Abstract
Influenza virus infection, induced experimentally in mice, was associated with marked changes in lung morphology viz. epithelial damage with focal areas of reactive papillary hyperplasia, infiltration of leukocytes and development of oxidative stress, as evidenced by increased superoxide radical production and lipid peroxidation (LPO) products by alveolar macrophages. These effects were observed on the 5th day after virus instillation. The levels of superoxide and LPO were measured spectrophotometrically by the nitroblue tetrazolium (NBT) assay and thiobarbituric acid reactive species (TBARS) assay, respectively. The former increased by 1.5–2 fold and the latter was raised by 85% when compared with normal control. Supplementation of intranasal viral instillation with the anti-oxidant, Quercetin, given orally, resulted in a significant decrease in the levels of both superoxide radicals and LPO products. There was also a significant decrease in the number of infiltrating cells. A mild to moderate protective effect was observed in lung morphology. Thus, Quercetin may be useful as a drug in reducing the oxidative stress induced by influenza virus infection in the lung, and protect it from the toxic effects of the free radicals.
Keywords: influenza virus, lipid peroxidation, Quercetin, anti-oxidant
Influenza virus is an important human pathogen, which causes worldwide epidemics and pandemics (Khanna & Kumar 2002). The pathogenesis of the influenza virus involves various factors and, despite considerable research over many decades, the exact mechanism(s) behind it still remain to be elucidated. The lung is one of the most widely investigated targets for influenza virus infection and potentially at high risk of injury mediated by oxygen-derived free radicals and lipid peroxidation products (Kornbrust & Mavis 1980; Cees et al. 1991). Infiltration by leukocytes in the lung produces reactive oxygen species (ROS), which are normally involved in killing of microorganisms that invade the body. The involvement of ROS in the pathogenesis of influenza virus infection has gained importance during the past decade (Oda et al. 1989; Akaike et al. 1990; Buffington et al. 1992). Oxidative stress is defined as an imbalance between anti-oxidants and pro-oxidants in favour of pro-oxidants. Treatment with anti-oxidant enzymes and Vitamin E reduces free radical formation and decreases the pathogenicity of the influenza virus (Christen et al. 1990; Hennet et al. 1992a; Jacoby & Choi 1994; Peterhans 1997a; Mileva et al. 2000). The mechanisms behind the protective action of anti-oxidants and their possible use for the prevention and treatment of influenza virus infection are areas in which researchers and clinicians have shown keen interest in the recent past (Jacoby & Choi 1994; Akaike et al. 1996; Peterhans 1997a). There are a few studies, which have reported that Quercetin, a bioflavonoid, prevents lipid peroxidation and scavenges superoxide radicals (Peter et al. 1995; Peterhans 1997b; Johnson & Loo 2000; O'Brien et al. 2000). However, its role in lung morphology during influenza virus infection is not well established.
In the present study, we investigated the histopathological changes in the lungs of influenza virus infected mice with and without oral supplementation with Quercetin. Simultaneously, we studied its effect on the levels of superoxide and lipid peroxidation products. It was anticipated that the results may throw some light on the usefulness of Quercetin as a protective agent in the lung pathology associated with influenza virus infection.
Materials and methods
Animal infection and treatment
BALB/c male mice (14–16 g) were fasted for 24 h before the study. These were distributed into four groups as follows: Group I (n = 6) – control group (healthy animals); Group II (n = 6) – mice infected with influenza virus A/Udorn/317/72(H3N2) 1.5 mg of LD50 by intranasal instillation; Group III (n = 6) – mice infected intranasally with influenza virus A/Udorn/317/72(H3N2) 1.5 mg of LD50 and supplemented orally with Quercetin in a dose of 1 mg/day for 5 consecutive days; Group IV (n = 6) – mice treated orally with Quercetin in a dose of 1 mg/day for 5 consecutive days.
Virulence of the influenza virus is well expressed on the 5th to 6th days after virus instillation (Oda et al. 1989; Gorbunov et al. 1992; Choi et al. 1996). Hence the animals were sacrificed on the 5th day.
Influenza virus A/Udorn/317/72(H3N2) was obtained from CDC Atlanta.
