Abstract
Acidosis is frequently associated with protein wasting and derangements in amino acid metabolism. As its effect on protein metabolism is significantly modulated by other abnormal metabolic conditions caused by specific illnesses, it is difficult to separate out the effects on protein metabolism solely due to acidosis. The aim of the present study was to evaluate, using a model of isolated perfused rat liver, the direct response of hepatic tissue to acidosis. We have compared hepatic response to perfusion with a solution of pH 7.2 and 7.4 (controls). Parameters of protein and amino acid metabolism were measured using both recirculation and single-pass technique with 4,5-[3H]leucine, [1–14C]leucine and [1–14C]ketoisocaproate (ketoleucine) as tracers and on the basis of difference of amino acid levels in perfusion solution at the beginning and end of perfusion. In liver perfused with a solution of pH 7.2, we observed higher rates of proteolysis, protein synthesis, amino acid utilization and urea production. Furthermore, the liver perfused with a solution of pH 7.2 released a higher amount of proteins to perfusate than the liver perfused with a solution of pH 7.4. Enhanced decarboxylation of ketoisocaproate in liver perfused by a solution of a lower pH indicates increased catabolism of branched-chain amino acids (leucine, valine and isoleucine), decreased reamination of branched-chain keto acids to corresponding essential amino acids and increased ketogenesis from leucine.
Keywords: acidosis, leucine, liver, protein synthesis, proteolysis
Acidosis is considered the principal cause of protein wasting in various pathologic situations such as renal and respiratory failure, diabetic coma and cardiac arrest. The effect of acidosis on protein metabolism, however, is significantly modulated by other abnormal metabolic conditions caused by specific illnesses. This can be proved by results of similar studies using different models of acidosis. In rats with chronic renal failure induced by 5/6 nephrectomy, a significant increase in whole-body proteolysis and leucine oxidation was observed (Holeček et al. 2001). On the other hand, in rats with acute renal failure induced by bilateral nephrectomy, we showed a marked decrease in proteolysis, protein synthesis and leucine clearance (Holeček et al. 2000).
Available data evaluating direct influence of acidosis on protein metabolism indicate a significant increase in whole-body proteolysis, amino acid oxidation and moderate increase in protein synthesis. May et al. (1992) demonstrated using primed continuous infusion of L-[1–14C]leucine a increase in whole-body protein degradation by 70%, protein synthesis by 55% and leucine oxidation by 100% in rats with acidosis induced by an oral administration of ammonium chloride. Similarly, a significant increase in protein turnover, leucine oxidation, plasma amino acid levels and negative protein balance were observed in dogs (Rodriguez et al. 1989) and rats (Šafránek et al. 2002) infused with hydrochloric acid.
More modest than data about the direct effect of acidosis on whole-body protein and amino acid metabolism in acidosis are data about the influence of acidosis on metabolism of proteins in specific tissues. Most studies refer to skeletal muscle and demonstrate significant increase in protein degradation and oxidation of amino acids, particularly leucine, and a moderate effect on protein synthesis (May et al. 1986, 1987; Williams et al. 1991). In the in vitro study using incubated soleus and extensor digitorum longus muscle, however, a decrease in pH by 0.1 in incubation medium had no effect on protein metabolism, while a decrease by 0.4 caused a decrease in proteolysis, protein synthesis and leucine oxidation (Šafránek et al. 2002). Data about the effect of acidosis on protein metabolism in other tissues are very sparse. The aim of the present study was to evaluate the effect of acidosis on protein and amino acid metabolism in liver.
Materials and methods
Animals
Male Wistar rats (Velaz, Prague, Czech Republic) were housed in standardized cages in quarters with controlled temperature and a 12 h light–dark cycle and received Velaz-Altromin 1320 laboratory chow and drinking water ad libitum. All procedures involving animals were performed according to guidelines set by the Institutional Animal Use and Care Committee of Charles University.
α-Keto[1–14C]isocaproate was purchased from Amersham (Buckinghamshire, UK), [14C]bicarbonate from DuPont-NEN (Bad Homburg, Germany) and 4,5-[3H]leucine from Lacomed (Prague, Czech Republic). Leucine and the sodium salt of α-ketoisocaproate (KIC) were purchased from Sigma Chemical (St Louis, MO, USA). Amino acid solution Aminoplasmal 15 was obtained from B. Braun Medicals (Kobenhaun, Germany). Hyamine hydroxide was obtained from Packard Instrument (Meriden, CT, USA). The remaining chemicals were purchased from Lachema (Brno, Czech Republic).
