Abstract
Alkaline phosphatase (AP) can be considered as a host defence molecule since this enzyme is able to detoxify bacterial endotoxin at physiological pH. The question emerged whether this anti-endotoxin principle is inducible in the glomerulus and if so, which glomerular cells might be involved in the expression of ectoAP after stimulation with pro-inflammatory agents. Therefore kidneys of rats treated with either lipopolysaccharide (LPS), E. coli bacteria or non-toxic monophosphoryl lipid A (MPLA) were examined for AP activity 6 or 24 h after challenge. In addition cultures of endothelial cells or mesangial cells were evaluated for AP activity after stimulation with either LPS, TNFα or IL-6, and mRNA for AP was studied in TNFα-stimulated and control mesangial cells. The results show significant up-regulation of glomerular AP in LPS- or E. coli-injected rats compared to rats injected with MPLA. Endothelial and mesangial cells in vitro showed significant up-regulation of AP activity following stimulation with LPS, TNFα or IL-6, whereas increased mRNA for AP was observed in mesangial cells after TNFα stimulation compared to non-stimulated control cells. Since it appeared that hydrolysis occurred when endotoxin was used as a substrate in the histochemical staining, we concluded that inducible glomerular ectoAP may reflect a local endotoxin detoxifying principle of the kidney.
Keywords: alkaline phosphatase, endothelium, endotoxin, glomerulus, inflammation, mesangium
To protect the glomerular microvasculature against toxic stimuli, various host defence principles are present within this structure. This glomerular self-defence machinery involves, for instance, inhibitors of inflammatory cytokines (Suzuki et al. 1994; Kitamura & Fine 1999), proteinase inhibitors (Shankland et al. 1996), oxygen radical scavengers (Marklund 1984) as well as ectoenzymes able to hydrolyse extracellular nucleotides (Bakker et al. 1993). Besides glomerular ectonucleotidases, which are potentially able to degrade pro-thrombotic and pro-inflammatory nucleotides (Poelstra et al. 1991; Bakker et al. 1993), also alkaline phosphatase (AP) is abundantly present in kidney tissue, although predominantly in tubular brush borders and vessel walls, whereas glomeruli are essentially negative (McComb et al. 1979,Poelstra et al. 1997a;).
The physiological function of AP has remained unknown for long time. This is partly due to the fact that AP has a rather unphysiological pH optimum, i.e. pH 10.5. However, we have shown in 1997 that AP activity is detectable at physiological pH when endotoxin is used as a substrate. Thus, after contact with AP, endotoxin is dephosphorylated (Poelstra et al. 1997a; Poelstra et al. 1997b). It is well known that removal of even one phosphate moiety from this highly toxic molecule, leading to the formation of monophosphoryl lipid A (MPLA), is sufficient to detoxify endotoxin completely (Gustafson & Rhodes 1992; Rietschel et al. 1994).
In addition, it has been demonstrated that administration of lipopolysaccharide (LPS) to experimental animals induces increased systemic up-regulation of AP, in particular in the liver and in neutrophils (Xu et al. 2002). Similarly, enhanced AP has been observed in leucocytes from patients with Gram-negative sepsis (Chikkappa 1992). These data suggest that AP may be considered as an important host defence enzyme (Kaplan 1986; Poelstra et al. 1997a; Poelstra et al. 1997b), the expression of which may be triggered by LPS.
Because the glomerular microcirculation is a relatively sensitive site for toxic agents due to its filtration function, the question was raised whether inducible AP may also belong to the local self-defence system of the glomerular tuft. Therefore we now investigated the expression of glomerular AP in the rat after either administration of LPS or E. coli in vivo, using histochemical methods. In vitro, AP expression and/or activity of endothelial or mesangial cells after stimulation with LPS or proinflammatory cytokines was evaluated. In addition studies were conducted to detect the mRNA signal for AP in mesangial cells after stimulation with TNFα vs. non-stimulated control cells.
It is shown that in the rat injection of LPS or E. coli in contrast to injection with MPLA induces up-regulation of glomerular AP within 6 h. Furthermore, endothelial and mesangial cells showed up-regulation of AP expression and enhanced AP activity after stimulation with LPS or cytokines in vitro, whereas mRNA for AP was increased in TNFα-stimulated mesangial cells. In view of the endotoxin detoxifying nature of AP, it is suggested that inducible glomerular AP reflects a local host defence principle against this highly toxic agent.
