Abstract
Transforming growth factor-β (TGF-β) is a multifunctional regulator of cell growth and differentiation, whose actions are highly cell type specific. To study the role of the TGF-β1 autocrine loop in regulating growth and myogenic differentiation in the human rhabdomyosarcoma cell line, RD, an attempt was made to establish a framework for the expression of several components of TGF-β1/Smad signalling pathway at the mRNA and protein levels by reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analysis in RD cells compared with the normal myoblasts. Higher exogenous concentration of TGF-β1 was necessary to reach a growth-inhibition effect, whereas TGF-β1 downregulated the expression of myosin heavy-chain mRNA at lower concentrations than that was required for growth inhibition. Treatment with TGF-β1 significantly decreased the number of sarcomeric actin and myosin-expressing cells. In this study, we have shown that RD cells displayed higher expression of TβRI, TβRII, Smad2 and Smad4 at both the mRNA and protein levels than myoblasts. Smad3 and Smad7 mRNA were expressed at higher level in RD cells than in myoblasts. The staining patterns of TβR and Smads suggest that they may transduce different TGF-β1 signalling in RD cells than in myoblasts. TGF-β1 signalling induced a rapid relocation of Smad2 to the nucleus; in contrast, Smad4 remained localized to the cytoplasm unless it was coexpressed with Smad2. These studies suggest that signalling from the cell surface to the nucleus through Smad proteins is a required component of TGF-β1-induced cell response in RD cells. The RD cell line is a suitable model to study the TGF-β autocrine loop involved in growth and differentiation of RMS.
Keywords: differentiation, growth, RD, rhabdomyosarcoma, signal pathway, transforming growth factor-β
Rhabdomyosarcoma (RMS) is the most commonly occurring soft tissue sarcoma in children, accounting for 4–8% of all paediatric cancers (Pappo et al. 1995). Most RMS tumour cells are characterized by the expression of several muscle-specific markers, such as the myogenic promoting transcription factor MyoD (Weintraub et al. 1991). Although the expression of such factors typically correlates with myogenic differentiation, RMS cells fail to undergo terminal differentiation into skeletal muscle (Parham 1994). Some autocrine growth factor loops can be expressed by RMS cells and are likely to be involved in the growth and differentiation of these cells in vitro (Schweigerer et al. 1987; El-Badry et al. 1990; Minniti et al. 1994). These cells are a suitable model to study autocrine loops involved in proliferation and/or differentiation of solid tumours and to set up a differentiation therapy approach based on the blockade of autocrine loops (De Giovanni et al. 1996).
Transforming growth factor-β (TGF-β), the prototypic member of a superfamily of structurally related cytokines, exerts a variety of biological effects in different cell types, including cell growth, differentiation, apoptosis and migration (Massague 1990; Roberts & Sporn 1993). TGF-β family members transduce signals from the plasma membrane to the nucleus via heteromeric complex formation of serine/threonine kinases of TGF-β type I receptor (TβRI) and TGF-β type II receptor (TβRII) and their downstream effectors, the Smad family of proteins (Feng & Derynck 1996). When the ligand binds to TβRII, it recruits and phosphorylates TβRI, and the signal is then propagated downstream through the receptor-mediated phosphorylation of the Smads (Heldin et al. 1997).
The Smad family of proteins plays key roles in transducing signals from cell-surface serine/threonine kinase receptors for TGF-β superfamily proteins to nuclear target genes. To date, eight vertebrate Smad proteins have been identified, and these can be grouped into three classes. The first class is the receptor-regulated Smads. Among them, Smad1, Smad5 and Smad8 mediate bone morphogenetic protein (BMP) signalling, whereas Smad2 and Smad3 transduce activin and TGF-β signals. These receptor-regulated Smads transiently interact with, and become phosphorylated by, specific activated type I receptors. Once phosphorylated, they associate with a common partner, Smad4 (also known as DPC4), which is the only known member of the second class of Smad proteins in vertebrates. The Smad complex then translocates to the nucleus and regulates the transcription of target genes through interaction with specific DNA sequences and other DNA-binding proteins. For example, the Smad2–Smad4 complex has been shown to associate with the winged helix transcription factor FAST and thereby interact with activin-responsive elements. Smad6 and Smad7 form the third class of Smad proteins, the inhibitory Smads. They act as inhibitors of BMP, activin and TGF-β signalling by competing with the receptor-regulated Smads for binding to the type I receptors or by competing with Smad4 for binding to the receptor-regulated Smads. Thus, the diverse biological actions of TGF-β stem from its capability to regulate the transcription of specific sets of target genes (Nakao et al. 1997b; Derynck et al. 1998).