Histopathological analysis of the lungs
The lungs were fixed by intratracheal instillation of buffered 20% formaldehyde solution. Lung sections were embedded in paraffin, sectioned and stained with haematoxylin and eosin.
Preparation and purification of alveolar macrophages
Alveolar macrophages were collected as described by Richard et al. (1984). Briefly, 0.5 mL of 0.5 m PBS was injected into the lungs and the return fluid was collected with a sterile syringe. This process was repeated until the lung was lavaged with a total of 5 mL of PBS. The collected fluid was pooled, and centrifuged at 650 g for 10 min at 4 °C and the supernatant was discarded. Alveolar macrophage enrichment was achieved by a one-step adherence procedure as described by Holt (1979). In brief, cell suspensions were plated into 60 mm plastic Petri dishes and incubated at 37 °C with 5% CO2 for 1.5 h. The monolayer was washed vigorously in medium with a Pasteur pipette to remove non-adherent cells. Alveolar macrophages were scraped and the pellet was re-suspended in RPMI, and viability counts were performed by trypan blue exclusion.
Assessment of superoxide radical production
Nitroblue tetrazolium (NBT) reduction assay was carried out to assess the superoxide radical generation capacity of alveolar macrophages, as described by Baehner & Nathan (1968). In brief, 500 µL of cell suspension adjusted to a density of 1 × 106 macrophage/mL of each of the various animal groups (Control, Infected, Infected + Quercetin treated, Quercetin treated) was mixed with 500 µL of 0.2% NBT in PBS and incubated for 15 min at 37 °C in a humid chamber. Tubes were spun down at 650 g for 5 min at 4 °C. The supernatant was discarded and 1 mL of N,N-dimethyl formamide was added to the pellet. The pellet was dispensed and placed at 56 °C for 60 min. The supernatant was collected and absorbance was recorded at 520 nm.
Lipid peroxidation
Mice lung microsomes used for lipid peroxidation studies were prepared by the method of Ernster et al. (1968). In brief, freshly excised mouse lung was suspended in 0.25 m sucrose solution and homogenized to obtain a 30% homogenate, which was centrifuged at 10 000 g for 30 °C min at 4 °C. The supernatant was spun at 100 000 g for 1 h and the surface of pelleted microsomes was rinsed with 0.15 m KCl and resuspended in 0.15 m KCl. Protein was measured by the method of Lowry et al. (1951). The homogenate was dissolved in PBS to contain 1 mg/mL of protein.
Endogenous LPO products reacting with 2-thiobarbituric acid (TBA-reactive substances, TBARS) were measured spectrophotometrically (λmax = 532 nm) as described by Asakawa & Matsushita (1980).
Statistical analysis
The data were analysed with one-way analysis of variance (anova) followed by Student t-test. All the results are expressed as mean ± SD.
Results
Effect of instillation of influenza virus and supplementation of Quercetin on lung morphology
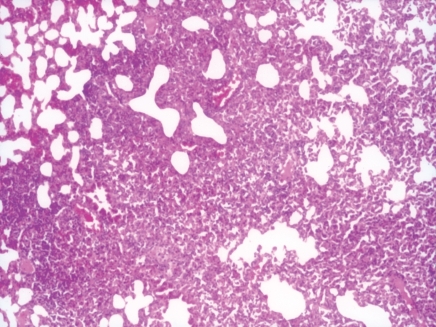
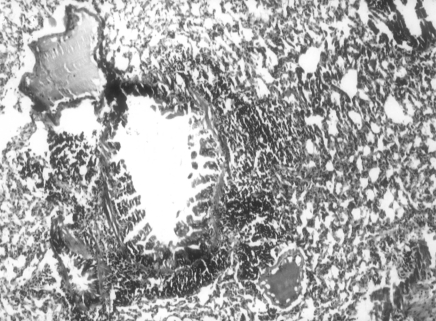
BALB/c mice were infected intranasally with influenza A/Udorn/317/72(H3N2) 1.5 mg of LD50. The first symptoms of disease, such as piloerection, reduced food intake and lethargy were apparent by 5 days post instillation. The lung fluid was influenza positive by the haemagglutination test (HA) and the bacterial culture was negative. Histopathological examination of lungs revealed epithelial damage with focal areas of reactive papillary hyperplasia (Fig 1.). The parenchymal response consisted of both interstitial and intra-alveolar exudate of neutrophils and macrophages leading to focal areas of consolidation. In a few places, dense peribronchial lymphoid aggregates were seen (Fig. 2).
