression | Function |
|---|---|---|---|
| E-selectin | Vascular endothelial surface | Transcriptionally induced by pro-inflammatory mediators and LPS. | Eosinophils, neutrophils and monocyte adhesion during acute inflammation. Facilitates migration of T cells to skin. |
| P-selectin | Platelet α granules and endothelial cell Weibel-Palade bodies | Granule/plasma membrane fusion in response to thrombin, histamine and substance P. | Eosinophils, neutrophils monocyte adhesion during acute inflammation and thrombosis. |
| L-selectin | Leukocytes | Expressed until leukocytes are activated, then shed. | Acute neutrophil-mediated inflammation, peripheral lymph node homing. |
(1) E-selectin (expressed on endothelial cells)
E-selectin is also known as endothelium leukocyte molecule ELAM-1 or CD62E. E-selectin is barely expressed by the inactivated endothelium, but its expression is up-regulated during inflammation if vascular endothelium become activated by pro-inflammatory cytokines or lipospolysaccharides. As well as facilitating the emigration of leukocytes into tissues, most notably neutrophil endothelial cell interaction, it also promotes adhesion of resting CD4 memory cells to activated endothelium (Shimizu et al. 1991). Interferon γ (IFN-γ) appears to stabilize E-selectin surface expression without prolonging its duration of synthesis (Doukas & Pober 1990).
While the majority of endothelial cell surface E-selectin is thought to be removed from the cell surface by internalization, some of it is shed in a circulating soluble form (Patel et al. 1995).The expression of E-selectin in the form of circulating soluble adhesion molecule is up-regulated by transcription from TNF-α, IL-1 and LPS in inflammatory bowel disease (IBD).
E-selectin is not expressed in the normal liver (Bevilacqua et al. 1989). However, during endotoxaemia or septic shock its mRNA expression is observed in large vessel endothelial cells but to a lesser degree on sinusoidal lining (Adams et al. 1994). A similar induction is found in liver from alcoholic hepatitis, primary biliary cirrhosis (PBC) and acute allograft rejection (Mueller et al. 1996., Wong et al. 1997).
(2) P-selectin (expressed on endothelial cells and platelets)
P-selectin is stored in Weibel–Palade bodies of endothelial cells and produced by alpha granules of platelets (Diacovo et al. 1996). It is released after activation of platelets with histamine or thrombin, during clotting, and mediates adhesion between leukocytes and platelets. During inflammation, endothelial P-selectin acts to recruit leukocytes into postcapillary venules while platelet associated P-selectin promotes aggregation of leukocytes with platelets to form thrombi. Endothelial cells can also synthesize and express P-selectin in response to endotoxin or cytokines. However, important species differences have been observed in response to these stimuli. TNFα and endotoxin increase expression of P-selectin in murine endothelial cells, but they do not do so in human endothelial cells (Gotsch et al. 1994). This differential response may be related to differences in P-selectin promoter among species (Weller et al. 1992).
(3) L-selectin (expressed on leukocytes)
The majority of B cells, virgin T cells, most neutrophils, monocytes and eosinophils express L-selectin (also known as LECAM-1, LAM-1, Mel-14 antigen, gp90mel, and Leu8/TQ-1 antigen). L-selectins are the smallest of the vascular selectins and are important in lymphocyte homing and adhesion to high endothelial venules of peripheral lymph nodes (Collett & Munro 1999), contributing largely to the capture of leukocytes during the early phases of the adhesion cascade. Following capture, L-selectins are shed from the leukocyte surface after chemoattractant stimulation, which limits the ability of these cells to roll on endothelial cells. They have been implicated in the binding and tethering of neutrophils to activated endothelium via different vascular adhesion molecules (Sitrin et al. 2001). Interferon α is the only cytokine reported to increase surface density of L-selectin, correlating with increased m-RNA levels (Evans et al. 1993; Seiter et al. 1999; Martin-Henao et al. 2000).
(B) The immunoglobulin (Ig) superfamily
The Ig superfamilies are calcium independent trans-membrane glycoproteins. Each Ig superfamily has extracellular domains, which contains several Ig-like disulphide bonded loops with condensed cysteine residues, a trans-membrane and an intracellular domain, which interacts with the cytoskeleton. The Ig superfamily includes ICAM-1, ICAM-2, VCAM-1, and MAdCAM-1. (Table 2). All members of this family are expressed, or inducible on vascular endothelium and only ICAM-1 and ICAM-2, very rarely VCAM (but not MAdCAM-1) may also be expressed by leukocytes.
Table 2.
Adhesion super-immunoglobulin glycoproteins involved in leukocyte–endothelial interactions in the liver and bowel
| Adhesion molecule | Alternative | Localization | Constitutive | Inducible | Ligand | Function |
|---|---|---|---|---|---|---|
| ICAM-1 | CD54 | Endothelium | Yes | Yes | LFA-1, Mac-1, CD43 | Adherence/Emigration |
| VCAM-1 | CD106 | Endothelium | V small | Yes | VLA-4 | Adherence |
| MAdCAM-1 | Endothelium | Yes | Yes | L-selectin, α4β7 | Adherence/Emigration |
(1) Intercellular adhesion molecule-1 and 2 (ICAM-1, ICAM-2)
Both ICAM-1 and ICAM-2 are receptors for LFA-1. They are either constitutively present on capillary endothelium or may be induced by the cytokines TNFα, IL-1, IFN-γ and LPS (Ayres et al. 1993). ICAM-1 is also expressed on activated T cells, B cells, and monocytes. In these cells ICAM-1 contributes to the adhesion between interacting lymphocytes, and between lymphocytes and antigen-presenting cells or target cells. ICAM-1 may be up-regulated at sites of immune reaction where it controls the enhanced movement of lymphocytes into sites of inflammation. ICAM-1 binds to the b2 integrins, lymphocyte function-associated antigen-1 (LFA) and also has a binding site for certain viruses, e.g. rhinovirus (Gahmberg 1997).
(2) Vascular cell adhesion molecule-1 (VCAM-1)
VCAM-1 appears to be important in controlling lymphocyte migration. VCAM-1 is less widely distributed than ICAM-1 and is expressed by germinal centre dendritic cells, interdigitating cells, Kupffer cells, synovial lining cells and renal proximal tubule cells. VCAM-1 is induced on the endothelium by cytokines with a similar time course to ICAM-1. VCAM-1 is primarily involved in lymphocyte and monocyte–endothelial cell interactions and binds to an integrin of the VLA-4 class expressed on all leukocytes except neutrophils. T cell activation leads to kinase-induced conformational changes of VLA-4, increasing the avidity of binding with VCAM-1.
(3) PECAM-1 (CD31)
PECAM-1 is found on platelets, leukocytes and endothelial cells. Its expression is stimulated by cytokines, e.g. TNF-α, IL-1 and IFN-γ (Bujan et al. 1999). Endothelial cells express PECAM-1 on their lateral cell membranes. This distribution suggests three main functions: involvement in endothelial cell junction, mediation of leukocyte movement between endothelial cells and binding of platelets to injured endothelium.