Materials and methods
Animals and tissue sampling
Wistar rats (females, 180–200 g) were obtained from Harlan CPB (Zeist, The Netherlands). Rats were housed under specific pathogen-free conditions and fed with standard chow (Hope Farms Woerden, The Netherlands) and received water ad libitum. All experiments were approved by the Animal Care and Use Committee of the University of Groningen, The Netherlands.
Kidney tissue samples from these animals were fixed in formalin or snap-frozen in isopentane and kept at −80 °C until use.
Experimental design
Animals were injected i.v. with LPS from E. coli serotype 055:B5 (Sigma Chemical Co., St. Louis, MO, USA) in saline (2.5 mg/kg body weight; n = 9), or i.p. with E. coli bacteria in saline (0.5 × 1010 CFU/mL; n = 6) (E. coli bacteria cultures (ATCC 25922) prepared by K. Kooi, Department of Surgery, University Hospital Groningen, The Netherlands). Control rats were injected i.v. with MPLA (List Biological Laboratories Inc., Campbell, CA, USA) in saline (2.5 mg/kg body weight, n = 7).
LPS-treated rats were sacrificed after 6 h (n = 5) or after 24 h (n = 4). Rats treated with E. coli were also sacrificed after 6 (n = 3) or 24 h (n = 3), whereas control rats were sacrificed 24 h following MPLA injection. Kidney tissue was fixed with formalin for conventional staining or snap-frozen in isopentane and kept at −80 °C until preparation for immunostaining or histochemistry.
To evaluate the potential expression of AP following stimulation with LPS, endothelial or mesangial cell cultures were incubated with LPS or TNFα and tested for the expression of the relevant molecules in cytospin preparations of these cell cultures. Thus, cytochemical or immunostaining for AP was carried out upon endothelial or mesangial cell preparations after incubation with LPS, MPLA or TNFα. All kidney sections as well as cytospins were counterstained with haematoxylin.
The mRNA signal for AP was evaluated in mesangial cells stimulated with TNFαin vitro and compared with unstimulated cells. Finally, the activity of AP in LPS-stimulated endothelial cells vs. non-stimulated cells was assayed biochemically.
Histochemical staining of kidney sections
Cryostat sections (4 µm) were fixed with acetone and stained for AP activity using 3 mm β-glycerophosphate (β-gP) as a substrate according to the cerium based Gomori method with minor modifications (Van Goor et al. 1989). In addition histochemical staining for AP was also carried out using endotoxin as a substrate (5 mg/mL; Sigma). The latter method allows evaluation of the dephosphorylation capacity of tissue AP, since the reaction product reflects hydrolysis of phosphate moieties from endotoxin, which leads to detoxification of the molecule (Poelstra et al. 1997a; Poelstra et al. 1997b).
To check the specificity of the reaction also incubations were done in the presence of the specific AP inhibitor levamisole (1 mg/mL; Sigma) (Tillyer et al. 1994).
Quantification of glomerular reaction product for AP detoxification activity
Reaction product detected after histochemical staining for AP using endotoxin as a substrate, was semiquantitatively evaluated in a double blind fashion by scoring 50 glomeruli (in two sections) per individual animal. Each glomerulus covered by reaction product for at least 50% was scored positive. The number of positive glomeruli compared to the total number of scored glomeruli in each individual animal was calculated as a ratio and the arithmetic means of these ratios were expressed as arbitrary units.
Stimulation of endothelial cells by LPS in vitro
Human umbilical cord venous endothelial cells (HUVEC) obtained from G. Molema (Endothelial Cell Facility Groningen University/Academic Hospital Groningen, The Netherlands) were cultured in RPMI 1640 (BioWhittaker Inc., Walkersville, MD, USA) supplemented with 20% foetal calf serum (FCS; batch 5-60403; Integro, Dieren, The Netherlands) under standard conditions (Jaffe et al. 1973). Only low passage numbers (1–3) in six-well plates (Costar, Cambridge, MA, USA) were used throughout the experiments. Before stimulation with LPS the culture medium was supplemented with 1% normal human serum (NHS) to provide a human source for soluble CD14 enabling proper stimulation with LPS (Pugin et al. 1993).
Cells were stimulated with LPS from E. coli (10 ng/mL) or with MPLA (10 ng/mL) at 37 °C for 24 h in duplicate. Control cultures were incubated with vehicle alone.
After 24 h the monolayers were washed with Hank's balanced salt solution (HBSS), and carefully detached using a disposable cell scraper. Subsequently cytospins were prepared according to standard methods.
