Abstract
Whilst factors controlling the site at which joints form within the developing limb are recognised, the mechanisms by which articular element separation occurs during the formation of the joint cavity have not been determined. Herein, we review the relationships between early limb patterning, embryonic movement, extracellular matrix composition, local signalling events and the process of joint cavity formation. We speculate that a pivotal event in this process involves the demarcation of signalling boundaries, established by local mechano-dependent modifications in glycosaminoglycan synthesis. In our opinion, studies that examine early patterning and also focus on local developmental alterations in tissue architecture are required in order to help elucidate the fundamental principals regulating joint formation.
Keywords: Joint, development, hyaluronan, movement, extracellular matrix, extracellular signal-regulated kinase
Introduction
Relationship between limb patterning events and joint cavitation
In the broadest terms, studies that address joint development can be subdivided into two related yet recognisably different categories. Firstly, those that examine initial formation and configuration of the primitive precursors of specific limb structures. These aim to define the mechanisms that control patterning of cartilaginous anlagen and interposed joint regions; representing the earliest in what is often considered a two-phase process (Bernays 1878). Secondly, those studies that aim to determine how the behaviour of cells and the composition of the extracellular matrix (ECM) in the intervening non-cartilaginous regions is modified during the subsequent joint cavity formation process. It is increasingly evident that an understanding of both of these phases, and moreover an appreciation of how they are related, is essential if the mechanisms controlling joint development are to be fully defined.
The formation of synovial joint cavities must eventually generate two opposing non-adherent surfaces, which can facilitate painless and almost frictionless joint articulation, by a process involving the creation of a cell-free, fluid-filled, separation. This must occur between the ends of the predetermined cartilaginous skeletal elements to create surfaces that are effectively continuous with the synovium and associated structures, such as menisci which provide cushioning and help disperse impact during movement. The opposing joint surfaces are supported and held in close proximity by a sophisticated arrangement of musculature and ligaments. All of these structures combine to produce the diarthrodial joint that functions to provide the range of movement required for efficient locomotion.
The processes of joint specification or patterning (those determining where a joint will form) and joint cavity formation (how a joint forms) both require precise regulation. Different diarthrodial joints have distinct anatomical organizations and thus it is clear that these two events have to be meticulously controlled. For example, mechanisms must exist that ensure joint shape, dictate whether an articular surface will be convex or concave, and control the degree of congruity. Classic limb patterning studies have concentrated on the events that define skeletal organisation within a framework of positional information (Wolpert 1990; Capdevila & Johnson 1998; Sanz-Ezquerro & Tickle 2001; Wolpert 2002). Within this framework, it is now clear that regulation of the relative location of each joint within the limb is crucial for correct skeletogenesis, with recent evidence highlighting the importance of Wnt-14 in this process (Hartmann & Tabin 2000). Moreover, it is clear that once the broadly defined location of each joint is specified there is also precise spatial control over the position, within this location, at which separation between the elements will occur (the ‘plane of cleavage’). It is argued that these two events are likely to be related, however, there is no specific characteristic of the cells within early presumptive joint regions that unequivocally identifies them as those that will contribute to this plane of cleavage, and as such, relationships between these events remain enigmatic.
It is well established that early embryonic limb patterning involves a dynamic relationship between a thickened region of the embryonic ectoderm (apical ectodermal ridge; AER) and the underlying distal limb mesenchyme. These mesenchymal cells are responsible for limb outgrowth and are maintained in an undifferentiated, proliferative state (progress zone). There has also been much support for the view that during limb outgrowth these mesenchymal cells depart from the influence of the AER and become committed to specific fates. Until recently it was the general view that this depended on the time and location at which they left this progress zone (Tickle 1995; Duprez et al. 1996). However recent studies have questioned this model, and have proposed that different limb segments are ‘specified’ as distinct domains; with subsequent development involving only an expansion of these progenitor populations before their differentiation (Dudley et al. 2002). Thus, cells in the distal mesenchyme become progressively determined, or irreversibly fixed, and exhibit an increasingly limited range of potential proximodistal fates. These studies provide a basis for understanding the mechanisms by which the location of specific skeletal structures is defined and it is clear that any relationships, which exist with the ensuing joint cavity-forming processes, need to be considered within these models.
Although also a subject of some controversy, it has long been the opinion that joint cavity formation occurs within an apparently uninterrupted extracellular matrix (Bernays 1878). The synovial joints form between opposing discrete regions of mesenchymal expansion whose location and length is predetermined by previous limb patterning events (Thorogood & Hinchliffe 1975). These regions expand rapidly by chondroblastic apposition to form a pair of opposing cartilaginous anlagen. During this expansion from perichondrial inner surfaces (the most peripheral layer of cells) and proliferation of ECM, a region of intervening primitive blastemal mesenchyme persists (becomes trapped) between the ends of these paired chondrogenic expansions. This region, which appears to remain isolated from, or indeed actively antagonizes stimuli that promote neighbouring chondrogenesis, is retained as densely packed, flattened cells known as the interzone (Lizarraga et al. 2002). There is however, a difference in opinion regarding the earliest time at which interzones can be distinguished from neighbouring structures (Mitrovic 1977; Mitrovic 1978; Archer et al. 1994; Francis-West et al. 1999). Some investigators adhere to the notion that they appear at specific times at predetermined sites within an otherwise ‘continuous cartilaginous rod’ (Craig et al. 1987). At the same time, many consider these interzonal regions to be distinct at much earlier times; failing to recognize that they pass through a stage when they consist of cartilage and are homogenous and continuous with the neighbouring cartilaginous elements.
As development progresses, the interzonal regions become increasingly flattened and attenuated by continued expansion of the cartilaginous skeletal elements. At later times, the peripheral presumptive joint capsule that is initially continuous with the interzone becomes vascularized, as does the presumptive synovium at its periphery. Tissue separation begins within the avascular centre of this interzone and it is clear that cells in this region behave individually in order to facilitate the precise differentiation that is essential for joint space formation (Edwards et al. 1994; Pitsillides et al. 1995). For this reason, closer examination of the interzone is required.
Cells of the interzone form three morphologically identifiable layers: a pair of outer chondrogenic layers at the interface of the interzone and the cartilaginous epiphyses, which contribute to cartilage expansion and are effectively continuous with perichondrium (see above); and an intermediate looser cell layer. There has also been reference to a transition zone, containing cells of an intermediary phenotype (‘in-between’ chondrocytes and the intermediate looser cell layer), that differentiate into spindle-shaped cells lining the joint cartilage, and which contribute to the formation of the joint menisci. Many studies have alluded to distinctive attributes that are peculiar to cells of the interzone, however, none have successfully identified factors that are specific to cells at the presumptive joint line, e.g. parathyroid hormone-related protein and stanniocalcin (Lee et al. 1996; Stasko & Wagner 2001). A detailed understanding of interzone structure may help define the characteristics that distinguish cells at this line of cleavage.
