Abstract
Previous clinical studies of the combination of local intravesical hyperthermia with cytostatic drugs for the treatment of Superficial Transitional Cell Carcinoma of the urinary bladder (STCCB) showed encouraging results both in reducing recurrence rate to 20–30% within 2 years and in ablative success rate of 79%. Our objectives were to evaluate bladder tissue and adjacent organs during and following hyperthermia treatment. An intravesical catheter equipped with a radio-frequency antenna (Synergo® SB-TS 101.1 System) was used for hyperthermia and intravesical chemotherapy (mitomycin C) was instilled in vivo for 60 min in two anaesthetized sheep. Thirteen to fifteen thermocouples were sewn surgically on the internal and external surfaces of the bladder wall and on adjacent organs to monitor the temperature during the treatment. We expected the intravesical temperature to be under 46°C and the external layers below 45°C. The bladder was filled with 50 mL of chemotherapeutic solution (400 µg/mL of mitomycin C in distilled water). The sheep were sacrificed at the end of the treatment. Three other sheep, which underwent thoracic surgery, served as control group. Histological changes in both groups showed foci of oedema and haemorrhage with inflammation in the lamina propria and serosa. Foci of desquamation of the epithelium were noticed in the treated sheep. Histological analysis of the treated group showed no significant differences from the control group. The control group showed similar changes, some less pronounced. The combined treatment of hyperthermia with mitomycin C did not cause major damage to the urinary bladder or adjacent organs. All changes were superficial and reversible, and the control group showed similar changes, some less pronounced. Although this is an experimental model based on one single session treatment, rather than repeated treatments, it suggests that the approach may be useful in future studies both in models and man.
Keywords: urinary bladder, superficial TCC, hyperthermia, mitomycin C, Synergo®
Superficial Transitional Cell Carcinoma of the urinary bladder (STCCB) accounts for approximately 90% of all bladder tumours (Silverberg et al. 1997; Amira et al. 2002). Epidemiological data show that 50–70% of patients treated only with transurethral resection (TURBT) for STCCB have a propensity to develop recurrences within 2 years, with progression of tumour grade and stage in 10–15% of cases (Yan et al. 2002). A combination of TURBT and adjuvant intravesical chemotherapy (ICT) or immunotherapy has a beneficial contribution to lowering the recurrence rate to 20–40%. However, the progression rate seems to have remained unchanged in spite of local adjuvant treatment (Nomata et al. 2002).
The use of combined therapeutic strategies, such as hyperthermia, radiotherapy and chemotherapy, to enhance the anti-cancer effect has been advocated for a long time (Mauroy et al. 1999). However, the lack of suitable technology has so far prevented the implementation of this anti-cancer regimen (Colombo et al. 1998).
Recently advances in the engineering development of radio frequency (RF) delivering devices have allowed the application of local hyperthermia in combination with anti-neoplastic agents to treat several malignant tumours. Local hyperthermia administration at temperatures of 40–44°C in combination with selected cytostatic drugs (Mauroy et al. 1999), results in a synergistic anti-tumoral effect. This may be offered as a therapeutic option for STCCB, because of its endocavitary superficial location, and the ease of accessibility via the urethra.
The Synergo® SB-TS 101.1 System has been devised to deliver local bladder hyperthermia concomitant with intravesical chemotherapy as a prophylactic or ablative therapy of STCCB (Colombo et al. 2001). A disposable catheter system allows intravesical administration of the anti-neoplastic agent, and includes a dipole antenna delivering hyperthermia via 915 MHz RF radiation to the bladder internal walls. A multiple thermocouple probe monitors the temperature at different locations of the internal bladder walls and at the feeding antenna cable at the urethral level.
Our aim in this study was to evaluate the effect of this hyperthermia system on bladder and adjacent organs in an experimental model.
Materials and methods
Five adult female sheep were used for this study. The sheep urinary bladder was evaluated for its resemblance to the human organ with regard to its shape, contour and thickness. Two sheep were treated with the Synergo® SB-TS 101.1 System for temperature mapping and pathological evaluation and three served as controls for the pathological evaluation.
The following parameters were used:
Temperature measurements on the internal layer of the bladder wall.