Figure 1.
Photomicrograph showing intense infiltration of inflammatory cells into alveolar walls with focal areas of consolidation, 5 days after influenza virus instillation in lungs of mice (Group II). H&E 100×.
Figure 2.
Peribronchial lymphoid infiltration in influenza virus infected lungs of mice, 5 days post-instillation (Group II). H&E 100×.
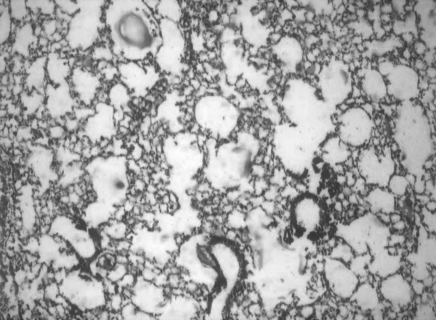
Oral supplementation with Quercetin reduced the severity of infection. The epithelial damage and leukocytic influx were significantly reduced (Fig. 3).
Figure 3.
Photomicrograph showing reduced number of infiltrated cells in influenza virus infected lung of mice following Quercetin supplementation (Group III). H&E 100×.
Level of superoxide production
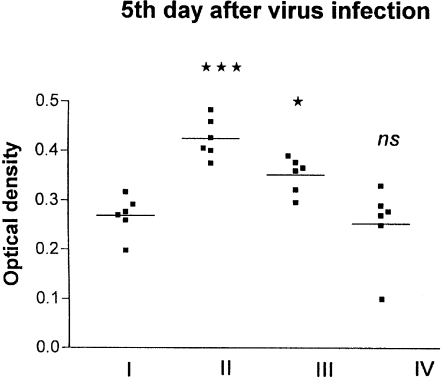
The 86% purified alveolar macrophages were obtained by the adherence procedure. Superoxide production by alveolar macrophages was measured by NBT assay. The amounts of formazan formed by 1 × 106 macrophages were expressed as optical densities. NBT activities of macrophages in influenza-infected mice were increased 1.5–2 fold compared to the normal control. Supplementation with Quercetin significantly reduced the formazan-positive cells (Fig. 4).
Figure 4.
Effect of influenza virus infection on the NBT activities of macrophages before and after supplementation with Quercetin. I, control; II, influenza virus infection; III, influenza virus infection + Quercetin treated; IV, Quercetin treated. ***P < 0.001 vs. Group I; *P < 0.05 vs. Group II; ns, non significant vs. Group I.
Level of lipid peroxidation
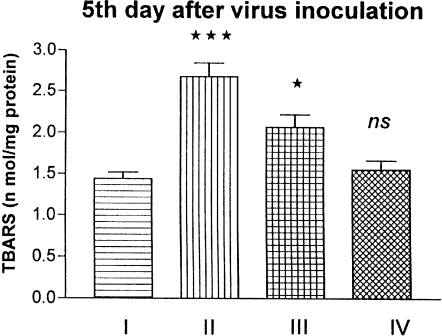
The effect of influenza virus on lipid peroxidation (LPO) products in lungs was measured by thiobarbituric acid reactive species (TBARS). LPO levels were raised by 85% in the influenza group (2.67 nmol/mg protein) compared to the normal control group (1.44 nmol/mg protein). The raise was significant (P < 0.001). A significant (P < 0.05) decrease of LPO was observed after supplementation with Quercetin. The TBARS level was brought down to 43% (2.06 nmol/mg protein) after Quercetin supplementation. Supplementation with Quercetin alone showed no alterations in the LPO level (Fig. 5).
Figure 5.
Effect of influenza virus infection on the level of LPO products in the lung of mice. I, control; II, influenza virus infection; III, Quercetin treated + influenza virus infection; IV, Quercetin treated. *P < 0.05 vs. Group II; ***P < 0.001 vs. Group I; ns, non significant vs. Group I.