The commonly held view is that the primitive blastemal cells, which subsequently form the interzone, are ‘committed’ to specific fates during the early limb patterning events prior to joint cavitation. It was originally proposed that joint formation was dependent upon specific influences from surrounding tissues, and that cartilaginous expansion dictated such differentiation of the interzone (Fell & Canti 1934). However, it has been shown that the early stages of joint formation are unaffected by the removal of these surrounding cartilaginous tissues (Holder 1977), confirming the view that interzonal cells receive specific intrinsic stimuli that influence their fate. The corollary of this also appears to be the case, as removal of the interzonal regions results in joint fusion, seemingly through the relatively unrestricted expansion and the eventual union of the opposed cartilage segments. Thus, despite experiments suggesting that all regions of the skeleton are individually preselected (Holder 1977; Dudley et al. 2002), the broadly held view is that the cellular origins of skeletal elements are initially homologous, and only lose some of their capacity for change once they have responded to exclusive differential stimuli (Edwards & Francis-West 2001). Thus, under normal circumstances the removal of a specific distinct population of cells will result in the absence of the tissues for which they represent a progenitor pool.
The likelihood that such predetermination is important in limb development raises many issues: it would require that cells of the interzone lose their primitive mesenchymal phenotype prior to joint formation; that cartilaginous and interzone cell populations cannot be interchanged (Holder 1977); and that mesenchymal cells of the presumptive interzone can respond to local cues in order to efficiently contribute to the joint-forming process. Such considerations are of vital importance at the present time, since research on joint formation is currently divided between studies into joint specification or patterning and joint cavity formation. It is clear that this division is undesirable as it imposes a purely acad- emic gap between the two continuous and related phases.
Relationship between movement and joint cavitation
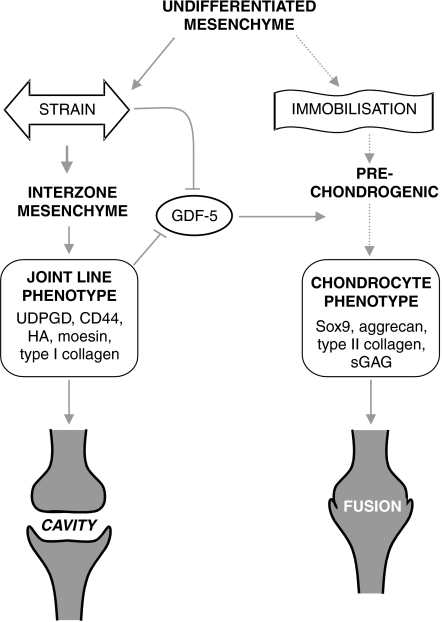
Among the factors that have an essential role in co-ordinating differentiation of the interzonal regions is skeletal movement (Lelkes 1958; Drachman & Sokoloff 1966; Ward et al. 1999; Hall & Miyake 2000; Mikic et al. 2000; Osborne et al. 2002). Under normal circumstances, movement is essential for appropriate joint formation and element configuration. We have described that in ovo immobilisation for periods as short as 3 days prior to joint cavitation irreversibly alters differentiation within the interzone and results in the fusion of opposing anlagen (Osborne et al. 2002). Clearly, an understanding of how movement regulates the behaviour of these cell populations is necessary. It is possible that such immobilisation-induced failures in joint formation involve loss of some discrete population, over-expansion of others or a change in phenotype within prede- termined cell populations (Fig. 1).
Fig. 1.
Events taking place at the joint line that lead to cavitation, and effects of immobilisation and mechanical stimuli upon development of this joint line phenotype and its replacement with a cartilaginous fusion (→) represents a positive influence and (⊣) a negative influence.
The importance of embryonic movement in skeletal development has been emphasised for over 70 years and models that provide the basis for dissecting its complex role are now available. Historically, immobilisation has been achieved in one of three ways: by culturing embryonic limbs in vitro (Niven 1933; Fell & Canti 1934); by grafting of the limb bud onto the chorioallantoic membrane (Murray & Selby 1930), or into the coelomic cavity (Hamburger & Waugh 1940); or by sustained ‘long-term’ in ovo administration of neuromuscular blocking agents (Hamburger & Waugh 1940; Mitrovic 1982; Hosseini & Hogg 1991a; Hosseini & Hogg 1991b; Osborne et al. 2002).
All of these models deprive the limb of muscular movement and result in the fusion of opposing skeletal elements and the absence of a joint cavity. This has been interpreted as an indication that mechanical influences provide an ‘extrinsic’ stimulus that significantly alters the predetermined pattern of behaviour of joint interzone cells. It also supports the view that early limb patterning events may impart upon interzonal cells an ability to adapt their phenotype to suit their physical environment. Cartilaginous and bony fusion can occur after prolonged periods of such immobilisation, suggesting that joint interzone cells may significantly modify their behaviour (Mikic et al. 2000; Murray & Selby 1930; Niven 1933; Hamburger & Waugh 1940; Mitrovic 1982; Hosseini & Hogg 1991a; Hosseini & Hogg 1991b). Immobilisation also significantly affects the formation of secondary structures of the joint, such as menisci and ligaments (Mikic et al. 2000). This supports the notion that adaptive change in cellular phenotype, or their ‘plasticity’, is a necessary component in the normal response to the physical environment during these early developmental stages. Although immobility affects joint cavity formation, no effect on shape and organisation of the joint has been reported. This would suggest that whilst intrinsic patterning events regulate cellular differentiation, some components such as shape are not susceptible to extrinsic control. Recent assessments of growth and deposition of cartilage and bone in embryonic limbs, immobilised for short periods during cavitation, showed that limb length was modified and that decreases in epiphyseal widths were more marked and most pronounced distally. The bone volume in these elements remained unchanged, whereas cartilage volume decreased significantly, suggesting that chondrogenic but not osteogenic events in the embryo are particularly sensitive to mechanical stimulation at this stage of development (Osborne et al. 2002).
Another consideration is whether such plasticity in cellular behaviour exhibits stage-selective expression. That is, whether the precise timing of the paralysis and type of immobility imposed can result in differential effects on cavity formation. We have found that the imposition of flaccid paralysis (by in ovo administration of pancuronium bromide for 3 days) at times after joint space generation, has more pronounced beneficial effects on joint cavity maintenance than rigid paralysis (induced by decamethonium bromide). However, immobility induced by either drug prior to the onset of cavitation results in similar joint fusions (Osborne et al. 2002). This suggests that the cells responsible for development and maintenance of the joint space exhibit differential phase-selective sensitivity to distinct components of their mechanical environment (Osborne et al. 2002). The fine details of such shifts in sensitivity are unresolved, but their determination is likely to provide novel insights into the processes regulating joint cavity formation and also those responsible for maintaining such ‘spaces’, once established (Fig. 1).
Relationship between changes in local ECM and joint cavitation
Whatever the influence engendered by the removal of movement, the joint cavitation process must nonetheless involve qualitative and pivotal changes in the local architecture of the extracellular matrix (ECM). Definition of the cartilaginous articular surfaces must take place where opposing skeletal elements meet, and their surfaces must become separated by a matrix that facilitates their almost frictionless motion relative to one another. Conversely, a period of immobilization that inhibits cavitation in developing chicks must engender local changes in these ECM components during fusion, as the ECM acquires properties appropriate to this novel (abnormal) mechanical milieu. These observations apart, it is clear that mechanisms regulating joint cavitation must lead to the formation of a non-adherent plane of cleavage, which involves both a local, precisely defined loss of tensile strength within the fragile interzone. Several types of event could mediate this, such as: (i) a mechanical or enzymatic degradation of the previously coherent elements of the matrix; (ii) changes in local synthesis and the selective secretion of non-coherent ECM components with low tensile strength; or (iii) a combination of both events.