Temperature measurements on the external layer of the bladder with assessment of the temperature gradient over the thickness of the bladder wall.
Heating the internal bladder wall to 1° – 2°C above the permissible levels of the Synergo® System (over 45°C) with verification that the external layers of the bladder were still within safe temperature range (below 45°C), and then assessing the pathological outcomes of the treatment upon the bladder walls by performing random, full thickness wall samples and comparing them to control samples.
Assessing the pathological outcomes of the treatment upon the bladder-adjacent tissues, searching for any possible damage, and comparing them to control samples.
Anaesthesia of animals
Five adult female sheep were pre-medicated with an injection of Ketamin 600 mg and Xylasin 12 mg. Induction was performed with intravenous injection of Pentotal 500 mg and intubations. Maintenance was performed with Isofluran 1.5–2% and inhalation according to the sheep's physiological response to anaesthesia with Oxigen 700 mL × 12/min.
Treated group
Temperature mapping
The two treated sheep's bladders were emptied of any residual urine using a 10Fr Foley catheter. Thereafter, the urethra was dilated to allow introduction of the Synergy® System catheter into the bladder. The catheter was anchored to the bladder by filling the catheter balloon with 15 mL of distilled water. Subsequently, the lower abdomen was surgically incised and the bladder exposed and dissected along the mid ventral length. At the heart of the Synergo® System is a 915-MHz RF generator that heats the bladder walls to an optimal temperature via an intravesical antenna that is an integral component. The bladder was filled with 50 mL of a chemotherapy agent solution (400 µg/mL of mitomycin C in distilled water) through the Synergo transurethral catheter. A small peristaltic pump circulated the chemotherapeutic solution via the catheter between thermally regulated drug reservoirs. The catheter also contained thermocouples that monitored temperature at the superficial layer of both bladder walls and the urethra. The information was then transmitted to an embedded computer driven by Synergo proprietary software that monitored and controlled all system functions.
A set of 13 thermocouples for ‘sheep 1’ and 15 thermocouples for ‘sheep 2’ were surgically sewn on the internal and external surface of the bladder wall and on adjacent organs. Each thermocouple junction was glued to a tiny piece of latex to ease the implementation procedure. Moreover, proper care was taken to ensure that the junctions were always facing the tissue and that they were relatively isolated from the liquid environment. This way the measurement of tissue surface temperatures, with minimal external interference, was secured. Five out of 13 and three out of 15 thermocouple junctions failed the implementation procedure in the ‘sheep 1’ and ‘sheep 2’ experiments, respectively, because of their fragile nature. Therefore, a total of eight (8) thermocouples for ‘sheep 1’ (three internal wall – thermocouples number 4, 6, 7, three external wall – thermocouples number 10, 11, 12, and two adjacent organs – thermocouples number 14, 16) and 12 (12) thermocouples for ‘sheep 2’ (six internal – thermocouples number 3, 4, 5, 6, 7, 9, four external wall – thermocouples number 10, 11, 12, 13, and two adjacent organs – thermocouples number 14, 16) were used for data processing. A total of about 2880 temperature data points were measured in each experiment and registered over the 60 min treatment session for 16 fixed thermocouple channels. The temperatures measured were correlated with the three original Synergo® System catheter thermocouple temperature readings. A number of thermocouples were sewed on adjacent organs, uterus, omentum and pelvic fat. Following the placement of the thermocouples, the bladder and abdominal cavity were closed and a treatment session with the Synergo® System commenced. The bladder was filled with 50 mL of a chemotherapeutic solution (400 µg/mL of mitomycin C in distilled water) and RF energy was emitted in order to achieve treatment conditions.
About 30 min after the start of the treatment session, the RF energy emission was temporarily shut off, the chemotherapeutic solution was evacuated from the bladder, and a fresh solution instilled into the bladder. The RF energy was then resumed and the treatment session continued for another 30 min. The thermocouple temperatures were sampled every 20 s and the device performance was registered on a personal computer log-file.