TGF-β is a well-known inhibitor of myogenic differentiation, as well as an autocrine product of RMS cells (Massague et al. 1986). It has been shown that the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) induces growth arrest and myogenic differentiation in RMS cells, which constitutively express muscle regulatory factors, and TPA inhibits the activation of secreted latent TGF-β, thus decreasing the concentration of active TGF-β to which the cells are exposed. This event is mediated by TPA-induced alteration of the urokinase plasminogen activator/plasminogen activator inhibitor (uPA/PAI) serine protease system (Bouche et al. 2000). To date, no systematic analysis of the TGF-β signalling pathway of RMS cells has been carried out. Little is known of the intracellular signals that mediate TGF-β action in RMS cells. In the present study, we observed that TGF-β1 downregulates the expression of several myogenic differentiation markers in the RD cell line. We use this model system to investigate the involvement of TGF-β signalling components, including the two TGF-β receptors and some of the Smad proteins.
Materials and methods
Cell culture
RD cells were obtained from the American Type Culture Collection (McAllister et al. 1969). Normal human primary skeletal myoblasts were obtained from a 16-week gestation human embryo. The cells were grown in Dulbecco's modified Eagles' medium (DMEM) containing 10% fetal calf serum (FCS) at 37 °C in 5% CO2. The human hepatocarcinoma cell line SMMC-7721, used as a positive control expressing TGF-β1 and TβR (Zhang et al. 2000), was obtained from the Cell Bank of Chinese Academic of Science and maintained in RPMI-1640 supplemented with 10% FCS.
Determination of cell growth
The effect of TGF-β1 on cell growth was evaluated by the cell count of harvested cultures and viability assay. RD cells and myoblasts were seeded at a density of 5 × 104 cells/well in 6-well plates and 5 × 103 cells/well in 96-well plates, respectively. After 24 h of incubation, cells were washed twice with phosphate-buffered saline (PBS), and medium containing 1% FCS with various concentrations of recombinant human TGF-β1 (Gibco, Life Technologies, Grand Island, NY, USA) was added. Cell numbers and viability were assessed with Trypan blue staining and haemocytometer counting and MTT [3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide] assay for 6 days at appropriate time points. Briefly, 20 µl of 5 mg/ml MTT (Sigma, St Louis, MO, USA) was added to each well (96-well plates). Plates were then incubated, and metabolically active cells were allowed to produce formazan product for 4 h at 37 °C. After replacing the solutions with 150 µl of dimethylsulfoxide (DMSO), cell viability was determined by measuring absorbance at 570 nm in an ELISA reader (Model 550, BIO-RAD Hercules, CA, USA). The change in optical density (OD), which correlates with change in viable cell numbers (Gomez et al. 1997), was plotted against time.
Total RNA isolation and RT-PCR
The mRNA for TGF-β1, TGF-β receptors and Smads expressed by RD cells and myoblasts were evaluated by reverse transcription-polymerase chain reaction (RT-PCR). Cells were harvested and processed for RNA isolation. Initially, total RNA was isolated using Trizol reagent (Gibco-BRL, Grand Island, NY, USA), according to the manufacturer's recommended protocol. Then, RT-PCR was performed using 2 µg of total RNA and the Qiagen RT-PCR kit (Hilden, Germany), according to the manufacturer's instructions. The following primers were used: TGF-β1, TβRI and TβRII (Kim et al. 1996), GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Maier et al. 1990) and Smad2, Smad3, Smad4, Smad7 and myosin heavy chain (MyHC). The following thermal cycler conditions were used: reverse transcription at 50 °C for 30 min and initial PCR activation step at 95 °C for 15 min. After RT, PCR was performed using the following conditions: 94 °C for 1 min and then incubation for 1 min at annealing temperature and 72 °C for 1 min for 35 cycles, followed by a 10 min incubation at 72 °C. PCR product samples were separated electrophoretically on a 1.5% agarose gel stained with ethidium bromide for visualization. The results shown are from one representative experiment of three performed. The sequence-specific primers used for detecting the various transcripts and expected size of the fragment amplified are summarized in Table 1.
Table 1.
Reverse transcription-polymerase chain reaction (RT-PCR) primers.
| Gene | Primer sequence 5′−3′ | Size of PCR fragment (bp) | Annealing temperature (°C) |
|---|---|---|---|
| TGF-β1(S) | CGGAGTTGTGCGGCAGTGGTTGA | 448 | 55 |
| TGF-β1(As) | GCGCCCGGGTTATGCTGGTTGTA | ||
| TβRI(S) | TTGCTGGACCAGTGTGCTTCG | 987 | 55 |
| TβRI(As) | CCATCTGTTTGGGATATTTGGCC | ||
| TβRII(S) | AGCAGAAGCTGAGTTCAACCTGGG | 702 | 55 |
| TβRII(As) | GGAGCCATGTATCTTGCAGTTCCC | ||
| Smad2(S) | GAATTTGCTGCTCTTCTGGCTCAG | 322 | 60 |
| Smad2(As) | GCCATAGGGACCACAACACAATG | ||
| Smad3(S) | GAGGGCAGGCTTGGGGAAAATG | 281 | 62 |
| Smad3(As) | GGGAGGGTGCCGGTGGTGTAATAC | ||
| Smad4(S) | AAAGGTGAAGGTGATGTTTGGGTC | 268 | 58 |
| Smad4(As) | CTGGAGCTATTCCACCTACTGATCC | ||
| Smad7(S) | CATCACCTTAGCCGACTCTG | 227 | 57 |
| Smad7(As) | GTCTTCTCCTCCCAGTATGC | ||
| MyHC(S) | GATATCGCAGAATCTCAAGTC | 183 | 50 |
| MyHC(As) | GAGTCACATGGACATTAAGT | ||
| GAPDH(S) | CCACCCATGGCAAATTCCATGGCA | 597 | 70 |
| GAPDH(As) | TCAAGACGGCAGGTCAGGTCCACC |
As, antisense; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MyHC, myosin heavy chain; S, sense.
