Abstract
We recently described a transgenic mouse strain overexpressing hamster αA-crystallin, a small heat shock protein, under direction of the hamster vimentin promoter. As a result myelin was degraded and axonal dystrophy in both central nervous system (especially spinal cord) and peripheral nervous system occurred. Homozygous transgenic mice developed hind limb paralysis after 8 weeks of age and displayed progressive loss of myelin and axonal dystrophy in both the central and peripheral nervous system with ongoing age. Pathologically the phenotype resembled, to a certain extent, neuroaxonal dystrophy. The biochemical findings presented in this paper (activity of the enzymes superoxide dismutase, catalase and transglutamase, myelin protein zero expression levels and blood sugar levels) confirm this pathology and exclude other putative pathologies like Amyothrophic Lateral Sclerosis and Hereditary Motor and Sensory Neuropathy. Consequently, an excessive cytoplasmic accumulation of the transgenic protein or a disturbance of the normal metabolism are considered to cause the observed neuropathology. Therefore, extra-ocular αA-crystallin-expressing transgenic mice may serve as a useful animal model to study neuroaxonal dystrophy.
Introduction
The eye lens protein α-crystallin consists of two closely related subunits, αA- and αB-crystallin, which can form homo- and hetero-oligomers of approximately 400–800 kDa (Bloemendal 1977). Nowadays it is known that αB-crystallin occurs in many tissues outside the lens, whereas αA-crystallin seems to be restricted virtually to the eye lens (Bhat & Nagineni 1989). Considering the high sequence homology between αA- and αB-crystallin (about 60%) it is hypothesized that αA-crystallin might behave like αB-crystallin when expressed in nonlenticular tissues. To verify this hypothesis, transgenic mice were generated expressing the hamster αA-crystallin gene under direction of the hamster vimentin promoter.
In a previous paper (de Rijk et al. 2000) we reported that these transgenic mice develop hindlimb paralysis after 8 weeks of age. In the spinal cord of those animals many eosinophilic bodies of varying size are present representing swollen axons with accumulation of neurofilaments, mitochondria, dense bodies, vesicular elements, tubular structures and thinning myelin sheaths. Immunohistochemical analysis revealed large deposits of αA-crystallin in astrocytes of the spinal cord and in the Schwann cells of dorsal roots and sciatic nerves. Many of the areas in brain and spinal cord, in which overexpression of αA-crystallin is found, run parallel to the mouse pyramidal tract, known to be involved in motor nerve functioning (Kuypers 1982).
The above pathology shows resemblance to neuroaxonal dystrophy. Inherited neuroaxonal dystrophies in man constitute a group of neurodegenerative disorders that share a common pathologic feature – the presence of segmental dystrophic swellings or ‘spheroids’ especially in the distal portions of axons. Ultrastructurally, such axonal spheroids contain a myriad of membranous arrays, dense axoplasmic matrices, electron-lucent clefts, and variously admixed organelles and other axoplasmic components (Wolfe et al. 1995).
Although the generated phenotype resembled neuroaxonal dystrophy, other pathologies that show only a minor resemblance to our mouse model had to be excluded. One of the known human diseases taken into consideration is Amyotrophic Lateral Sclerosis (ALS) (Kato et al. 1997). ALS is characterized by degeneration and loss of large motor neurones in the cerebral cortex, brainstem, and cervical and lumbar spinal cord (Tandan & Bradley 1985; Aizawa et al. 2000) as well as vacuoles in axons and dendrites, derived from degenerating mitochondria (Wong et al. 1995).
The second pathology, Hereditary Motor and Sensory Neuropathy (HMSN) (Dyck et al. 1993) also known as Charcot Marie Tooth disease (CMT) (Kulkens et al. 1993) is a heterogeneous group of disorders. HMSN is the most common hereditary neuropathy in humans. Many genetic defects, like aberrant peripheral myelin protein 22 expression (Suter et al. 1992) are known to cause HMSN. Distal muscular atrophy and weakness, progressive muscular atrophy and sensory loss in the distal extremities and reduced nerve conduction velocities characterize this disease. Furthermore, symptoms of de- and re-myelination are found in peripheral nerve biopsies in case of HMSN type I (Dyck et al. 1993; Kulkens et al. 1993). Since we observed hind limb paralysis, HMSN should be taken into account.
Therefore, the aim of this study was to biochemically characterize the transgenic mouse strain and possibly link the observed phenotype to neuroaxonal dystrophy as found in man.
To elucidate the pathogenesis of the central nervous system (CNS) and peripheral nervous system (PNS) in the transgenic mouse strain, and to unravel the possible role for αA-crystallin over-expression in this pathological process, mice were thoroughly analysed. Blood sugar levels of control and transgenic mice as well as enzyme activities of superoxide dismutase (SOD), catalase and transglutaminase in spinal cord extracts were carefully compared. Furthermore, integration of the transgene into the mouse genome was established and also the expression levels of myelin protein zero (P0) which is located near the integration site of the transgene and known to be involved in HMSN (Kulkens et al. 1993) were examined.