A stand-alone, multi-channel temperature measuring system was devised. The thermocouples employed were of a T-type, AWG-40 copper-constantan (Physitemp Ltd), specified by a ± 0.1°C absolute accuracy. Up to 36 thermocouple channels could be connected to a multiplexer and interrogated by a DMM/scanner system. The multiplexer box also contained a thermistor cold junction for calibration of the thermocouples. Custom electronics included a high accuracy isolation amplifier and high pass filter for signal conditioning and low frequency noise reduction. A dedicated software system was used to ensure automatic computer control of temperature measurements and data acquisition, via an IEEE 488 communication protocol. The speed of data acquisition and transfer was evaluated to be 33 ms/channel or about 0.5 s to complete a full cycle of 15-channels scans. For the chosen temperature-sampling rate (every 20 s) and 15 channels, a total of 2700 temperature points were registered over a 60-min treatment session.
Control group
The three control sheep underwent a thoracic operation. Their urinary bladder and abdominal organs were intact. They were anaesthetized and sacrificed with a pentobarbitol overdose.
Pathological examination
Evisceration of the urinary bladder and organs adjacent to the bladder was performed. The urinary bladder and adjacent organs were excised, dissected and fixed in 4% buffered formaldehyde solution.
Treated group
The harvested organs were macroscopically and histologically examined. Transmural biopsies were taken from all implantation sites of thermocouples and other random sites (total of 20 sites). Adjacent organs were also examined including uterus, omentum and pelvic fat.
Control group
Random transmural biopsies of the urinary bladder were taken (total of 36 sites). The same adjacent organs as in the treated group were examined.
The tissue samples were embedded in paraffin blocks and slides of 4 µ thick section were cut and stained with Haematoxylin and Eosin. A microscopic examination of the samples was performed to compare the condition of the treated tissues with the control.
Ethical considerations (GLP)
The Ethics Committee for animal experimentation at Beilinson Campus Hospital approved the protocol for the Synergo® System study prior to commencing the study.
Results
Table 1 and Table 2 summarize the temperature measurements by treatment time for the various thermocouples for the treated sheep.
Table 1.
Temperatures of the thermocouples during the treatment
| Internal Wall | External Wall | Adjacent | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Junction* | (3) | (4) | (5) | (6) | (7) | (9) | (10) | (11) | (12) | (13) | (14) | (16) |
| Sheep 1 | ||||||||||||
| TI (°C) | 44.3 | 45.6 | 42.9 | 39.8 | 38.3 | 38.9 | 36.7 | 37.9 | ||||
| TII (°C) | 41.7 | 45.7 | 39.9 | 37.5 | 37.2 | 37.8 | 36.8 | 37.2 | ||||
| Sheep 2 | ||||||||||||
| TI (°C) | 44.3 | 45.1 | 42.9 | 44.7 | 43.6 | 41.4 | 39.2 | 41.1 | 39.1 | 39.2 | 39.0 | 38.0 |
| TII (°C) | 46.1 | 46.3 | 44.7 | 46.1 | 44.8 | 43.2 | 41 | 42.6 | 40.8 | 40.3 | 40.1 | 38.0 |
Junction numbers do not correspond to the same locations in the two experiments.
Table 2.