Western blot analysis
Cells were seeded in T25 flasks and cultured for 24 h. At 50–70% confluence, cells were harvested and lysed with sample buffer and boiled for 5 min. Equal amounts of total protein were electrophoresed on 8% SDS polyacrylamide gels and electrotransferred onto a polyvinylidine diflouride (PVDF) membrane. The membranes were blocked with 1×Tris-buffered saline (TBS) containing 5% nonfat powdered milk (w/v), 0.02% sodium azide and 0.02% Tween-20 and then incubated with antibodies. TβRI, TβRII, Smad2 and Smad4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-TβRI, anti-TβRII and anti-Smad4 were used at 1 : 300 dilution. Anti-Smad2 was used at a dilution of 1 : 50. Peroxidase-conjugated rabbit anti-goat immunoglobulin G (IgG) or goat anti-rabbit IgG was used at 1 : 2000 dilution as the secondary antibody. Detection was performed using an enhanced chemiluminescence system (Santa Cruz Biotechnology).
Immunofluorescence staining
RD cells were cultivated on coverslips in DMEM containing 10% FCS. After 24 h, the cells were treated with TGF-β1 (1 ng/ml) for 96 h in DMEM containing 1% FCS. Parallel cells were cultured in 1% FCS DMEM. Coverslips were then washed with PBS and fixed with 4% paraformaldehyde for 15 min at 4 °C. After washing with PBS, the cells were blocked with 5% rabbit or goat serum in 1% bovine serum albumin (BSA)–PBS at 37 °C for 30 min. Then, the cells were incubated at 4 °C overnight with the primary antibodies in PBS containing 1% BSA. The antibodies used were sarcomeric actin (Dako, Glostrup, Denmark), MyHC (Zymed, San Francisco, CA, USA), MyoD1 (Dako) and Smad2 and Smad4 (Santa Cruz Biotechnology). Cells were washed with PBS again, and a 1 : 50 dilution of fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG (for mouse anti-sarcomeric actin, MyHC and MyoD1), FITC-conjugated rabbit anti-goat IgG (for goat anti-Smad2) and rhodamine-conjugated goat anti-rabbit IgG (for rabbit anti-Smad4) were applied as the secondary antibody for 30 min at room temperature. The slides were then washed, air dried, coverslipped and photographed with laser scanning confocal microscope (LSCM). At least 300 cells/slide in random fields were counted at ×20 for determining the percentage of sarcomeric actin-, MyHC- and MyoD1-positive cells. Each experiment was repeated at least three times.
Statistical analysis
Analyses were carried out using spss 10.0 (SPSS Inc, Chicago, IL, USA). Differences between the control samples and TGF-β1-treated samples or among different cell lines were compared using either the Mann–Whitney U-test, if the data were not normally distributed, or the Student's t-test, if the observations were consistent with a sample from a normally distributed population. P-Values reported are two sided; P < 0.05 was considered statistically significant.