Methods
Animals
All animals tested were approximately six months old.
Mice of the FVB/N strain obtained from the Netherlands Cancer Institute (Amsterdam) were used for the generation of transgenic mice. The hamster αA-crystallin gene under direction of the hamster vimentin (pVim-αA) promoter was microinjected into the pronucleus of fertilized eggs to obtain homozygous transgenic animals (de Rijk et al. 2000). Transgenic mice as well as control mice were housed in macrolon cages with sterilized wood chip bedding. Environmental conditions were standardized, light/dark cycle of 12 h, room temperature of 20–25 °C and relative humidity of 55%. Several weeks after birth mice were screened for the integration of the transgene by isolating toe-DNA essentially as described by Blin & Stafford (1976). The extracted chromosomal DNA was restricted with EcoR1 and analysed for the presence of the transgene by Southern blotting and hybridization with an αA-crystallin cDNA probe.
Blood sugar levels
β-D-glucose levels were analysed by using capillary blood from the tail artery. Gluco card memory 2 blood glucose test meter (A. Menarini diagnostics) was used in combination with accompanying reagent strips. Animals were tested after one night of food deprivation.
Fluorescence in situ hybridization of pVim-αA
Bone marrow cells obtained from transgenic animals were grown in RPMI1640 (Gibco BRL, Gaithersburg, MO, USA) containing 10% FCS, 113 U/mL penicillin (Gibco BRL) and 113 U/mL streptomycin (Gibco BRL) and retained in metaphase using a final concentration of 0.2 µg/mL colchicin (Gibco BRL). The gene construct, to generate the transgenic mice, pVim-αA, was used as probe for fluorescence in situ hybridization (FISH) essentially as described by Suijkerbuijk et al. (1994). A whole chromosome 1 specific painting probe (a kind gift of Dr José Coco Martin, Dutch Cancer Institute, NKI, Amsterdam, the Netherlands) was used to unambiguously identify the chromosome hybridizing to pVim-αA.
Southern and Western blot analysis of myelin protein zero
A Southern blot already available from the initial screenings was hybridized with a cDNA probe for myelin protein zero (P0) (kindly provided by Dr Frank Baas, Academic Medical Center, University of Amsterdam, the Netherlands).
Following 13% SDS-PAGE of peripheral nerve extracts obtained from control and transgenic mice Western blots were stained with a polyclonal affinity-purified antibody directed against αA-crystallin (1: 2000) and a monoclonal antibody directed against P0 (1 : 1000) (a kind gift of Prof Juan Jose Archelos, University Hospital for Neurology, Graz, Austria). 30 and 5 µg of peripheral nerve extract was used for the detection of αA-crystallin and P0, respectively.
Protein content:
The protein content in samples was calculated by the bicinchoninic acid (BCA) method (PIERCE).
Determination of enzyme activities
Superoxide dismutase assay SOD activity in spinal cord extracts was initially determined by analysis of a 13% acrylamide gel stained with Nitro Blue Tetrazolium (NBT) (Research Organics Inc, Cleveland, OH, USA) essentially as described by Beauchamp & Fridovich (1971). Spinal cord extracts (40 µg) of 5 positive and 2 negative mice were run on a basic urea gel (as described by Schoenmakers et al. (1969). As a negative control 5 µg of α-crystallin was used. The gel was stained by soaking it for 20 min in 2.45 × 10−3 M NBT followed by an incubation in 0.028 m TEMED (Merck, Whitehouse Station, NJ, USA), 2.8 × 10−5 M riboflavin (Sigma-Aldrich, St. Louis, MO, USA) and 0.036 m potassium phosphate at pH 7.8 until, at the position of SOD, destaining could be observed. To analyse the SOD activity in more detail, the inhibition of oxidation of xanthine (Sigma-Aldrich) by xanthine oxidase (Sigma-Aldrich) was measured. The generation of O2– by xanthine oxidase provides the basis for this assay. The reaction mixture contained 340 µL of 0.25 U/mL xanthine oxidase, 1 × 10−4 M xanthine, 2.5 × 10−5 M NBT 1 × 10−4 M EDTA and 0.05 m sodium carbonate of pH 10.2, 560 µL potassium phosphate at pH 7.8 and 100 µL of spinal cord extract, the enzyme solution. Spinal cords were homogenized in 0.036 m potassium phosphate at pH 7.8. The supernatant after centrifugation was used as enzyme solution and the activity was corrected for protein content. Protein concentration was determined as described above.
The activity was recorded at 550 nm using a Perkin-Elmer Lambda 2 UV/VIS spectrophotometer equipped with a thermostated circulating water-bath at 25 °C and a thermocouple to register the temperature of the sample.