Immunostaining of endothelial and mesangial cells for AP expression
In order to evaluate the endothelial or mesangial AP expression in vitro following stimulation with either LPS (10 ng/mL) or TNFα (10 ng/mL), respectively; cytospins were stained using polyclonal rabbit-anti-AP IgG (Rockland, Gilbertsville, PA, USA), biotin conjugated swine-anti-rabbit antibody (DAKO A/S, Glostrup, Denmark) and finally incubated with peroxidase-conjugated streptavidin (DAKO A/S). Reaction product was visualized using 3-amino-9-ethyl-carbazole (Warnke & Levy 1980).
Cytochemical staining of endothelial and mesangial cells for AP activity
Stimulation of cells with LPS was carried out as mentioned under ‘stimulation of endothelial cells by LPS in vitro’ except that in these experiments a higher LPS concentration was used (100 ng/mL). In these experiments confluent cultures were kept on gelatine-coated chamber-slides (Nalge Nunc International, Naperville, IL, USA).
After 24 h monolayers were washed with HBSS twice, air-dried, fixed with acetone and stained according to the modified method of Gomori (Van Goor et al. 1989), using 27.7 mm β-gP as a substrate.
Identical staining was carried out upon cytospins of mesangial cells.
Quantification of cytochemical staining for AP activity
In each LPS-stimulated endothelial, or TNFα-stimulated mesangial cell cultures vs. non-stimulated cultures, cells were randomly selected (three low-power fields per slide) in slides of stimulated or non-stimulated cells; the total cell number within representative low-power fields (approximately 1000 cells per field) and the number of AP-positive cells within these fields were counted. Exclusively cells with a clear brown cytoplasmic reaction product were considered as AP-positive. The results were calculated as ratios indicating the number of positive cells over the total number of counted cells in each culture, and expressed as staining indexes.
Scoring of cell numbers in all cytospins or chamber-slides of both endothelial and mesangial cells throughout the present study was carried out in a double-blind fashion with two independent observers.
Biochemical assay of stimulated endothelial cells
In addition to the immunocytochemistry upon cytospins of stimulated and control endothelial cells in vitro, we also assayed AP activity biochemically according to standard methods with 4-nitrophenylphosphate as a substrate (Courdouhji et al. 1988). Thus, non-stimulated endothelial cells or endothelial cells stimulated with LPS (10 ng/mL) or with a LPS-associated cytokine IL-6 (Bailly et al. 1990) (10 ng/mL; Sigma) were cultured at 37 °C for 24 h in duplicate. The cultures were subsequently washed with saline twice and incubated with 4-nitrophenylphosphate 1.28 mm for 60 min. In one set of assays levamisole (Sigma; 0.2 mm) was added to the incubation mixture. The phosphate release was detected by photospectometry at 405 nm.
Stimulation of mesangium cells by TNFα in vitro
Human mesangial cells (HMC) were kindly provided by L. A. Monnens (Department of Paediatrics, University Hospital Nijmegen, The Netherlands) and E. de Heer (Department of Pathology, Leiden University Medical Centre, The Netherlands). The identity of the cultures was established by morphological criteria and positive staining for smooth muscle cell actin, fibronectin and negative staining for factor VIII. The mesangium cells, used in passage number 6–7, were cultured in RPMI 1640 (BioWhittaker Inc.) supplemented with 10% FCS (Integro, Dieren, The Netherlands) and 10% NHS, 100 U/mL penicillin – 100 µg/mL streptomycin (BioWhittaker Inc.) using tissue culture flasks (25 cm2 bottom surface area Costar) which were previously coated with 1% gelatin (Sigma).
As mesangial cells stimulated with LPS showed inconsistent activation in our hands, probably due to the lack of CD14 receptors (Yang et al. 1999), we used TNFα for stimulation of mesangial cell cultures throughout the present study. Before incubation with either TNFα (Sigma; 10 ng/mL) or vehicle, cells were washed with RPMI 1640 twice to remove serum present in the culture medium and incubated with TNFα overnight in serum-free conditions at 37 °C.
Cells were subsequently detached using trypsin/EDTA and cytospins were prepared according to standard methods.
Some of the stimulated and control HMC cultures were thoroughly washed and frozen until use for isolation of mRNA.
Detection of mRNA for AP in stimulated and non-stimulated mesangial cells
The frozen mesangium cells were used for the isolation of RNA. Total RNA was isolated from stimulated and non-stimulated cells with Trizol (Life Technologies Inc., Gaithersburg, MD, USA).
RNA isolation was followed by DNAse treatment according to the protocol from the MessageClean kit (GenHunter Corp., Nashville, TN, USA). Quality of the isolated total RNA was checked on a 1% agarose gel. The DNAse treatment was tested by PCR using a dinucleotide primer set D11S875 specific for genomic DNA (5′-ACTGTCCTCTCATCCTACTG-3′ and 5′-TACAGAGCTGAGTTTGTAGC-3′) and only those cases for which no PCR product was obtained were used for further analysis.