In an attempt to define which events contribute to joint cavitation, much work has aimed to define the temporospatial changes in local ECM composition during joint formation. Despite evidence to the contrary, it is relevant to point out that the cavitation process does not appear to involve merely a liquefaction of the ground substance or cells (Whillis 1940; Mitrovic 1978; Abu-Hijleh et al. 1997). Rather, this process seems to involve a series of continuous, progressive changes in cell differentiation status leading to the distinct range of connective tissues of the joint (Hamerman et al. 1970).
It is therefore appropriate to consider the fine details of the ECM in each of the developing joint's compartments before, during and after the cavity forming process has taken place. Such studies have established that prior to the chondrogenic condensation, which forms the discrete foci for each of the limb elements, the mesenchyme contains type I collagen; thereafter, chondrocytic differentiation within these condensations coincides with their expression of type II collagen, whilst the surrounding cells of the perichondrium retain type I collagen expression (Pacifici et al. 2000; Lizarraga et al. 2002). More recently it has become clear that the cartilaginous tissues of the developing joint region can be further differentiated on the basis of their ECM composition; with cartilage in the developing epiphyses containing matrillin-1, whilst in contrast the chondrogenic regions contain type I, III and V collagen but not matrillin-1 (Kavanagh & Ashhurst 1999).
Closer examination of the joint line had previously shown local increases in chondroitin sulphates A and C in the interzone before joint cavitation (Andersen & Bro-Rasmussen 1961), and that there was also a loss of type II collagen and keratan sulphate-containing proteoglycans from the joint interzone, along with the appearance of type I collagen at the time of joint cavitation (Craig et al. 1987; Archer et al. 1994). This apparent loss of particular components may be paradoxical to those reports that suggest a predetermination of interzonal cell populations, as it suggests that the ECM elaborated by these cells resembles that associated with their neighbouring cartilaginous counterparts in many respects. This supports the notion that these cells are not distinct from each other, at least in terms of their local ECM, until cavitation is underway.
In addition, in situ hybridization studies support increases in procollagen type IIa and collagen type I mRNA levels in the joint interzone prior to cavitation (Nalin et al. 1995). These significant alterations in the ECM content may be interpreted as local changes in the tensile strength of the interzonal tissue, however, they are more likely to have broader implications for controlling local cell behaviour. Such changes in ECM synthesis prior to cavitation are likely to exert an essential role, as evidence for the local degradation of the ECM at the presumptive joint line is limited (Edwards et al. 1994; Edwards et al. 1996).
Our findings support the likelihood that cells within these interzones also express a range of characteristics that are consistent with their direct contribution to the increases in ECM fluidity of this region. One of these key constituents is the unsulphated glycosaminoglycan (GAG), hyaluronan (HA). HA is synthesized at the plasma membrane and is extruded directly into the extracellular compartment (Prehm 1984; Itano et al. 1999). The mechanisms for regulating HA synthesis remain enigmatic; nonetheless supply of the UDP-glucuronate monosaccharide may constitute a key point in controlling its rate of synthesis (Ward et al. 1999). In addition, antibodies raised against a HA synthase-associated protein used in developing chick joint sections suggest that HA synthase (HAS) is also likely to contribute to the regulation of HA synthesis at the joint line (Pitsillides et al. 1995).
HA has many characteristics that are clearly suited to facilitating cell-cell separation and providing a degree of fluidity to this interzonal region (see Camenisch & McDonald 2000). Normal adult synovial joints contain high concentrations of large molecular weight HA (Balazs et al. 1967; Balazs 1974; Pitsillides et al. 1994). Several studies have also documented the appearance of free HA at presumptive joint lines during cavitation suggesting that local HA synthesis and its release may be pivotal to joint cavitation (Pitsillides et al. 1995; Craig et al. 1990; Archer et al. 1994; Edwards et al. 1994). Furthermore, tissues in which there is a high concentration of HA are generally regarded as soft with a very high swelling potential, these are characteristics that may contribute to increases in ECM fluidity at the developing joint interzone (Laurent & Fraser 1992; Oster et al. 1985).
To address the possibility that HA may diffuse from other regions of the developing limb, an in situ-based microbiochemical approach has provided strong evidence for the local synthesis of HA at the presumptive joint line prior to cavitation (Pitsillides et al. 1995). These studies established that interzonal cells express high levels of uridine diphospho-glucose dehydrogenase (UDPGD) activity, which provides the UDP-glucuronate for both HA and chondroitin sulphate synthesis (De Luca & Castellani 1984; Pitsillides & Blake 1992; Wilkinson et al. 1992; Pitsillides et al. 1993; Ward et al. 1999). The research outlined above also confirmed low levels of radiolabelled sulphate incorporation at these interzonal sites, suggesting that the UDP-glucuronate synthesized by these cells is preferentially incorporated into HA and not sulphated GAGs, such as chondroitin sulphate (Pitsillides et al. 1995).
In order to determine whether these joint line- associated characteristics may be enhanced by mechanical factors, we examined the response of cultured chick articular surface cells to defined mechanical strain stimuli and found that levels of UDPGD and HAS-2, but not HAS-3 mRNA were significantly increased after mechanical stimulation (Lamb, unpublished data). This supports a role for the regulation of HA synthesis in the mechano-dependence exhibited by the joint cavitation process. Also present at the presumptive joint line prior to cavitation is the hyaladherin, CD44 the principal cell surface receptor for HA (Aruffo et al. 1990). The coexpression of CD44 and HA at presumptive joint line suggests that HA-receptor site saturation may contribute to the loss of cohesion between the developing elements, or their separation to form the fluid-filled synovial cavity (Underhill & Toole 1981; Dowthwaite et al. 1998). The direct role of HA–receptor:HA interactions in the joint forming process is also supported by the ability of exogenously applied HA-oligosaccharides to displace endogenous HA and disrupt joint cavitation events in ovo (Dowthwaite et al. 1998).
Polymerized actin along with one particular member of the actin-capping erzin, radixin and moesin (ERM) protein family, moesin, is also detected in cells at the developing joint line (Dowthwaite et al. 1998). The presence of these cytoskeletal components, and the requirement for CD44 to be associated with the cytoskeleton in order to achieve effective ligand-binding suggests that these cells are functionally engaged in binding to HA (Dowthwaite et al. 1998). Moreover, loss of both moesin and polymerised actin from these sites during in ovo immobilization further supports their intimate involvement in the joint cavity-forming process (Ward and Lamb, unpublished data).
As alluded to earlier, there are distinct opinions regarding the mechanism by which joint cavitation occurs. The first, which is still widely accepted, is that a partial cavity emerges within the interzone and becomes enlarged by mechanical factors (Whillis 1940; Andersen & Bro-Rasmussen 1961; Murray & Drachman 1969; Doskocil 1985). The second opinion is a revival of the concept that selective cell degeneration and cell death within the interzone is responsible for cavity formation (Abu-Hijleh et al. 1997; Whillis 1940; Mitrovic 1977; Mitrovic 1978; Nalin et al. 1995). A recent morphological, immunohistochemical and biochemical study concluded that there is no evidence of apoptosis in the interzone (Ito & Kida 2000). This supported studies that examined cavitating joints using TdT-mediated dUTP digoxigenin nick end labelling (TUNEL) staining techniques that failed to find a distribution of apoptotic cells within the interzonal region that might account for joint cavitation (Kavanagh et al. 2002). These studies conclude that apoptosis does not contribute to knee joint cavitation and that interzonal cells do not disappear, but are incorporated as constituents of the final joint structure. Nonetheless, it is well recognized that cells in each of the distinct layers of the interzone change in organisation and shape during the joint formation process. Thus, differentiation involving selective increases in the capacity to synthesise, export and bind HA, rather than apoptosis, is likely to contribute to these changes in the interzone.