Average temperatures (°C) with corresponding standard deviations
| Tint(±σn) | Text(±σn) | Tadj(±σ) | ΔTint − ext(±σ) | ΔTint − adj(±σ) | |
|---|---|---|---|---|---|
| Sheep 1 | |||||
| Max I | 44.3 (1.3) | 39.0 (0.8) | 37.3 (0.9) | 5.3 (1.3) | 7.0 (1.3) |
| Max II | 42.4 (3.0) | 37.5 (0.3) | 37.0 (0.3) | 4.9 (3.0) | 5.4 (3.0) |
| Sheep 2 | |||||
| Max I | 43.7 (1.2) | 39.6 (1.0) | 37.8 (1.0) | 4.1 (1.2) | 5.9 (1.2) |
| Max II | 44.4 (1.4) | 41.1 (1.0) | 38.6 (1.2) | 3.3 (1.4) | 5.8 (1.4) |
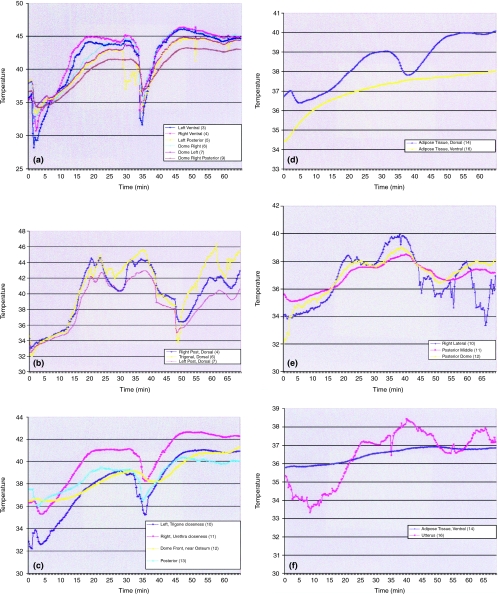
Good heating uniformity in the internal bladder was observed in the ‘sheep 2’ experiment junctions (3)–(4) and (5)–(6)–(7) in Figure 1a, whereas this homogeneity was less apparent in the ‘sheep 1’ experiment (Figure 1b). The temperatures measured by different junctions evolved about nearly parallel profiles, especially those of internal and external wall junctions. The change of the mitomycin C solution in the middle of the session with the RF power switched off corresponds with the dip in the temperature distribution starting after about 30 min of treatment in Figures 1a–d and after 44 min in Figures 1b, e and f.
Figure 1a.
In sheep no. 2, the internal bladder temperature range was 42°C−46°C depending on the thermocouple's location. The refreshing of the intravesical solution and RF discontinuation after 30 min of treatment corresponds to the dip in temperature distribution. During the second part of the experiment, a temperature above 46°C was intentionally employed for limited periods of time, and the maximal internal bladder temperature was then increased to 46°C. Rates of temperature rise were, as expected, higher in the right ventral (4), dome right (6) and left ventral (3) areas of the bladder compared to the posterior wall region, because of the closeness of these areas to the RF antenna. (b). In sheep no. 1 the internal bladder temperature range was 41°C−44.5°C, depending on the thermocouple's location. The refreshing of the intravesical solution and RF discontinuation after 44 min of treatment corresponds to the dip in temperature distribution. The difference in the temperature homogeneity in comparison to sheep no. 2 (Figure 1a) was caused by fluid leakage from the sheep's bladder during the study. As a result, the RF energy had to be altered often (due to variable bladder volume). (c). External bladder temperatures varied in a safety range of 39°C−43°C, even though a temperature above 46°C was intentionally employed for limited periods of time in the bladder. (d). Adjacent organ temperatures were mildly elevated to a maximal temperature value of 40°C even though a temperature above 46°C was intentionally employed for limited periods of time in the bladder. The maximum temperature reached in the adjacent organ is close to the average external bladder wall surface temperature. The rise in temperature in adipose tissue may be explained by the proximity (or even physical contact) of the dorsal adipose tissue with the external bladder wall. In contrast, the more distant (ventral) adipose tissue temperature evolved independently from the bladder wall temperature. (e). External bladder temperatures were raised up to 40°C for a limited period. During most of the study, external bladder temperature remained within a safety limit of 38°C. (f). Adjacent organ temperatures were mildly elevated to a maximal temperature value of 38.4°C. During most of the treatment temperature remained in a safety range of 37–38°C, being mainly influenced by body temperature variations that are assumed to be related to rich vascularization.