The cDNA synthesis was primed with oligo(dT) using the protocol provided by the manufacturer (Gibco BRL, Eggenstein, Germany). AP primers were selected from the sequence available in the GenBank (accession number J03572). Primer sequences used for the amplification were AP (f 5′-TCACATTTGGTGGCTACACCC-3′; r 5′- CATGGAGACGTTCTCTCGTTC-3′) and GAPDH (f 5′- CCATC ACTG CCACT CAGAA GACT-3′, r 5′-TTACTCCTTGGAGGCCATGTAGG-3′). PCR for all primer sets was performed with 1 unit of Taq-polymerase (Pharmacia Biotech) and the reaction buffer provided by the manufacturer. The PCR programme of AP consisted of 40 cycles with a denaturation step of 30 s at 94 °C, an annealing step of 40 s at 57 °C, and an extension step of 40 s at 72 °C. The first denaturing step lasted for 5 min and the final extension step lasted for 7 min. Amplification of GAPDH was performed for only 20 cycles and used as control to compare mRNA amounts.
Statistics
Statistical evaluation of the data was carried out by the Wilcoxon test, and P ≤ 0.05 was considered as significant.
Results
Glomerular AP activity of LPS-treated rats
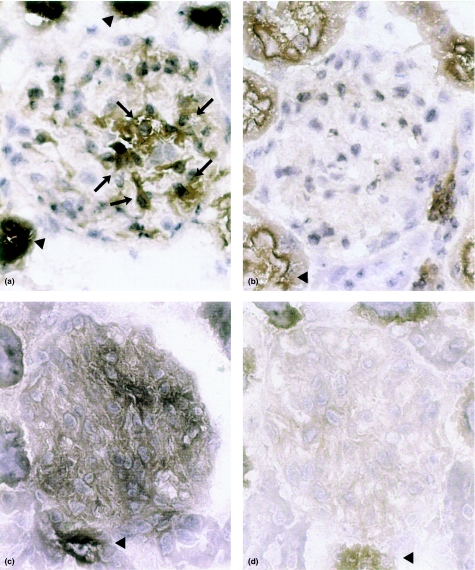
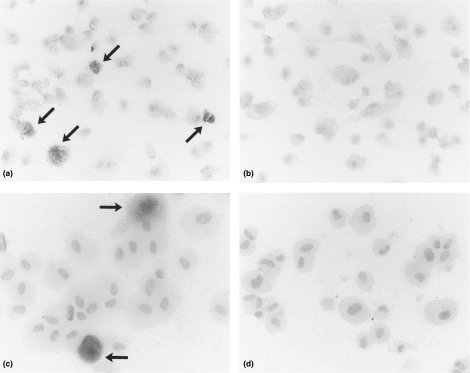
Kidney sections of rats sacrificed 6 or 24 h following treatment with either LPS or E. coli bacteria did not show histological alterations compared with control rats, using conventional staining of paraffin sections (HE or PAS staining; results not shown). In contrast, clear increase of reaction product after staining for AP activity was observed in glomeruli of LPS- or E. coli-treated animals (Figure 1). In kidneys from LPS- or E. coli-treated rats stained for AP activity using endotoxin as a substrate, diffuse glomerular staining was seen compared to kidney sections of the same rats stained for AP activity using conventional substrate, i.e. β-gP (Figure 1a and c). In the latter sections staining of the mesangial area and reaction product along the capillary walls was seen (Figure 1a, arrows). Tubules and blood vessels stained positive in LPS-treated animals and to a lesser extend also in control rats (Figure 1a–d, arrow heads). No reaction product was observed when the sections were pre-incubated with the AP inhibitor levamisole (results not shown). Figure 2 shows the results of quantitative evaluation of reaction product after staining for AP using endotoxin as a substrate.
Figure 1.
Photomicrographs of rat kidney cortex stained for AP by the cerium-based Gomori technique. Brown reaction product is present in glomeruli from LPS-treated animals after 6 h a, arrows, and c, whereas glomeruli of control rats stain negative, b and d. Sections were incubated with either β-gP, a and b, or endotoxin, c and d, as a substrate. In addition to glomerular reaction product, up-regulation of tubular AP can be seen, a and c vs. b and d; arrowheads. Final magnification × 300.
