Abstract
Small intestinal mucosal T cell activation results in villous atrophy and crypt hyperplasia. There is conflicting evidence as to whether a Th1 IFN-γ response may be involved. Using a murine intestinal transplant model of T cell-mediated enteropathy we aimed to study the role of IFN-γ on the development of villous atrophy and crypt hyperplasia. Isografts or allografts of foetal small intestine from 129SV−/− IFN-γ receptor knockout mice or wild type mice were implanted under the kidney capsule of Balb/c recipient mice. Grafts were examined histologically at intervals from 2 to 9 days post implantation for signs of rejection. Quantitative rtPCR for IFN-γ, TNFα and IL-4 was conducted on grafts at 5 and 9 days post implantation. In allografts, rejection accompanied by the development of villous atrophy and crypt hyperplasia, occurred in a time-dependent manner. However this process was markedly slower in the IFN-γ receptor knockout grafts compared to the wild type grafts at 5 days (χ2 = 10.08, P = 0.007) and 9 days post implantation (χ2 = 13.25, P = 0.004). There were also significantly fewer TNFα transcripts in allografts of IFN-γ−/− intestine than in wild type allografts (P = 0.02). IFN-γ has a partial, but not obligatory, role in the development of villous atrophy and crypt hyperplasia during T cell mediated rejection of intestinal allografts.
Keywords: interferon gamma, tumour necrosis factor alpha, allograft, intestine, murine
Ex vivo and animal studies support the hypothesis that activated lamina propria T cells are pivotally involved in the pathogenesis of villous atrophy and crypt hyperplasia (enteropathy) (Guy-Grand & Vassalli 1986; Lionetti et al. 1993; Wakelin et al. 1994; Maric et al. 1996). T cell activation has been demonstrated in the small intestinal mucosa of untreated coeliac disease (Halstensen et al. 1993), and this is associated with a Th1 IFN-γ response (Kontakou et al. 1994). Studies in a human foetal intestinal explant model have demonstrated that direct stimulation of lamina propria T cells can result in either villous atrophy/crypt hyperplasia or complete mucosal destruction that is prevented by inhibition of T cell activation (MacDonald & Spencer 1988; Ferreira et al. 1990; Lionetti et al. 1993). Although Th1 responses in this model are important in the genesis of the lesions, the final effector molecules of mucosal change are growth factors and matrix metalloproteinases released by cytokine activated gut stromal cells (Bajaj-Elliot et al. 1997, 1998; Pender et al. 1996, 1997).
The role of interferon-γ in gut diseases is still not clear. Although numbers of CD4 cells secreting this cytokine are invariably elevated in animal models of inflammatory bowel disease (Strober et al. 1998; Fish et al. 1999), some studies have suggested that gut damage is actually worse in the absence of interferon-γ (IFN-γ) (Dohi et al. 1999).
Transplantation of genetically incompatible tissues into immunocompetent recipients invariably leads to tissue rejection, a process known to be T cell mediated. Allografts of murine foetal gut implanted under the kidney capsule of recipient mice are rejected in a time-dependent fashion in a predictable way that varies according to which combinations of strains of mice are used (Ferguson & Parrott 1972; 1973; Elves & Ferguson 1975). During the rejection process the morphology of the grafts changes from essentially normal with tall villi, and short crypts, to partial villous atrophy and crypt hyperplasia, and eventual tissue destruction. Since the role of IFN-γ in this model is not known, and since generally the role of IFN-γ in enteropathy has not been resolved, we have used allografts of foetal intestine from IFN-γ receptor knockout mice to directly assess whether IFN-γ has a direct effect on transplanted tissue during the rejection process.
Materials and methods
Animals
Six to eight weeks old mice, weighing at least 20 g were used. Timed-mated pregnant female 129SV+/+ mice were purchased from Harlan, UK. A stock of 129SV−/− IFN-γ receptor knockout mice was maintained in the Biological Services Unit at St. Bartholomew's and the Royal London School of Medicine and Dentistry.