Transglutaminase assay
Transglutaminase activity in spinal cord homogenates (in 50 mm Tris-HCl, pH 8.0) was based on the incorporation of [3H]-putrescine (Amersham Pharmacia Biotech) into N,N,dimethylated casein (Sigma-Aldrich).The reaction mixture of 700 µL for determination of enzyme activity contained 1.5 mm CaCl2, 10 mm dithiothreitol (Sigma-Aldrich), 1 µL [3H]-putrescine (1 mCi/mL), 50 mm Tris-HCl pH 8.0, 28 mm N,N,dimethylated casein and 100 µg spinal cord extract. The negative control did not contain casein. The reaction mixtures were incubated for 30 min at 37 °C. Reactions were terminated by adding 0.3 mL of 20% TCA, and the insoluble material was dissolved in 20 µL 1 m Tris-HCl, pH 8.0. The radioactivity in the dissolved pellets was determined by using a liquid scintillation spectrometer (Beckman LS3801, Beckman Instruments Inc., Palo Alto, CA, USA). Transglutaminase activity was determined by subtracting the value in the sample without casein from the activity in the sample containing casein.
Catalase assay
To determine catalase activity in spinal cord extracts the decrease in extinction at 240 nm of H2O2 (Sigma-Aldrich) was analysed. Catalase activity was measured according to Beers & Sizer (1952) by spectrometric recording of the reduction of H2O2. The reaction mixture of 1 mL contained 0.023 m H2O2 in 0.05 m phosphate buffer, pH 7.0 and 5 µL of spinal cord extract. The activity was recorded for 10 min at 240 nm using a Perkin-Elmer Lambda 2 UV/VIS spectrophotometer equipped with a thermostated circulating water-bath at 28 °C and a thermocouple to register the temperature of the sample. Activity was corrected for protein content. Spinal cord homogenates were made in 0.05 m phosphate buffer at pH 7.8.
Results
Blood sugar levels
Light and electron microscopic analysis of the spinal cord of the transgenic mice pointed to neuroxonal dystrophy since axonal swellings and degenerating axons containing enlarged mitochondria and tubulovesicular arrays were observed (de Rijk et al. 2000).
Linden et al. (1989) observed that a lysosomal storage disease, a cause of neuroaxonal dystrophy, is characterized by a deficiency in α-N-acetylgalactosaminidase activity. This pathology coincided with a urinary accumulation of α-N-acetylgalactosaminyl-containing oligosaccharides and glycopeptides. To test the accumulation of oligosaccharides in transgenic mice compared to control mice, blood sugar levels were measured with the aid of the Gluco Card Memory Meter. Table 1 shows that after one night of food deprivation the transgenic mice displayed a slightly but significantly higher blood sugar level (one-sided t-test with a p-value of 0.04 and 95% confidence interval) compared to control animals.
Table 1.
Blood sugar levels of transgenic and control mice.
| N | standard deviation | Mean mmol/L | highest value mmol/L | lowest value mmol/L | |
|---|---|---|---|---|---|
| transgenic mice | 32 | 0.87 | 4.0 | 6.1 | 2.8 |
| control mice | 26 | 0.80 | 3.7 | 5.7 | 2.6 |
Chromosomal localization of pVim-αA.
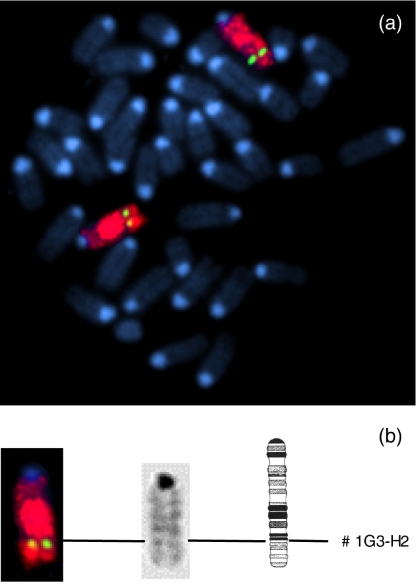
HMSN is a disease that is known to result from a defect in one or more genes. Determination of the integration site of the transgene by FISH analysis might exclude disrupting one of the genes known to be involved in HMSN or point to a possible cause of the observed pathology. For this reason mouse bone marrow cells were grown in tissue culture flasks and arrested in metaphase. A fluorescent probe containing the full transgene was used to map the integration site to chromosome 1Q G3-H2 (Fig. 1). The identification of the chromosome was confirmed by using a whole chromosome 1-specific painting probe. Since the endogenous αA-crystallin mouse gene is mapped to chromosome 17 (Skow & Donner 1985), which was not stained by our probe (not shown), it specifically identified the transgene. Notably, our transgene integrated near the position of myelin protein zero (P0), which is located at position 1Q H3 (You et al. 1991) and is involved in Charcot Marie Tooth Disease type 1B (Kulkens et al. 1993).