Relationship between signalling events and joint cavity formation
There are several facets of joint specification and cavity formation that must involve intracellular and intercellular signalling events. For this reason, many of the studies that address local signalling during limb patterning have done so in a manner that aims to determine how the integration required for early developmental events is achieved (Lizarraga et al. 2002). These, for the most part have again concentrated on the signalling events involved in the early patterning phase and few address how signalling events might contribute to how a joint forms during cavitation.
Several elegant reviews have focused on the signalling events that regulate limb patterning (Francis-West et al. 1999). One aspect addressed extensively is the role of epidermal growth factor (EGF) or scatter factors (also known as hepatocyte growth factor, HGF/SF). Receptor binding of EGF is antagonistic to bone morphogenetic protein (BMP) function and acts as an inhibitor of chondrogenesis in chick mandible and limb mesenchyme (Coffin-Collins & Hall 1989; Dealy et al. 1998). Treatment of embryonic mouse mandible explants with antisense EGF oligonucleotides produced dysmorphogenesis of cartilage, suggesting that endogenous autocrine and/or paracrine EGF and EGF-like proteins regulate the size and shape of cartilage (Shum et al. 1993). In addition, when BMP-4 and EGF soaked beads were implanted in juxtaposition within embryonic day 10 mouse mandibles, the incidence and amount of ectopic cartilage, Sox9 and type II collagen expression induced by BMP-4 were significantly reduced by EGF in a dose-dependent manner (Nonaka et al. 1999). Similarly, in serum-free chick micromass cultures, expression of constitutively active BMP receptor type-IB by replication competent avian retrovirus system promoted the rate and extent of chondrogenesis, whilst exogenous EGF attenuated this effect. In micromass cultures, BMP signalling resulted in nuclear translocation and accumulation of Smad11, whereas the addition of EGF inhibited this event. These findings suggest that BMP-4 and EGF function antagonistically, yet are coupled, in the regulation of initial chondrogenesis (Kretzschmar et al. 1997; Nonaka et al. 1999). Embryonic chick forelimbs infected with a dominant-negative Smad1 exhibit reduced cartilage formation (Zhang et al. 2002, see fig. 2).
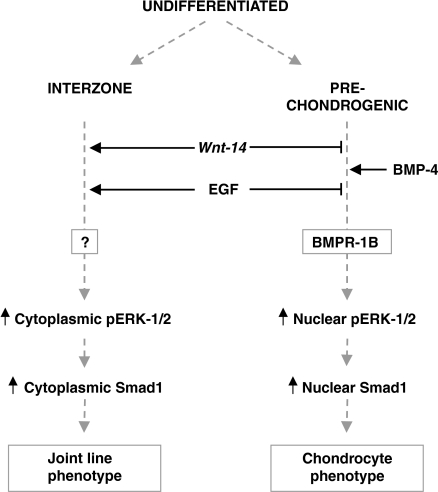
Figure 2.
The role of ERK activation in the signalling events controlling determination of joint line or chondrocyte phenotypes.
Smad1 may therefore serve as a point of convergence for the integration of these two growth factor signalling pathways during chondrogenesis (Nonaka et al. 1999), indicating the complexity with which multiple signals contribute to the control of joint specification and development2. Bmp-2, − 4 and scatter factor are all co-expressed in the developing joint (Takebayashi et al. 1995; Hogan 1996; Rosen et al. 1996; Macias et al. 1997; Zou et al. 1997) and, assuming that the appropriate receptors are co-expressed, their effects on joint development are likely to be modified intracellularly (Francis-West et al. 1999). It is therefore intriguing that studies conducted by Nonaka (Nonaka et al. 1999) have described an accumulation of Smad1 in nuclear and cytoplasmic compartments following exogenous BMP-4 and EGF treatment, respectively. Thus, regulation of BMP expression within the developing limb may result in differential subcellular localization of Smad1, thereby differentiating the underlying chondrocytes from those cells found on the articular surface (Fig. 2).
Relatively recently much attention has been focused on mitogen-activated protein kinase (MAPK) cascades as a means of mediating the transduction of many signals from the cytosol to the nucleus. In the ‘classical’ MAPK cascade, which like the Smad-1 cascade can also act as a point of convergence for many signals, Ras binds directly to Raf and recruits it to the membrane, where it undergoes activation. Activated Raf in turn phosphorylates and activates MEK, which phosphorylates and activates ERK-1/2. Activation of ERK-1/2 is essential for several Ras-induced cellular responses, including transcriptional activation of a number of genes (Mulder 2000). Major targets of these MAPK signalling pathways are the transcription factors, activator protein-1 (AP-1) (Whitmarsh & Davis 1996) and Elk (Yang et al. 1998).
EGF is a potent activator of membrane-localized Raf in a Ras-dependent manner, resulting in the phosphorylation of the dual-specificity kinase MEK-1/2, and subsequently ERK-1/2. Sequence analysis of a recently cloned homologue of the Drosophila Mad gene (rSmad1;1 (Yue et al. 1999)) concluded that it contained 4 potential ERK phosphorylation sites, similar to human Smad1 (Mulder 2000). Further analysis showed that an inhibitor of MEK (PD98059) significantly decreased the ability of both TGF-β and BMP to induce phosphorylation of endogenous Smad1. This reduced phosphorylation of Smad1 resulted in a decreased level of activation of the Smad-binding element, suggesting that under these circumstances Smad1 had a limited capacity to enhance transcription (Mulder 2000). This indicates that the classical Ras/MEK/ERK pathway is partially responsible for TGFβ-mediated Smad1 activation and transcription. Together, these findings may indicate that EGF's antagonistic effect on BMP signalling is mediated through cytoplasmic interaction of ERK and the Smad1 receptor.
With a view to understanding the role of these signalling events in the process of joint cavitation, our studies have demonstrated a distinct presumptive joint line-selective expression of phosphorylated ERK-1/2 (pERK-1/2; active (Lamb et al. 2002)). This expression of pERK-1/2 is visible in joints three days prior to cavitation; therefore developing at the same time that interzone cells can be readily differentiated from the developing anlagen. The expression of pERK-1/2 initially is comparatively diffuse with several layers of cells of the presumptive joint line expressing phosphorylated ERK-1/2, correlating with the wide interzonal band present at this stage in development. As a presumptive joint progresses towards cavitation, a greater degree of specific cell differentiation has occurred and the localisation of pERK-1/2 is retained in a region only one to two cells thick at the presumptive ‘plane of cleavage’. This expression becomes restricted to cells occupying the distinctive arcs of the developing articular surface in the cavitating joint. Once these joints have cavitated pERK-1/2 expression is greatly diminished, however, the degree of pERK-1/2 labelling remains high where the opposing surfaces are in very close proximity. This joint line expression of pERK-1/2 is mechano-dependent as its expression is rapidly lost in immobilised limbs. It is also important to appreciate the relationship of these findings to those reported by Ward, in which other known joint line- associated factors, such as CD44, UDPGD activity and predominance of HA are also lost with immobilization (Ward et al. 1999). This confirms a mechano-dependent expression of these components with prolonged embryonic immobilization preventing cavity formation and promoting joint fusion (Drachman & Sokoloff 1966; Mitrovic 1982).