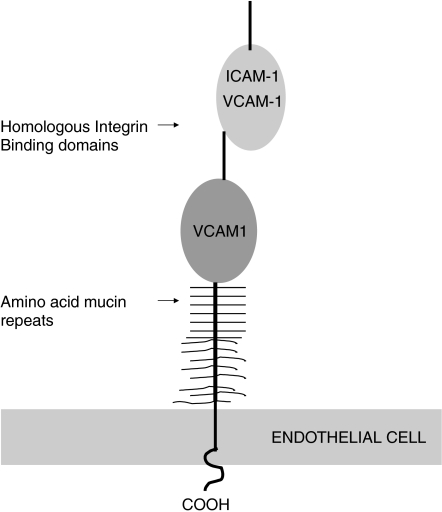
(4) Mucosal addressin cell adhesion molecule-1 (MAdCAM-1)
Human MAdCAM-1 is a 60-kDa endothelial cell adhesion molecule, which combines two immunoglobulin like domains and a mucin-like region between Ig domain 2, and the endothelial surface (Figure 3) (Tan et al. 1998; Streeter et al. 1988). It can bind two distinct lymphocyte receptors, L-selectin and the α4β7 integrin (Erle et al. 1994). Thus, within Peyer's patches MAdCAM-1 is covered with unique oligosaccharide determinants, which allow it to serve as a ligand to L-selectin and mediate rolling of naïve lymphocytes. This is in contrast to the vasculature of the lamina propria where initial rolling interactions between activated lymphocytes and endothelial cells are apparently mediated by binding of α4β7 and immunoglobulin-like domains of MAdCAM-1.
Figure 3.
Human MAdCAM-1 super-immunoglobulin.
MAdCAM-1's important role is in regulating lymphocyte trafficking to both normal and inflamed mucosal tissues, specifically in the maintenance of mucosal immunity and the regulation of inflammation of the gastrointestinal tract (Briskin et al. 1997). It participates in directing lymphocyte traffic into the lamina propria of the small and large intestine, the inflamed pancreas and the lactating mammary gland. Its constitutive expression is restricted to endothelium lining a subset of blood vessels and mucosal-associated lymphoid tissue in stomach, small intestine, large bowel, pancreas, gall bladder, marginal zone of the spleen and inflamed choroid plexus (Auth et al. 1993).
Up to 20% of thymocytes are CD4 or CD8, reacting with soluble MAdCAM-1, via α4β7. However, after birth, soluble MAdCAM-1 reactive thymocytes are rapidly down-regulated and MAdCAM-1 expression in thymic blood vessels disappears. In the developing gastrointestinal tract of rodents MAdCAM-1 is expressed widely in the venules of lamina propria and follicular dendritic cells in neonatal Peyer's patches (Iizuka et al. 2000). It is also expressed transiently in non-mucosal tissues during foetal life of rats, e.g. vascular endothelial cells in the skin. Recent work indicates that immune cell trafficking in utero and early human life is dominated by MAdCAM-1 (Salmi et al. 2001). Unlike in adults, MAdCAM-1 is widely expressed from week 7 onwards of embryogenesis and gradually becomes polarized to mucosal vessels after birth.
The micro-environmental factors that maintain preferential expression of MAdCAM-1 on gut endothelial cells are elusive. Human microvasculature endothelial cell cultures (HIMEC) established from the small intestine appear to lose their expression of MAdCAM-1 within a few days in vitro (Haraldsen et al. 1996). Two endothelial cell lines bEND.3 (brain) and SVEC (high endothelium) have been used to study the signal pathways that regulate MAdCAM-1 in response to TNF-α (Takeuchi & Baichwal 1995), which induces MAdCAM-1 mRNA and protein in a dose- and time-dependant manner (Oshima et al. 2001). This induction is tyrosine kinase (TK), p42/44, p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB/poly ADP ribose polymerase (PARP) dependent. MAdCAM-1 expression requires NF-κB translocation through both p42/44 and p38 MAPK pathways in high endothelial cells.
MAdCAM-1 is induced in both gut and liver inflammation. In the gut it is present on endothelial cells, in the lamina propria, mesenteric nodes and on follicular dendritic cells of the mucosal lymphoid organs (Peyer's patches). We and others have demonstrated MAdCAM-1 to be expressed in inflammatory liver disease, e.g. primary sclerosing cholangitis in which portal inflammation may be a prominent feature (Hillan et al. 1999; Ala et al. 2001; Grant et al. 2001). Its expression is restricted to the portal vasculature, particularly the portal veins, peribilary capillary plexus and the specialized but not exclusively HEVs, as well as at sites of portal tract lymphoid follicle formation, where it may be localized to dendritic cells, like its related super-Ig family member VCAM-1. Thus, these observations suggest that MAdCAM-1 may be important in inflammation, where it is involved in the recruitment of lymphocytes to the liver.
(C) Integrins
Integrins form a diverse family of proteins that mediate cell-matrix and cell–cell interactions. Integrins are constitutively expressed molecules on unstimulated lymphocytes (LFA-1, α4β1, VLA-4, α4β7, αEβ7) and/or up-regulated on rolling lymphocytes in response to chemokines on endothelial surfaces. They become activated to enhance rolling and lead to tethering of the cells. Integrins consist of two heterodimers, with non-covalently associated αβ chains and short cytoplasmic tails (Berman & Kozlova 2000). They ‘integrate’ the activation of the cytoskeleton with the extracellular matrix by transducing messages via classical signal pathways, and are important in cellular processes, e.g. proliferation, apoptosis and differentiation (Arao et al. 2000; Masumoto et al. 1999).
Naïve lymphocytes express low levels of α4β7 integrin, but upon activation a significant amount appears on the lymphocyte surface in a functionally active form. The α4β7 defines a discrete subpopulation of memory T cells (CD4+, CD8+) and probably B cells involved in mucosal immunity. Monoclonal antibodies to MAdCAM-1 or α4β7 effectively inhibit migration of gut-derived thoracic duct blasts and memory/effector T cells to the intestine. Unlike naïve lymphocytes, gut homing blasts and memory cells express very low levels of L-selectin or lack it completely. They do not need L-selectin to home to Peyer's patches or the appendix. Instead, they can bind directly to endothelium via an activated α4β7. Of interest, α4β7's principal functions at several steps of the adhesion cascade are interacting with MAdCAM-1, where it acts as a rolling receptor and mediates firm adhesion to endothelium.
The LFA-1 appears to be instrumental in the contact between cytotoxic T lymphocytes and target cells, where it binds to ICAM-1; in the gut (Peyer's patches and the lamina propria) the combination of α4β7 integrin–MAdCAM-1 interaction and possibly LFA-1 ensure firm lymphocyte adhesion.
(D) E-cadherin
E-cadherin is a member of the large cadherin superfamily. It is the predominant intercellular adhesion molecule expressed by intestinal epithelial cells. It is a calcium-dependent trans-membrane protein, which forms a key component of the zone adherens. E-cadherin molecules form dimers at the cell surface, which interdigitate with other E-cadherin molecules on adjacent epithelial cells. The functions of E-cadherin are mediated through actin cytoskeleton linkage via a number of cytoplasmic catenin plaque proteins, leading to signal transduction. E-cadherin gene knockout mice have confirmed (Schon et al. 1999) its critical importance to normal development and tissue function. Low or absent levels of E-cadherin are associated with a variety of epithelial malignancies arising from mutations in the E-cadherin promoter (Palmer et al. 2001) These abnormalities are accompanied with increased tumour invasion, metastasis and poor survival. Decreased membranous expression of E-cadherin molecules have been found in a number of gastrointestinal solid organ malignancies in man, e.g. gastric, oesophageal, pancreas, liver, colon as well as breast, bladder and prostate. Direct correlation between E-cadherin and grade of tumour differentiation has been observed in some gastric tumours (Grabsch et al. 2001), e.g. in a multivariate retrospective study, E-cadherin positive tumours had significantly better survival rates than E-cadherin negative tumours.
