Abstract
In this study we have examined the effect of systemic administration of T helper (Th) 2 cytokines on reovirus type-2 (Reo-2)-triggered Th1-mediated autoimmune insulitis with impaired glucose tolerance (IGT) in DBA/1J suckling mice. We have demonstrated clearly that the systemic administration of both interleukin (IL)-4-expressing plasmid DNA (pIL-4) and recombinant IL-4 (rIL-4) inhibited the development of insulitis with IGT in a dose dependent manner as compared to untreated groups in Reo-2-infected DBA/1J suckling mice. The inhibitory effects of IL-4 on the development of insulitis with IGT and the advantages of pIL-4 as compared to rIL-4 in this model are discussed.
Keywords: gene therapy, IL-4, insulitis, Th1, Th2, Reo-2
Introduction
The selective and specific destruction of insulin-producing pancreatic β-cells is classified into Type I insulin dependent diabetes mellitus (DM), and has been associated with several different mechanisms which lead to insulin deficiency (Kuzuya & Matsuda 1997; Alberti & Zimmet 1998). Cell-mediated immunity mediated by CD4+ T helper (Th) 1 cells, which secrete cytokines especially interferon-γ and interleukin (IL)-2, have been seen in the histological lesions, where inflammatory cell infiltrates are observed in the pancreatic islets (autoimmune insulitis) (Campbell & Harrison 1989; Rabinovitch 1994; Tisch & McDevitt 1996; Von Herrath & Oldstone 1997). Moreover IL-12 in the presence of IL-18 induces Th1-specific immune responses (Manetti et al. 1993; Matsui et al. 1997) and accelerates autoimmune diabetes in mice (Tremleau et al. 1995).
In reovirus type-2 (Reo-2)-triggered mild diabetes (Hayashi et al. 1998, 2001) the initial damage to β cells, without hyperglycaemia, is induced by direct Reo-2 infection and release of β cell antigens from the damaged β cells. Subsequently, systemic Th1-related (IL-12-induced and IL-18-activated) and Th1 immune reactions against β cell antigens may occur (Hayashi et al. 2001). Lymphocytic insulitis with impaired glucose tolerance (IGT) was found around 10–14 days post infection (d.p.i), and the IGT is still apparent in some animals several weeks after infection (Onodera et al. 1990). Therefore Reo-2-infected mice serve as a useful experimental animal model for the study of virus-induced autoimmune insulitis.
Recently a trial of gene therapy has been reported in an autoimmune diabetes animal model based on the notion of a mutual inhibitory mechanism between Th1 and Th2 cytokines (Mosmann & Coffman 1989). Hyporesponsiveness in regulatory Th2 cells may favour the development of a Th1 cell-mediated environment in the pancreas leading to loss of immunologic tolerance to β cell autoantigens and onset of overt disease in non-obese diabetic (NOD) mice, which is another model for autoimmune type I DM (Jaramillo et al. 1994). It has been shown that IL-4 expressing plasmid leads to long-term systemic expression of therapeutic proteins and produces remarkable beneficial effects in other animal models of autoimmune type I DM (Lee et al. 2002). Adenovirus vector expressing IL-4 has prevented not only insulitis but also the onset of diabetes in NOD mice (Cameron et al. 2000), and IL-4-based retroviral gene has therapeutic effects on the development of diabetes in NOD mice (Yamamoto et al. 2001). Cytokine expressing plasmid DNA such as IL-10 (pIL-10) has reduced the incidence of diabetes in NOD mice (Nitta et al. 1998), and combined administration of plasmids encoding IL-4 (pIL-4) and pIL-10 prevented the development of autoimmune diabetes in NOD mice (Ko et al. 2001). However there is no report of the effect of pIL-4 or recombinant IL-4 (rIL-4) on the virus-triggered form of insulitis with IGT.
In the present study we have examined the effect of the systemic administration of either pIL-4 or rIL-4 on the development of insulitis with IGT in suckling DBA/1 J mice infected with Reo-2.
Materials and methods
Mice
Suckling DBA/1 J mice, which have a disease susceptibility locus (Vas 1) involved in autoimmune type I DM (Vyse & Todd 1996) of either sex (Kyudo, Saga, Japan) were used as described previously (Hayashi et al. 1998, 2001). All experiments were approved by the Animal Research Ethics Board of the Faculty of Agriculture, Yamaguchi University, Japan.