Results
Inhibitory effect of TGF-β1 on the growth of RD cells in a dose-dependent manner
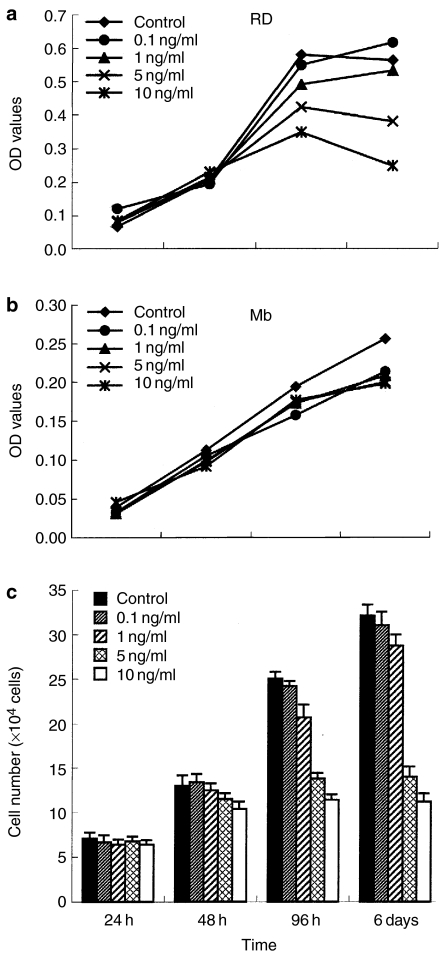
To investigate the TGF-β1 effect on cell growth, RD cell line and myoblasts were treated with various concentrations of TGF-β1 (0.1, 1, 5 and 10 ng/ml) in the presence of 1% FCS, and cell viability or numbers were analysed at 24 h, 48 h, 96 h and 6 days. The results obtained by MTT reduction assay showed significant differences in the viability of RD cells with increasing amounts of TGF-β1 (Figure 1a) but not in myoblasts (Figure 1b). As shown in Figure 1a, compared with control cells, treatment with TGF-β1 induced a decrease in OD values in a dose-dependent manner; in particular, TGF-β1 concentrations ranging from 0.1 to 1 ng/ml did not exert a significant inhibition, whereas a higher concentration of TGF-β1 (5 and 10 ng/ml) led to 28–56% reduction of cell viability in RD cells. The inhibitory effect of TGF-β1 on the growth of RD cells was additionally supported by cell counting (Figure 1c); significant differences in cell numbers were observed between untreated and TGF-β1-treated cells in the presence of high concentrations after 96 h and day 6 of in vitro culture (P < 0.01). Although the growth of RD cells was suppressed by TGF-β1 treatment, few cell death was observed in cultures, as assayed by the uptake of Trypan blue, and there was no significant difference in the percentage of dead cells between control and treated groups throughout the time course of the study. TGF-β1 induced G0/G1 accumulation in RD cells by flow cytometric analysis (data not shown). These results indicate that the inhibitory effect of TGF-β1 was due to cell-cycle arrest rather than cell death.
Figure 1.
The effect of transforming growth factor-β1 (TGF-β1) on growth of cells in vitro. (a) Dose–response curve for TGF-β1 treatment of RD cell growth. Cells were seeded and treated with TGF-β1 (0.1, 1, 5 and 10 ng/ml). Cell viability was quantified by MTT [3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide] assay as described in Materials and methods. Each experiment was performed three times, and representative results are shown. (b) Normal myoblasts (Mb) were treated with TGF-β1. (c) RD cells were treated with varying concentrations of TGF-β1 in the presence of 1% fetal calf serum (FCS). Cell numbers were counted at 24 h, 48 h, 96 h and 6 days using a haemocytometer. Data represent means of triplicate assay; bars, standard deviation.
Suppression of myogenic differentiation by TGF-β1 treatment in RD cells
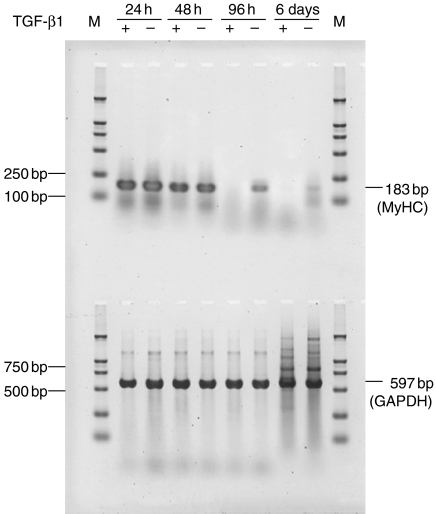
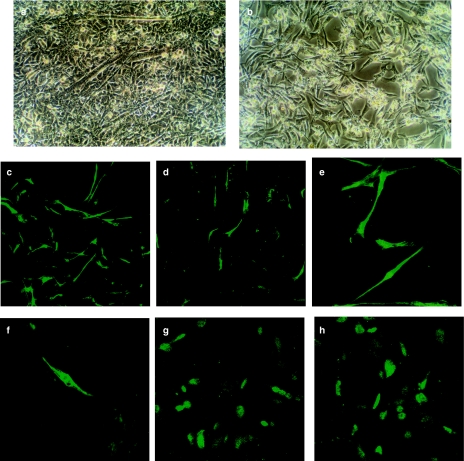
Myogenic differentiation is characterized by a marked increase in the expression of genes encoding muscle-specific proteins, including sarcomeric actin and 3% MyHC (Malone 1993). Myogenic differentiation was analysed as the mRNA level of MyHC by RT-PCR. The result showed that exogenous TGF-β1 downregulated the expression of MyHC mRNA at lower concentrations (1 ng/ml), which is not compatible with the growth-inhibition effect (Figure 2). In a parallel experiment, immunofluorescent staining was used to detect the expression of sarcomeric actin and MyHC. RD cells showed 5% sarcomeric actin- and MyHC-positive cells in TGF-β1-treated cultures, whereas the low concentration of FCS-containing medium allowed the occurrence of about 21% sarcomeric actin- and 10% MyHC-positive cells. As shown in Figure 3, treatment with TGF-β1 significantly decreased the number of sarcomeric actin- and MyHC-expressing cells when compared with the control cells (P < 0.05), suggesting that TGF-β1 signal transduction plays an important role in myogenic differentiation of RMS cells. TGF-β1 treatment did not change the expression of MyoD1, indicating that TGF-β1 may inhibit myogenic differentiation independent of its effects on MyoD1 (Figure 3).