Grafts
Allogenically mismatched donors and recipients were used to study the rejection process. Donor 129SV mice are of the H-2b haplotype and Balb/c mice are H-2d. 129SV+/+ wild type grafts and 129SV−/− ‘IFN-γ receptor knockout’ foetal grafts were implanted into adult allogenic Balb/c recipients (39 mice 129SV+/+ Balb/c, 18 mice 129SV−/−Balb/c). 129SV−/− isografts were also implanted (13 mice). The time course of rejection with these combinations of strains of mice was determined by harvesting grafts at intervals following implantation (Ferguson & Parrott 1973).
Foetal gut was obtained and implanted under the kidney capsule of recipient mice as previously described (Ferguson & Parrott 1972). One graft was implanted under the capsule of each kidney. Grafts were retrieved from recipient mice from 2 to 9 days after implantation. From each mouse, one graft was stored for histological examination, and one for rtPCR cytokine analysis. Samples were stored at − 70 °C.
Snap-frozen grafts were cryostat-cut into 5 µm sections, mounted on poly L-lysine coated glass slides and stained with haematoxylin and eosin. Histological changes within the grafts were graded according to the classification of Ferguson and Parrott in which the degree of mucosal injury varied from none (‘normal’) to complete destruction (‘submucosa’) (Ferguson & Parrott 1973). Intermediate grades ‘L+, L++, and flat’ indicated lamina propria lymphocytic infiltration, reduction in villous height, and subtotal villous atrophy with crypt hyperplasia, respectively.
Graft cytokine expression
Quantitative rtPCR was performed as previously described (Eckmann et al. 1996). In brief, RNA was extracted from biopsies using a monophasic solution of phenol and guanidine isothiocyanate and chloroform, followed by isopropyl precipitation. Serial dilutions of standard RNA were co-reverse transcribed with target RNA. Standard RNA was derived from plasmids encoding primer sequences for all of the cytokines studied. A mixture of four plasmids, pMCQ1, pMCQ2, pMCQ3, and pMCQ4 was used that contained primer sites for the cytokines under investigation. Plasmids were kindly provided by MF Kagnoff, Department of Medicine, University of California, San Diego. Using the same primer set, rtPCR of the standard RNA produces a PCR product of a different size to that of the target tissue, thus allowing quantification of specific cytokine mRNA transcripts. PCR products were electrophoresed on agarose gels and bands were visualized by ethidium bromide staining. Band intensities were quantified by densitometry, and RNA quantified from the ratios of band intensities from standard RNA and target RNA.
Statistics
Chi-squared analysis was used for comparison of the distribution of histological grades of rejection between groups. Cytokine mRNA data were compared using Cuzick's test for trend.
Results
One hundred and thirty-eight grafts were implanted, of which 126 (91%) were subsequently identified and retrieved for analysis. Fifty-four out of 65 (83%) samples intended for histological analysis were adequate for this purpose (19 129SV+/+ →Balb/c, 21 129SV−/−→Balb/c, and 14 129SV−/− isografts). The ones which were not adequate were those in which there was no detectable graft tissue, presumably because of problems during implantation. The combinations of strains used, the number of grafts analysed, and time points of analysis are summarized in Table 1
Table 1.
Samples suitable for histological analysis
| Mouse strain | Number of grafts | |||||
|---|---|---|---|---|---|---|
| Graft | Recipient | Day 2 | Day 4 | Day 5 | Day 6 | Day 9 |
| 129SV+/+ | Balb/c | 3 | 3 | 4 | 3 | 6 |
| 129SV−/− | Balb/c | 10 | 11 | |||
| 129SV−/− | 129SV−/− | 6 | 8 | |||
A 9-day study period was chosen, based on previous data in which complete loss of mucosa occurred in H-2 genetically mismatched grafts by that time (Ferguson & Parrott 1973). The time course of the rejection process was plotted in the experiments with 129SV+/+ allografts implanted into Balb/c recipients, and 5 days was chosen as an intermediate time point for subsequent studies.