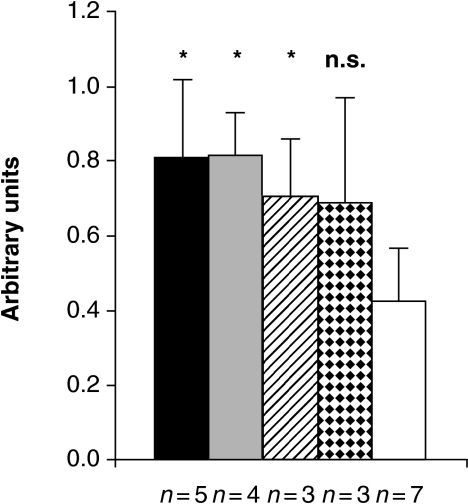
Figure 2.
Semiquantitative evaluation of glomerular staining for AP activity using endotoxin as a substrate in kidneys from rats treated with either LPS, E. coli bacteria or MPLA. The columns represent arithmetic mean scores of glomerular reaction product in sections of either LPS-treated (solid and grey columns), E. coli-treated (hatched and stippled columns) or MPLA-treated animals (open column). Animals were sacrificed either 6 h (solid and hatched columns) or 24 h after endotoxin or E. coli injection (grey and stippled columns). It can be seen that significantly increased staining occurs in LPS-treated animals compared to MPLA-treated control rats. Glomeruli of E. coli-treated rats also show increased staining, although the difference in the 24 h group compared to control rats was not statistically significant (hatched and stippled columns) (*P ≤ 0.05 vs. control, Wilcoxon); n.s. = statistically not significant; n = number of animals per group. Bars represent standard deviation.
Identical statistical differences (as illustrated in Figure 1a and b) were observed when β-gP was used as a substrate (results not shown).
AP expression in endothelial or mesangial cells
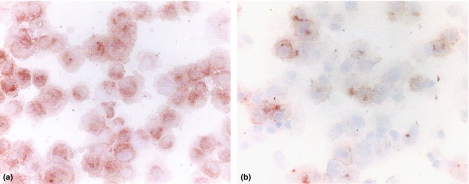
Figure 3 shows immunostaining for AP upon endothelial cells following incubation with either LPS or saline. It can be seen that an increased amount of reaction product was present in stimulated cultures (Figure 3a) in contrast to saline-treated control cells (Figure 3b). Control cells showed weak expression of AP (Figure 3b) and a similar level of background staining of AP could be seen in cultures incubated with MPLA (results not shown).
Figure 3.
Photomicrographs of cultured endothelial cells stained for AP expression by anti-AP IgG antibody using the indirect peroxidase method. It can be seen that predominant AP staining occurs in cells after stimulation of LPS, a, compared with unstimulated cells, in which only faint staining can be observed, b; final magnification × 300.
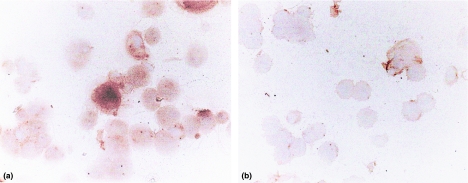
In addition, mesangial cells following stimulation in vitro showed increased staining for expression of AP compared to non-stimulated cells (Figure 4); again mesangial cells stimulated with MPLA showed the same level of staining for AP compared with saline-treated cultures (results not shown). As can be deduced from Figure 5, AP mRNA level was up-regulated in stimulated vs. non-stimulated cells.
Figure 4.
Photomicrographs of cultured mesangial cells stained for AP expression by anti-AP IgG antibody using indirect peroxidase method. It can be seen that predominant AP staining occurs in mesangial cells after stimulation with TNFα, a, compared with unstimulated cells, b; final magnification × 300.
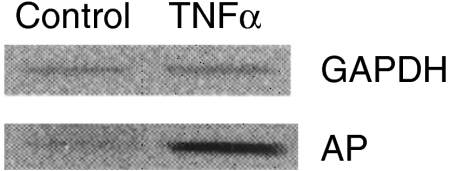
Figure 5.
Gel electrophoresis of AP RT-PCR products from stimulated vs. non-stimulated mesangium cells (lower panel). The upper panel shows RT-PCR products from GAPDH (housekeeping gene). Clear up-regulation of AP expression following TNFα stimulation vs. non-stimulated cells can be seen in the lower panel.
AP activity in endothelial and mesangial cells
In addition to AP expression in endothelial and mesangial cells in vitro, as shown by immunostaining, cytochemical staining for AP activity was carried out. It can be seen from Figure 6 that both stimulated endothelial cells (Figure 6a) as well as cytospots prepared from mesangial cells (Figure 6c), showed relatively more positive cells compared with non-stimulated cells (Figure 6b and d). Cells stimulated with MPLA also stained negative (results not shown).