Experimental design
Overnight fasted rats were anaesthetized with sodium pentobarbital (35 mg/kg body weight intraperitoneally), and the livers were prepared for perfusion as described in detail recently (Holeček et al. 1997). Briefly, after laparotomy, the bile duct was cannulated and 1000 IU/kg of heparin was injected into the saphenous vein. Then, the portal vein was cannulated with a polyethylene catheter (internal diameter = 1.5 mm) and the hepatic artery was ligated. During portal perfusion with Krebs–Henseleit solution (20 °C), the liver was quickly removed. The perfusion was carried out at 37 °C in a thermostatically controlled cabinet. A peristaltic pump took the perfusate from the reservoir through an oxygenator and a bubble trap to the liver. Flow rates were maintained at 3.5 ml/g/min. Viability of the perfused livers was monitored by their appearance, concentration of minerals and hepatic enzymes in perfusate, by the stability of the bile flow and oxygen consumption.
The perfusion medium consisted of Krebs-Henseleit bicarbonate buffer, 6 mm glucose and amino acids at about the normal plasma concentration in rats. Perfusate pH was adjusted by HCl to 7.4 in control and 7.2 in acidotic group. Prior to starting the experimental protocol, livers were perfused with tracer-free perfusion medium for 15 min to ensure stabilization of the liver and washout of endogenous hormones. Two separate studies were performed.
Study 1
In the first study, we analysed using the recirculation technique (30 min) the effect of acidosis on protein synthesis, proteolysis and the net uptake or release of individual amino acids. Proteolysis was estimated as [3H]leucine release from the liver of rats labelled in vivo by an intraperitoneal injection of 4,5-[3H]leucine (0.8 mCi/kg body weight) 6 h prior to the perfusion experiment. Protein synthesis was measured by determining incorporation of [1–14C]leucine into liver proteins (16 µCi/l was added to the perfusate at the beginning of the recirculation). Protein concentrations in the liver and perfusate were measured according to the method described by Lowry et al. (1951). Amino acid exchanges were calculated using following formula:
where Ct16 and Ct46 are amino acid concentrations at the end and at the beginning of the recirculation phase of the study, respectively, V the total volume of perfusate in litres, t the duration of recirculation in hours and Wdry the liver dry weight in grams. Results are expressed as µmol/g dry liver/h. Negative values indicate net amino acid uptake and positive values net release.
Study 2
In the second study, we analysed the effect of acidosis on leucine metabolism in liver using the single-pass technique using KIC as a tracer (Patel et al. 1981). At the sixteenth minute (after stabilization), the perfusion medium containing [1–14C]KIC (2 µCi/l) KIC, 1.26 mm leucine and 1 mm KIC was infused for 15 min. Our preliminary experiments, in agreement with other studies (Patel et al. 1981; Blonde-Cynober et al. 1995), showed that 1 mm KIC was necessary for the substrate to be nonlimiting during the flux study. Samples of the effluent perfusate were collected in 20 ml flasks equipped with stoppers and centre wells containing 0.4 ml of methylbenzethonium hydroxide at 1 min intervals to monitor 14CO2 production. Labelled CO2 in the perfusate which was produced from [1–14C]KIC was released by injecting 0.5 ml of 5 N sulfuric acid through the stopper into the flasks. Oxidation rates of KIC were calculated as follows:
where O is the substrate oxidation (µmol substrate oxidized/g dry liver/h), R the radioactivity of 14CO2 in the effluent perfusate (dpm/ml), F the flow rate of perfusion medium through the liver (ml/h), RF the recovery factor, SA the specific activity of KIC in perfusion medium (dpm/µmol) and W the liver dry weight in grams. The 14CO2 recovery estimated by [14C]bicarbonate was 97% in both groups.
Other techniques
Amino acid concentrations were determined using high-performance liquid chromatography (Waters, Milford, MA, USA) after precolumn derivatization with o-phthaldialdehyde. The radioactivity of the samples was measured using liquid scintillation radioactivity counter LS 6000 (Beckman Instruments, Fullerton, CA, USA). Glucose concentration and activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and gamma-glutamyl transferase (GGT) were measured using commercial tests (Boehringer, Mannheim, Germany and Lachema, Brno, Czech Republic). Na+ and K+ were determined using ion-selective electrodes on AVL 983-S (Graz, Austria).
Statistical analysis
Results are expressed as mean ± SE. Statistical analysis was performed using Mann–Whitney test. A difference was considered significant at P < 0.05. Statistical software ncss 6.0 was used for the analysis.