Higher temperature rates were measured in the trigone, dome and lateral areas of the bladder walls compared to the posterior wall region. This was due to the specific sheep urethra anatomy, such as larger diameter and shorter length, compared to the human urethra, forcing the RF antenna to a closer position against the bladder neck. This finding is emphasized in the second half of the treatment session of the ‘sheep 1’ experiment, due to pulling of the catheter to avoid leakage. The antenna's position also explains the relatively higher temperatures measured by junction (11) (Figure 1c), which was disposed closer to the urethral region on the external bladder wall. In contrast, the lowest temperatures measured on the internal posterior bladder wall by junction (9) (Figure 1a), point to its position, away from the peak RF radiation field. The same effect is clearly observed when comparing the thermocouple (6) vs. thermocouples (4) and (7) measurements (Figure 1b), during the first and second session treatments. The maximum temperatures reached in the adjacent organ junction (14), as seen in Figure 1d, are close to the average external bladder wall surface temperature (Table 1). This may be explained by the close proximity (or even physical contact) of the adipose tissue with the external bladder wall. In contrast, the more distant (ventral) adipose tissue temperatures of junctions (16) (Figure 1d) and (14) (Figure 1f) evolved independently from the bladder wall temperatures. These reached a maximum of around 37–38°C, being mainly influenced by body temperature variations. The uterus junction measurements (16) (Figure 1f) showed independent temperature variations that are assumed to be related to rich vascularization.
Sampling of the different measured temperatures of the thermocouples at fixed times during the treatment have been performed. Table 1 presents maximum temperatures of the various thermocouples, registered in the first (TI) and second (TII) treatment periods of the two experiments. Temperatures above 46°C were deliberately employed for limited time periods in the ‘sheep 2’ experiment to demonstrate the worst case and its possible influence outside the bladder. Moreover, the thickness of the sheep's bladder wall of about 7 mm on average (for empty bladder and thinner for filled bladder) is thinner than in humans, which provides us with an additional worst case condition, as far as the temperatures developed outside the bladder wall are concerned (Table 1).
Sampled temperature values, for the same fixed time points, have been averaged over the regions measured (internal bladder wall, external bladder wall and adjacent organs). Table 2 shows these average temperatures with corresponding standard deviations (σn), and averaged temperature differences of the internal wall − external wall and internal wall − adjacent organs. The average of maximum internal bladder wall temperatures was confirmed by the average temperatures measured by the three internal bladder thermocouples of the Synergo® System catheter (44–45°C) and is available from the treatments log files.
In summary, temperature differences of ΔTint − ext = 3.3–5.3°C and ΔTint − adj = 5.4–7.0°C were developed on the average over the whole treatment session. These temperature differences were checked by measuring in samplings at other fixed times and confirmed as accurate average figures. Comparison of two pairs of thermocouple junctions (5)–(12) and (6)–(11) in the ‘sheep 2’ experiments that were implemented at near (internal − external wall) topological coincidence was performed. The temperature differences sensed were ΔT(5) – (12) = 3.9°C and ΔT(6) – (11) = 3.6°C, respectively, and were well within the average values of ΔTint − ext shown above.
Pathological examination
Macroscopic
On macroscopical examination of the treated sheep's bladder the mucosal (urothelial) surface was smooth and seemed intact. Small foci of haemorrhage were noticed. The serosal site also showed small areas of fresh haemorrhage. The fatty tissue adjacent to the urinary bladder and uterus showed no macroscopic alterations.
In the control group, the urinary bladders, the fatty tissue adjacent to the urinary bladder and uterus showed no macroscopic alterations.
Microscopic
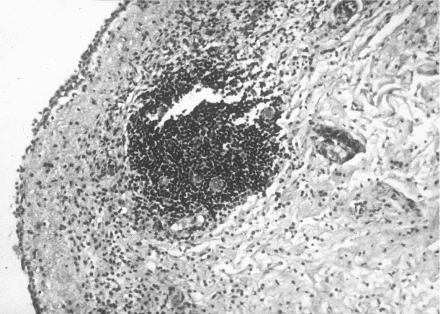
A summary of the pathological features of the urinary bladders of the treated group is presented in Table 3. In the random samples taken from different sites of the urinary bladder the surface transitional epithelium was preserved (Figure 2). There were foci with partial, superficial sloughing of the epithelium, but at those sites also, more than 50% of the urothelial thickness was preserved. The lamina propria showed mild oedema with mild inflammatory infiltrate, acute and chronic, in all samples. The muscularis propria was intact. The serosa showed foci of oedema with mild inflammation, acute and chronic.
Table 3.