Infection protocol of mice with Reo-2
The BN-77 strain of Reo-2 isolated from a cow with diarrhoea (Kurogi et al. 1980) was used as described previously (Hayashi et al. 1998, 2001). Each one day old mouse (body weight (BW) approximately 1 g) was infected intraperitoneally (i.p) with 5 × 106 plaque- forming units (p.f.u) in 0.05 mL of Eagle's minimal essential medium (Nissui, Tokyo, Japan).
Plasmid DNA encoding cDNA of mouse IL-4 (pIL-4)
pIL-4 was purchased from the Japan Health Sciences Foundation (Tokyo, Japan). In brief, the cDNA coding region of IL-4 was amplified by polymerase chain reaction based on the cDNA sequence of IL-4 (Todryk et al. 1999). The mouse IL-4 fragment was inserted into BamHI and E. Coli-filled in pcDNA3.1 (+) under the TPA leader sequence.
IL-4 treatment
The infected mice were treated intraperitoneally (i.p.) either with pIL-4 (1 or 10 µg/mouse; n = 6 or 8 in each group) or empty plasmid (1 or 10 µg/mouse; n = 6 in each group) as control in 0.05 mL saline at 7 d.p.i., since the development of insulitis begins approximately 5–6 d.p.i. (induction phase of insulitis; Hayashi et al. 1998). Infected mice were also treated with rIL-4 (50 or 500 units/mouse; EC Ltd, London, UK; n = 6 in each group), which is the effective dose for suppression of diabetes in NOD mice (Rapoport et al. 1993) or saline as control (n = 6).
Viral titration
Pancreatic tissue (10 mg) was homogenised in 1 mL of Eagle's minimal essential medium (Nissui, Tokyo, Japan). For the determination of the infectious titre, 0.1 mL volumes of 10-fold dilutions (n = 4 in each group) were cultured on HmLu-1 cells in duplicate, as described previously (Onodera et al. 1990) at 12 d.p.i.
Blood sampling and blood glucose tolerance tests (GTTs)
Mice were anaesthetised by ketamine (45 mg/kg BW; Sankyo Co., Tokyo, Japan) and xylazine (8 mg/kg BW; Bayer Co., Tokyo, Japan) i.p. Whole blood was obtained from the heart puncture. GTTs of the blood (serum) were made by injecting glucose (2 mg/g BW, i.p) into non-fasted animals (n = 6 or 8 in each group), and the glucose concentrations in the serum were determined 60 min later as described previously (Onodera et al. 1990). More than four standard error (SE) of the mean in age matched uninfected mice (n = 10; 96 ± 44 mg/dL) was diagnosed as IGT. All assays were done 12 d.p.i., since infected mice show the peak of insulitis 10–14 d.p.i. (Onodera et al. 1990).
Histology
Four µm thickness sections of pancreas in each mouse (n = 6 or 8 in each group) were stained with haematoxylin and eosin (HE). For each mouse, the incidence (%) of insulitis (inflammatory cell infiltration into pancreatic islets) including peri-insulitis in the islets was evaluated, and an ‘islet score index (SI)’ was calculated as described previously (Hayashi et al. 1998). The severity of the cellular infiltration was evaluated by light microscopy, and an insulitis score was given to each islet on a 0–4 scale (0 = no peri-insulitis or insulitis; 1 = peri-insulitis; 2 = insulitis in <25% of islet area; 3 = insulitis in 25–50% of islet area; and 4 = insulitis in >50% of islet area). The grade of insulitis/mouse was expressed as the average score (score index = total score/total number of islets). An average of about 10 different pancreatic islets was scored blindly by two different observers.
Statistics
Data are expressed as the mean ± SE (±SEM). Statistical analysis was performed with unpaired Student's t-test (two-tailed). A value of P < 0.05 was considered significant.