Discussion
The morphological and biochemical studies on mouse lungs 5 days after instillation of influenza virus A/Udorn/317/72(H3N2) suggest that in the mouse model of experimental influenza virus infection, there is progressive damage of alveolar cells with acute inflammatory reaction and development of pneumonitis and bronchitis. Lung morphology revealed high recruitment of neutrophils and macrophages along with epithelial damage. The massive infiltration of leukocytes into alveolar walls and spaces (Fig. 1) was due to the pathogenic effects of the virus only as no pathogenic bacterium or mycoplasma was isolated from the lungs. The lung tissue from the control animals showed no such pathological features.
The infiltrated cells produce free radicals and cytokines leading to inflammation in the lungs (Hers et al. 1958; Wyde & Cate 1978; Hennet et al. 1992a, 1992b). The early phase of influenza virus infection is associated with the development of oxidative stress in lung. Accumulated leukocytic cells in the lung, a characteristic feature of influenza, may produce free radicals and pro-oxidants cytokines, which may contribute to virus pathogenesis. Infiltration of phagocytic cells (mainly polymorphonuclear leukocytes) occurs in a sequential fashion, in which several factors and adhesion molecules are involved (Hennet et al. 1992a, 1992b; Pahl & Baeuerle 1995). Peterhans et al. (1987) have shown that the influenza virus is capable of activating a respiratory burst in a phagocytic cell. Rodgers & Mims (1981), Oda et al. (1989) and Buffington et al. (1992) have reported that intranasal infection of influenza virus in mice increased the alveolar macrophage population and superoxide production.
In the present study, it was demonstrated that the influenza virus A/Udorn/317/72(H3N2) caused a marked increase of superoxide radical production in lung macrophages. There were increases in the levels of LPO products also (Figs 4 and 5). These data indicated the potential role of the active products of free radical processes and lipid peroxidation in the development of such disorders in the lung under acute influenza virus infection. The enhanced LPO level on the 5th day after influenza virus infection is in concordance with other studies (Cees et al. 1991; Akaike et al. 1996; Choi et al. 1996; Mileva et al. 2002). The increased levels of superoxide and LPO provide evidence for the presence of oxidative stress during influenza virus infection.
It has been reported that Quercetin, a bioflavonoid, possessed anti-oxidant and free radical scavenging activity (Blackburn et al. 1987; Afanas'ev et al. 1989). It occurs naturally in fruits, vegetables, nuts, seeds, red wine, tea and flowers (Hertog et al. 1992; Hertog et al. 1993; McAnlis et al. 1999). Other studies have shown that Quercetin prevented lipid peroxidation and scavenged superoxide radicals (Peter et al. 1995; Peterhans 1997b; Johnson & Loo 2000; O'Brien et al. 2000).
The histopathological study of the lung after supplementation with Quercetin revealed a reduced infiltration of polymorphonuclear leukocytes (Fig. 3). There was also a decrease in the severity of infection. Thus, Quercetin may protect cells from the cytopathological effects of the influenza virus.
The results show that there is an increase in the level of LPO products and an increase in generation of free radicals during influenza virus infection. A previous study (Hennet et al. 1992a) reported that activities of anti-oxidant enzymes from the lungs of influenza virus infected mice increased significantly, probably due to the abnormal generation of superoxide radicals in lungs. Supplementation of Quercetin resulted in significant decreases in superoxide production and LPO products. It was also reported (Hayek et al. 1997; Mileva et al. 2000) that Vitamin E supplementation resulted in a significant increase of anti-oxidant content in blood as well as in lung. Concurrently, there was a significant decrease of LPO products. The present results are in agreement with the published reports. Additionally, they show that supplementation of Quercetin alleviates the toxic effects of free radicals, induced during influenza virus infection. Several studies report that Quercetin delays oxidative pathway mediated cell injury by scavenging free radicals, affording protection against lipid peroxidation and chelating metal ions (Peter et al. 1995; Johnson & Loo 2000; O'Brien et al. 2000).