Another important observation made in these studies (Lamb et al. 2002) is that the subcellular localisation of activated ERK exhibits dramatic differences across the distinct regions of the developing joint. We found that immunolabelling for pERK-1/2 was localised to the nucleus in neighbouring chondrocytes of the opposing anlagen. This may reflect an association between normal BMP-induced chondrogenesis in these regions and Smad1/Raf/Ras/MEK/ERK-mediated control of chondrocyte transcription and differentiation. However, in stark contrast, cells at the developing articular surface exhibit a colocalization of pERK-1/2 with polymerised actin and a distribution that is restricted to the cytoplasmic compartment of these cells. The function of this cytoplasmic enrichment of active ERK to cells that are found only at the developing joint line is currently unclear. However, EGF is known to both antagonize the expression of BMP in the developing joint and to potently activate the classical MAPK cascade. It is therefore tempting to speculate that EGF-mediated antagonism of the BMP-induced nuclear translocation of ERK contributes to active ERK's cytoplasmic accumulation, and that this acts to restrict local chondrogenic differentiation of these articular surface cells and promotes their joint line-selective differentiation. Clearly, this may be an over-simplification, but it may nonetheless emphasize the need for us to re-examine the findings from early ‘patterning’ studies in the light of those more closely associated with the joint cavity formation process (Fig. 2).
In this regard, it is relevant to highlight ERK's activation in response to mechanical stimuli, which we have shown both through its loss in immobilised limbs in vivo and its activation in cultured articular surface cells exposed to a period of mechanical strain in vitro (Lamb et al. 2002). This suggests that ERK's early activation at the joint line directly contributes to joint specification and acts to punctuate the developing limb with future articulation points, in a manner that is dependent upon the functional demands made during development. Furthermore, the cytoplasmic accumulation of pERK-1/2 in a specific subset of cells within the interzone appears to be the first characteristic that unequivocally identifies cells that will contribute to the joint's plane of cleavage, and as such may also provide a novel means by which the relationship between limb patterning and joint cavitation events may be established.
Defining boundaries during joint cavitation
Despite the importance of both limb patterning and the subsequent generation of joint spaces, it is clear that the relevant molecular processes are only now becoming clear (Hall & Miyake 1995; Francis-West et al. 1999; Hall & Miyake 2000). Until recently there have been no genes reported that appear to have the ability to initiate the entire process of joint formation. For example, both growth/differentiation factor-5 (Gdf-5) and Wnt-4 (a member of a large family of secreted protein growth factors) conserve a distribution consistent with the potential to act in this manner, with expression relatively restricted to the joint interzonal regions in the developing limb (Hartmann & Tabin 2000; Storm et al. 1994; Kawakami et al. 1999). Further analysis however, revealed that their role in skeletal development was to promote chondrogenesis rather than interzonal specification (Hartmann & Tabin 2000). Similarly, several other genes have been implicated in the control of chondrogenesis suggesting that the interzones may serve as signalling centres to control chondrogenesis, e.g. Wnt-5a, Wnt-5b and Wnt-4 (Rudnicki & Brown 1997; Hartmann & Tabin 2000); Wnt-5a (Kawakami et al. 1999; Yamaguchi et al. 1999); and Hox genes (Zakany & Duboule 1999).
It has also been reported that another member of the Wnt gene family, Wnt-14, is highly expressed specifically in joint forming regions (Hartmann & Tabin 2001). However, in this case Wnt-14 ms-expression induces morphological and molecular signs of joint formation, therefore confirming its integral role in joint development. Hartman and Tabin showed Wnt-14 mRNA specifically located in wide bands of expression within the mesenchymal condensations between the developing phalangeal elements (Hartmann & Tabin 2001). This expression in the interzone and the neighbouring non-chondrogenic cells appears to dictate the position of future joints in the element. In contrast, Wnt-14 over-expression resulted in the abnormal formation, or the complete absence of cartilage elements. Importantly, CD44 was also shown to exhibit Wnt-14 induced up-regulation in the developing limbs. This therefore, is the first study to successfully link a key factor that exerts a role during joint specification (Wnt-14) to one that appears to play a direct role in joint cavitation (CD44 (Dowthwaite et al. 1998)). Furthermore, this interzonal expression of Wnt-14 correlates spatially with the joint line-related increases in UDPGD activity, and CD44 and pERK-1/2 expression; but is likely to precede them temporally, suggesting that Wnt-14 may promote the acquisition of the joint cavity- forming phenotype (Fig. 2).
Over-expression of Wnt-14 in micromass cultures did not prevent the formation of precartilage aggregates but did inhibit the differentiation of these aggregates into cartilage nodules. It also regulated the expression of other interzone-selective markers such as Gdf-5, autotaxin and chordin, thus confirming that Wnt-14 promotes the interzone phenotype in prechondrogenic cells and probably acts upstream of Gdf-5, autotaxin and chordin. Hartmann and Tabin also reported that such neo- interzone formation induced by Wnt-14 in vivo was accompanied by the inhibition of interzone formation between nearby elements, suggesting that Wnt-14 also contributes to exact spacing of joints and, in turn, for the formation of the correct number of bones in the limb skeleton (Hartmann & Tabin 2001; Spitz & Duboule 2001).
The capacity to form joints may therefore be an intrinsic property of all prechondrogenic cells in mesenchymal condensations. Extrinsic stimuli like skeletal movement may simply direct these prechondrogenic cells to form an interzone, and if so it would be expected that Wnt-14 expression would exhibit a sensitivity to in ovo immobilization. It is postulated that the release of inhibitory molecules by interzone cells would thus prevent the formation of a second interzone too close to the first. The second interzone would then be formed only by prechondrogenic cells that were sufficiently far away to be unaffected by these inhibitory factors. In any case, even if the formation of one joint is necessary to determine the position of the next, induction of the first interzone still needs to be explained. Regardless, Wnt-14 is likely to be a key player in both segmental patterning and joint formation in the limb.
What becomes clear from these considerations is that appropriate limb development may also be modulated by the creation of distinct signalling boundaries. These might act to regulate the influence of such intercellular cross-talk. The construction of such boundaries may act to conserve and reaffirm the consequences of preceding developmental events, and it is possible that they involve the elaboration of specific ECM architecture and composition. Intriguingly, studies on the Drosophila gene, sugarless that encodes a homologue of vertebrate UDPGD, may exemplify this. Hacker found that mutant embryos deficient in sugarless (UDPGD activity) developed with aberrant segment polarity phenotypes that were similar to those induced by the loss of either wingless or hedgehog signalling, that sugarless mutations impaired wingless signalling, and that overexpression of wingless could bypass this requirement for sugarless. They concluded that UDPGD (or sugarless) might regulate wingless signalling by restricting its diffusion (Hacker et al. 1997).