E-cadherin is thought to be exclusively involved in cell–cell adhesion and hence cancer cell metastasis and invasion. However, evidence suggests a heterophilic interaction with αEβ7 integrin, which is on the surface of predominately intraepithelial lymphocytes and only on a minority of circulating lymphocytes (Taraszka et al. 2000). This receptor ligand interaction may therefore be important in mediating the retention of lymphocytes within the mucosal epithelium.
(E) CD44
CD44 is a multifunctional adhesion proteoglycan molecule able to mediate lymphocyte rolling on hyaluronate and to activate LFA-1. Lymphocyte binding to mucosal endothelium can be partially blocked by anti-CD44 antibodies in vitro (Shepley & Racaniello 1994); CD44 may not, however, be central in the homing of lymphocytes to normal mucosal sites, as CD44-deficient mice have not been reported to have abnormalities in their mucosa-associated lymphatic tissues (Steeber et al. 1996).
(F) Vascular adhesion protein (VAP-1)
VAP-1 was discovered in the early 1990s (Salmi & Jalkanen 1992). It is an inducible human endothelial protein, which supports shear-dependant lymphocyte binding to HEV in peripheral and mesenteric lymph nodes. Intriguingly, it has a dual role as it is also a semicarbazide-sensitive mono-amine oxidase (SSAO). The adhesion function involves binding to an uncharacterized lymphocyte counter-receptor by the oligosaccharide moiety of VAP-1. As an enzyme, VAP-1 can convert soluble primary amines into corresponding aldehydes and hydrogen peroxide, in a reaction that results in the formation of biologically active products (Kurkijarvi et al. 2000). At high concentrations these compounds are cytotoxic and might contribute to the pathogenesis of different vasculopathies, as indicated by studies demonstrating that SSAO inhibition ameliorates the development of atherosclerotic lesions in diabetic models.
The recent molecular characterization and cloning of VAP-1 shows it to be a homodimeric 170–180 kDa glycoprotein, consisting of two 90-kDa subunits, held together by disulphide bonds (Salmi et al. 1997). VAP-1 has a large extracellular domain, a single trans-membrane domain, and a short cytoplasmic tail. The molecule has abundant sialic acid decorations that are essential to its adhesive function. In vivo studies support its involvement in rolling mechanisms on mesenteric vessels, although its receptor on lymphocytes is presently uncharacterized.
VAP-1 has been found to be expressed constitutively in high concentrations on hepatic endothelium, which normally fails to express adhesion molecules such as selectins, and mediates capture of lymphocytes in the liver. The expression of VAP-1 under normal conditions is most prominent in endothelium of lymph nodes, although in the setting of chronic inflammation it is up-regulated in vessels from a variety of tissues including synovium, tonsil, gut and skin. Its constitutive expression in the liver and particularly the sinusoidal and vascular endothelia suggests that it could function as an addressin, to direct lymphocytes onto hepatic endothelium.
Although VAP-1 is only expressed at low concentrations in vessels in the non-inflamed gut, it is up-regulated in inflammatory bowel disease, which suggests that lymphocytes using VAP-1 to enter the liver (Kurkijarvi et al. 1998) could also use VAP-1 to enter inflamed mesenteric vessels. Thus, a proportion of mucosal T cells and T cells activated in the liver have the ability to migrate to both the liver and gut, providing immune surveillance to both sites.
Regulation and synthesis of endothelial adhesion molecules
Stimuli such as lipopolysaccharide (LPS) and proinflammatory cytokines enhance expression of different endothelial cell adhesion molecules. For example, LPS and the cytokines TNF-α, IL-1β, Il-4 and IFN-γ can substantially enhance the transcription of genes for ICAM-1, V-CAM-1, P-selectin and E-selectin (Lidington et al. 1999). P- and E-selectin expression on HUVEC monolayers and in vivo usually reach a maximal level at 3–5 h, in comparison to VCAM-1, which peaks at 6 h and ICAM-1 at 12 h after endothelial cell stimulation. E-selectin expression returned to basal values within a few hours after stimulation, whereas P-selectin, VCAM-1 and ICAM-1 persisted for 24–48 h after a single stimulus.
NF-κB transcription factors appear to be the most relevant in inflammatory bowel and liver diseases, as they are important in the regulation of endothelial cell adhesion molecules. Unlike most transcription factors, NF-κB normally resides in the cytoplasm and must translocate into the nucleus to elicit a response. Many signals, including bacterial and viral pathogens and inflammatory cytokines elicit NF-κB translocation.
Binding sites for NF-κB have been identified in the promoter regions of the genes for VCAM-1, ICAM-1 and E-selectin. Activation of VCAM-1 requires two tandem binding sites for NF-κB, which are located on the basal VCAM-1 promoter. Of the four positive regulatory domains identified in the promoter region of the E-selectin gene, three require NF-κB binding for inducibility.
Adhesion molecules play their major functional role by being expressed on cell surfaces, but a small amount is cleaved from sites of expression and appears to be present in biological fluids. This is probably by enzymatic action, since mRNA encoding alternatively spliced soluble forms of adhesion molecules has not been reported in man. The first molecule to be recognized in soluble form was ICAM-1, and soluble forms of other molecules including E-selectin and VCAM-1 were later identified. The shed molecules may represent only a small proportion of the total expression of E-selectin, as most of the molecules are internalized and subsequently degraded or recycled. The circulating isoforms of the adhesion molecules retain their ability to bind to their respective ligands in spite of lacking trans-membranous and intra-cytoplasmic domains; however, little is known about their functional ability.
Adhesion molecules in gut and liver disease
Many different families of adhesion molecules are up-regulated during inflammation in gastrointestinal and liver diseases accompanied by the coordinated recruitment of leukocytes. In the gastrointestinal tract, lymphocytes are localized in the lamina propria, Peyer's patch, and are increased in number, particularly in the lamina propria and the epithelial cells in ulcerative colitis, Crohn's disease, coeliac disease and many other conditions. The liver has a massive population of leukocytes principally lymphocytes, which are recruited in response to insults such as injury, infection, and inflammation in viral hepatitis, primary biliary cirrhosis (PBC) and autoimmune hepatitis. In alcoholic liver diseases there is also a prominent neutrophil recruitment.
Gastrointestinal infection
Bacterial products initiate or amplify gastrointestinal inflammation with the recruitment and activation of leukocytes during inflammation of the colon (e.g. Clostridium difficile) and stomach (e.g. Helicobacter pylori).
There is an accumulation of neutrophils in the lamina propria in response to an acute gastrointestinal infection with invasive bacteria (Huang et al. 1996). Intestinal epithelial cells play an important role in the recruitment of these inflammatory cells to the site of infection through the secretion of adhesion molecules. ICAM-1 may function to maintain neutrophils, which have transmigrated through the epithelium in close contact with the intestinal epithelium, thereby reducing further invasion of colonic mucosa by invading pathogens. Activation of the leukocyte adhesion glycoprotein LFA-1 and subsequent interaction with constitutively expressed endothelial ICAM-1 may help this, causing increased vascular permeability further aided by leukocyte–platelet aggregation. The infiltration of leukocytes caused by Clostridium difficile toxin A is protracted if the LFA-1 and P-selectin expression induced indirectly by the toxin is blocked.