Figure 1.
Detection of the transgene pVim-αA (green label) on chromosome 1 (red label) by fluorescence in situ hybridization. Panel (a) Probe pVim-αA was hybridized to a metaphase spread of chromosomes derived from bone marrow cells. Panel (b) Partial karyotype; localization of the transgene to chromosome 1. The integration site was determined at position G3-H2, as indicated.
Southern and Western blot analysis of myelin protein zero.
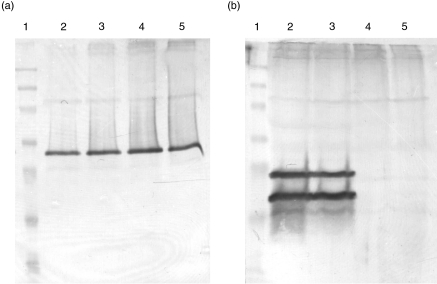
Although FISH analysis provides a strong indication of the position of the transgene, analyses of the exact location was still necessary. Southern hybridization (not shown) confirmed that the transgene did not incorporate into the P0 gene, which is a candidate gene in certain forms of HMSN (Giese et al. 1992), excluding a direct effect of integration on P0. However, the fact that the P0 gene appeared not to be altered does not guarantee a normal P0 expression. Upstream regulating promoter elements still might be affected. However, using Western blotting, no differences in immunoreactivity between control and transgenic mice for P0 were observed (Fig. 2A), implying that the normal expression of P0 was not disturbed. αA-Crystallin is, as could be expected, present in the peripheral nerves of the transgenic mice but absent in the nerves of the control animals (Fig. 2B).
Figure 2.
Western blots after SDS-PAGE of peripheral nerve proteins stained with a monoclonal antibody directed against P0 (a) and a polyclonal antibody directed against αA-crystallin (b). Lane (1) prestained marker (NOVEX); lane (2) transgenic mouse F13 48 male; lane (3) transgenic mouse F13 60 female; lane (4) control mousse F13 78 male and lane (5) control mouse F13 81 female.
Determination of enzyme activity
Although the observed pathology resembled neuroaxonal dystrophy to a much greater degree than ALS or HMSN, one should rule out these diseases by analysing parameters known to be characteristics for these pathologies.
Superoxide dismutase.
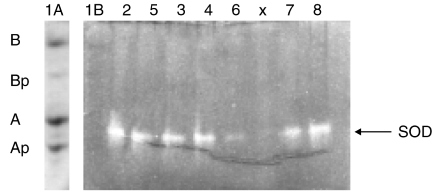
Initially to test if there was any SOD activity at all, spinal cord protein was subjected to basic urea gel electrophoresis. Superoxide dismutase (SOD) activity (Fig. 3) was detected in the region of αA-crystallin (compare lane 1 A to 2–8 of Fig. 3) All spinal cord extracts showed SOD activity, the gel became uniformly blue due to Nitro Blue Tetrazolium (NBT) staining, except at positions containing SOD activity. To examine the enzyme activity more accurately, the inhibition of oxidation of xanthine by xanthine oxidase was followed by means of a decrease in the reduction speed of NBT. In the presence of air the transfer of oxygen to NBT is inhibited by SOD giving rise to a decreased reduction speed of NBT. Spinal cord extracts of 8 animals provided the source of SOD. No differences in SOD activity between control animals and transgenic animals could be observed (not shown).
Figure 3.
Basic urea-polyacrylamide gel electrophoresis of spinal cord extract. Except for lane 1 A SOD activity was detected by disappearance of blue staining. Lane (1) bovine α-crystallin, containing the non-phosphorylated (αA and αB) and mono-phosphorylated α-crystallins (αAp and αBp): lane 1A stained with amido black, showing the positions of the α-crystallin subunits on a urea-gel run in parallel; lane (1B) bovine α-crystallin corresponding to lane 1 A; lane (2) transgenic mouse F7 1 female; lane (3) control mouse F7 2 female; lane (4) control mouse F7 3 female; lane (5) transgenic mouse F7 17 male; lane (6) transgenic mouse F7 18 male; lane (7) transgenic mouse F7 98 male; lane (8) transgenic mouse F7 99 male. X = blank.
Catalase activity.
Since no differences in SOD activity could be detected catalase a scavenger for H2O2, the reaction product of SOD activity (O2– + O2– + 2H+(r) H2O2 + O2) was tested subsequently. A decrease in extinction due to removal of H2O2 by catalase was followed at 240 nm. The rate of decrease of the H2O2 extinction was corrected for the amount of protein present in 5 µL spinal cord homogenate. No decrease of catalase activity in spinal cord extracts could be observed in transgenic mice compared to control mice (data not shown).
Transglutaminase activity.