Results
Effect of pIL-4 or rIL-4 on IGT, incidence and SI of insulitis in Reo-2-infected mice
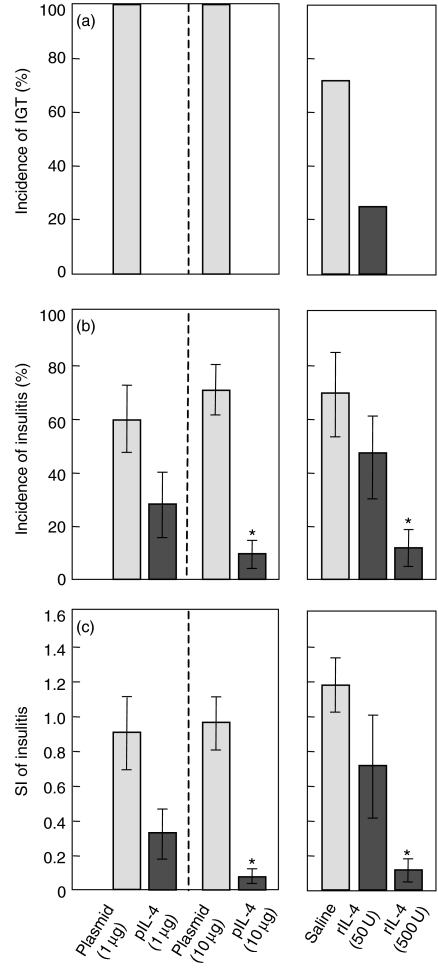
Treatment with both pIL-4 and rIL-4 inhibited elevation of the blood glucose concentrations (Fig. 1a), the incidence of disease (Fig. 1b) and SI (Fig. 1c) of insulitis compared to empty plasmid or saline only treated groups (control), respectively, in a dose dependent manner at 12 d.p.i.
Figure 1.
Effects of intraperitoneal injection of empty plasmid only (n = 6), pIL-4 (n = 6 or 8, respectively), saline (n = 6) or rIL-4 (n = 6) on IGT (a), incidence (b) and SI (c) of insulitis. Suckling mice were infected with Reo-2 were treated with either pIL-4 (1 or 10 µg/mouse) or rIL-4 (50 or 500 units/mouse) at 7 d.p.i. Assays were done 12 d.p.i. One-day-old suckling mice were inoculated with Reo-2. Data shown are mean ± SE. Statistically different between treated and control group (*P < 0.01).
The incidences of IGT in the empty plasmid-treated controls at doses of both 1 µg and 10 µg were 100% (6 of 6 animals, respectively), whereas they were 0% (0 of 6 animals or 0 of 8 animals, respectively) at doses of both 1 µg and 10 µg in pIL-4-treated groups. Also the mean incidence of insulitis in each control at a dose of 1 µg or 10 µg was 60% and 71%, respectively, whereas it was 28% (1 µg) and 9% (10 µg; P < 0.01 comparison with the corresponding control) in the pIL-4-treated group. The mean SI was 0.90 (1 µg) or 0.96 (10 µg), respectively, in the controls, whereas it was 0.32 (1 µg) or 0.09 (10 µg; P < 0.01 comparison with the corresponding control) in the groups administered with the pIL-4. Fig. 1
The incidence of IGT in the saline-treated control group was about 70% (4 of 6 animals), whereas it was about 30% (50 units; 2 of 6 animals) or 0% (500 units; 0 of 6 animals), respectively, in rIL-4-treated groups. The mean incidence was 69% in the control, whereas it was 45% (50 units) or 12% (500 units; P < 0.01 comparison with the control), respectively, in the rIL-4-treated group, and the mean SI was 1.18 in the control, whereas it was 0.70 (50 units) or 0.12 (500 units; P < 0.01, comparison with the control) in the rIL-4-treated groups (Fig. 1).
Effect of pIL-4 or rIL-4 on histology
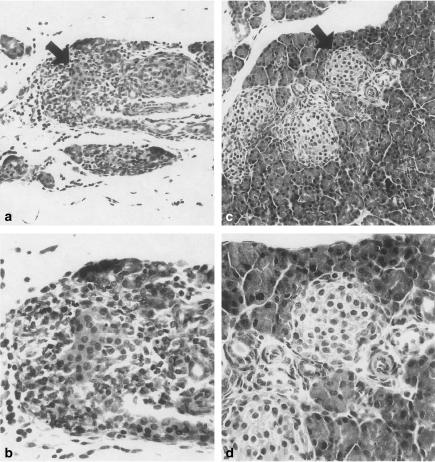
There was mild to severe cellular infiltration in and/or around pancreatic islets showing swelling and death of cells, though the changes varied between islets and between mice in the control mice treated without pIL-4 or rIL-4 (Figs 2a.b; empty plasmid, 10 µg). Those changes were also seen around intrapancreatic ducts. Reacting cells consisted of mononuclear cells (mostly lymphocytes) mixed with some polymorphonuclear leucocytes. Cellular infiltration with oedema and hyperaemia in the interstitial connective tissue, and necrosis of some exocrine pancreatic tissues were also seen. Those changes seen in the infected groups were prominently reduced in the pIL-4-treated or rIL-4-treated groups (Figs 2c.d; pIL-4, 10 µg).