Penetration of the gut mucosa by pathogens expressing invasive genes occurs prominently through M cells, located in Peyer's patches. However, alternative mechanisms exist for bacterial uptake in mucosal tissues such as that proposed for dendritic cells (Rescigno et al. 2001). Dendritic cells appear to open the tight junction between epithelial cells, send dendrites outside the epithelium and directly sample bacteria. This mechanism may involve interactions with the tight junctions trans-membrane protein occludin as well as other cell adhesion related molecules, including E-cadherin and B-catenin. It will be of interest to know if similar mechanisms exist in the liver.
Coeliac disease
In coeliac disease, a T-cell mediated response to dietary gluten causes chronic inflammation, increased epithelial cell proliferation and architectural distortion in the small upper bowel. Lymphocytes accumulate within the lamina propria, and also a distinct subpopulation within the epithelial cell layer. ICAM-1 and LFA-1 are expressed in the former site but not the latter in active disease, although on short-term gluten challenge in treated coeliac patients ICAM-1 expression was not altered whilst VCAM-1 and E-selectin expression increased over hours (Sturgess et al. 1990; Kelleher et al. 1994).
Radiation enteritis
Radiation enterocolitis is a sequel to radiotherapy for pelvic and abdominal malignancy. Early changes after exposure include increased cell death, loss of villous height, and the extensive loss of intestinal function leading to fluid and electrolyte imbalance. Late complications develop from endarteritis obliterans leading to intestinal ischaemia and gangrene.
Cellular adhesion molecules may play an important role in the pathogenesis of radiation enteropathy. After gamma radiation, the nuclear transcription factor NF-κB induces oxygen radicals, causing endothelial cell activation and the release of inflammatory mediators. There follows up-regulation of CD11/CD18 on leukocytes accompanied by NF-κB dose-dependent increased expression of ICAM-1 and E-selectin (Handschel et al. 1999). This increase occurs by binding p50 and p65 nuclear proteins, with release of proteases and reactive oxygen metabolites accounting for most of the neutrophil-mediated radiation-induced tissue damage (Handschel et al. 1999).
As a result of endothelial adhesion molecule up-regulation there is recruitment of adherent and emigrating leukocytes in postcapillary venules. In vitro studies show that cultured monolayers of endothelial cells respond to ionizing radiation by increasing mRNA and cell surface expression of ICAM-1 and E-selectin (Panes et al. 1995; Heckmann et al. 1998). Indeed, ICAM-1 may remain elevated as long as 10 days after one single dose of radiation.
Medical therapy of radiation enteritis is limited. Antibiotics may be of use for bacterial overgrowth and local topical therapy to patients with proctitis. Anti-adhesion therapy could have a therapeutic role in controlling the local inflammatory processes, e.g. blocking LFA-1 and ICAM-1 has been shown to be beneficial in treating post-irradiation syndrome in rats (Horie et al. 1996).
Inflammatory bowel disease
Mucosal changes in ulcerative colitis (UC) and Crohn's disease (CD) are characterized by the development of ulcerative lesions and prominent inflammatory infiltrates of leukocytes in the bowel wall consisting of T, B lymphocytes, macrophages, plasma cells and granulocytes. The aetiology is unknown but a failure to control mucosal immune responses to antigens in the lumen is likely to play a role. Recruitment of cells to diseased gut mucosa appears essential to the initiation and perpetuation of IBD. The recruitment involves activation of a number of adhesion molecules and their ligands, on leukocytes and vascular endothelium.
MAdCAM-1 plays a central role in the aetiology of IBD through its ability to direct circulating lymphocytes to enter gut-associated lymphoid tissue and the gut interstitium. Furthermore, although MAdCAM-1 is constitutively expressed in some regions of the gut and mesenteric lymph nodes, the expression of MAdCAM-1 in these tissues is significantly increased during active colitis. Briskin et al. (1997) reported that in small intestine and colon from patients with Crohn's disease, the pattern of MAdCAM-1 expression paralleled that found constitutively in normal intestinal tract, but the relative area of endothelium expressing MAdCAM-1 was greater in IBD.
In active IBD, ICAM-1 expression is increased on the cell membranes primarily by activated endothelial venular cells at the base of ulcerations with dense cellular infiltration. ICAM-1 is also expressed by infiltrating leukocytes and is up-regulated in early lesions of CD, e.g. aphthous ulcerations. The blocking of β2 integrins may initiate some intracellular mechanisms, which make neutrophils insensitive to ICAM-1, and these mechanisms seem to be impaired in UC, in which integrins have also been shown to inhibit ICAM-1 induced neutrophil locomotion (Nielsen & Vainer 2000). The chemotactic properties of ICAM-1 have been further explored in UC, where the effect of prednisolone was shown to include both anti-chemotactic effects towards ICAM-1 and pro-chemokinetic properties, which might explain the rebound symptoms of UC during the tapering of prednisolone.
In IBD VCAM-1 is found to be membrane-bound on endothelial cells, monocyte/macrophages, lymphoid and non-lymphoid tissues. VCAM-1 is functionally active in the chronic inflammation of UC and CD. It is important in the trafficking of recirculating lymphocytes into inflamed bowel mucosa, but does not appear to be active in the acute inflammatory response.
Expression of the PECAM-1 is greater in normal colonic tissue than in the small intestine. A significant increase in PECAM-1 positive vessels is seen in active UC (Vainer & Nielsen 2000) as compared with non-inflamed areas adjacent to the inflamed tissue, indicating that PECAM-1 is involved in the earliest phases of IBD; its up-regulation seems to a preconditioning for the development of inflammatory lesions. It is significantly increased in mucosal vessels of uninvolved UC compared with healthy volunteers.
E-selectin may provide a potential target for in vivo detection and quantification of disease activity in IBD, as its expression is limited to activated endothelium. Interestingly, radiolabelled E-selectin has been used to image IBD-affected bowel by scintigraphy, which appears to show good correlation with results of colonoscopy and barium studies (Bhatti et al. 1998). This study and others (Nielson et al. 1996) also report a positive correlation between disease activity in IBD and serum levels of soluble, presumed shed E-selectin, although other studies (Goggins et al. 2001) have not confirmed this relationship.
(A) Entero-hepatic recirculation and extra-intestinal manifestations of IBD
(1) Co-existing liver and bowel disease
Inflammatory bowel disease may be associated with a variety of extraintestinal manifestations. Some are transient and generally associated with active disease (e.g. joint, skin and eye inflammation). Other conditions may precede or follow episodes of inflammation, e.g. liver involvement with sclerosing cholangitis. Grant et al. (2002) proposed an attractive rationale for simultaneous expression of α4β7-mediated responses in gut and liver which therefore allows the entero-hepatic recirculation of lymphocytes and provides surveillance to protect the liver against gut-derived organisms; this pathway could well promote the development of liver inflammation in association with IBD. Effector lymphocytes would have the capacity to migrate to both the gut and liver due to the common expression of adhesion molecules. T cell activation in the gut during episodes of IBD would thus lead to the production of primed lymphocytes with the ability to bind both hepatic and mucosal endothelium. Furthermore, some of these cells will persist as long-lived memory T cells even after resolution of IBD. Such a mechanism could involve VAP-1 and/or MAdCAM-1 acting as common addressins shared by the liver and bowel. If hepatic inflammation subsequently develops in response to an appropriate insult or infection, these memory cells will be rapidly recruited via interactions with MAdCAM-1 or VAP-1 on portal endothelium and with VAP-1 on hepatic sinusoidal endothelium. If cross-reactive self antigens in the liver activate these cells, they could presumably survive and promote the development of chronic inflammation, in liver diseases such as primary sclerosing cholangitis (PSC) or autoimmune hepatitis. The involvement of long-lived memory T cells from the gut in the pathogenesis of PSC could explain why IBD and PSC do not always occur at the same time and why PSC can present for the first time in patients with quiescent UC and in people whose colons were removed many years before. However, although this model is attractive, it does not explain all the features of PSC and indeed why the association is greater with UC than Crohn's disease.