The present study supports the suggestion that oxidative stress is involved in the pathogenesis of influenza virus infection of the lung and shows that Quercetin reduces the damage caused by the free radicals produced. Thus, Quercetin seems promising as an antiviral drug therapy in alleviating the oxidative stress induced by influenza virus infection.
Acknowledgments
Financial support from the Department of Science and Technology, Government of India, is gratefully acknowledged.
References
- Afanas'ev IB, Doronznko AI, Brodskii VA, Potapoviteu AI. Chelating and free radical scavenging mechanism of inhibitory action of rutin and Quercetin in lipid peroxidation. Biochem. Pharmacol. 1989;38:1763–1768. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- Akaike T, Ando M, Oda T, et al. Dependence on O2− generation by xanthine oxidase of pathogenesis of influenza virus infection. J. Clin. Invest. 1990;85:739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T, Noguchi S, Ijiri K, et al. Pathogenesis of influenza virus induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa T, Matsushita S. Thiobarbituric acid test for detecting lipid peroxides. Lipids. 1980;14:401–406. [Google Scholar]
- Baehner RL, Nathan DG. Quantitative nitro-blue tetrazolium test in chronic granulomatous disease. N. Eng. J. Med. 1968;278:971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Blackburn WD, Jr, Blackburn WD, Heck LW, Wallace RW. The bioflavonoid Quercetin inhibits neutrophil degranulation, superoxide production and the phosphorylation of specific neutrophil proteins. Biochem. Biophys. Res. Commun. 1987;144:1229–1236. doi: 10.1016/0006-291x(87)91442-2. [DOI] [PubMed] [Google Scholar]
- Buffington GD, Christen S, Peterhans E, Stocker R. Oxidative stress in lungs of mice infected with influenza A virus. Free Rad. Res. Commun. 1992;16:99–110. doi: 10.3109/10715769209049163. [DOI] [PubMed] [Google Scholar]
- Cees J, Doelman A, Aalt B. Oxygen radicals in lung pathology. Free Rad. Biol. Med. 1991;9:381–400. doi: 10.1016/0891-5849(90)90015-b. [DOI] [PubMed] [Google Scholar]
- Choi AM, Knobil K, Otterbein SL, Eastman DA, Jacobi DB. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am. J. Physiol. 1996;271:L383–L391. doi: 10.1152/ajplung.1996.271.3.L383. [DOI] [PubMed] [Google Scholar]
- Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc. Natl. Acad. Sci. USA. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L, Nordenbrand K, Orrenius S, Das ML. Microsomal lipid peroxidation. Hoppe Seylers Z. Physiol. Chem. 1968;349:1604–1605. [PubMed] [Google Scholar]
- Gorbunov NV, Volgararev AV, Brailovskaia IV, Bikova NO, Avrova NF, Kiselev OI. The activation of free radical reaction and modulation of antioxidant activity in blood under toxic experimental influenza infection. Bull. Exp. Biol. Med. 1992;114:42–44. [PubMed] [Google Scholar]
- Hayek MG, Tancheva L, Bakalova R, Galabov A, et al. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J. Infect. Dis. 1997;176:273–276. doi: 10.1086/517265. [DOI] [PubMed] [Google Scholar]
- Hennet T, Peterhans E, Stocker R. Alteration in antioxidant defense in lung and liver of mice infected with influenza A virus. J. General Virol. 1992a;73:39–46. doi: 10.1099/0022-1317-73-1-39. [DOI] [PubMed] [Google Scholar]
- Hennet T, Ziltener HJ, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J. Immunol. 1992b;149:932–939. [PubMed] [Google Scholar]
- Hers JF, Masurel PHN, Mulder J. Bacteriology and histopathology of the respiratory tracts and lungs in fatal Asian influenza. Lancet. 1958;2:1141–1143. doi: 10.1016/s0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food. Chem. 1992;40:2379–2383. [Google Scholar]
- Hertog MGL, Hollman PCH, Putte B. Content of potentially anticarcinogenic flavonoids of tea infusion, wines and fruit juices. J. Agric. Food. Chem. 1993;41:1242–1246. [Google Scholar]