Figure 2.
Reverse transcription-polymerase chain reaction (RT-PCR) for the expression of myosin heavy-chain mRNA in transforming growth factor-β1 (TGF-β1)-treated RD cells (upper panel). Equal amounts of total RNA, prepared from RD cells cultured for different periods of time, both in the absence and presence of TGF-β1 (1 ng/ml), were loaded in each lane. Amplification products were from simultaneous sets of RT-PCR reactions, and 8 µl of PCR products was run on the same 1.5% agarose gels. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA expression was used as an internal control standard (lower panel). M, molecular weight marker (DL 2,000, TaKaRa, Tokyo, Japan).
Figure 3.
Inhibition of myogenic differentiation by transforming growth factor-β1 (TGF-β1). Morphology of RD cells in Dulbecco's modified Eagles' medium (DMEM) containing 1% fetal calf serum (FCS) with (b) or without (control, a) TGF-β1 for 96 h. Note the formation of myotubes in the untreated controls, the inhibition of this process by TGF-β1 (magnification, ×200). Immunofluorescence analysis of untreated RD cells (c, e and g) or RD cells treated with TGF-β1 (d, f and h). After 6 days, cells were stained with anti-sarcomeric actin (c and d, ×100), anti-myosin heavy chain (e and f, ×400) and anti-MyoD1 (g and h, ×400). Positive cells were detected and quantified as described in Materials and methods.
Expression of TGF-β1, TβRI, TβRII and Smads in RD cells
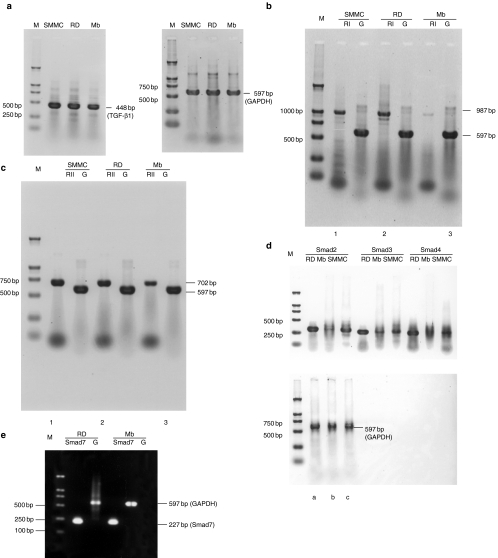
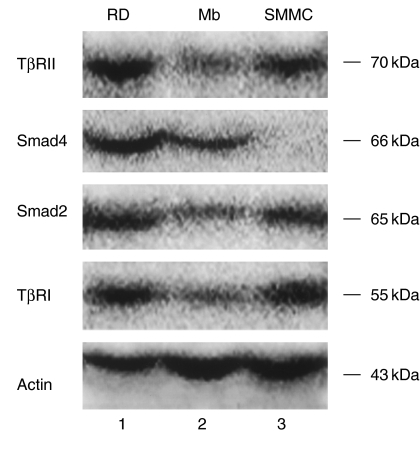
To further investigate the differences of TGF-β1 signalling pathway between RMS cells and normal myoblasts, we analysed the expression of TGF-β1, TβRI, TβRII and several components of Smads at the mRNA and protein levels. Meanwhile, RD cells were studied to establish the profile of expression of TGF-β1 signalling pathway. These were accomplished using RT-PCR with sequence-specific primers and Western blot and immunofluorescence with specific antibodies. In this study, we have shown that all of these signalling components are expressed in RD cells. RD cells displayed higher expression of TβRI, TβRII, Smad2 and Smad4 at both the mRNA and protein levels than normal myoblasts. mRNAs for TGF-β1, Smad3 and Smad7 were all detected in RD cells. Smad3 and Smad7 mRNA, however, were expressed at higher level in RD cells than in myoblasts (Figures 4 and 5). The staining patterns of TβRs and Smads suggest that they may transduce TGF-β1 signalling in RD cells. Although the expression of TGF-β1 mRNA was not higher in the RD cell line than in myoblasts, both type I and type II receptor were expressed at higher levels, indicating that RD cells may exhibit a higher susceptibility to TGF-β1 signalling.
Figure 4.
Expression of transforming growth factor-β1 (TGF-β1), TβRI, TβRII, Smad2, Smad3, Smad4 and Smad7 in RD cell line and myoblasts. Reverse transcription-polymerase chain reaction (RT-PCR) for the expression of TGF-β1 mRNA (a), TβRI mRNA (b) and TβRII mRNA (c) in three cell lines, and equal total RNA was obtained from SMMC-7721 (lane 1, the positive control cell line), RD (lane 2) or myoblasts (lane 3). Top, target genes; bottom, GAPDH gene (as an internal control standard). Expression of Smad2 (lane 1), Smad3 (lane 2), Smad4 (lane 3) mRNA (d) in RD (a), myoblasts (b) or SMMC-7721 (c). Top, target genes; bottom, GAPDH gene. Expression of Smad7 mRNA (e) in RD (lane 1) or myoblasts (lane 2). Top, GAPDH gene; bottom, target genes. The PCR primers used and the expected sizes of the PCR products are indicated in Table 1. M, molecular weight marker (DL 2,000, TaKaRa). RI, TβRI; RII, TβRII; G, GAPDH; Mb, myoblasts.