Figure 2.
Infiltration of mononuclear cells at 12 d. p.i. in and around a pancreatic islet of the mouse treated with empty plasmid (10 µg) (a, and b; higher magnification of arrow in a), whereas cellular infiltration in and around islets in the mouse treated with the pIL-4 (10 µg) is prominently reduced (c, and d; higher magnification of arrow in c). Representative histological changes are shown. HE. ×50 (a and c), ×200 (b and d).
Discussion
We have reported previously that plasmid encoding Th1 cytokines such as IFN-γ exacerbated Reo-2-triggered autoimmune insulitis (Hasegawa et al. 2002). Therefore, we tried to employ Th2 cytokines especially IL-4, which has been usually used for the prevention of Th1-mediated autoimmune diabetes (Cameron et al. 2000; Yamamoto et al. 2001; Lee et al. 2002). The present study clearly demonstrated that the systemic treatment of either the pIL-4 or the rIL-4 inhibited the development of Reo-2-triggered insulitis with IGT in a dose dependent manner. Though one might suppose that these treatments exert an effect upon viral multiplication in the pancreas, the effect must be negligible, since there was no difference in pancreatic viral titre with these treatments (less than 102 p.f.u. in all groups; data not shown) at 12 d.p.i.
Although currently the main focus on plasmid DNA is as a possible means of vaccination, an alternative use may be to achieve immunomodulation in diseases such as autoimmune diabetes (Cameron et al. 2000; Nitta et al. 1998; Ko et al. 2001; Yamamoto et al. 2001; Lee et al. 2002). Our results add support to this possibility. A serum half-life of murine rIL-4 administered intravenously in mice, which is inactivated by protease and secreted into the urine rapidly, was about 19 ± 2 min (Conlon et al. 1989–90). Thus large doses of rIL-4 administration or several time treatments are necessary to maintain the concentration of IL-4 in the blood (Rapoport et al. 1993; Cameron et al. 1997; Tominaga et al. 1998). Also the effective dose (500 units) in inhibition of the development of the insulitis with IGT in this model was 10 times higher, on a BW basis and the experimental procedure, than that in NOD mice that are usually used (Rapoport et al. 1993). On the other hand only one or two treatments with the pIL-4 or adenovirus vector expressing IL-4 mRNA results in IL-4 protein secretion for several weeks (Cameron et al. 2000; Lee et al. 2002). It is also relevant that the mammalian cells transfected with plasmid carrying cDNA of IL-4 used in the present study secrete IL-4 protein in vitro for several weeks (unpublished observation). Taken together, compared to the administration of the rIL-4, the administration of the pIL-4 may be useful for the prevention of a Th1 dependent autoimmune insulitis with IGT.
The present study showed drastically reduced or no insulitis with IGT by the administration of the pIL-4 or the rIL-4. This may be due to the fact that the treatment was done at the beginning of the induction phase of the development of insulitis. The findings are similar to NOD mice where rIL-4 administration from earlier age (preinsulitis phase; 2 weeks of age), prevented insulitis and overt diabetes (Arreaza et al. 1996; Cameron et al. 1997). Thus the presence of IL-4 during the antigen response of naive T cells in the induction phase of insulitis may cause the systemic development of Th2 cells (Mosmann & Coffman 1989), resulting in the reduction in the local Th1 dependent autoimmune response in the islets. In addition Cameron et al. (1997) have suggested that rIL-4 treatment may interfere with the migration of autoreactive effector T cells expressing CD44high during the inductive phase of autoimmune DM in NOD mice, since CD44 plays a role as an adhesion receptor, and islet-infiltrating T cells posses a high level of CD44 expression (Faveeuw et al. 1994). However, an alternative view is that treatment with Th2 cytokine (e.g. pIL-10 or rIL-4) in the developing phase of insulitis suppressed diabetes, but not insulitis, in adult NOD mice (5–6 weeks of age) (Nitta et al. 1998; Tominaga et al. 1998). They suggest that it may be due to the alteration of the Th1/Th2 balance in pancreatic islets by facilitating the development of autoreactive Th2 cells, which play a minor role in the destruction of pancreatic islet cell, resulting in antagonising the Th1 cells in the islets. This is supported with the observation that long-term administration of rIL-4 during the development of the disease facilitates the infiltration of Th2-like CD4+ T cells in the islets (Tominaga et al. 1998). Taken together, though there are several possibilities, systemic Th2 immune deviation may be the key occurrence in this model.