Recent studies conducted in zebrafish to investigate cell signalling during cardiac valve formation have added unique support to this proposed role for UDPGD. Cardiac valves form at chamber boundaries and function to prevent retrograde blood flow through the heart. Large-scale screens of zebrafish identified several mutations that affect cardiac valve formation, the most severe of which is the recessive mutation Jekyll (Stainier et al. 1996). Jekyll mutant embryos exhibit pericardial oedema and toggling of blood between the two heart chambers. Together these phenotypes are generally indicative of defective atrioventricular (AV) valve function and are consistent with previous findings that Jekyll mutant hearts lack valve tissue (48 h post- fertilization). To gain further insight into this Jekyll valve defect, Walsh and Stainier isolated the disrupted gene by synteny cloning and disclosed that the Jekyll mutation disrupts UDPGD, the homologue of Drosophila sugarless that is required for the synthesis of heparan sulphate, chondroitin sulphate and HA (Walsh & Stainier 2001). Walsh and Stainier concluded that cells at the AV border do not differentiate from their neighbours in Jekyll mutants, implicating a vital role for UDPGD activity in the cell signalling events that establish a boundary between the atrium and ventricle. This is consistent with the hypothesis that UDPGD creates a cell–signalling boundary that demarcates the valve-forming region as distinct from atrium and ventricle. The view that GAGs derived from this UDPGD activity function in such boundary definition is further supported by the observation that HA-synthase deficient (HAS2-/–) mutant mice also fail to develop AV valves and that this can be corrected by exogenously administered HA in vitro (Camenisch et al. 2000).
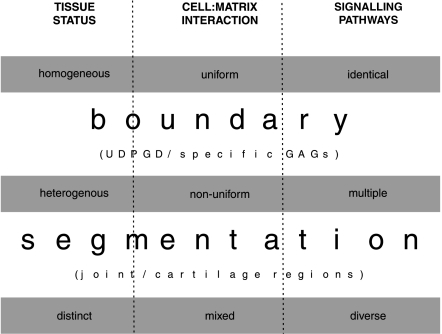
This data may therefore correlate with the expression and activity of UDPGD found between the developing elements of the joint during cavitation (Pitsillides et al. 1995; Pitsillides 1998). Together these results imply that the role of UDPGD is more sophisticated than simply providing the building blocks for HA production. It may also play an essential role in specifying the boundary between the interposed skeletal elements. Whether this is achieved solely by a mechanism that involves its contribution to regulating GAG synthesis is the subject of current investigations (Fig. 3).
Figure 3.
Importance of boundary formation in the segmentation of signalling events, that are required for cell fate determination during joint specification and development.
Moreover, mutations in the gene encoding UDPGD disrupt both the synthesis of GAGs and Wnt signalling (Binari et al. 1997; Hacker et al. 1997; Haerry et al. 1997). Since the supply of the UDP-glucuronate (product of UDPGD activity) appears rate limiting in GAG synthesis, it has been proposed that any modification of UDPGD activity might influence proteoglycan structure and function (Cumberledge & Reichsman 1997). This confirms the possible association between early patterning of interzonal regions and the subsequent changes in GAG synthesis that these cells exhibit during joint cavity formation.
Conclusion
In conclusion, it is evident that the process by which developmental mechanisms form a functionally competent joint has for some time been divided into two distinct phases. This appears to be based purely on an academic desire to divide this process into ‘bite-sized’ phases, which allow us to examine parts of the process without reference to others. This has meant that we often examine only part of the process, yet choose to make speculations regarding others. For a number of reasons, this division appears to have contributed to confusing our understanding of the mechanisms by which efficient articulation is achieved. It appears that many of the current experimental embryological approaches that aim to define the mechanisms that control joint formation, in fact only examine events related to the first of these phases (‘limb patterning’). It is our desire to ensure that we bridge the gap between these two continuous phases, and that we emphasize that the second of these phases, ‘joint formation’, has at its core the elaboration of a cell-free fluid extracellular matrix bordered by joint surfaces and synovium, which facilitates efficient movement through articulation. In our opinion studies that strive to unify both the early and late phases of this process will help to elucidate the fundamental principals controlling joint formation.
Footnotes
SMADS: a family of transcription factors that mediate many TGF-β growth factor superfamily signals. The term Smad is derived from the original members of this group, the Drosophila protein MAD (Mothers against Decapentaplegic) and the Caenorhabditis elegans protein SMA (small body size).
It may be pertinent to emphasize that GDF-5 is also capable of signalling through Smad1 (Aoki et al. 2001) and the branchypodism mice expressing mutations in Gd-5 exhibit alterations in the number and lengths of bones in the limb (Storm et al. 1994; Buxton et al. 2001)
References
- Abu-Hijleh G, Reid O, Scothorne RJ. Cell death in the developing chick knee joint. I. Spatial and temporal patterns. Clin. Anat. 1997;10:183–200. doi: 10.1002/(SICI)1098-2353(1997)10:3<183::AID-CA4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Andersen H, Bro-Rasmussen F. Histochemical studies in the histogenesis of the joint in human fetuses with special reference to the development of joint cavities in the hand and foot. Am. J. Anat. 1961;108:111–122. [Google Scholar]
- Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, Miyazono K. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J. Cell Sci. 2001;114:1483–1489. doi: 10.1242/jcs.114.8.1483. [DOI] [PubMed] [Google Scholar]
- Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthrodial joints and articular cartilage. J. Anat. 1994;184:447–456. [PMC free article] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Balazs EA. The physical properties of synovial fluid and the special role of hyaluronic acid. In: Helfet A, editor. Disorders of the Knee. Philadelphia: Lippincott; 1974. pp. 63–75. [Google Scholar]
- Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967;10:357–376. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- Bernays A. Die Entwicklungsgeschichte des kniegelenkes des menschen, mit bemerkungen uber die gelenke im allegemeinen. Morph Jahrb. 1878;4:403–446. [Google Scholar]
- Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS. Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997;124:2623–2632. doi: 10.1242/dev.124.13.2623. [DOI] [PubMed] [Google Scholar]
- Buxton P, Edwards C, Archer CW, Francis-West P. Growth/differentiation factor-5 (GDF-5) and skeletal development. J. Bone Joint Surg. Am. 2001;83-A:S23–30. [PubMed] [Google Scholar]
- Camenisch TD, McDonald JA. Hyaluronan: is bigger better? Am. J. Respir Cell Mol Biol. 2000;23:431–433. doi: 10.1165/ajrcmb.23.4.f201. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, Johnson RL. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol. 1998;197:205–217. doi: 10.1006/dbio.1997.8824. [DOI] [PubMed] [Google Scholar]