Figure 5.
Western blot analysis for the expression of TβRI, TβRII, Smad2 and Smad4 in RD (lane 1), myoblasts (lane 2) and SMMC-7721 (lane 3). The same lysates were also used to evaluate the expression of actin as loading control.
TGF-β1 induces nuclear translocation of Smad2 and Smad4 in cultured RD cells
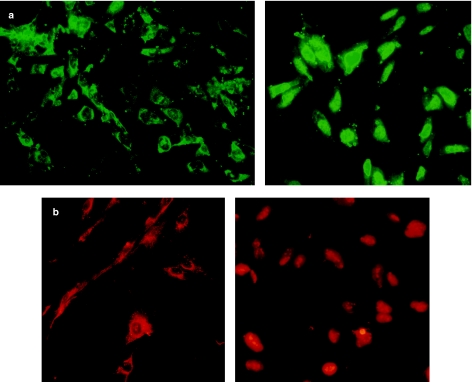
It is likely that, as in other cellular systems, TGF-β receptors are activated by TGF-β and are able to phosphorylate Smad2 or Smad3, allowing them to bind to Smad4 and migrate to the nucleus. In contrast, Smad1 is not TGF-β sensitive. To determine the cellular localization of Smad2 and Smad4 proteins in RD cells, immunofluorescent staining of Smads was performed on cells. In most cells, staining of Smad2 and Smad4 was greater in the cytoplasm, with the greatest staining in the perinuclear area. To determine whether Smad proteins respond to TGF-β1 signalling, cultured RD cells were treated with exogenous TGF-β1. TGF-β1 signalling induced a rapid relocation of Smad2 to the nucleus. In contrast, Smad4 remained localized to the cytoplasm unless it was coexpressed with Smad2. Ninety-six hours after TGF-β1 treatment, TGF-β1 inhibited RD cell differentiation, and at this time, Smad2 and Smad4 all showed nuclear localization in a subset of RD cells (Figure 6).
Figure 6.
Transforming growth factor-β1 (TGF-β1) signalling induces the translocation of Smad proteins to the nucleus in RD cells. RD cells were treated with (right panels) or without (left panels) TGF-β1 for 96 h and then fixed with 4% paraformaldehyde and stained with anti-Smad2 using the fluorescein isothiocyanate (FITC) filter (green) (a) or anti-Smad4 using the rhodamine filter (red) (b). Photographs were taken on laser scanning confocal microscope. (magnification, ×400).
Discussion
Human RMS has a complex machinery of growth factor production and receptor expression that makes possible both autocrine effects and susceptibility to exogenous growth factors provided by the microenvironment (De Giovanni et al. 1996). These data represent the demonstration that a single-autocrine loop, the TGF-β1 loop, is responsible for the growth and suppression of differentiation in RMS cells. In fact, it has already been shown that the simultaneous blockade of all the autocrine loops by surmin in these cells results in growth arrest and muscle differentiation; the specific inhibition of certain single-autocrine loops, such as epidermal growth factor (EGF), insulin-like growth factor (IGF) and basic fibroblast growth factor (bFGF), impairs cell proliferation but does not lead to muscle differentiation (De Giovanni et al. 1995).
Although TGF-β1 has been reported to negatively regulate muscle cell and RMS cell growth and myogenic differentiation (Massague et al. 1986; Brennan et al. 1991; Bouche et al. 2000), little is known about the molecular mechanisms by which this occurs. It has been shown that treatment of RD cells with the phorbol ester TPA induces growth arrest and myogenic differentiation in these cells, which constitutively express muscle regulatory factors. TPA inhibits the activation of secreted latent TGF-β1, thus decreasing the concentration of active TGF-β1 to which the cells are exposed. This event is mediated by the TPA-induced alteration of the uPA/PAI serine protease system (Bouche et al. 2000). In this study, we have shown that RD cells displayed the expression of TGF-β1 at both the protein and mRNA levels. The cells also significantly expressed cell-surface receptors that could bind to TGF-β1. Furthermore, exogenous TGF-β1 showed a growth-inhibitory effect in a dose-dependent manner; TGF-β1 concentrations ranging from 0.1 to 1 ng/ml exerted a very low level of inhibition, whereas a higher concentration of TGF-β1 (5 and 10 ng/ml) was necessary to reach a growth-inhibition effect. The neutralizing antibody abolished the negative growth effects of endogenous TGF-β1, resulting in cell growth. These results indicate that TGF-β1 acts as an autocrine growth and differentiation regulator in RD cells.