It is known that pIL-10 injected into skeletal muscle is taken up by myocytes and the gene carried by the plasmid can be subsequently expressed for more than two months at the site of infection (Wolff et al. 1990), and IL-10 protein was detectable in the sera for more than two weeks after intramuscular injection of pIL-10 in mice (Nitta et al. 1998). Thus IL-4-expressing plasmid DNA injected i.p. may be taken up the peritoneal cells especially macrophages, since it is known that DNA from bacteria containing unmethylated CpG dinucleotides, interacts with Toll-like receptors, which are expressed on a variety of immune cells, including monocytes and dendritic cells (Modin 2000). Further studies are needed to identify the cell type which takes up plasmid in the peritoneal cells.
In conclusion, the present study indicates that IL-4 can inhibit the development of insulitis with IGT, suggesting that down-regulation of antigen-driven Th1-mediated responses by Th2 cytokine is important to control the disease. We are now investigating the effect of suppressing mechanisms on the development of insulitis by pIL-4, including how secreted IL-4 protein affects IL-4 receptor, since IL-4 receptor α-chain is a component of both the IL-4 and the IL-13 receptor (Mohrs et al. 1999). The systemic treatment of pIL-4 may be advantageous for cytokine therapy for virus-induced autoimmune insulitis with IGT.
References
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional rfeport of a WHO consultation. Diabet. Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Arreaza GA, Cameron MI, Delovitch TL. Interleukin-4: Potential immunoregulatory agent in therapy of insulin-dependent diabetes mellitus. Clin. Immunother. 1996;6:251–260. [Google Scholar]
- Cameron MJ, Arreaza GA, Waledhauser L, Gauldie J, Delovitch TL. Immunotherapy of spontaneous type I diabetes in nonobese diabetic mice by systemic interleukin-4 treatment employing adenovirus vector-mediated gene transfer. Gene Ther. 2000;1:111–123. doi: 10.1038/sj.gt.3301309. [DOI] [PubMed] [Google Scholar]
- Cameron MJ, Arreaza GA, Zucker P, Chensue SW, Strieter RM, Chakrabarti S, Delovitvh TL. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in nonobese diabetic mice by potentiation of regulatory T helper-2 cell function. J. Immunol. 1997;159:4686–4692. [PubMed] [Google Scholar]
- Campbell IL, Harrison LC. Viruses and cytokines: evidence for multiple roles in pancreatic beta cell destruction in Type I insulin-dependent diabetes mellitus. J. Cell Biochem. 1989;40:57–66. doi: 10.1002/jcb.240400107. [DOI] [PubMed] [Google Scholar]
- Conlon PJ, Tyler S, Grabstein KH, Morrissery P. Interleukin-4 (B-cell stimulatory factor-1) auguments the in vivo generation of cytotoxic cells in immunosuppressed animals. Biotechnol. Thec. 1989–90;1:31–41. [PubMed] [Google Scholar]
- Faveeuw C, Gagnerault MC, Lepault F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J. Immunol. 1994;152:5969–5978. [PubMed] [Google Scholar]
- Hasegawa K, Hayashi T, Maeda K, Onodera T. Plasmid encoding interferon-γ exacerbates reovirus type-2-induced diabetes in DBA/1 suckling mice. J. Comp. Path. 2002;127:1–6. doi: 10.1053/jcpa.2002.0584. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Morimoto M, Iwata H, Onodera T. Interferon-γ plays a role in pancreatic islet-cell destruction of reovirus type 2-induced diabetes–like syndrome in DBA/1 suckling mice. Int. J. Exp. Path. 1998;79:313–320. doi: 10.1046/j.1365-2613.1998.670398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Morimoto M, Iwata H, Onodera T. Possible involvement of IL-12 in reovirus type-2-induced diabetes in newborn DBA/1 mice. Scand. J. Immunol. 2001;53:572–578. doi: 10.1046/j.1365-3083.2001.00907.x. [DOI] [PubMed] [Google Scholar]
- Jaramillo A, Gill BM, Delovitch TL. Insulin- dependent diabetes mellitus in the non-obese diabetic mouse: a disease mediated by T cell anergy? Life Sci. 1994;55:1163–1177. doi: 10.1016/0024-3205(94)00655-5. [DOI] [PubMed] [Google Scholar]