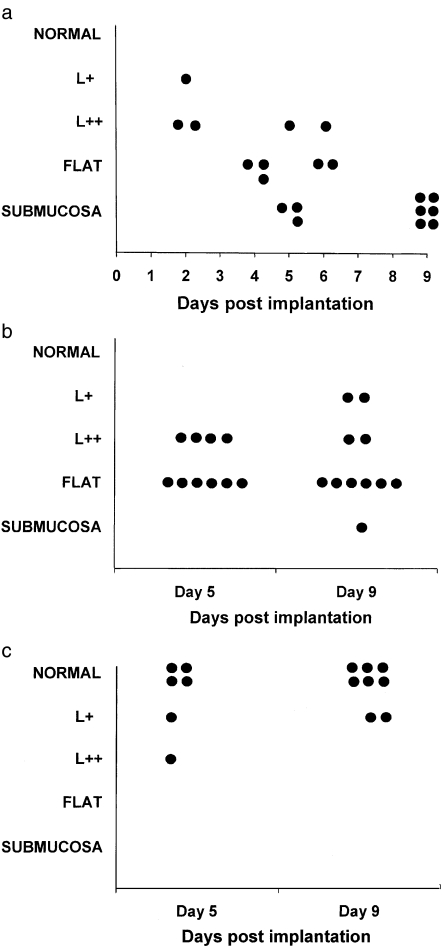
In the 129SV+/+→Balb/c experiments, complete rejection occurred by day 9 with complete loss of mucosa, and intermediate stages of rejection at earlier time points (Figs 1 and 2). In the IFN-γ receptor knockout experiments (129SV−/−→Balb/c) rejection also occurred, but to a lesser degree, by 9 days, with the majority of grafts displaying subtotal villous atrophy. Isograft control 129SV−/−→129SV−/− were nearly all normal, although at each time point there was some injury, probably due to the grafting procedure. Comparison of the three experimental groups revealed significant differences in the degree of mucosal injury at 5 days (χ2 = 30.38, P = 0.0002) and 9 days (χ2 = 39.08, P < 0.0001) post implantation. Comparison of the two allograft groups revealed a reduction in the degree of mucosal injury with the IFN-γ receptor knockout grafts compared to the ‘wild type’ grafts at 5 days (χ2 = 10.08, P = 0.007) and 9 days post implantation (χ2 = 13.25, P = 0.004)
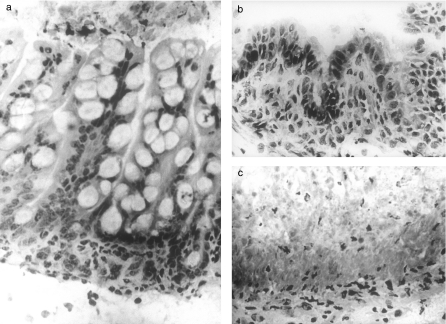
Figure 1.
(a) ‘Normal’ grade (Ferguson & Parrott 1973) foetal intestinal mucosa. Mucosal architecture is preserved. 129SV−/− graft→129SV−/− host. 9 days post implantation. Original magnification ×400. (b) ‘Flat’ grade (Ferguson & Parrott 1973) enteropathy with subtotal villous atrophy. There is loss of villi and lengthening of crypts. 129SV−/− graft→Balb/c host. 9 days post implantation. Original magnification ×400. (c) ‘Submucosa’ grade (Ferguson & Parrott 1973) enteropathy with complete mucosal destruction. Necrotic tissue is seen overlying the muscularis mucosa. 129SV+/+ graft→Balb/c host. 5 days post implantation. Original magnification ×400.
Figure 2.
(a) 129(S)V+/+ grafts implanted into Balb/c mice: mucosal rejection process at intervals to 9 days post implantation. Mucosal injury varied from none (‘normal’) to complete destruction (‘submucosa’) (Ferguson & Parrott 1973). Intermediate grades ‘L +, L ++, and flat’ indicated lamina propria lymphocytic infiltration, reduction in villous height, and subtotal villous atrophy with crypt hyperplasia, respectively. (b) 129(S)V−/− grafts implanted into Balb/c mice: mucosal rejection process at 5 and 9 days post implantation. Histological grading as described by Ferguson & Parrott (1973). (c) 129(S)V−/− grafts implanted into 129SV−/− mice: mucosal rejection process at 5 and 9 days post implantation. Histological grading as described by Ferguson & Parrott (1973).
rtPCR analysis was conducted at 9 days post implantation, when the greatest histological differences were noted in the rejection process between the three groups of experiments. Graft tissue was analysed in 5129SV+/+→Balb/c, 7129SV−/−→Balb/c, and 8129SV isografts. In the two allograft groups (129SV+/+→Balb/c and 129SV−/−→Balb/c) there was a marked inflammatory response with the cytokines IFN-γ and TNFα, but no IL-4 response (Table 2). No IFN-γ, TNFα and IL-4 transcripts were detected in the isografts. However there were significantly fewer TNFα transcripts in the allografts from IFN-γ receptor knockout mice than in allografts from wild type mice. IFN-γ transcripts were also reduced, but due to wide variation in the samples did not reach statistical significance.