Results
The perfused liver had a uniform colour, and there was no indication of uneven flow distribution. There were no differences between control and acidotic group in liver weight, the dry-to-wet liver weight ratio, the bile flow, oxygen consumption and concentrations of glucose, hepatic enzymes and minerals in perfusate at the end of perfusion (Tables 1 and 2).
Table 1.
Parameters of isolated perfused rat liver (recirculation study)
| Perfusate pH | ||
|---|---|---|
| 7.4 (n = 8) (control) | 7.2 (n = 9) | |
| Body weight (g) | 263 ± 3 | 254 ± 4 |
| Liver weight | ||
| Wet (g/kg body weight) | 36.1 ± 1.1 | 36.4 ± 1.4 |
| Dry (g/kg body weight) | 8.6 ± 0.2 | 8.4 ± 0.2 |
| Dry/wet (%) | 23.8 ± 0.4 | 23.1 ± 0.4 |
| Bile flow (mg/g dry liver/h) | 79 ± 4 | 83 ± 6 |
| O2 consumption (µmol/g dry liver/h) | 218 ± 14 | 267 ± 18 |
| Glucose release (µmol/g dry liver/h) | 23.4 ± 4.1 | 15.6 ± 5.4 |
| Na+ (mmol/l) | 146 ± 1 | 145 ± 1 |
| K+ (mmol/l) | 4.29 ± 0.01 | 4.23 ± 0.00 |
| Aspartate aminotransferase (µkat/l) | 0.40 ± 0.01 | 0.40 ± 0.01 |
| Alanine aminotransferase (µkat/l) | 0.23 ± 0.00 | 0.25 ± 0.01 |
| Lactate dehydrogenase (µkat/l) | 3.05 ± 0.50 | 2.36 ± 0.32 |
| Gamma-glutamyl transferase (µkat/l) | 0.28 ± 0.00 | 0.28 ± 0.00 |
Values are mean ± SE. Mann–Whitney test.
Table 2.
Parameters of isolated perfused rat liver (single-pass study).
| Perfusate pH | ||
|---|---|---|
| 7.4 (n = 9)(control) | 7.2 (n = 6) | |
| Body weight (g) | 257 ± 6 | 247 ± 5 |
| Liver weight | ||
| Wet (g/kg body weight) | 39.9 ± 1.4 | 38.7 ± 1.6 |
| Dry (g/kg body weight) | 10.7 ± 0.5 | 10.6 ± 0.4 |
| Dry/wet (%) | 26.8 ± 0.8 | 28.0 ± 0.8 |
| Bile flow (mg/g dry liver/h) | 95 ± 17 | 125 ± 9 |
| O2 consumption (µmol/g dry liver/h) | 199 ± 12 | 234 ± 23 |
Values are mean ± SE. Mann–Whitney test.
Perfusion of liver with a medium of a lower pH resulted in a higher release of urea, [3H]leucine and proteins to perfusate and also in an increased incorporation of [14C]leucine to liver proteins (Table 3).
Table 3.
Effect of perfusate pH on leucine and protein metabolism in isolated perfused rat liver (recirculation study)
| Perfusate pH | ||
|---|---|---|
| 7.4 (n = 8) (control) | 7.2 (n = 9) | |
| Urea release (µmol/g dry liver/h) | 21.5 ± 2.8 | 42.3 ± 7.5* |
| [3H]Leucine release into perfusate (dpm/g dry liver/h) | 134 325 ± 11 031 | 189 307 ± 17 913* |
| Protein release (µg/g dry liver/h) | 1243 ± 407 | 2613 ± 496* |
| [3H]Leucine in released proteins | ||
| dpm/g dry liver/h | 28 096 ± 7552 | 50 689 ± 15 092* |
| dpm/mg protein | 27 717 ± 4126 | 19 483 ± 3165 |
| [14C]Leucine in liver proteins (dpm/mg protein/h) | 307 ± 78 | 1039 ± 291* |
Values are mean ± SE. Mann–Whitney test.
P < 0.05 vs. perfusion solution of pH 7.4 (control).
Table 4 demonstrates that a net uptake of amino acids by hepatic tissue was observed in both groups. The net release of ornithine is caused by its absence in the prepared solution. The uptake was higher in the acidotic group. The significant increase showed serine, glutamic acid, leucine and histidine.
Table 4.