Since differences in SOD and catalase activity were undetectable, activity of transglutaminase (TG), a marker for tissue degeneration (Fesus et al. 1988) was also analysed. TG activity is up-regulated within minutes after ischemic infarction (Fujita et al. 1998). In our transgenic animals, however, no up-regulation of TG activity could be observed (data not shown).
Discussion
Transgenic mice expressing hamster αA-crystallin develop a severe pathology which morphologically (de Rijk et al. 2000) as well as biochemically (data presented here) showed striking resemblance to neuroaxonal dystrophy. Since older animals clearly display more and more severe lesions than younger mice, we decided to only describe animals of 6 months of age (control as well as transgenic).
In case of Sietelberger's disease (Schindler et al. 1989) both central and peripheral nerves system are affected. Furthermore, large dystrophic axons can be observed that display tubulovesicular arrays and swollen mitochondria which are also observed in neuroaxonal dystrophy (Seitelberger 1986). However, the pathology resembled, to a certain extent, ALS and HMSN. To discriminate between these diseases and to unravel the underlying mechanism the animals were subjected to thorough examination.
Although the pathological resemblance to ALS is marginal, it had to be excluded. ALS, essentially a disease of the CNS, is characterized by degeneration and loss of motor neurones and reduction in number of large myelinated fibers in the cervical and lumbar ventral roots. Furthermore, a decrease in size of the ventral roots can be observed (Tandan & Bradley 1985; Aizawa et al. 2000). In the αA-crystallin transgenic mice, however, the dorsal roots are thinning (de Rijk et al. 2000). These data together with the vacuoles seen in axons and dendrites in a mouse model (Wong et al. 1995) made ALS a weak but possible candidate.
Although vacuolisation of the axons seems to be the only phenotypic similarity, enzyme activities that are often altered in ALS like SOD, catalase (Przedborski et al. 1992; Gsell et al. 1995) and transglutaminase (Fujita et al. 1995) had to be analysed. Fujita et al. (1998) showed that in transgenic mice with a mutated SOD gene, paralysis in one or more limbs could be observed, as well as motor neurone loss from the spinal cord. Mice suffering from this mutation died prematurely. Furthermore, increased transglutaminase activity is associated with the early stages of postischemic infarction and is a marker for tissue degeneration (Fujita et al. 1995). Therefore, transglutaminase is a candidate enzyme as well.
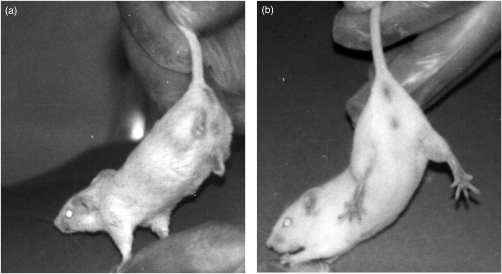
However, no differences in enzyme activities (present in the spinal cord protein fraction) between control and transgenic mice could be observed. Furthermore, the phenotype did not appear lethal and hind limb paralysis (Fig. 4) was not detected until 8 weeks of age (de Rijk et al. 2000). Upon analysing these data we concluded that the possibility of ALS should be excluded.
Figure 4.
(a) Transgenic mouse showing hind limb paralysis at 8 weeks of age. (b) Control mouse.
HMSN caused by alterations in, for instance, the peripheral myelin protein 22, P0, Sox 10, connexin 30 or connexin 32 was also taken into account. FISH analysis revealed that our transgene is mapped near to the position of P0 (1Q H3) on chromosome 1Q G3-H2. Since P0 is a major peripheral protein involved in compaction of the multilamellar myelin sheet (Kulkens et al. 1993) disturbance of the expression of P0 might, at least partially, cause the observed pathology. With respect to the other known genes that may cause HMSN, it seems unlikely that the transgene interferes with their expression, since they are all mapped to other chromosomes (Schwarz et al. 1992; Suter et al. 1992; Dahl et al. 1996; Herbarth et al. 1998; and Herrmann et al. 1998). However, direct effects of protein–protein interactions remain elusive. Furthermore, Southern and Western analysis revealed that the transgene had not integrated into the gene encoding P0. Therefore, a defect in P0, which results in demyelination of peripheral nerves, is not causing the pathology as observed in our transgenic mice. Althoughs HMSN normally is not causing the severe CNS pathology (Hoffmann 1889; Dyck et al. 1992) it has not been completely rejected.
Biochemically, the transgenic mice, due to the high number of animals tested, show a marginally, but significantly increased mean blood sugar level as compared to control animals (4.0 ± 0.87 mmol/L vs. 3.7 ± 0.8 mmol/L). In man, elevated blood sugar levels (above 15 mmol/L, Dutch Diabetic Foundation, Amersfoort, The Netherlands) are known to cause neuroaxonal dystrophy. However, in contrast to our mice, Ikegami et al. (2000) describe mice with high blood sugar levels (6.5–7.6 mmol/L) displaying a mild spontaneous peripheral neuropathy, while their littermates with decreased blood sugar levels (2.4–5.4 mmol/L) develop an exacerbated neuropathy.