The growth-inhibitory effect of the TGF-β superfamily in a variety of cell types appears to be mediated by the arrest of the cell cycle in the G1 phase. In addition to causing cell-cycle arrest, TGF-β can induce apoptosis in certain cell types (Massague et al. 2000). We sought to determine whether the reduction in cell numbers was a consequence of a similar increase in the rate of death in RD cells. The Trypan blue studies demonstrated, however, that the vast majority of RD cells remained viable over the duration of the experiment. Analyses for up to 6 days after TGF-β1 application also revealed no classical morphological features of apoptosis, including chromosomal condensation and nuclear fragmentation. Furthermore, we found no differences in RD numbers below starting levels following the highest doses of TGF-β1 that we studied. In contrast, TGF-β1 led to a significant cell-cycle arrest in RD cells (data not shown). These findings suggest that for higher exogenous dose of TGF-β1 treatment, RD cells do not undergo high levels of apoptotic or necrotic death but enter a period of prolonged cell-cycle arrest.
The RD cell line retains the rhabdomyoblastic phenotype and undergoes very limited myogenic differentiation, even though the cells express the muscle regulatory factor MyoD1 (Tapscott et al. 1993). In this experiment, some of the RD cells express muscle-specific proteins, including sarcomeric actin and MyHC, in low-serum differentiation medium. Treatment with TGF-β1 significantly decreased the expression of MyHC mRNA and the number of sarcomeric actin or MyHC-expressing cells when compared with the control cells. It is worth noting that the active TGF-β1 concentration in RD cells (1 ng/ml) is compatible with the anti-differentiative effect but not with the growth-inhibition effect. Although differentiation of human primary myoblasts is inhibited by TGF-β1, a much higher concentration of the factor is needed to exert its effect in myoblasts than in RD cells; this suggests that myoblasts are less sensitive to TGF-β1 signalling. TGF-β1 is an inhibitor of myogenic differentiation as well as an autocrine product of RMS cells. The intracellular molecular mechanisms that mediate TGF-β-induced inhibition of differentiation, however, remain poorly defined.
Smads comprise a novel family of signalling mediators downstream of TGF-β1 receptors. They transmit signals directly from the cell surface to the nucleus (Attisano & Wrana 1998; Massague & Chen 2000). Although the biochemical characterization of these Smads has brought remarkable achievements in the elucidation of the intracellular signalling pathway of TGF-β (Watanabe et al. 2000; Xu et al. 2000), little is known about their expression and functions in RMS cells. In the present study, we examined the expression of Smads in RD cells compared with human primary skeletal myoblasts.
The staining patterns of Smads suggest that they may transduce TGF-β signalling in RD cells. Smad2, Smad3, Smad4 and Smad7 mRNA and Smad2 and Smad4 proteins were expressed at higher levels in RD cells than in myoblasts. Smad2 and Smad4 exhibited nuclear translocation in response to exogenous TGF-β1 treatment. Because Smad7 has an antagonistic effect on TGF-β signalling (Hayashi et al. 1997; Nakao et al. 1997a), the induction of Smad7 mRNA by TGF-β suggests that Smad7 may participate in a negative feedback loop to control TGF-β responses in RMS cells. Although Smad7 mRNA was also elevated in RD cells compared with myoblasts, R-Smads such as Smad2 and Smad3, especially Smad2, were expressed at higher level than Smad7. Thus, upregulation of Smad7 would not further interfere with the TGF-β-induced suppression of differentiation in RD cells. Furthermore, these studies suggest that signalling from the cell surface to the nucleus through Smad proteins is a required component of TGF-β1-induced cell response in RD cells. Considering that TGF-β1 plays an important role in the suppression of differentiation in RMS cells, it is possible that TGF-β1 signalling in RMS cells is important to keep these cells committed to undifferentiation or poor differentiation.
In summary, our current study shows that TGF-β1 receptors and Smad proteins are expressed in RD cells at high levels and are functional in a TGF-β1 signalling pathway, leading to the activation of gene expression. The changes of TβRs and Smads, which are different from myoblasts, may contribute to TGF-β-induced inhibition of differentiation and progression to malignancy. Furthermore, these studies provide a framework for understanding interactions between the Smad proteins signalling pathway and the pathways leading to cellular differentiation and for understanding the complex roles of TGF-β1 in modulating RD cell function. The RD cell line is a suitable model to study TGF-β autocrine loop involved in myogenic differentiation of RMS.
Acknowledgments
This work was supported by the China Medical Board of New York (CMB 00-722), National Natural Science Foundation of China (39670293).