Table 2.
Quantitative rtPCR analysis of cytokine mRNA expression in graft tissue at 9 days post implantation
| Graft/Recipient | n | IFN-γ | TNFα | IL-4 |
|---|---|---|---|---|
| 129SV+/+ | 5 | 64 000 | 217 960 | 0 |
| →Balb/c | (0–204 559) | (44 207−1.53 × 106) | (0–131 793) | |
| 129SV−/− | 7 | 42012 | 5584 | 0 |
| →Balb/c | (0–0.5 × 106) | (0–335 378) | (0–27 476) | |
| 129SV−/− | 8 | 0 | 0 | 0 |
| →129SV−/− | (0–3752) | (0–2064) | (0–0) | |
| NS | P = 0.02 | NS |
Results expressed as number of cytokine RNA transcripts per µg total sample RNA: median (inter-quartile range). Statistics: Cuzick's test for trend applied to the three study groups. NS = not significant.
Discussion
Histological examination of intestinal grafts from genetically mismatched donor and hosts (129SV+/+→Balb/c) revealed as expected a time-dependent rejection process, with enteropathy progressing to complete mucosal destruction (Ferguson & Parrott 1972). In genetically similar mice (129SV−/−→129SV−/−), grafts were not rejected and appeared normal. Implantation of foetal gut from IFN-γ receptor knockout mice into a genetically dissimilar host (129SV−/−→Balb/c) also resulted in rejection of the grafts, with partial or complete villous atrophy by 5 days post implantation, but no further change by 9 days. Our data have demonstrated that IFN-γ has, at least, a partial role in the enteropathic process in this model. Quantitative rtPCR for cytokine mRNA confirmed an IFN-γ response in the rejection process in both allograft models, which is to be expected since the infiltrating recipient T cells are immunologically normal. Protective effects of the absence of IFN-γ receptor expression are therefore not due to host T cell recognition of the grafts, but to events further downstream. In the graft gut both epithelial cells and immune cells of graft origin were present. Foetal gut is immunologically immature however, and the degree of rejection in response to IFN-γ is likely to have been dependent on the presence or absence of the IFN-γ receptor on graft epithelial cells.
In man and animals, transplantation of intestine into a genetically incompatible host results in graft rejection with mucosal enteropathic changes morphologically similar to those in coeliac disease (Cerf-Bensussan et al. 1990). We have used a graft rejection model of enteropathy in which the resultant enteropathic changes in the graft have been shown to be T cell dependent (MacDonald & Ferguson 1976). Studies in other animal models of enteropathy, and studies of human pathological specimens, are confounded by the presence of the normal intestinal bacterial flora. In this work we have used foetal small intestine, which has the advantage of being sterile. In addition, foetal mouse small intestine is immunologically immature and any changes in the graft are therefore the result of host immune rejection mechanisms against the donor tissue.
IFN-γ is a glycosylated protein produced by CD4+ and CD8+ T cells, and NK cells. It is the major cytokine involved in Th1 T cell responses. IFN-γ inhibits Th2 cytokine production, and IL-4 and IL-10 reciprocally inhibit Th1 responses including IFN-γ production. IFN-γ stimulates T cells and is a powerful macrophage activator Macrophages, thus activated, exert their tumoricidal, microbicidal and tissue damaging effects by the production of cytokines, including IL-1, TNF-α, IL-6 and IL-8, and increased production of reactive oxygen and nitrogen intermediates such as nitric oxide and hydrogen peroxide. Predictably, IFN-γ has no effect on IFN-γ receptor deficient cells, such as in IFN-γ receptor-knockout mice (Huang et al. 1993). In our graft rejection model of enteropathy IFN-γ, produced by the host T cells in response to the graft, would therefore have no effect on the graft tissue. However it clearly will have an effect on host cells which non-specifically migrate into the tissues as a consequence of the grafting process. When small intestinal grafts are implanted into the highly vascular sub-capsular region of the kidney, there is rapid revascularization of the tissue from the host. These vascular and endothelial cells will be normal and capable of responding to IFN-γ by up-regulating adhesion molecules, thus allowing further extravasation of host T cells and macrophages into the graft. The quantitative changes we observed in this study in terms of reduced injury and lower TNFα transcripts suggests that there is less inflammatory infiltrate into the IFN-γ receptor knockout grafts, but this awaits further study.