Since the blood sugar levels that we observed are in the range of normal lower levels (Tsutsumi et al. 2003), and show less difference in the mean values as observed by Ikegami et al. (2000), we concluded that the phenotype displayed by our mouse strain is not likely to be caused by differences in this value. Furthermore, elevated human blood and urinary sugar levels are observed in case of a neuropathology due to diabetes or a defect in α-N-acetyl galactosaminidase (Desnick & Bishop 1989; Linden et al. 1989; Wolfe et al. 1995). However, a defect in α-N-acetyl galactosaminidase due integration of the transgene is not expected, since the gene encoding in α-N-acetyl galactosaminidase is mapped to mouse chromosome 15 (Herrmann et al. 1998), whereas our transgene integrated in chromosome 1.
Simple over-expression of a gene might also induce the observed pathology. A mouse strain expressing the human MDR-gene under direction of the same vimentin promoter (Smit et al. 1996) as used in the construct to generate the mouse strain described here showed slowed motor nerve conduction and demyelination of their peripheral nerves, which resulted in paresis of the hind legs. Furthermore, the MDR transgenic mice showed, in addition to the peripheral nerve pathology, visible alterations in eyes and muscle. Light microscopical analysis of the spinal cord showed no apparent abnormalities. However, in our transgenic mouse strain no alterations were observed in muscle, while a pathology was found in the spinal cord (de Rijk et al. 2000). The similarities, demyelination of peripheral nerves and paresis of the hind legs observed in the transgenic mouse strains, might be due to the fact that both the transgenic proteins are expressed in the same tissues as a consequence of usage of the same promoter. However, the differences might also be explained by the fact that MDR is a large membrane protein (van den Heuvel-Eiberink et al. 2000) that might be expected to interfere with normal membrane composition or membrane wrapping of Schwann cells, whereas αA-crystallin is known to be a cytosolic protein (Bloemendal 1977).
The above data led us to conclude that overexpression or alteration of protein expression in a well balanced environment like Schwann cells or axons will eventually result in neuroaxonal dystrophy. Therefore, in our transgenic mouse strain the neuroaxonal pathology is most likely caused by changes in metabolism as is found in the case of diabetic neuropathies (Schmidt et al. 1998), Seitelberger disease, Hallervorden-Spatz disease, neuroaxonal leukodystrophy (Seitelberger 1986) and for a deficient activity of the lysosomal α-N-acetylgalactosaminidase (Wolfe et al. 1995). These different forms are distinguished by age of onset, main localization of changes, dynamics of the process and genetic characters (Seitelberger 1986).
Although overexpression of αA-crystallin is normally not occurring, it might hint to processes causing neuroaxonal dystrophy in man. With this paper we tried to understand the mechanisms underlying a severe neuropathology which might in the future prove to be useful to man. However, further research will be necessary to elucidate if all neuroaxonal dystrophies result from disturbances in the metabolism of Schwann cells and how to prevent the resulting pathology.
Acknowledgments
We would like to thank Henk Arnts for excellent animal care, Ron Wehrens of the Department of Chemometrics for the statistical analysis and Pieter Wesseling for fruitful discussions and critical reading. This work was supported by EC-BIOMED (contract no. B14H4-CT96-1593) and in part by the Alcon Research Institute Award to one of us (H.B).