- Coffin-Collins PA, Hall BK. Chondrogenesis of mandibular mesenchyme from the embryonic chick is inhibited by mandibular epithelium and by epidermal growth factor. Int. J. Dev Biol. 1989;33:297–311. [PubMed] [Google Scholar]
- Craig FM, Bayliss MT, Bentley G, Archer CW. A role for hyaluronan in joint development. J. Anat. 1990;171:17–23. [PMC free article] [PubMed] [Google Scholar]
- Craig FM, Bentley G, Archer CW. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987;99:383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Cumberledge S, Reichsman F. Glycosaminoglycans and WNTs: just a spoonful of sugar helps the signal go down. Trends Genet. 1997;13:421–423. doi: 10.1016/s0168-9525(97)01275-4. [DOI] [PubMed] [Google Scholar]
- De Luca G, Castellani AA. Regulatory aspects of glycosaminoglycan biosynthesis. Acta Biol. Hung. 1984;35:109–121. [PubMed] [Google Scholar]
- Dealy CN, Scranton V, Cheng HC. Roles of transforming growth factor-alpha and epidermal growth factor in chick limb development. Dev Biol. 1998;202:43–55. doi: 10.1006/dbio.1998.8988. [DOI] [PubMed] [Google Scholar]
- Doskocil M. Formation of the femoropatellar part of the human knee joint. Folia Morph. 1985;33:38–47. [PubMed] [Google Scholar]
- Dowthwaite GP, Edwards JC, Pitsillides AA. An essential role for the interaction between hyaluronan and hyaluronan binding proteins during joint development. J. Histochem. Cytochem. 1998;46:641–651. doi: 10.1177/002215549804600509. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Sokoloff L. The role of movement in embryonic joint development. Dev Biol. 1966;14:401–420. [Google Scholar]
- Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418:539–544. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
- Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev. 1996;57:145–157. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Francis-West PH. Bone morphogenetic proteins in the development and healing of synovial joints. Semin Arthritis Rheum. 2001;31:33–42. doi: 10.1053/sarh.2001.24875. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Wilkinson LS, Jones HM, Soothill P, Henderson KJ, Worrall JG, Pitsillides AA. The formation of human synovial joint cavities: a possible role for hyaluronan and CD44 in altered interzone cohesion. J. Anat. 1994;185:355–367. [PMC free article] [PubMed] [Google Scholar]
- Edwards JC, Wilkinson LS, Soothill P, Hembry RM, Murphy G, Reynolds JJ. Matrix metalloproteinases in the formation of human synovial joint cavities. J. Anat. 1996;188:355–360. [PMC free article] [PubMed] [Google Scholar]
- Fell H, Canti R. Experiments on the development in vitro of the avian knee joint. Proc. R. Soc. 1934;B1176:316–351. [Google Scholar]
- Francis-West PH, Parish J, Lee K, Archer CW. BMP/GDF–signalling interactions during synovial joint development. Cell Tissue Res. 1999;296:111–119. doi: 10.1007/s004410051272. [DOI] [PubMed] [Google Scholar]
- Hacker U, Lin X, Perrimon N. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development. 1997;124:3565–3573. doi: 10.1242/dev.124.18.3565. [DOI] [PubMed] [Google Scholar]
- Haerry TE, Heslip TR, Marsh JL, O'Connor MB. Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila. Development. 1997;124:3055–3064. doi: 10.1242/dev.124.16.3055. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int. J. Dev Biol. 1995;39:881–893. [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Waugh M. The primary development of the skeleton in nerveless and poorly innervated limb transplants of chick embryos. Physiol. Zoo. 1940;13:367–380. [Google Scholar]
- Hamerman D, Rosenberg LC, Schubert M. Diarthrodial joints revisited. J. Bone Joint Surg. Am. 1970;52:725–774. [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Holder N. An experimental investigation into the early development of the chick elbow joint. J. Embryol Exp Morph. 1977;39:115–127. [PubMed] [Google Scholar]
- Hosseini A, Hogg DA. The effects of paralysis on skeletal development in the chick embryo. II. Effects on histogenesis of the tibia. J. Anat. 1991a;177:169–178. [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Hogg DA. The effects of paralysis on skeletal development in the chick embryo. I. General effects. J. Anat. 1991b;177:159–168. [PMC free article] [PubMed] [Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- Ito MM, Kida MY. Morphological and biochemical re-evaluation of the process of cavitation in the rat knee joint: cellular and cell strata alterations in the interzone. J. Anat. 2000;197(Part 4):659–679. doi: 10.1046/j.1469-7580.2000.19740659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh E, Abiri M, Bland YS, Ashhurst DE. Division and death of cells in developing synovial joints and long bones. Cell Biol. Int. 2002;26:679–688. doi: 10.1006/cbir.2002.0918. [DOI] [PubMed] [Google Scholar]
- Kavanagh E, Ashhurst DE. Development and aging of the articular cartilage of the rabbit knee joint: Distribution of biglycan, decorin, and matrilin-1. J. Histochem. Cytochem. 1999;47:1603–1616. doi: 10.1177/002215549904701212. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Wada N, Nishimatsu SI, Ishikawa T, Noji S, Nohno T. Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Dev Growth Differ. 1999;41:29–40. doi: 10.1046/j.1440-169x.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Lamb KJ, Kavanagh E, Osborne AC, Wheeler-Jones CPD, Pitsillides AA. Active ERK-1/2 selectively co-localises with polymerised actin at developing joint surfaces. Trans. Orthop Res. Soc. 2002;27:123. [Google Scholar]
- Laurent TC, Fraser JR. Hyaluronan. Faseb J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- Lee K, Lanske B, Karaplis AC, Deeds JD, Kohno H, Nissenson RA, et al. Parathyroid hormone-related peptide delays terminal differentiation of chondrocytes during endochondral bone development. Endocrinology. 1996;137:5109–5118. doi: 10.1210/endo.137.11.8895385. [DOI] [PubMed] [Google Scholar]
- Lelkes G. Experiments in vitro on the role of movement in the development of joints. J. Embryol Exp Morph. 1958;6:183–186. [PubMed] [Google Scholar]
- Lizarraga G, Lichtler A, Upholt WB, Kosher RA. Studies on the role of Cux1 in regulation of the onset of joint formation in the developing limb. Dev Biol. 2002;243:44–54. doi: 10.1006/dbio.2001.0559. [DOI] [PubMed] [Google Scholar]
- Macias D, Ganan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM. Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development. 1997;124:1109–1117. doi: 10.1242/dev.124.6.1109. [DOI] [PubMed] [Google Scholar]
- Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J. Rehabil Res. Dev. 2000;37:127–133. [PubMed] [Google Scholar]
- Mitrovic DR. Development of the metatarsophalangeal joint of the chick embryo: morphological, ultrastructural and histochemical studies. Am. J. Anat. 1977;150:333–347. doi: 10.1002/aja.1001500207. [DOI] [PubMed] [Google Scholar]
- Mitrovic D. Development of the diarthrodial joints in the rat embryo. Am. J. Anat. 1978;151:475–485. doi: 10.1002/aja.1001510403. [DOI] [PubMed] [Google Scholar]
- Mitrovic D. Development of the articular cavity in paralyzed chick embryos and in chick embryo limb buds cultured on chorioallantoic membranes. Acta Anat. 1982;113:313–324. doi: 10.1159/000145566. [DOI] [PubMed] [Google Scholar]