Figure 6.
Photomicrographs of endothelial cells, a and b, and mesangial cells, c and d, histochemically stained for AP activity by the cerium-based Gomori technique using β-gP as a substrate. Reaction product occurs in either LPS-stimulated endothelial cells, a; arrows, and TNFα-stimulated mesangium cells, c, arrows; in contrast to non-stimulated endothelium or mesangium cells, b and d. Final magnification ×300.
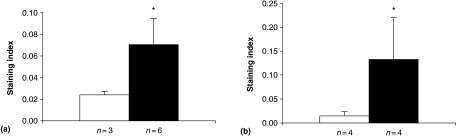
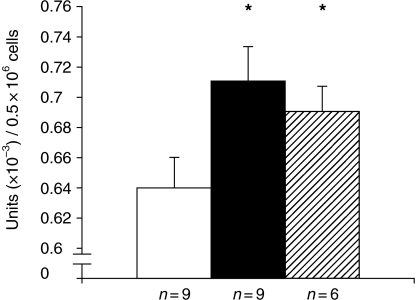
Semi-quantitative scoring of relative numbers of positive cells in stimulated vs. control cultures is shown in Figure 7. It can be seen in both endothelial and mesangial cell preparations that a significant increase in positive cells occurred (Figure 7a and b). In Figure 8 the results of a biochemical assay are depicted. These data confirm the findings in endothelial cells, showing a significantly increased amount of AP activity in LPS- or IL-6-stimulated cells vs. control cells. Addition of levamisole to the reaction mixture abolished the phosphate release completely (results not shown).
Figure 7.
Semi-quantitative evaluation of staining for AP activity of LPS-stimulated vs. non stimulated endothelial cells, a, and TNFα-stimulated vs. non-stimulated mesangium cells, b. Columns represent arithmetic means of ratios expressed as indexes, i.e. relative number of positive cells, from stimulated (solid column) vs. control cultures (open columns). It is shown that significantly higher numbers of cells stain positive for AP in stimulated vs. non-stimulated cultures (*P ≤ 0.05; Wilcoxon). n = number of endothelial or mesangial cell cultures per group. Bars represent standard deviation.
Figure 8.
Measurement of AP activity using AP assay on endothelial cells stimulated for 24 h with either LPS (solid column), IL-6 (hatched column) vs. unstimulated cells (open column). The columns represent arithmetic means of AP activity within each group of cell cultures. It can be seen that the LPS and IL-6 stimulated cells (solid and hatched columns) show significant higher amount of activity compared with the control cultures (open column) (*P < 0.01; Wilcoxon). Units: AP activity in units per 0.5 × 106 cells. n = number of endothelial cell cultures per group. Bars represent standard deviation.
Discussion
The aim of the present study was to test whether AP activity could be induced in glomeruli of the rat kidney following challenge with LPS or E. coli in vivo. It was shown histochemically that as soon as 6 h after challenge with LPS or E. coli bacteria significant up-regulation of this ectoenzyme occurs (Figures 1 and 2). In addition the functional activity of this glomerular AP was evaluated. It appeared that glomerular AP was able to detoxify endotoxin when used as a substrate in vitro (Figure 1c and d) (Poelstra et al. 1997b).
The reaction product may be localized in the mesangial area as well as along the capillary loops (Figure 1c). Because of the inevitable loss of morphological detail following enzyme histochemistry upon cryostat sections, as well as differences in the staining pattern relating to the substrate used, precise localization of the reaction product is difficult to establish (Figure 1a and c). Immunohistology at the ultrastructural level and in situ hybridization are needed to settle this issue. However, the primary aim of the present communication relates to the LPS detoxifying activity of glomerular AP as a protective principle rather than to the exact location of the enzyme within the glomerular microvasculature.
AP activity occurs both as an ectoenzyme and as a soluble enzyme present in the circulation (Miki et al. 1986). Therefore, it is conceivable that the enhanced presence of glomerular AP activity in LPS-treated rats (Figures 1 and 2) may reflect intra-glomerular trapping of circulating AP rather than in situ up-regulation of AP. To settle this issue the most straightforward approach would be to check for up-regulation of the mRNA signal for AP in glomeruli of LPS-treated vs. control animals. Indeed, in kidney cortex of diseased rats we found a tendency towards increase of the mRNA signal for AP (using standard RT-PCR techniques with relevant probes), compared to control animals, although a statistically significant difference could not be observed (results not shown). This is probably caused by the abundant presence of AP in tubular brush-borders of the rat kidney (McComb et al. 1979), leading to a relative high background signal, and consequently to quenching of differences of the glomerular mRNA signal for AP in diseased vs. control rats. Laser microdissection techniques and real time RT-PCR may clarify this issue.