- Ko KS, Lee M, Koh JJ, Kim SW. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol. Ther. 2001;4:313–316. doi: 10.1006/mthe.2001.0459. [DOI] [PubMed] [Google Scholar]
- Kurogi H, Inaba Y, Takahashi E, Sato K, Omori T. Serological relationship between Japanese strain of reovirus of bovine origin and type 2 reovirus strains. Natl. Inst Anim. Health Q. (Japan) 1980;20:32–33. [PubMed] [Google Scholar]
- Kuzuya T, Matsuda A. Classification of diabetes on the basis of etiologies versus degrees of insulin deficiency. Diabetes Care. 1997;20:219–229. doi: 10.2337/diacare.20.2.219. [DOI] [PubMed] [Google Scholar]
- Lee M, Koh JJ, Han S, Ko KS, Kim SW. Prevention of autoimmune insulitis by delivery of interleukin-4 plasmid using a soluble and biodegradable polymeric carrier. Pharm. Res. 2002;19:246–249. doi: 10.1023/a:1014478515005. [DOI] [PubMed] [Google Scholar]
- Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulating factor (interleukin 12, IL-12) induces T helper type 1 (Th1) -specific immune responses and inhibits the development of IL-4 producing Th cells. J. Exp. Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashino K. Propionibacterium acnes treatment diminishes CD4+NK1, 1+ T cells but induces type 1 T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cell. J. Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- Modin RL. A toll for DNA vaccines. Nature. 2000;408:659–660. doi: 10.1038/35047207. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Ledermann B, Kohler G, Gessner A, Brom Bacher F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 1999;162:7302–7308. [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nitta Y, Tashiro F, Tokui M, Shimada A, Takei I, Takabayashi K, Miyazaki J. Systemic delivery of interleukin 10 by intramuscular injection of expression plasmid DNA prevents autoimmune diabetes in nonobese diabetic mice. Hum. Gene. Ther. 1998;9:1701–1707. doi: 10.1089/hum.1998.9.12-1701. [DOI] [PubMed] [Google Scholar]
- Onodera T, Taniguchi T, Yoshihara S, Shimizu M, Sato M, Hayashi T. Reovirus type 2 induced diabetes prevented by immunosuppression and thymic hormone. Diabetologia. 1990;33:192–196. doi: 10.1007/BF00404795. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A. Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM. Therapeutic intervention by immunostimulation. Diabetes. 1994;43:613–621. doi: 10.2337/diab.43.5.613. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, Jaramille A, Zipris D, Lazarus AH, Serrezed V, Leiter EH, Cyopick P, Danska JS, Delovitch TL. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J. Exp. Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J. Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- Tominaga Y, Nagata M, Yasuda H, Okamoto N, Arisawa K, Moriyama H, Miki M, Yokono K, Kasuga M. Administration of IL-4 prevents autoimmune diabetes but enhances pancreatic insulitis in NOD mice. Clin. Immunol. Immunpathol. 1998;86:209–218. doi: 10.1006/clin.1997.4471. [DOI] [PubMed] [Google Scholar]
- Tremleau S, Penna G, Bosi E, Mprtara A, Gately MK, Adorini L. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J. Exp. Med. 1995;181:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Herrath MG, Oldstone MBA. Interferon-γ is essential for destruction of β cells and development of insulin-dependent diabetes mellitus. J. Exp. Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyse TJ, Todd JA. Identification of checkpoints in immunity by genetic analysis of autoimmune diseases. Cell. 1996;85:311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Yamamoto AM, Chernajovsky Y, Lepault F, Podhajcer O, Feldmann M, Bach JF, Chatenoud L. The activity of immunoregulatory T cells mediating activity tolerance is potentiated in nonobese diabetic mice by an IL-4-based retroviral gene therapy. J. Immunol. 2001;166:4973–4980. doi: 10.4049/jimmunol.166.8.4973. [DOI] [PubMed] [Google Scholar]