(2) Arthropathy
About 25% of IBD patients suffer from extraintestinal inflammatory complications, of which arthropathy is the commonest. The manifestations have been attributed to the presence of putative cross-reactive antigens between gut and liver, shared susceptibility genes, and to circulating immune complexes or lipopolysaccharide. Leukocyte trafficking between gut and joints suggests an alternative mechanism. Salmi et al. (2001) showed that mucosal T cells bind to inflamed synovial high endothelial venules in vitro, an occurrence which is not just a result of inflammation, because the same activated mucosal T cells bind poorly to inflamed peripheral lymph nodes.
MAdCAM-1 is absent from synovial vessels. Activated IBD gut immunoblasts may rely on VAP-1 for synovial recognition, because blocking VAP-1 significantly inhibits binding of these leukocyte subsets to joint vessels (Salmi & Jalkanen 2001).
(B) Modulating adhesion molecule expression and therapeutic applications of anti-adhesion therapy in IBD
Interrupting cellular recruitment into IBD lesions is an important therapeutic aim. Several approaches have been used to probe the role of adhesion molecules in vivo, including blocking antibodies (Podolsky et al. 1993; Briskin et al. 1997) chimeric selectin–immunoglobulin proteins and the study of humans and animals with genetically determined adhesion deficiencies. These studies demonstrate that blocking one or more adhesion molecules can effectively inhibit inflammation; however, there appear to be clear differences in the adhesion requirements for particular types of inflammation.
Anti-α4 monoclonal antibodies with recognition of α4β7 and α4β1 relieved gut inflammation and reduced leukocyte accumulation in a cotton-top tamarin model of inflammatory colitis (Podolsky et al. 1993). Studies of transgenic mice have shown that α4-deficient transgenic mice are unable to recruit T cells to Peyer's patches and similarly, Peyer's patches are absent in β7-integrin-deficient animals, suggesting that both these integrins are essential for the formation of gut-associated lymphoid tissue. Studies of other animal models of colitis have also shown intravenous administration of monoclonal antibodies to α4, or β7 integrin significantly attenuates colonic inflammation (Picarella et al. 1993).
More recently, following the positive therapeutic effect of anti-α4 antibodies in experimental colitis, a trial of humanized monoclonal antibody to α4 integrin was performed. Although therapeutic efficacy was low, there was evidence of interaction with endothelial VCAM-1, as shown by reduced serum-soluble VCAM-1 concentrations after antibody blockade of α4 integrins (Gordon et al. 2001; Gordon et al. 2002).
In other approaches, antisense oligonucleotides (Anti-S) have been used, preventing the synthesis of adhesion molecule proteins in IBD patients. Anti-S have been produced to block the production of specific subunits of NF-κB. Reduced production of NF-κB p65 subunit mRNA had profound effects on cell adhesion. Anti-S to p65/p50 also reduces the expression of Mac-1 on stimulated monocytic HL60 cells and the expression of E-selectin, ICAM-1 and VCAM-1 on stimulated human umbilical vein endothelial cells.
ISIS 2302, an antisense phosphorothioate to human ICAM-1 selectively inhibits cytokine-induced ICAM-1 expression in a variety of human cells both in vitro and in vivo. A murine analogue was active in multiple models of inflammation, including experimental murine colitis (Scheurmann et al. 2000), allograft rejection and endotoxin-induced neutrophils in the lung. Treatment of stimulated umbilical vein endothelium cells with Anti-S directed against ICAM-1, E-selectin or VCAM-1 resulted in selective inhibition of protein synthesis (Bennett et al. 1994).
ISIS 2302 in steroid-dependent Crohn's disease (Yacyshyn et al. 1998) confirmed safety, but not efficacy; critical analysis of the data indicated that whilst some down-regulation of ICAM-1 was achieved it was only partial. More recently, Schreiber et al. (1998) also showed, using subcutaneous antisense ICAM-1 in chronic active Crohn's disease, that although safe, there was no clinical efficacy based on the primary endpoint, steroid-free remission at 14 weeks of treatment. Whether adequate delay of the anti-S into the diseased tissue was achieved in vivo remains unclear.
Liver disease
Lymphocyte–endothelial interactions take place in sinusoidal vessels under conditions of low-velocity blood flow (Table 3). Normal liver sinusoids contain large granular lymphocytes, which are intimately associated with other sinusoidal (pit) cells. Lymphocyte interactions with sinusoidal endothelium differ from those involved with postcapillary venules in, e.g. lymphoid tissue, in some important aspects. The characteristic rolling phase of primary adhesion seen in HEV is not observed, and adhesion to sinusoidal endothelium is unaffected in animals lacking endothelial selectins. Retention of leukocytes in the hepatic sinusoids is however, greatly reduced, although not abolished, in animals lacking certain cellular adhesion molecules, e.g. ICAM-1. Inflammation in the liver causes portal vessels to express P-selectin, E-selectin and VCAM-1 (none of which are detected constitutively on non-inflamed portal endothelium) and high levels of ICAM-1.
Table 3.
The super-immunoglobulins (members of the immunoglobulin superfamily) in normal, inflamed and the rejecting liver
| Normal | Inflammation/Rejection | |
|---|---|---|
| ICAM-1 | Sinusoidal endothelia | Sinusoidal endothelia |
| Kupffer cells | Kupffer cells | |
| Portal vein/arterial endothelia | ||
| Central vein endothelia | ||
| Hepatocytes | ||
| Bile duct epithelia | ||
| VCAM-1 | Kupffer cells (portal vein endothelia) | Portal vein/arterial endothelia |
| Central vein endothelia | ||
| Kupffer cells | ||
| Interstitial cells/macrophages | ||
| Sinusoidal endothelia (hepatocytes) |
In some forms of chronic liver diseases, particularly those associated with ductopenia, such as PBC and primary sclerosing cholangitis (PSC), immunological mechanisms involving lymphocyte-mediated damage are important in the characteristic bile duct damage. PSC and PBC are both characterized by the presence of cytotoxic T-lymphocytes within bile epithelium. The up-regulation and maintenance of T cell cytoxicity requires lymphocyte adhesion to target cells. ICAM-1 is strongly expressed on inflammatory interlobular bile ducts in PSC and PBC, in contrast to bile ducts in normal livers, which do not express ICAM-1 (Adams et al. 1991., Volpes et al. 1990., Bloom et al. 1995). In PBC, medium sized ducts, which are spared by the disease, do not express ICAM-1. Whether ICAM-1 expression on biliary epithelia cells is an early event in the pathogenesis of bile-duct damage or a secondary response to inflammation is not clear. This is highlighted by Bloom et al. (1995), who showed ICAM-1 expression only in advanced PSC, making a primary role for ICAM-1 in early disease less likely.