However since cultured endothelial and mesangial cells per se are able to up-regulate AP following stimulation with proinflammatory agents like LPS or TNFαin vitro (Figures 3 and 4), it is highly likely that also in vivo in situ induction of glomerular AP may occur.
In addition, also mesangial cells show enhancement of the mRNA signal for AP after stimulation with TNFα (Figure 5).
The activity of endothelial or mesangial AP following stimulation with LPS or TNFα is shown cytochemically (Figures 6a, c and 7) and biochemically (Figure 8). In the latter assay it can be seen that next to LPS or TNFα, also IL-6 is able to induce up-regulation of AP activity. This is in line with the notion that both IL-6 and TNFα can be released by LPS-stimulated monocytes (Bailly et al. 1990), which may contribute to the up-regulation of this detoxifying enzyme (Nakazato et al. 1997).
Although it should be kept in mind that glomerular endothelium is not equal to HUVEC and that human endothelial or mesangial cells are not identical to their cellular equivalents in the rat, these results support the suggestion that in the rat similar up-regulation of AP in situ may occur, following appropriate stimulation in vitro. These data await further confirmation using the relevant rat cells in vitro to rule out potential species differences.
The present data focus on inducible up-regulation of glomerular AP after challenge with LPS in vivo. Because this locally induced ectoenzyme was able to detoxify LPS, it is suggested that this glomerular ectoAP subserves a protective function preventing LPS-related injury to the glomerular microvasculature. Such a locally induced protective response may be considered as a variation of systemic inducible responses upon challenge with Gram-negative bacteria or LPS in the liver or in leucocytes (Chikkappa 1992; Xu et al. 2002). Because other inflammatory models, i.e. anti-Thy-1 nephritis or acute transplant rejection, do not show significant up-regulation of glomerular AP activity (unpublished results), it is likely that this glomerular response is characteristic for LPS. (Glomerular AP activity in humans following E. coli or LPS challenge is unknown; unfortunately we were not able to test AP in autopsy tissue and renal biopsies from these patients are lacking). In other inflammatory conditions in humans, caused by either acute, chronic rejection or systemic lupus erythematosus, preliminary studies do not show significant up-regulation of glomerular AP.
Potential capacity of up-regulation of AP as an endotoxin scavenger in the glomerular tuft may be considered from a teleological viewpoint as highly important for the continuation of blood flow during ultrafiltration in the kidney. The glomerular filtration barrier is characterized by its high permeability to electrolytes and small plasma molecules, whereby its fenestrated endothelium promotes close contact between blood and the glomerular basement membrane. Consequently this structure is highly sensitive to damaging stimuli like inflammatory, pro-thrombotic and toxic agents. As reviewed recently by Kitamura and Fine (Kitamura & Fine 1999), several protective principles, including glomerular ectoATP diphosphohydrolase along the capillary walls, belong to the host defence machinery of the glomerulus to prevent local microthrombus formation and activation of PMNs (Bakker et al. 1994).
We feel that the potential capacity of the glomerular tuft to up-regulate AP after challenge with products of Gram-negative bacteria, may contribute a great deal to the nonspecific limb of the host defence system located in the kidney.
Acknowledgments
We thank Henk Moorlag for preparing endothelial cell cultures and Siep Noorman for performing the microphotography. The technical assistance of Geert Harms is greatly appreciated.