- Mulder KM. Role of Ras and Mapks in TGFbeta signaling. Cytokine Growth Factor Rev. 2000;11:23–35. doi: 10.1016/s1359-6101(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Murray PD, Drachman DB. The role of movement in the development of joints and related structures: the head and neck in the chick embryo. J. Embryol Exp Morph. 1969;22:349–371. [PubMed] [Google Scholar]
- Murray PDF, Selby D. Intrinsic and extensive factors in the primary development of the skeleton. Wilhelm Roux Arch. Fur Entwicklungsmechanik Organismen. 1930;122:629–649. doi: 10.1007/BF00573594. [DOI] [PubMed] [Google Scholar]
- Nalin AM, Greenlee TK, Jr, Sandell LJ. Collagen gene expression during development of avian synovial joints: transient expression of types II and XI collagen genes in the joint capsule. Dev Dyn. 1995;203:352–362. doi: 10.1002/aja.1002030307. [DOI] [PubMed] [Google Scholar]
- Niven JSF. The development in vivo and in vitro of the avian patella. Wilhelm Roux Arch. Fur Entwicklungsmechanik Organismen. 1933;128:480–501. doi: 10.1007/BF00649861. [DOI] [PubMed] [Google Scholar]
- Nonaka K, Shum L, Takahashi I, Takahashi K, Ikura T, Dashner R, et al. Convergence of the BMP and EGF signaling pathways on Smad1 in the regulation of chondrogenesis. Int. J. Dev Biol. 1999;43:795–807. [PubMed] [Google Scholar]
- Osborne AC, Lamb KJ, Lewthwaite JC, Dowthwaite GP, Pitsillides AA. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J. Musculoskel Neuron Interact. 2002;2:448–456. [PubMed] [Google Scholar]
- Oster GF, Murray JD, Maini PK. A model for chondrogenic condensations in the developing limb: the role of extracellular matrix and cell tractions. J. Embryol Exp Morph. 1985;89:93–112. [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M, Gentili C. Development of articular cartilage: what do we know about it and how may it occur? Connect Tissue Res. 2000;41:175–184. doi: 10.3109/03008200009005288. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA. The role of hyaluronan in joint cavitation. In: Archer CW, Caterson B, Ralphs J, Benjamin M, editors. Biology of the Synovial Joint. London: Harwood Academics; 1998. pp. 41–61. [Google Scholar]
- Pitsillides AA, Archer CW, Prehm P, Bayliss MT, Edwards JC. Alterations in hyaluronan synthesis during developing joint cavitation. J. Histochem. Cytochem. 1995;43:263–273. doi: 10.1177/43.3.7868856. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Blake SM. Uridine diphosphoglucose dehydrogenase activity in synovial lining cells in the experimental antigen induced model of rheumatoid arthritis: an indication of synovial lining cell function. Ann. Rheum Dis. 1992;51:992–995. doi: 10.1136/ard.51.8.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsillides AA, Wilkinson LS, Mehdizadeh S, Bayliss MT, Edwards JC. Uridine diphosphoglucose dehydrogenase activity in normal and rheumatoid synovium: the description of a specialized synovial lining cell. Int. J. Exp Pathol. 1993;74:27–34. [PMC free article] [PubMed] [Google Scholar]
- Pitsillides AA, Worrall JG, Wilkinson LS, Bayliss MT, Edwards JC. Hyaluronan concentration in non-inflamed and rheumatoid synovium. Br. J. Rheumatol. 1994;33:5–10. doi: 10.1093/rheumatology/33.1.5. [DOI] [PubMed] [Google Scholar]
- Prehm P. Hyaluronate is synthesized at plasma membranes. Biochem. J. 1984;220:597–600. doi: 10.1042/bj2200597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen V, Thies RS, Lyons K. Signaling pathways in skeletal formation: a role for BMP receptors. Ann. N Y Acad. Sci. 1996;785:59–69. doi: 10.1111/j.1749-6632.1996.tb56244.x. [DOI] [PubMed] [Google Scholar]
- Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol. 1997;185:104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- Sanz-Ezquerro JJ, Tickle C. ‘Fingering’ the vertebrate limb. Differentiation. 2001;69:91–99. doi: 10.1046/j.1432-0436.2001.690203.x. [DOI] [PubMed] [Google Scholar]
- Shum L, Sakakura Y, Bringas P, Jr, Luo W, Snead ML, Mayo M, et al. EGF abrogation-induced fusilli-form dysmorphogenesis of Meckel's cartilage during embryonic mouse mandibular morphogenesis in vitro. Development. 1993;118:903–917. doi: 10.1242/dev.118.3.903. [DOI] [PubMed] [Google Scholar]
- Spitz F, Duboule D. Development. The art of making a joint. Science. 2001;291:1713–1714. doi: 10.1126/science.1059665. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- Stasko SE, Wagner GF. Possible roles for stanniocalcin during early skeletal patterning and joint formation in the mouse. J. Endocrinol. 2001;171:237–248. doi: 10.1677/joe.0.1710237. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Takebayashi T, Iwamoto M, Jikko A, Matsumura T, Enomoto-Iwamoto M, Myoukai F, et al. Hepatocyte growth factor/scatter factor modulates cell motility, proliferation, and proteoglycan synthesis of chondrocytes. J. Cell Biol. 1995;129:1411–1419. doi: 10.1083/jcb.129.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood PV, Hinchliffe JR. An analysis of the condensation process during chondrogenesis in the embryonic chick hind limb. J. Embryol Exp Morph. 1975;33:581–606. [PubMed] [Google Scholar]
- Tickle C. Vertebrate limb development. Curr. Opin. Genet Dev. 1995;5:478–484. doi: 10.1016/0959-437x(95)90052-i. [DOI] [PubMed] [Google Scholar]
- Underhill CB, Toole BP. Receptors for hyaluronate on the surface of parent and virus-transformed cell lines: binding and aggregation studies. Exp Cell Res. 1981;131:419–423. doi: 10.1016/0014-4827(81)90248-2. [DOI] [PubMed] [Google Scholar]
- Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science. 2001;293:1670–1673. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- Ward AC, Dowthwaite GP, Pitsillides AA. Hyaluronan in joint cavitation. Biochem. Soc. Trans. 1999;27:128–135. doi: 10.1042/bst0270128. [DOI] [PubMed] [Google Scholar]
- Whillis J. The development of synovial joints. J. Anat. 1940;74:227–283. [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Pitsillides AA, Worrall JG, Edwards JC. Light microscopic characterization of the fibroblast-like synovial intimal cell (synoviocyte) Arthritis Rheum. 1992;35:1179–1184. doi: 10.1002/art.1780351010. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Signals in limb development: STOP, GO, STAY and POSITION. J. Cell Scisupplement. 1990;13:199–208. doi: 10.1242/jcs.1990.supplement_13.18. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Limb patterning: reports of model's death exaggerated. Curr. Biol. 2002;12:R628–R630. doi: 10.1016/s0960-9822(02)01137-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yang SH, Whitmarsh AJ, Davis RJ, Sharrocks AD. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 1998;17:1740–1749. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Hartsough MT, Frey RS, Frielle T, Mulder KM. Cloning and expression of a rat Smad1: regulation by TGFbeta and modulation by the Ras/MEK pathway. J. Cell Physiol. 1999;178:387–396. doi: 10.1002/(SICI)1097-4652(199903)178:3<387::AID-JCP13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Zakany J, Duboule D. Hox genes in digit development and evolution. Cell Tissue Res. 1999;296:19–25. doi: 10.1007/s004410051262. [DOI] [PubMed] [Google Scholar]
- Zhang D, Schwarz E, Puzas E, Rosier R, O'Keefe R. Smad1 signalling is critical for normal skeletal development and joint formation. Trans. Orthop Res. Soc. 2002;27:344. [Google Scholar]
- Zou H, Wieser R, Massague J, Niswander L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]