A soluble isoform of ICAM-1 can be detected in the bile as well as sera of normal subjects, with greatly elevated levels detected in chronic liver disease (Adams et al. 1992). Soluble ICAM-1 from bile samples may arise as a result of increased production by hepatocytes, bile epithelial cells or from changes in ICAM-1 elimination. The use of soluble isoforms of ICAM-1 as systemic markers of endothelial cell ICAM-1 expression from work on murine and human endothelial cells has been proposed. Messenger RNA which specifically encodes soluble isoforms of ICAM-1 in mouse plasma after TNF-α administration may be dissociated from ICAM-1 expression on endothelial cells of lung, intestine and other organs. Serum ICAM-1 levels are increased in intra- and extra-hepatic cholestasis (Polzien & Ramadori 1996). The rise appears to be dependant on the degree of disease. Determination of serum ICAM-1 concentrations in advanced PBC seems to be more reliably correlated to the histologically proved degree of intra-hepatic cholestatsis than any of the other classical parameters of cholestasis.
In alcoholic cirrhosis, there is activation of the immune response with increased expression of adhesion molecules, e.g. ICAM-1, LFA-3 and MAC-1, on the surface of circulating lymphocytes, which is more intense in patients with advanced disease (Luna-Casado et al. 1997).
There is emerging evidence of MAdCAM-1's role in inflammatory liver disease. We have observed MAdCAM-1 is expressed in livers taken at transplantation from patients with PSC and PBC (Figure 3) — by definition at an advanced stage of disease — mainly in vascular structures adjacent to biliary ductules and also within lymphoid aggregates (Ala et al. 2001). Grant et al. (2001) also demonstrated using adhesion assays that leukocytes would specifically adhere via α4β7 receptors to human PSC liver, and that α4β7 lymphocytes could be demonstrated adjacent to MAdCAM-1 on portal vessels. These findings indicate that the MAdCAM-1/α4β7 system is likely to be of functional significance in the inflammatory pathophsiology of PSC.
Liver allograft rejection
Acute (cellular) rejection, the most common form of rejection, is a cell-mediated immune injury. The characteristic histological triad of cellular rejection includes portal inflammation, bile duct damage and endothelialitis, although the last is not present in all cases. During acute liver allograft rejection there is up-regulation of ICAM-1 expression on bile ducts, endothelium and perivenular hepatocytes, compared to donor livers, patients with stable transplants, or those with non-rejection complications (Adams et al. 1989). The tissue distribution of ICAM-1 during rejection resembles that seen for HLA DR although ICAM-1 expression rather than MHC antigen expression seems more specific for rejection.
The up-regulation of ICAM-1 on tissues may be an important step in the development of acute rejection, determining which cells are the targets of immune damage. Most patients with acute cellular rejection will respond to treatment with high dose immunosuppression, but approximately 15% do not and progress to chronic irreversible rejection. Chronic (ductopenic) rejection (‘vanishing bile duct syndrome’) is defined as obliterative vasculopathy and loss of bile ducts occurring 60 days or longer after transplantation. The expression of ICAM-1 on bile ducts and hepatocytes is greatest in chronic, irreversible rejection. Persistent expression of ICAM-1 may promote a continuing inflammatory response resulting in their destruction. However, why these patients fail to respond to immunosuppression is unknown. The reduction of ICAM-1 expression seen after successful treatment with high-dose corticosteroids suggests that the action of these drugs may in part be mediated by ICAM-1 regulation.
Ischaemia/Reperfusion
Ischaemia/Reperfusion (I/R) has been implicated in the pathogenesis of a number of gastrointestinal disorders such as IBD and necrotizing enterocolitis. Leukocytes are key mediators of I/R injury in several splanchnic organs, including stomach, intestine, liver, and pancreas (Dulkanchainun et al. 1998). Leukocytes accumulate in the post-ischaemic gut, and I/R-induced inflammatory responses (leukocyte adhesion, increased vascular permeability) may be mimicked in vitro by exposing endothelial cell monolayers to hypoxia and oxygenation. I/R also results in the up-regulation and activation of the P65/P50 heterodimer of NF-κB in endothelial monolayers. The firm adhesion and emigration of leukocytes elicited by I/R involves an interaction between Mac-1 on leukocytes and ICAM-1 on endothelial cells. Antibody-blocking studies show that P-selectin contributes to the rapid rolling of leukocytes. There is a biphasic response that peaks at 30 min with a second peak in surface expression at 4–6 h after re-oxygenation. A similar biphasic response occurs with very rapid increase in E-selectin. Several chemical mediators have been implicated in the increased expression of P- and E-selectin, with leukotrienes, histamine and reactive oxygen metabolites all postulated as mediators.
Conclusions
We have gained an improved understanding into the molecular mechanisms underlying lymphocyte homing mechanisms in the closely related tissues of the liver and bowel, but the list of ligands and receptors involved in intercellular adhesion continues to grow. Most of the studies described represent only a snapshot view of the cell adhesion and migration cascade; in particular, the real time dynamics of adhesion molecule expression and activation remain ill defined. Even so, we have come a long way in our appreciation of the interactions between cellular adhesion molecules linking the liver and gut. The wider potential for therapeutic adhesion molecule inhibition is now being realized; further work is needed however, before anti-adhesion therapy becomes a realistic therapeutic option.
Acknowledgments
Dr Aftab Ala is a Wellcome Trust Training Fellow.
References
- Adams DH, Burra P, Hubscher SG, Elias E, Newman W. Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology. 1994;19:588–594. doi: 10.1002/hep.1840190308. [DOI] [PubMed] [Google Scholar]
- Adams DH, Hubscher SG, Shaw J, et al. Increased expression of intercellular adhesion molecule 1 on bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 1991;14:426–431. [PubMed] [Google Scholar]
- Adams DH, Hubscher SG, Shaw J, Rothlein R, Neuberger JM. Intercellular adhesion molecule 1 on liver allografts during rejection. Lancet. 1989;8672:1122–1125. doi: 10.1016/s0140-6736(89)91489-x. [DOI] [PubMed] [Google Scholar]
- Adams DH, Mainolfi E, Burra P, et al. Detection of circulating intercellular adhesion molecule-1 in chronic liver diseases. Hepatology. 1992;16:810–814. doi: 10.1002/hep.1840160330. [DOI] [PubMed] [Google Scholar]
- Ala A, Standish R, Khan K, et al. Mucosal addressin cell adhesion molecule (MAdCAM-1) expression in primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC) Gut. 2001;49(Suppl. 111):3043. [Google Scholar]
- Arao S, Masumoto A, Otsuki M. Beta 1 integrins play an essential role in adhesion and invasion of pancreatic carcinoma cells. Pancreas. 2000;20:129–137. doi: 10.1097/00006676-200003000-00004. [DOI] [PubMed] [Google Scholar]
- Ayres RC, Neuberger JM, Shaw J, Joplin R, Adams DH. Intercellular adhesion molecule-1 and MHC antigens on human intrahepatic bile duct cells: effect of pro-inflammatory cytokines. Gut. 1993;34:1245–1249. doi: 10.1136/gut.34.9.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CF, Condon TP, Grimm S, Chan H, Chiang MY. Inhibition of endothelial cell adhesion molecule expression with antisense oligonucleotides. J. Immunol. 1994;152:3530–3540. [PubMed] [Google Scholar]
- Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–185. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- Berman AE, Kozlova NI. Integrins: structure and functions. Membr. Cell Biol. 2000;13:207–244. [PubMed] [Google Scholar]
- Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bhatti M, Chapman P, Peters M, Haskard D, Hodgson HJ. Visualising E-selectin in the detection and evaluation of inflammatory bowel disease. Gut. 1998;43:40–47. doi: 10.1136/gut.43.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S, Fleming K, Chapman R. Adhesion molecule expression in primary sclerosing cholangitis and primary biliary cirrhosis. Gut. 1995;36:604–609. doi: 10.1136/gut.36.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 1993;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Bujan J, Gimeno MJ, Prieto A, Pascual G, Bellon JM, Alvarez-Mon M. Modulation of PECAM-1 (CD31) expression in human endothelial cells: effect of IFN gamma and IL-10. J. Vasc. Res. 1999;36:106–113. doi: 10.1159/000025632. [DOI] [PubMed] [Google Scholar]
- Collett C, Munro JM. Functional distribution and further characterization of human endothelial ligand for cellular 1-selectin. Tissue Cell. 1999;31:39–44. doi: 10.1054/tice.1998.0018. [DOI] [PubMed] [Google Scholar]
- Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- Doukas J, Pober JS. IFN-gamma enhances endothelial activation induced by tumour necrosis factor but not IL-1. J. Immunol. 1990;145:1727–1733. [PubMed] [Google Scholar]
- Dulkanchainun TS, Goss JA, Imagawa DK, Shaw GD, Anselmo DM. Reduction of hepatic ischaemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann. Surg. 1998;227:832–840. doi: 10.1097/00000658-199806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erle DJ, Briskin MJ, Butcher E. Expression and function of the MAdCAM-1 receptor integrin α4β7 on human leukocytes. J. Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- Evans SS, Collea RP, Appenheimer MM, Gollnick SO. Interferon-alpha induces the expression of the 1-selectin homing receptor in human B lymphoid cells. J. Cell Biol. 1993;123:1889–1898. doi: 10.1083/jcb.123.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg CG. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr. Opin. Cell Biol. 1997;9:643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Monzon C, Sanchez-Madrid F, Garcia-Buey L, Garcia-Arroyo A, Garcia-Sanchez A, Moreno-Otero R. Vascular adhesion molecule expression in viral chronic hepatitis: evidence of neoangiogenesis in portal tracts. Gastroenterology. 1995;108:231–241. doi: 10.1016/0016-5085(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Goggins MG, Goh J, O'Connell MA, Weir DG, Kelleher D, Mahmud N. Soluble adhesion molecules in inflammatory bowel disease. Ir. J. Med. Sci. 2001;179:107–111. doi: 10.1007/BF03168821. [DOI] [PubMed] [Google Scholar]
- Gordon FH, Lai CW, Hamilton MI, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn's disease. Gastroenterology. 2001;121:268–274. doi: 10.1053/gast.2001.26260. [DOI] [PubMed] [Google Scholar]
- Gordon FH, Hamilton MI, Donoghue S, et al. A pilot study of treatment of active ulcerative colitis with natalizumab, a humanized monoclonal antibody to alpha-4 integrin. Aliment. Pharmacol. Ther. 2002;16:699–705. doi: 10.1046/j.1365-2036.2002.01205.x. [DOI] [PubMed] [Google Scholar]
- Gotsch U, Jager U, Dominis M, Vestweber D. Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-alpha in vivo. Cell Adhes. Commun. 1994;2:7–14. doi: 10.3109/15419069409014198. [DOI] [PubMed] [Google Scholar]
- Grabsch H, Takeno S, Noguchi T, Hommel G, Gabbert HE, Mueller W. Different patterns of beta-catenin expression in gastric carcinomas: relationship with clinicopathological parameters and prognostic outcome. Histopathology. 2001;39:141–149. doi: 10.1046/j.1365-2559.2001.01177.x. [DOI] [PubMed] [Google Scholar]
- Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359:150–157. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschel J, Prott FJ, Sunderkotter C, Metze D, Meyer U, Joos U. Irradiation induces increase of adhesion molecules and accumulation of beta2-integrin-expressing cells in humans. Int. J. Radiat. Oncol. Biol. Phys. 1999;45:475–481. doi: 10.1016/s0360-3016(99)00202-3. [DOI] [PubMed] [Google Scholar]
- Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J. Immunol. 1996;156:2558–2565. [PubMed] [Google Scholar]
- Heckmann M, Douwes K, Peter R, Degitz K. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp. Cell Res. 1998;238:148–154. doi: 10.1006/excr.1997.3826. [DOI] [PubMed] [Google Scholar]
- Hillan KJ, Hagler KE, MacSween RN, et al. Expression of the mucosal vascular addressin, MAdCAM-1, in inflammatory liver disease. Liver. 1999;19:509–518. doi: 10.1111/j.1478-3231.1999.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Horie Y, Wolf R, Miyasaka M, Anderson DC, Granger DN. Leukocyte adhesion and hepatic microvascular responses to intestinal Ischemia/reperfusion in rats. Gastroenterology. 1996;111:666–673. doi: 10.1053/gast.1996.v111.pm8780571. [DOI] [PubMed] [Google Scholar]
- Huang GT, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial expression and neutrophil adhesion. J. Clin. Invest. 1996;98:572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka T, Tanaka T, Suematsu M, et al. Stage-specific expression of mucosal addressin cell adhesion molecule 1 during embryogenesis in rats. J. Immunol. 2000;164:2463–2471. doi: 10.4049/jimmunol.164.5.2463. [DOI] [PubMed] [Google Scholar]
- Kelleher D, Murphy A, Lynch S, O'Farrelly C. Adhesion molecules utilized in binding of intraepithelial lymphocytes to human enterocytes. Eur. J. Immunol. 1994;24:1013–1016. doi: 10.1002/eji.1830240437. [DOI] [PubMed] [Google Scholar]
- Kurkijarvi R, Adams DH, Leino R, Mottonen T, Jalkanen S, Salmi M. Circulating form of human vascular adhesion protein-1 (VAP-1): increased serum levels in inflammatory liver diseases. J. Immunol. 1998;161:1549–1557. [PubMed] [Google Scholar]
- Kurkijarvi R, Yegutkin GG, Gunson BK, et al. Circulating soluble vascular adhesion protein 1 accounts for the increased serum monoamine oxidase activity in chronic liver disease. Gastroenterology. 2000;119:1096–1103. doi: 10.1053/gast.2000.18163. [DOI] [PubMed] [Google Scholar]
- Lidington EA, Moyes DL, McCormack AM, Rose ML. A comparison of primary endothelial cells and endothelial cell lines for studies of immune interactions. Transpl. Immunol. 1999;7:239–246. doi: 10.1016/s0966-3274(99)80008-2. [DOI] [PubMed] [Google Scholar]
- Luna-Casado L, Diez-Ruiz A, Gutierrez-Gea F, et al. Increased peripheral mononuclear cells expression of adhesion molecules in alcoholic cirrhosis: its relation to immune activation. Hepatology. 1997;27:477–483. doi: 10.1016/s0168-8278(97)80351-0. [DOI] [PubMed] [Google Scholar]
- Martin-Henao GA, Quiroga R, Sureda A, et al. 1-selectin expression is low on CD34+ cells from patients with chronic myeloid leukemia and interferon-a up-regulates this expression. Haematologica. 2000;85:139–146. [PubMed] [Google Scholar]
- Masumoto A, Arao S, Otsuki M. Role of beta 1 integrins in adhesion and invasion of hepatocellular carcinoma cells. Hepatology. 1999;29:68–74. doi: 10.1002/hep.510290146. [DOI] [PubMed] [Google Scholar]
- Mueller AR, Platz KP, Haak M, et al. The release of cytokines, adhesion molecules, and extracellular matrix parameters during and after reperfusion in human liver transplantation. Transplantation. 1996;62:1118–1126. doi: 10.1097/00007890-199610270-00017. [DOI] [PubMed] [Google Scholar]