The various histopathological findings in the examined urinary bladders of the treated sheep and control group
| Control sheep 36 sites | |||||
|---|---|---|---|---|---|
| Site Lesions | Treated sheep 20 sites | No. | % | No. | % |
| Epithelium | Desquamation | 14 | 70 | 0 | 0 |
| Lamina propria | Oedema | 20 | 100 | 15 | 42 |
| Inflammation | 7 | 35 | 3 | 8 | |
| Haemorrhage | 3 | 15 | 0 | 0 | |
| Muscularis propria | Oedema | 9 | 45 | 4 | 11 |
| Inflammation | 0 | 0 | 0 | 0 | |
| Haemorrhage | 0 | 0 | 0 | 0 | |
| Serosa | Oedema | 18 | 90 | 10 | 28 |
| Inflammation | 9 | 45 | 2 | 6 | |
| Haemorrhage | 4 | 20 | 1 | 3 | |
Figure 2.
Urinary bladder mucosa of treated sheep no. 2. There is focal sloughing of the epithelium, and mild inflammatory infiltrate in the lamina propria with a focus of lymphocytic aggregation. H&E ×100.
Samples taken from the implantation sites of the thermocouples showed intact transitional epithelium. In the lamina propria there were foci of disintegrated tissue.
No changes were observed in the muscularis propria. The serosal site showed severe oedema with fresh haemorrhage and foci of mild inflammation. These foci were small, focal and adjacent to normal tissue. The fatty tissue adjacent to the urinary bladder was normal. The uterus was normal as well with only mild oedema of the mucosa.
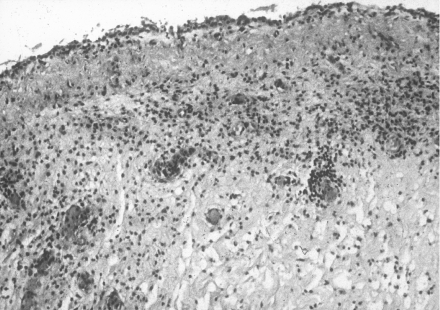
A summary of pathological features of the urinary bladders of the control group is presented in Table 3. Random samples from the bladders of this group showed a normal urothelium. There were foci of congestion and mild oedema with mild inflammatory infiltrate in lamina propria (Figure 3). Muscularis propria and the serosa were normal, as was the adjacent fatty tissue. Mild congestion was found in the uterus.
Figure 3.
Urinary bladder mucosa of control sheep no. 1. The surface epithelium is intact. A mild inflammatory infiltrate is seen in the lamina propria. H&E ×100.
Discussion
For some time hyperthermia alone or in combination with radiotherapy, immunotherapy or chemotherapy has been considered a promising method for treating several kinds of solid tumours (Cavaliere et al. 1967; Matsushita et al. 1993; Colombo et al. 1995; Oldhafer et al. 1998; Mauroy et al. 1999; Paroni et al. 2001). However, hyperthermia can affect the normal tissues adjacent to the treated tumour when localized, or various other normal tissues when whole body hyperthermia is applied.
Fajardo (1984) reviewed studies of the effects of hyperthermia on various tissues of several mammals and humans. In his review hyperthermia of the urinary bladder of dogs and rabbits between 35°C and 44.5°C showed no gross or microscopic alterations.
Our experiment consisted of a treatment session with Synergo® device. The bladder was filled with 50 mL of chemotherapeutic agent solution (400 µg/mL of mitomycin C in distilled water) and 60 min heating with the Synergo® temperature set between 41°C and 44°C.
There were no significant differences, macroscopical or microscopical, in the urinary bladder wall and adjacent organs of the treated sheep or the control group. Neither heated sheep's bladder nor the control group showed irreversible changes. It should be stressed that the pathological findings are only the acute effects described after a single application of the treatment and, as a consequence, these results cannot be directly translated into clinical practice, where many (6–12) operative sessions are generally performed, and therefore pathology must be considered as a cumulative effect.
The design of the study does not allow for discriminating between the degree of the histological effects in treated sheep caused by local hyperthermia and mitomycin C chemotherapy.