Effect of pH of perfusion solution on net amino acid uptake (–) or release (+) by isolated perfused rat liver (recirculation study)
| Perfusate pH | ||
|---|---|---|
| 7.4 (n = 9) (control) | 7.2 (n = 6) | |
| Aspartate | −4.6 ± 0.5 | −5.4 ± 0.3 |
| Threonine | −2.2 ± 0.3 | −2.4 ± 0.1 |
| Serine | −0.6 ± 0.2 | −1.9 ± 0.2* |
| Glutamic acid | −3.2 ± 0.6 | −7.2 ± 0.8* |
| Glutamine | −11.0 ± 0.4 | −10.4 ± 0.6 |
| Glycine | −10.0 ± 1.3 | −11.5 ± 0.6 |
| Alanine | −14.8 ± 3.0 | −19.4 ± 0.5 |
| Valine | −0.6 ± 0.6 | −1.7 ± 0.6 |
| Methionine | −2.1 ± 0.3 | −2.5 ± 0.1 |
| Isoleucine | −0.2 ± 0.4 | −0.8 ± 0.3 |
| Leucine | −1.0 ± 0.6 | −4.1 ± 0.3* |
| Tyrosine | 0.8 ± 0.1 | 0.6 ± 0.0* |
| Phenylalanine | −1.7 ± 0.2 | −2.3 ± 0.1 |
| Ornithine | 0.8 ± 0.2 | 1.5 ± 0.3* |
| Lysine | −2.5 ± 0.3 | −3.1 ± 0.2 |
| Histidine | −2.1 ± 0.2 | −3.1 ± 0.2* |
| Arginine | −6.3 ± 0.2 | −6.7 ± 0.2 |
| Derived values | ||
| Branched-chain amino acids | −1.4 ± 1.6 | −6.6 ± 1.2* |
| Total amino acids | −39.0 ± 7.3 | −59.6 ± 3.3* |
Units are µmol/g dry liver/h. Data are given as mean ± SE. Mann–Whitney test.
P < 0.05 vs. perfusion solution of pH 7.4 (control).
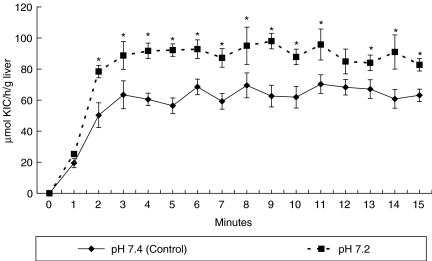
The results obtained using the single-pass method showed a significant increase in oxidation of KIC in liver perfused by the solution of a lower pH (Figure 1).
Figure 1.
Effect of perfusate pH on oxidation of ketoisocaproic acid (KIC) (single-pass study). Values are mean ± SE. Mann–Whitney test. *P < 0.05 vs. perfusion solution of pH 7.4 (control).
Discussion
The obtained results demonstrate that the decrease in extracellular pH by 0.2 has a significant effect on protein and amino acid metabolism in liver. We observed increased rates in proteolysis, protein synthesis, amino acid utilization, ketoisocaproic acid oxidation and urea production. These findings demonstrate a significant involvement of hepatic tissue in changes of whole-body protein metabolism, resulting in negative protein balance in conditions complicated by acidosis.
Changes induced by decreased pH in hepatic protein and amino acid metabolism differ from those observed in whole-body and skeletal muscle. The main difference seems to be the increased utilization of amino acids in protein synthesis. The finding of increased uptake of amino acid from the perfusion solution and increased incorporation of [14C]leucine to proteins of the liver perfused with a solution of a lower pH suggests that some amino acids released from skeletal muscle within the course of acidosis are used in hepatic tissue for protein synthesis in higher amounts than under normal conditions. Enhanced utilization of amino acids in protein synthesis is the probable cause of insignificant changes in glutamine level, although stimulatory effect of acidosis on glutamine synthesis in liver and its release to the blood has been demonstrated in several studies (Oliver et al. 1977; Haussinger et al. 1983).
Increased release of proteins to perfusion solution of a lower pH and insignificant difference in [3H]leucine radioactivity per mg of protein between experimental and control group indicate that the decrease in pH enhances the release of hepatic proteins to circulation. In terms of protein economy, a relatively short time was allowed for the liver to respond to the lower pH and to demonstrate increased incorporation of [14C]leucine not only to proteins of hepatic tissue but also to proteins released to perfusate. It should be noted that protein synthesis in liver is mainly under the control of several humoral factors (e.g. hormones and interleukins) and that pH is only one of many modulatory factors. Therefore, changes occurring under in vivo conditions can be quite different (Kaysen & Rathore 1996; Ulrich et al. 1999). We suppose that activated synthesis and release of proteins by the liver may be an important component of adaptive metabolic response of the body to acidosis. The response can increase the buffering capacity of the blood and prevent a rapid decrease in plasma proteins mediated by increased production of cytokines in various clinical conditions. Cytokines, particularly interleukin-1, may cause the decrease in plasma albumin concentration both by its decreased synthesis and by its enhanced escape through leaky capillaries (Ballmer et al. 1992).