- Nielsen OH, Vainer B. Beta 2 integrins inhibit ICAM-1 induced neutrophil locomotion in ulcerative colitis. Gastroenterology. 2000;118(Suppl. 12):1879. [Google Scholar]
- Nielson OH, Brynskov J, Vainer B. Increased mucosal concentrations of soluble intercellular adhesion molecule-1 (sICAM-1), sE-selectin, and interleukin-8 in active ulcerative colitis. Dig. Dis. Sci. 1996;41:1780–1785. doi: 10.1007/BF02088745. [DOI] [PubMed] [Google Scholar]
- Oshima T, Pavlick KP, Laroux FS, et al. Regulation and distribution of MAdCAM-1 in endothelial cells in vitro. Am. J. Physiol. Cell Physiol. 2001;281:C1096–C1105. doi: 10.1152/ajpcell.2001.281.4.C1096. [DOI] [PubMed] [Google Scholar]
- Pachynski RK, Wu SW, Gunn MD, Erle DJ. Secondary lymphoid-tissue chemokine (SLC) stimulates integrin alpha 4 beta 7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol. 1998;161:952–956. [PubMed] [Google Scholar]
- Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D (3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panes J, Anderson DC, Miyasaka M, Granger DN. Role of leucocyte endothelial cell adhesion in radiation microvascular dysfunction in rats. Gastroenterology. 1995;108:1761–1769. doi: 10.1016/0016-5085(95)90138-8. [DOI] [PubMed] [Google Scholar]
- Patel RT, Pall AA, Adu D, Keighley MR. Circulating soluble adhesion molecules in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 1995;7:1037–1041. doi: 10.1097/00042737-199511000-00005. [DOI] [PubMed] [Google Scholar]
- Picarella DE, Kratz A, Li CB, Ruddle NH, Flavell RA. Transgenic tumor necrosis factor (TNF)-alpha production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-alpha and TNF-beta transgenic mice. J. Immunol. 1993;150:4136–4150. [PubMed] [Google Scholar]
- Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B. E-selectin in-situ expression correlates with clinical, endoscopic and histological activity and outcome. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J. Clin. Invest. 1993;92:372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzien F, Ramadori G. Increased intercellular adhesion molecule-1 serum concentration in cholestasis. J. Hepatol. 1996;25:877–886. doi: 10.1016/s0168-8278(96)80292-3. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Salmi M, Alanen K, Grenman S, Briskin M, Butcher EC, Jalkanen S. Immune cell trafficking in uterus and early life is dominated by the mucosal addressin MAdCAM-1 in humans. Gastroenterology. 2001;121:853–864. doi: 10.1053/gast.2001.27968. [DOI] [PubMed] [Google Scholar]
- Salmi M, Hellman J, Jalkanen S. The role of two distinct endothelial molecules, vascular adhesion protein-1 and peripheral lymph node addressin, in the binding of lymphocyte subsets to human lymph nodes. J. Immunol. 1998;160:5629–5636. [PubMed] [Google Scholar]
- Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992;257:1407–1409. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- Salmi M, Jalkanen S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J. Immunol. 2001;166:4650–4657. doi: 10.4049/jimmunol.166.7.4650. [DOI] [PubMed] [Google Scholar]
- Salmi M, Tohka S, Berg EL, Butcher EC, Jalkanen S. Vascular adhesion protein 1 (VAP-1) mediates lymphocyte subtype-specific, selectin-independent recognition of vascular endothelium in human lymph nodes. J. Exp. Med. 1997;186:589–600. doi: 10.1084/jem.186.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurmann G, Rijcken E, Christian F, et al. ICAM-1 and VCAM-1 antisense oligonucleotides attenuate leukocyte adhesion and inflammation in a rat model of inflammatory disease. Gastroenterology. 2000;118(4) Suppl.:A577. [Google Scholar]
- Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J. Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiter K, Kancherla R, Yang L, et al. Adenovirus and retrovirus mediated interferon alpha gene transfer into CD34+ cells maintains regeneration capacity and enhances adhesion molecules in K562 cells. J. Invest. Med. 1999;47:414–424. [PubMed] [Google Scholar]
- Shepley MP, Racaniello VR. A monoclonal antibody that blocks poliovirus attachment recognizes the lymphocyte homing receptor CD44. J. Virol. 1994;68:1301–1308. doi: 10.1128/jvi.68.3.1301-1308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Shaw S, Graber N, Gopal TV, Horgan KJ, van Seventer GA. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991;349:799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Sitrin RG, Pan PM, Blackwood RA, Huang J, Petty HR. Cutting edge: evidence for a signalling partnership between urokinase receptors (CD87) and 1-selectin (CD62L) in human polymorphonuclear neutrophils. J. Immunol. 2001;166:4822–4825. doi: 10.4049/jimmunol.166.8.4822. [DOI] [PubMed] [Google Scholar]
- Springer T. Adhesion receptor of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Steeber DA, Green NE, Sato S, Tedder TF. Lymphocyte migration in 1-selectin-deficient mice. Altered subset migration and aging of the immune system. J. Immunol. 1996;157:1096–1106. [PubMed] [Google Scholar]
- Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Sturgess RP, Macartney JC, Makgoba MW, Hung CH, Haskard DO, Ciclitara PJ. Differential upregulation of intercellular adhesion molecule-1 in coeliac disease. Clin. Exp. Immunol. 1990;82:489–492. doi: 10.1111/j.1365-2249.1990.tb05477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Baichwal VR. Induction of the gene encoding mucosal vascular addressin cell adhesion molecule 1 by tumor necrosis factor alpha is mediated by NF-kappa B proteins. Proc. Natl. Acad. Sci. USA. 1995;92:3561–3565. doi: 10.1073/pnas.92.8.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Casasnova JM, Liu JH, et al. The structure of immunoglobulin superfamily domains 1 and 2 of MAdCAM-1 reveals novel features important for integrin recognition. Structure. 1998;6:793–801. doi: 10.1016/s0969-2126(98)00080-x. [DOI] [PubMed] [Google Scholar]
- Taraszka KS, Higgins JM, Tan K, Mandelbrot DA, Wang JH, Brenner MB. Molecular basis for leukocyte integrin alpha (E) beta (7) adhesion to epithelial (E)-cadherin. J. Exp. Med. 2000;191:1555–1567. doi: 10.1084/jem.191.9.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainer B, Nielsen OH. Changed colonic profile of P-selectin, platelet-endothelial cell adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and ICAM-3 in inflammatory bowel disease. Clin. Exp. Immunol. 2000;121:242–247. doi: 10.1046/j.1365-2249.2000.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Rocha MA, Asumendi A, Barbera-Guillem E. Role of periportal and perivenous sinusoidal endothelial cells in hepatic homing of blood and metastatic cancer cells. Semin. Liver Dis. 1993;13:60–71. doi: 10.1055/s-2007-1007338. [DOI] [PubMed] [Google Scholar]
- Volpes R, van den Oord JJ, Desmet VJ. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology. 1990;12:59–65. doi: 10.1002/hep.1840120110. [DOI] [PubMed] [Google Scholar]
- Weller A, Isenmann S, Vestweber D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. J. Biol. Chem. 1992;267:15176–15183. [PubMed] [Google Scholar]
- Wong J, Johnston B, Lee SS, et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J. Clin. Invest. 1997;99:2782–2790. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacyshyn BR, Bowen-Yacyshyn MB, Jewell L, et al. A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn's disease. Gastroenterology. 1998;114:1133–1142. doi: 10.1016/s0016-5085(98)70418-4. [DOI] [PubMed] [Google Scholar]