The mechanism by which IFN-γ contributes to the structural changes of crypt hyperplasia and mucosal remodelling, with reduction in villous height, is unknown. IFN-γ has diverse effects, including secondary generation of other macrophage-derived cytokines, including TNFα. In studies of normal adult mice, injection of a single dose of TNFα resulted in rapid development of enteropathy, which was enhanced by addition of IFN-γ (Garside et al. 1993). In a murine model of graft vs. host disease, inhibition of TNFα ameliorated the enteropathy (Brown et al. 1999). In vitro studies have demonstrated direct TNFα-mediated toxicity to intestinal crypt cells (Garside et al. 1993), and TNFα injection into mice results in apoptosis of villus epithelial cells (Guy-Grand et al. 1998). TNFα is a prime candidate for immune-mediated injury in the gut and the reduced TNFα mRNA expression in the IFN-γ receptor knockout grafts is consistent with this notion. The dramatic clinical effects of TNFα neutralization in inflammatory bowel disease (van Deventer 1999) further emphasizes its crucial role in T-cell mediated gut damage.
The concept of a bystander effect of mucosal T cell activation on gut stromal cells, epithelial cells and mucosal shape, is not new (Elson et al. 1977). Recent studies however, have begun to unravel the biochemical basis for this response. There is now increasing evidence that elevated cytokine concentrations in the mucosa up-regulate the production of epithelial growth factors and matrix remodelling enzymes (Bajaj-Elliot et al. 1998; Pender et al. 1997). In this scenario, it is not the immune cells that damage the gut, but the resident cells themselves. The data contained in this paper are consistent with this notion.
Acknowledgments
Andrew Veitch was supported by a Robin Brook Fellowship from the Barts Foundation for Research, Lisa Higgins by the Crohns in Childhood Research Association, and Mona Bajaj-Elliott by the Wellcome Trust.
References
- Bajaj-Elliot M, Breese E, Poulsom R, Fairclough PD, MacDonald TT. Keratinocyte growth factor in inflammatory bowel disease. Increased mRNA transcripts in ulcerative colitis compared with Crohn's disease in biopsies and isolated mucosal fibroblasts. Am. J. Pathol. 1997;151:1469–1476. [PMC free article] [PubMed] [Google Scholar]
- Bajaj-Elliot M, Poulsom R, Pender SL, Wathen NC, MacDonald TT. Interactions between stromal cell-derived keratinocyte growth factor and epithelial transforming growth factor in immune-mediated crypt cell hyperplasia. J. Clin. Invest. 1998;102:1473–1480. doi: 10.1172/JCI2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Lindberg G, Meddings J, Silva M, Beutler B, Thiele D. Tumour necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116:593–601. doi: 10.1016/s0016-5085(99)70181-2. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N, Brousse N, Jarry A, et al. Role of in vivo activated T cells in the mechanisms of villous atrophy in humans: study of allograft rejection. Digestion. 1990;46(Suppl. 2):297–301. doi: 10.1159/000200400. [DOI] [PubMed] [Google Scholar]
- van Deventer SJ. Review article: targeting TNF alpha as a key cytokine in the inflammatory processes of Crohn's disease – the mechanisms of action of infliximab. Aliment Pharmacol. Ther. 1999;13(Suppl. 4):3–8. doi: 10.1046/j.1365-2036.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- Dohi T, Fujihashi K, Rennert PD, Iwatani K, Kiyono H, McGhee JR. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper 2-type responses. J. Exp Med. 1999;189:1169–1180. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann L, Fierer J, Kagnoff MF. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella dublin. J. Immunol. 1996;156:2894–2900. [PubMed] [Google Scholar]
- Elson CO, Reilly RW, Rosenberg IH. Small intestinal injury in the graft versus host reaction: an innocent bystander phenomenon. Gastroenterology. 1977;72:886–889. [PubMed] [Google Scholar]