- Holt PG. Alveolar macrophages. I. A simple technique for the preparation of high numbers of viable alveolar macrophages from small laboratory animals. J. Immunol. Method. 1979;27:189–198. doi: 10.1016/0022-1759(79)90264-3. [DOI] [PubMed] [Google Scholar]
- Jacoby DB, Choi AM. Influenza virus induces expression of antioxidant genes in human epithelial cells. Free Rad. Biol. Med. 1994;16:821–824. doi: 10.1016/0891-5849(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Loo G. Effect of epigallocatechin gallate and Quercetin on oxidative damage to cellular DNA. Mutat. Res. 2000;459:211–218. doi: 10.1016/s0921-8777(99)00074-9. [DOI] [PubMed] [Google Scholar]
- Khanna M, Kumar P. Influenza: a serious global threat. J. Infect. Dis. Antimicro. Agents. 2002;19:25–39. [Google Scholar]
- Kornbrust D, Mavis D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation. Lipid. 1980;15:315–322. doi: 10.1007/BF02533546. [DOI] [PubMed] [Google Scholar]
- Lowry O, Rosenbrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:256–272. [PubMed] [Google Scholar]
- McAnlis GT, McEneny J, Pearce J, Youn IS. Absorption and antioxidant effects of Quercetin from onion. Eur. J. Clin. Nutr. 1999;53:92–96. doi: 10.1038/sj.ejcn.1600682. [DOI] [PubMed] [Google Scholar]
- Mileva M, Bakalova R, Tancheva L, Galabov A, Ribarov S. Effect of vitamin E supplementation on lipid peroxidation in blood and lung of influenza virus infected mice. Comp. Immunol. Microbiol. Infect Dis. 2002;25:1–11. doi: 10.1016/s0147-9571(01)00010-8. [DOI] [PubMed] [Google Scholar]
- Mileva M, Tancheva L, Bakalova R, Galabov A, Savov V, Ribarov S. Effect of vitamin E on lipid peroxidation and liver monooxygense activity in experimental influenza virus infection. Toxicol. Lett. 2000;114:39–45. doi: 10.1016/s0378-4274(99)00265-9. [DOI] [PubMed] [Google Scholar]
- O'Brien NM, Wods JA, Aherne SA, O'Callaghan YC. Cytotoxicity, genotoxicity, and oxidative reactions in cell-culture models: modulatory effects of photochemicals. Biochem. Soc. Trans. 2000;28:22–26. doi: 10.1042/bst0280022. [DOI] [PubMed] [Google Scholar]
- Oda T, Akaike T, Hamamoto T, Suzuki F, Hirano T, Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989;244:974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. Expression of influenza virus hemagglutinin activates transcription factor NF-kappa B. J. Virol. 1995;69:1480–1484. doi: 10.1128/jvi.69.3.1480-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter CHH, Jeanne HM, Devaris DL, Marcel JBM, Martijin BK. Absorption of dietary Quercetin glycosides and Quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995;62:1276–1282. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- Peterhans E. Reactive oxygen species and nitric oxide in viral diseases. Biol. Trace Element Res. 1997a;56:107–115. doi: 10.1007/BF02778986. [DOI] [PubMed] [Google Scholar]
- Peterhans E. Oxidant and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J. Nutr. 1997b;127:962S–965S. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- Peterhans E, Grob M, Burge TZ. Virus-induced formation of reactive oxygen intermediates in phagocytic cells. Free Rad. Res. Commun. 1987;3:39–46. doi: 10.3109/10715768709069768. [DOI] [PubMed] [Google Scholar]
- Richard M, McCarron A, Diana K, et al. Methods for the collection of peritoneal and alveolar macrophages. Method. Enzymol. 1984;108:273–284. doi: 10.1016/s0076-6879(84)08092-7. [DOI] [PubMed] [Google Scholar]
- Rodgers B, Mims CA. Interaction of influenza virus with mouse macrophages. Infect. Immun. 1981;31:751–757. doi: 10.1128/iai.31.2.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde PR, Cate TR. Cellular changes in lungs of mice infected with influenza virus: characterization of the cytotoxic response. Infect. Immun. 1978;22:423–429. doi: 10.1128/iai.22.2.423-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]