Temperature mapping of the urinary bladder and adjacent organs, and their pathological evaluation, clearly show that treatment with the Synergo® System can be administered safely with no risk of damage to the urinary bladder or adjacent tissues. This was further emphasized in sheep 2, in which a temperature of 46°C was deliberately employed with no subsequent pathological results to adjacent organs. Because the sheep bladder is thinner than the human bladder, injury to the surrounding tissue in humans is less likely.
Therefore, our conclusion is that the Synergo® System for combined hyperthermia–chemotherapy treatment is safe, because there was no irreversible damage to the deep layers of the urinary bladder and adjacent organs. This system is effective on the superficial layers with only partial changes that are not irreversible. The results are based on a restricted number of animals, and although it is evident that they need to be confirmed in a larger experimental series, nevertheless they are sufficiently encouraging to justify continuation with this approach.
References
- Amira N, Mourah S, Rozet F, et al. Non-invasive molecular detection of bladder cancer recurrence. Int. J. Cancer. 2002;101:293–297. doi: 10.1002/ijc.10561. [DOI] [PubMed] [Google Scholar]
- Cavaliere R, Ciocatto EC, Giovanella BC, et al. Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer. 1967;20:1351–1381. doi: 10.1002/1097-0142(196709)20:9<1351::aid-cncr2820200902>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Colombo R, Brausi M, Da Pozzo LA, et al. Thermo-chemotherapy and electromotive drug administration of mitomycin C in superficial bladder cancer eradication. A pilot study on marker lesion. Eur. Urol. 2001;39:95–100. doi: 10.1159/000052419. [DOI] [PubMed] [Google Scholar]
- Colombo R, Da Pozzo LF, Lev A, et al. Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumors. J. Urol. 1998;159:783–787. [PubMed] [Google Scholar]
- Colombo R, Lev A, Da Pozzo LF, Freschi M, Gallus G, Rigatti P. A new approach using local combined microwave hyperthermia and chemotherapy in superficial transitional bladder carcinoma treatment. J. Urol. 1995;153:959–963. [PubMed] [Google Scholar]
- Fajardo LF. Pathological effects of hyperthermia in normal tissues. Cancer Res. 1984;44:4826s–4835s. [PubMed] [Google Scholar]
- Matsushita S, Reynolds R, Urano M. Synergism between alkylating agent and cis-platin with moderate local hyperthermia: the effect of multidrug chemotherapy in an animal system. Int. J. Hyperthermia. 1993;9:285–296. doi: 10.3109/02656739309022541. [DOI] [PubMed] [Google Scholar]
- Mauroy B, Bonnal JL, Prevost B, et al. Study of the synergy of microwave hyperthermia/intravesical chemotherapy in the prevention of recurrences of superficial tumors of the bladder. Prog. Urol. 1999;9:69–80. [PubMed] [Google Scholar]
- Nomata K, Noguchi M, Kanetake H, et al. Intravesical adjuvant chemotherapy for superficial transitional cell bladder carcinoma: results of a randomized trial with epirubicin comparing short-term versus long-term maintenance treatment. Cancer Chemother. Pharmacol. 2002;50:266–270. doi: 10.1007/s00280-002-0487-6. [DOI] [PubMed] [Google Scholar]
- Oldhafer KJ, Frerker MK, Lang H, et al. High-dose mitomycin C in isolated hyperthermic liver perfusion for unresectable liver metastases. J. Invest. Surg. 1998;11:393–400. doi: 10.3109/08941939809032216. [DOI] [PubMed] [Google Scholar]
- Paroni R, Salonia A, Lev A. Effect of local hyperthermia of the bladder on Mitomycin C pharmacokinetics during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma. Br. J. Clin. Pharmacol. 2001;52:273–278. doi: 10.1046/j.0306-5251.2001.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg SG, DeLellis RA, Frable WJ. Principles and Practice of Surgical Pathology and Cytopathology. 3. New York: Churchill Livingstone; 1997. pp. 2191–2217. [Google Scholar]
- Yan Y, Andriole GL, Humphrey PA, Kibel AS. Patterns of multiple recurrences of superficial (Ta/T1) transitional cell carcinoma of bladder and effects of clinicopathologic and biochemical factors. Cancer. 2002;95:1239–1246. doi: 10.1002/cncr.10822. [DOI] [PubMed] [Google Scholar]