- Elves MW, Ferguson A. The humoral immune response to allografts of foetal small intestine in mice. Br. J. Exp Pathol. 1975;56:454–458. [PMC free article] [PubMed] [Google Scholar]
- Ferguson A, Parrot DMV. Growth and development of ‘antigen-free’ grafts of foetal mouse intestine. J. Pathol. 1972;106:95–101. doi: 10.1002/path.1711060205. [DOI] [PubMed] [Google Scholar]
- Ferguson A, Parrott DM. Histopathology and time course of rejection of allografts of mouse small intestine. Transplantation. 1973;15:546–554. doi: 10.1097/00007890-197306000-00005. [DOI] [PubMed] [Google Scholar]
- Ferreira RC, Forsyth LE, Richman PI, Wells C, Spencer J, MacDonald TT. Changes in the rate of crypt epithelial cell proliferation and mucosal morphology induced by a T-cell-mediated response in human small intestine. Gastroenterology. 1990;98:1255–1263. doi: 10.1016/0016-5085(90)90342-x. [DOI] [PubMed] [Google Scholar]
- Fish SM, Proujansky R, Reenstra WW. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–198. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P, Bunce C, Tomlinson RC, Nichols BL, Mowat AM. Analysis of enteropathy induced by tumour necrosis factor alpha. Cytokine. 1993;5:24–30. doi: 10.1016/1043-4666(93)90020-6. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D, Disanto JP, Henchoz P, Malassis-Seris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. Eur J. Immunol. 1998;28:730–744. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D, Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J. Clin. Invest. 1986;77:1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen TS, Scott H, Fausa O, Brandtzaeg P. Gluten stimulation of coeliac mucosa in vitro induces activation (CD25) of lamina propria CD4+ T cells and macrophages but no crypt-cell hyperplasia. Scand. J. Immunol. 1993;38:581–590. doi: 10.1111/j.1365-3083.1993.tb03245.x. [DOI] [PubMed] [Google Scholar]
- Huang S, Hendriks W, Althage A, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Kontakou M, Sturgess RP, Przemioslo RT, Limb GA, Nelufer GM, Ciclitira PJ. Detection of interferon gamma mRNA in the mucosa of patients with coeliac disease by in situ hybridisation. Gut. 1994;35:1037–1041. doi: 10.1136/gut.35.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti P, Breese E, Braegger CP, Murch SH, Taylor J, MacDonald TT. T-cell activation can induce either mucosal destruction or adaptation in cultured human fetal small intestine. Gastroenterology. 1993;105:373–381. doi: 10.1016/0016-5085(93)90710-t. [DOI] [PubMed] [Google Scholar]
- MacDonald TT, Ferguson A. Hypersensitivity reactions in the small intestine. 2. Effects of allograft rejection on mucosal architecture and lymphoid cell infiltrate. Gut. 1976;17:81–91. doi: 10.1136/gut.17.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Spencer J. Eviden\ce that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J. Exp Med. 1988;167:1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric D, Riddell RH, Steele-Norwood D, Borojevic R, Dragomir A, Croitoru K. Characterisation of enteropathy induced by in vivo T cell activation in Balb/c mice: role of TCRa/b and TCRg/d T cells. Gastroenterology. 1996;110:A957. [Google Scholar]
- Pender SL, Lionetti P, Murch SH, Wathan N, MacDonald TT. Proteolytic degradation of intestinal mucosal extracellular matrix after lamina propria T cell activation. Gut. 1996;39:284–290. doi: 10.1136/gut.39.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender SL, Tickle SP, Docherty AJ, Howie D, Wathan NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J. Immunol. 1997;158:1582–1590. [PubMed] [Google Scholar]
- Strober W, Ludviksson BR, Fuss IJ. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn's disease. Ann. Intern. Med. 1998;128:848–856. doi: 10.7326/0003-4819-128-10-199805150-00009. [DOI] [PubMed] [Google Scholar]
- Wakelin D, Goyal PK, Dehlawi MS, Hermanek J. Immune responses to Trichinella spiralis and T. pseudospiralis in mice. Immunology. 1994;81:475–479. [PMC free article] [PubMed] [Google Scholar]