References
- Aizawa H, Kimura T, Hashimoto K, Yahara O, Okamoto K, Kikuchi K. Basophilic cytoplasmic inclusions in a case of sporadic juvenile amyotrophiclateral sclerosis. J. Neurol. Sci. 2000;176:109–113. doi: 10.1016/s0022-510x(00)00321-x. [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. αB Subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biophys. Res. Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucl Acid Res. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. The vertebrate eye lens. Science. 1977;197:127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Dahl E, Mantey D, Chen Y, Schwarz HJ, Chang YS, Lalley PA, Nicholson BJ, Willecke K. Molecular cloning and functional expression of mouse connexin-30, a gap junction gene highly expressed in adult brain and skin. J. Biol. Chem. 1996;271:17903–17910. doi: 10.1074/jbc.271.30.17903. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Bishop DF. Fabry disease: α-N-galactosidase deficiency; Schindler disease: α-N-acetylgalactosaminidase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 6. New York: McGraw-Hill; 1989. pp. 1751–1798. [Google Scholar]
- Dyck PJ, Chance P, Lebo R, Carney JA. Hereditary motor and sensory neuropathies. In: Dyck PJ, Griffin JW, Low PA, Podulso JF, editors. Hereditary, Motor and Sensory Neuropathies. Philadelphia: W.B. Saunders; 1992. pp. 1094–1136. [Google Scholar]
- Fesus L, Thomazy V, Falus A. Reaching for function of tissue transglutaminase; its possible involvement in biochemical pathway of programmed cell death. Adv. Exp. Biol. 1988;231:119–139. doi: 10.1007/978-1-4684-9042-8_10. [DOI] [PubMed] [Google Scholar]
- Fujita K, Ando M, Yamauchi M, Nagata Y, Honda M. Alteration of transglutaminase activity in rat and human spinal cord after neuronal degeneration. Neurochem. Res. 1995;20:1195–1201. doi: 10.1007/BF00995383. [DOI] [PubMed] [Google Scholar]
- Fujita K, Shibayama K, Yamauchi M, Kato T, Ando M, Takahashi H, Iritani K, Yoshimoto N, Nagata Y. Alteration of enzymatic activities implicating neuronal degeneration in the spinal of the motor neuron degeneration mouse during postnatal development. Neurochem. Res. 1998;23:557–562. doi: 10.1023/a:1022442904179. [DOI] [PubMed] [Google Scholar]
- Giese KP, Martini R, Lemke G, Soriano P, Schachner M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71:565–576. doi: 10.1016/0092-8674(92)90591-y. [DOI] [PubMed] [Google Scholar]
- Gsell W, Conrad R, Hickethier M, Sofic E, Frolich L, Wichart I, Jellinger K, Moll G, Ransmayr G, Beckmann H, Riederer P. Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J. Neurochem. 1995;64:1216–1223. doi: 10.1046/j.1471-4159.1995.64031216.x. [DOI] [PubMed] [Google Scholar]
- Herbarth B, Pingault V, Bondurand N, Kuhlbrodt K, Hermans-Borgmeyer I, Puliti A, Lemort N, Goossens M, Wegner M. Mutation of Sry-related Sox10 gene in dominant megacolon, a mouse model for human Hirschsprung disease. Proc. Natl. Acad. Sci. USA. 1998;95:5161–5165. doi: 10.1073/pnas.95.9.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann T, Schindler D, Tabe H, Onodera O, Igarashi S, Polack A, Zehnpfennig D, Tsuji Shoji. Molecular cloning, structural organization, sequence, chromosomal assignment,and expression of the mouse alpha-N-acetylgalactosaminidase gene. Gene. 1998;211:205–214. doi: 10.1016/s0378-1119(98)00103-6. [DOI] [PubMed] [Google Scholar]
- van den Heuvel-Eiberink MM, Sonneveld P, Pieters R. The prognostic significance of membrane transport-associated multidrug resistance (MDR) proteins in leukemia. Int. J. Clin. Pharmacol. Ther. 2000;38:94–110. doi: 10.5414/cpp38094. [DOI] [PubMed] [Google Scholar]
- Hoffmann J. Ueber progressive neurotische Muskelatrophie. Arch. Psychiat Nervenkr. 1889;20:660–713. [Google Scholar]
- Ikegami H, Tabata H, Matsuzawa T, Suzuki H. The Exacerbating effect of insulin-induced hypoglycemia on spontaneous peripheral neuropathy in aged B6C3F1 mice. J. Toxicol. Sci. 2000;25:137–142. doi: 10.2131/jts.25.137. [DOI] [PubMed] [Google Scholar]
- Kato S, Hayashi H, Nakashima K, Nanba E, Katao M, Hirano A, Nakano I, Asayama K, Ohama E. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am. J. Pathol. 1997;151:611–620. [PMC free article] [PubMed] [Google Scholar]
- Kulkens T, Bolhuis PA, Wolterman RA, Kemp S, Te Nijenhuis S, Valentij LJ, Hensels GW, Jennekens FGI, De Visser M, Hoogendijk JE, Baas F. Deletion of the serine 34 codon from the major peripheral myelin protein P0 gene in Charcot-Marie Tooth disease type 1B. Nat Genet. 1993;5:35–38. doi: 10.1038/ng0993-35. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. A new look at the organization of the motor system. In: Kuypers HGJM, Martin GF, editors. Progress in Brain Research: Anatomy of the Descending Pathways to the Spinal Cord. Amsterdam: Elsevier; 1982. pp. 381–403. [Google Scholar]