References
- Bailly S, Ferrua B, Fay M, Gougerot-Pocidalo MA. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990;2:205–210. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- Bakker WW, Poelstra K, Barradas MA, Mikhailidis DP. Platelets and ectonucleotidases. Platelets. 1994;5:121–129. doi: 10.3109/09537109409005523. [DOI] [PubMed] [Google Scholar]
- Bakker WW, Poelstra K, Hardonk MJ. Relevance of adenine nucleotidases in the glomerular filtration barrier. Nephron. 1993;64:338–342. doi: 10.1159/000187351. [DOI] [PubMed] [Google Scholar]
- Chikkappa G. Control of neutrophil alkaline phosphatase synthesis by cytokines in health and diseases. Exp. Hematol. 1992;20:388–390. [PubMed] [Google Scholar]
- Courdouhji MK, Guelfi JF, Fontanilles AM, Grozdea JD, Vergnes HA. Characterization of alkaline phosphatase in polymorphonuclear neutrophils from normal sheep. Enzyme. 1988;40:217–222. doi: 10.1159/000469166. [DOI] [PubMed] [Google Scholar]
- Gustafson GL, Rhodes MJ. A rationale for the prophylactic use of monophosphoryl lipid A in sepsis and septic shock. Biochem. Biophys. Res. Commun. 1992;182:269–275. doi: 10.1016/s0006-291x(05)80140-8. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MM. Serum alkaline phosphatase – another piece is added to the puzzle. Hepatology. 1986;6:526–528. doi: 10.1002/hep.1840060334. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Fine LG. The concept of glomerular self-defense. Kidney Int. 1999;55:1639–1671. doi: 10.1046/j.1523-1755.1999.00425.x. [DOI] [PubMed] [Google Scholar]
- Marklund SL. Extracellular superoxide dismutase in human tissues and human cell lines. J. Clin. Invest. 1984;74:1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb RB, Bowers GN, Posen S. Distribution in nature. In: McComb RB, Bowers GN, Posen S, editors. Alkaline Phosphatase. New York: Plenum Press; 1979. pp. 27–152. [Google Scholar]
- Miki A, Tanaka Y, Ogata S, Ikehara Y. Selective preparation and characterization of membranous and soluble forms of alkaline phosphatase from rat tissues. A comparison with the serum enzyme. Eur J. Biochem. 1986;160:41–48. doi: 10.1111/j.1432-1033.1986.tb09937.x. [DOI] [PubMed] [Google Scholar]
- Nakazato H, Deguchi M, Fujimoto M, Fukushima H. Alkaline phosphatase expression in cultured endothelial cells of aorta and brain microvessels: induction by interleukin-6-type cytokines and suppression by transforming growth factor betas. Life Sci. 1997;61:2065–2072. doi: 10.1016/s0024-3205(97)00865-5. [DOI] [PubMed] [Google Scholar]
- Poelstra K, Bakker WW, Klok PA, Hardonk MJ, Meijer DK. A physiologic function for alkaline phosphatase: endotoxin detoxification. Laboratory Invest. 1997a;76:319–327. [PubMed] [Google Scholar]
- Poelstra K, Bakker WW, Klok PA, Kamps JA, Hardonk MJ, Meijer DK. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am. J. Pathol. 1997b;151:1163–1169. [PMC free article] [PubMed] [Google Scholar]
- Poelstra K, Baller JF, Hardonk MJ, Bakker WW. Demonstration of antithrombotic activity of glomerular adenosine diphosphatase. Blood. 1991;78:141–148. [PubMed] [Google Scholar]
- Pugin J, Schurer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- Shankland SJ, Ly H, Thai K, Scholey JW. Glomerular expression of tissue inhibitor of metalloproteinase (TIMP-1) in normal and diabetic rats. J. Am. Soc. Nephrol. 1996;7:97–104. doi: 10.1681/ASN.V7197. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Tomizawa S, Arai H, Seki Y, Maruyama K, Kuroume T. Purification of two types of TNF inhibitors in the urine of the patient with chronic glomerulonephritis. Nephron. 1994;66:386–390. doi: 10.1159/000187851. [DOI] [PubMed] [Google Scholar]
- Tillyer CR, Rakhorst S, Colley CM. Multicomponent analysis for alkaline phosphatase isoenzyme determination by multiple linear regression. Clin. Chem. 1994;40:803–810. [PubMed] [Google Scholar]
- Van Goor H, Gerrits PO, Hardonk MJ. Enzyme histochemical demonstration of alkaline phosphatase activity in plastic-embedded tissues using a Gomori-based cerium-DAB technique. J. Histochem. Cytochem. 1989;37:399–403. doi: 10.1177/37.3.2465338. [DOI] [PubMed] [Google Scholar]
- Warnke R, Levy R. Detection of T and B cell antigens with hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J. Histochem. Cytochem. 1980;28:771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]
- Xu Q, Lu Z, Zhang X. A novel role of alkaline phosphatase in protection from immunological liver injury in mice. Liver. 2002;22:8–14. doi: 10.1034/j.1600-0676.2002.220102.x. [DOI] [PubMed] [Google Scholar]
- Yang T, Sun D, Huang YG, Smart A, Briggs JP, Schnermann JB. Differential regulation of COX-2 expression in the kidney by lipopolysaccharide: role of CD14. Am. J. Physiol. 1999;277:F10–F16. doi: 10.1152/ajprenal.1999.277.1.F10. [DOI] [PubMed] [Google Scholar]