References
- Attisano L, Wrana JL. Mads and Smads in TGF-β signalling. Curr. Opin. Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- Bouche M, Canipari R, Melchionna R, Willems D, Senni M, Molinaro M. TGF-β autocrine loop regulates cell growth and myogenic differentiation in human rhabdomyosarcoma cells. FASEB J. 2000;14:1147–1158. doi: 10.1096/fasebj.14.9.1147. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Edmondson DG, Li L, Olson EN. Transforming growth factor-β represses the actions of myogenin through a mechanism independent of DNA binding. Proc. Natl. Acad. Sci. USA. 1991;88:3822–3826. doi: 10.1073/pnas.88.9.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giovanni C, Landuzzi L, Frabetti F, et al. Antisense epidermal growth factor receptor transfection impairs the proliferative ability of human rhabdomyosarcoma cells. Cancer Res. 1996;56:3898–3901. [PubMed] [Google Scholar]
- De Giovanni C, Melani C, Nanni P, et al. Redundancy of autocrine loops in human rhabdomyosarcoma cells: induction of differentiation by suramin. Br. J. Cancer. 1995;72:1224–1229. doi: 10.1038/bjc.1995.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-β responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- El-Badry OM, Minniti C, Kohn EC, Houghton PJ, Daughaday WH, Heilman LJ. Insulin-like growth factor II acts as an autocrine growth and motility factor in human rhabdomyosarcoma tumors. Cell Growth Differ. 1990;1:325–331. [PubMed] [Google Scholar]
- Feng XH, Derynck R. Ligand-independent activation of transforming growth factor-β signaling pathways by heteromeric cytoplasmic domains of TGF-β receptors. J. Biol. Chem. 1996;271:13123–13129. doi: 10.1074/jbc.271.22.13123. [DOI] [PubMed] [Google Scholar]
- Gomez LA, Alekseev AE, Aleksandrova LA, Brady PA, Terzic A. Use of the MTT assay in adult ventricular cardiomyocytes to assess viability: effects of adenosine and potassium on cellular survival. J. Mol. Cell. Cardiol. 1997;29:1255–1266. doi: 10.1006/jmcc.1996.0363. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Kim IY, Ahn H, Zelner DJ, et al. Genetic change in transforming growth factor β (TGF-β) receptor type I gene correlates with insensitivity to TGF-β1 in human prostate cancer cells. Cancer Res. 1996;56:44–48. [PubMed] [Google Scholar]
- Maier JAM, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1α antisense oligomer. Science. 1990;249:1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- Malone M. Soft tissue tumours in childhood. Histopathology. 1993;23:203–216. doi: 10.1111/j.1365-2559.1993.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor-β family. Annu. Rev. Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Massague J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Massague J, Cheifetz S, Endo T, Nadal-Ginard B. Type β transforming growth factor is an inhibitor of myogenic differentiation. Proc. Natl. Acad. Sci. USA. 1986;83:8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-β signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- McAllister RM, Melnyk J, Finkelstein JZ, Adams EC, Gardner MB. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969;24:520–525. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Minniti CP, Tsokos M, Newton WA, Helman LJ. Specific expression of insulin-like growth factor-II in rhabdomyosarcoma tumor cells. Am. J. Clin. Pathol. 1994;101:198–203. doi: 10.1093/ajcp/101.2.198. [DOI] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, et al. Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signalling. Nature. 1997a;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Nakao A, Imamura T, Souchelnytskyi S, et al. TGF-β receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997b;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappo AS, Shapiro DN, Crist WM, Maurer HM. Biology and therapy of pediatric rhabdomyosarcoma. J. Clin. Oncol. 1995;13:2123–2139. doi: 10.1200/JCO.1995.13.8.2123. [DOI] [PubMed] [Google Scholar]
- Parham DM. The molecular biology of childhood rhabdomyosarcoma. Semin. Diagn. Pathol. 1994;11:39–46. [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Schweigerer L, Neufeld G, Mergia A, Abraham JA, Fiddes JC, Gospodarowicz D. Basic fibroblast growth factor in human rhabdomyosarcoma cells: implications for the proliferation and neovascularization of myoblast-derived tumors. Proc. Natl. Acad. Sci. USA. 1987;84:842–846. doi: 10.1073/pnas.84.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott SJ, Thayer MJ, Weintraub H. Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science. 1993;259:1450–1453. doi: 10.1126/science.8383879. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Masuyama N, Fukuda M, Nishida E. Regulation of intracellular dynamics of Smad4 by its leucine-rich nuclear export signal. EMBO Reports. 2000;1:176–182. doi: 10.1093/embo-reports/kvd029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H, Davis R, Tapscott S, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Xu J, Chen YG, Massague J. The nuclear import function of Smad2 is masked by SARA and unmasked by TGF-β dependent phosphorylation. Nat. Cell Biol. 2000;2:559–562. doi: 10.1038/35019649. [DOI] [PubMed] [Google Scholar]
- Zhang SW, Lin WS, Ying XL, Zhu D, Guo MY, Gu JX. Effect of suppression of TGF-beta1 expression on cell-cycle and gene expression of beta-1,4-galactosyltransferase 1 in human hepatocarcinoma cells. Biochem. Biophys. Res. Commun. 2000;273:833–838. doi: 10.1006/bbrc.2000.3028. [DOI] [PubMed] [Google Scholar]