- Linden HU, Klein RA, Egge H, Peter-Katalinic J, Dabrowski J, Schindler D. Isolation and structural characterization of sialic-acid containing glycopeptides of the O-glycosidic type from the urine of two patients with a hereditary deficiency in α-N-acetylgalactosaminidase activity. Biol. Chem. Hoppe-Seyler. 1989;370:661–672. doi: 10.1515/bchm3.1989.370.2.661. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Kostic V, Carlson E, Epstein CJ, Cadet JL. Superoxide dismutase, catalase, and glutathione peroxidase activities in copper/zinc-superoxide dismutase transgenic mice. J. Neurochem. 1992;58:1760–1767. doi: 10.1111/j.1471-4159.1992.tb10051.x. [DOI] [PubMed] [Google Scholar]
- de Rijk EPCT, van Rijk AF, van Esch E, de Jong WW, Wesseling P, Bloemendal H. Demyelination and axonal dystrophy in alpha A-crystallin transgenic mice. Int. J. Exp. Pathol. 2000;81:271–282. doi: 10.1046/j.1365-2613.2000.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D, Bishop DF, Wolfe DE, Wang AM, Egge H, Lemieux RU, Desnick RJ. Neuroaxonal dystrophy due to lysosomal α-N-acetylgalactosaminidase deficiency. N. Engl. J. Med. 1989;320:1735–1740. doi: 10.1056/NEJM198906293202606. [DOI] [PubMed] [Google Scholar]
- Schmidt RE, Dorsey DA, Beaudet LN, Plurad SB, Williamson JR, Ido Y. Effect of sorbitol dehydrogenase inhibition on experimental diabetic autonomic neuropathy. J. Neuropathol. Exp. Neurol. 1998;57:1175–1189. doi: 10.1097/00005072-199812000-00010. [DOI] [PubMed] [Google Scholar]
- Schoenmakers JGG, Matze R, van Poppel M, Bloemendal H. Isolation of non-identical polypeptide chains of α–crystallin. Intern. J. Prot. Res. 1969;1:19–27. doi: 10.1111/j.1399-3011.1969.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Schwarz HJ, Chang YS, Hennemann H, Dahl E, Lalley PA, Willecke K. Chromosomal assignment of mouse connexin genes, coding for gap junctional proteins, by somatic cell hybridization. Somat. Cell. Mol. Genet. 1992;18:205–206. doi: 10.1007/BF01235758. [DOI] [PubMed] [Google Scholar]
- Seitelberger F. Neuroaxonal dystrophy: its relation to aging and neurological diseases. In: Vinken PJ, Bruyn GW, Klawans, editors. Handbook of Clinical Neurology, Series 5. Vol. 49. Amsterdam: Elsevier; 1986. pp. 391–415. [Google Scholar]
- Skow LC, Donner ME. The locus encoding alpha A-crystallin is closely linked to H-2K on mouse chromosome 17. Genetics. 1985;110:723–732. doi: 10.1093/genetics/110.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JJM, Baas F, Hoogendijk JE, Jansen GH, van der Valk MA, Schinkel AH, Berns AJM, Acton D, Nooter K, Burger H, Smith SJ, Borst P. Peripheral neuropathy in mice transgenic for a human MDR3 P-glycoprotein mini-gene. J. Neurosci. 1996;16:6386–6393. doi: 10.1523/JNEUROSCI.16-20-06386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suijkerbuijk RF, Sinke RJ, Weghuis DE, Roque L, Forus A, Stellink F, Siepman A, van de Kaa C, Soares J, Geurts van Kessel AH. Amplification of chromosome subregion 12p11.2-p12.1 in a metastasis of an i(12p) -negative seminoma: Relationship to progression? Cancer. Genet. Cytogenet. 1994;78:145–152. doi: 10.1016/0165-4608(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Suter U, Welcher AA, Ozcelik T, Snipes GJ, Kosaras B, Francke U, Billings-Gagliardi S, Sidman RL, Shooter EM. Trembler mouse carries a point mutation in a myelin gene. Nature. 1992;356:241–244. doi: 10.1038/356241a0. [DOI] [PubMed] [Google Scholar]
- Tandan R, Bradley WG. Amyotrophic lateral sclerosis: Part 1. Clinical features, pathology, and ethical issues in management. Ann. Neurol. 1985;18:271–280. doi: 10.1002/ana.410180302. [DOI] [PubMed] [Google Scholar]
- Tsutsumi T, Kobayashi S, Liu YY, Kontani H. Anti-hyperglycemic Effect of Fangchinoline Isolated from Stephania Tetrandra Radix in Streptozotocin-Diabetic Mice. Biol. Pharm. Bull. 2003;26:313–317. doi: 10.1248/bpb.26.313. [DOI] [PubMed] [Google Scholar]
- Wolfe DE, Schindler D, Desnick RJ. Neuroaxonal dystrophy in infantile α-N-acetylgalactosaminidase deficiency. J. Neurol. Sci. 1995;132:44–56. doi: 10.1016/0022-510x(95)00124-k. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- You KH, Hsieh CL, Hayes C, Stahl N, Francke U, Popko B. DNA sequence, genomic organization, and chromosomal localization of the mouse peripheral myelin protein zero gene: identification of polymorphic alleles. Genomics. 1991;9:751–757. doi: 10.1016/0888-7543(91)90370-t. [DOI] [PubMed] [Google Scholar]