Increased release of urea together with enhanced oxidation of KIC indicates increased catabolism of amino acids, at least of essential branched-chain amino acid. Activated oxidation of KIC is caused by increased activity of the hepatic branched-chain keto acid (BCKA) dehydrogenase. The enzyme enables irreversible decarboxylation of BCKAs derived from branched-chain amino acids (leucine, isoleucine and valine) to thioesters of coenzyme A (CoA). These are, through a series of reactions, converted to acetoacetate and acetyl-CoA (leucine), propionyl-CoA and acetyl-CoA (isoleucine) and succinyl-CoA (valine). Significant increase of hepatic BCKA dehydrogenase has been observed also in liver mitochondrial extracts of rats in which acidosis was induced by ingestion of 0.28 m ammonium chloride solution for 10 days (Rodriguez-Bayona & Peragon 1998).
From rates of 14CO2 production from [1–14C]KIC and [U-14C] KIC by isolated hepatocytes, it has been shown that only 10% of flux of KIC through BCKA dehydrogenase was oxidized to CO2, the remainder was released as acetoacetate (Corkey et al. 1982). In the liver, in contrast to other tissues, not all enzymes essential for the synthesis of acetyl-CoA from acetoacetate, the reaction necessary for the oxidation of ketone bodies, are expressed. Therefore, increased oxidation of KIC in liver perfused by a solution of a lower pH indicates also enhanced ketogenesis from leucine in acidosis. This finding can give new information regarding the pathogenesis of enhanced hepatic decarboxylation of BCKA and enhanced contribution of leucine in ketogenesis from amino acids in diabetes (May et al. 1980; Kulaylat et al. 1988).
BCKA can be produced by specific aminotransferase reaction in liver and delivered to the liver from peripheral tissues, particularly from skeletal muscle and adipose tissue (Harper et al. 1984). The importance of BCKA, particularly KIC, delivered by blood stream is supported by a number of studies. Livesey & Lund (1980) demonstrated that the liver can extract a quantity of BCKA equivalent to that released by muscle. These BCKAs delivered to the liver can be oxidized and converted to branched-chain amino acid. The ability of the liver to reaminate BCKA and release the corresponding branched-chain amino acid into the blood stream is the basis for use of BCKA in the treatment of chronic renal failure. Our results demonstrate that acidosis decreases the efficiency of conversion of BCKA to the corresponding essential amino acids (valine, leucine and isoleucine). This finding can be of practical importance when BCKAs are administered to prevent negative nitrogen balance, particularly in chronic renal failure.
Acknowledgments
The study was supported by a grant of the Grant Agency of the Czech Republic, number 305/01/0578.
References
- Ballmer PE, Ballmer-Hofer K, Repond F, Kohler H, Studer H. Acute suppression of albumin synthesis in systemic inflammatory disease: an individually graded response of rat hepatocytes. J. Histochem. Cytochem. 1992;40:201–206. doi: 10.1177/40.2.1552164. [DOI] [PubMed] [Google Scholar]
- Blonde-Cynober F, Plassart F, De Bandt JP, et al. Metabolism of α-ketoisocaproic acid in isolated perfused liver of cirrhotic rats. Am. J. Physiol. 1995;268:E298–E304. doi: 10.1152/ajpendo.1995.268.2.E298. [DOI] [PubMed] [Google Scholar]
- Corkey B, Martin-Requero A, Walajtys-Rode E, Williams RJ, Williamson JR. Regulation of the branched chain α-ketoacid pathway in liver. J. Biol. Chem. 1982;257:9668–9676. [PubMed] [Google Scholar]
- Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Haussinger D, Gerok W, Sies H. Regulation of flux through glutaminase and glutamine synthetase in isolated perfused rat liver. Biochim. Biophys. Acta. 1983;755:272–278. doi: 10.1016/0304-4165(83)90214-3. [DOI] [PubMed] [Google Scholar]
- Holeček M, Šprongl L, Skopec F, Andrýs C, Pecka M. Leucine metabolism in TNF-α- and endotoxin-treated rats: contribution of hepatic tissue. Am. J. Physiol. 1997;273:E1052–E1058. doi: 10.1152/ajpendo.1997.273.6.E1052. [DOI] [PubMed] [Google Scholar]
- Holeček M, Šprongl L, Tilšer I, Tichý M. Leucine and protein metabolism after bilateral nephrectomy in rats: the role of hepatic tissue. Res. Exp. Med. 2000;200:53–65. [PubMed] [Google Scholar]
- Holeček M, Šprongl L, Tilšer I, Tichý M. Leucine and protein metabolism in rats with chronic renal insufficiency. Exp. Toxicol. Pathol. 2001;53:71–76. doi: 10.1078/0940-2993-00171. [DOI] [PubMed] [Google Scholar]
- Kaysen GA, Rathore V. Derangements of protein metabolism in chronic renal failure. Blood Purif. 1996;14:378–381. doi: 10.1159/000170289. [DOI] [PubMed] [Google Scholar]
- Kulaylat MN, Frexes-Steed M, Geer R, Williams PE, Abumrad NN. The role of leucine in hepatic ketogenesis. Surgery. 1988;103:351–360. [PubMed] [Google Scholar]
- Livesey G, Lund P. Enzymic determination of branched-chain amino acids and 2-oxoacids in rat tissues. Biochem. J. 1980;188:705–713. doi: 10.1042/bj1880705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- May RC, Hara Y, Kelly RA, Block KP, Buse MG, Mitch WE. Branched-chain amino acid metabolism in rat muscle: Abnormal regulation in acidosis. Am. J. Physiol. 1987;252:E712–E718. doi: 10.1152/ajpendo.1987.252.6.E712. [DOI] [PubMed] [Google Scholar]
- May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J. Clin. Invest. 1986;77:614–621. doi: 10.1172/JCI112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May ME, Mancusi JJ, Aftring RP, Buse MG. Effects of diabetes on oxidative decarboxylation of branched-chain keto acids. Am. J. Physiol. 1980;239:E215–E222. doi: 10.1152/ajpendo.1980.239.3.E215. [DOI] [PubMed] [Google Scholar]
- May RC, Masud T, Logue B, Bailey J, England BK. Chronic metabolic acidosis accelerates whole body proteolysis and oxidation in awake rats. Kidney Int. 1992;41:1535–1542. doi: 10.1038/ki.1992.223. [DOI] [PubMed] [Google Scholar]
- Oliver J, Koelz AM, Costello J, Bourke E. Acid–base induced alterations in glutamine metabolism and ureogenesis in perfused muscle and liver of the rat. Eur. J. Clin. Invest. 1977;7:445–449. doi: 10.1111/j.1365-2362.1977.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Patel TB, DeBuysere MS, Barron LL, Olson M. Studies on the regulation of the branched chain α-keto acid dehydrogenase in the perfused rat liver. J. Biol. Chem. 1981;256:9009–9015. [PubMed] [Google Scholar]
- Rodriguez NR, Miles JM, Schwenk WF, Haymond MW. Effects of acute metabolic acidosis and alkalosis on leucine metabolism in conscious dogs. Diabetes. 1989;38:847–853. doi: 10.2337/diab.38.7.847. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bayona B, Peragon J. Stimulation of rat-liver branched-chain alpha-keto acid dehydrogenase activity by chronic metabolic acidosis. Int. J. Biochem. Cell Biol. 1998;30:529–534. doi: 10.1016/s1357-2725(97)00158-1. [DOI] [PubMed] [Google Scholar]
- Šafránek R, Holecek M, Kadlcíková J, Chládek J, Šprongl L. Effect of acute acidosis on protein and amino acid metabolism in rats. Clin. Nutr. 2002;21(Suppl. 1):65. doi: 10.1016/s0261-5614(03)00041-4. [DOI] [PubMed] [Google Scholar]
- Ulrich C, Kruger B, Kohler H, Riegel W. Effect of acidosis on acute phase protein metabolism in liver cells. Miner. Electrolyte Metab. 1999;25:228–233. doi: 10.1159/000057453. [DOI] [PubMed] [Google Scholar]
- Williams B, Layward E, Walls J. Skeletal muscle degradation and nitrogen wasting in rats with chronic metabolism acidosis. Clin. Sci. Lond. 1991;80:457–462. doi: 10.1042/cs0800457. [DOI] [PubMed] [Google Scholar]