Abstract
The purpose of this investigation was to determine whether changes in myosin heavy chain (MHC) expression and atrophy in rat skeletal muscle are observed during transition from cardiac hypertrophy to chronic heart failure (CHF) induced by aortic stenosis (AS). AS and control animals were studied 12 and 18 weeks after surgery and when overt CHF had developed in AS animals, 28 weeks after the surgery. The following parameters were studied in the soleus muscle: muscle atrophy index (soleus weight/body weight), muscle fibre diameter and frequency and MHC expression. AS animals presented decreases in both MHC1 and type I fibres and increases in both MHC2a and type IIa fibres during late cardiac hypertrophy and CHF. Type IIa fibre atrophy occurred during CHF. In conclusion, our data demonstrate that skeletal muscle phenotype changes occur in both late cardiac hypertrophy and heart failure; this suggests that attention should be given to the fact that skeletal muscle phenotype changes occur prior to overt heart failure symptoms.
Keywords: aortic stenosis, atrophy, heart failure, muscle fibre types, myosin heavy chains, Wistar rats
Chronic heart failure (CHF) is characterized by a reduced tolerance to exercise because of early fatigue and dyspnea; this may be in part because of skeletal muscle myopathy, with atrophy and shift from type I ‘slow’ to type II ‘fast’ fibres (Lipkin et al. 1988; Sullivan et al. 1990; Mancini et al. 1992; De Sousa et al. 2000). CHF induces skeletal muscle myosin heavy chain (MHC) isoform expression towards the fast isoform (Simonini et al. 1996; Vescovo et al. 1998a), which is related to CHF severity (Spangenburg et al. 2002).
Few investigations have studied skeletal muscle MHC isoform expression and atrophy during cardiac hypertrophy and heart failure, although they are well described during CHF (Vescovo et al. 1998a; Coirault et al. 1999). According to Vescovo et al. (1998a), compensated right ventricle hypertrophy is not accompanied by biochemical or morphological skeletal muscle changes. Alterations in metabolism (Chati et al. 1994) and impaired fatigue resistance (Levy et al. 1996) and performance (Coirault et al. 1999) of limb skeletal muscle, however, have been reported in experimental cardiac volume overload, without failure; this suggests that skeletal muscle myopathy may develop during cardiac hypertrophy and could also confirm that biochemical changes occur prior to muscle atrophy (Vescovo et al. 1998b).
The purpose of this investigation was to determine whether changes in MHC expression and atrophy of skeletal muscle are present during transition from cardiac hypertrophy to CHF failure induced by aortic stenosis (AS).
Materials and methods
Experimental model
Forty weaned male Wistar rats (3–4 weeks old; 80–100 g) were obtained from the Central Animal House at São Paulo State University. AS was induced in 20 rats by placing a 0.6-mm internal diameter silver haemoclip on the ascending aorta via thoracic incision (AS group), according to the method described by Feldman et al. (1993). Twenty age-matched control rats underwent the same procedure without clip placement (C group). Aortic banded rats and age-matched sham-operated control rats were studied 12 and 18 weeks after surgery and at 28 weeks when the AS group had developed overt heart failure. This experimental model of heart failure has been well characterized and is widely accepted (Feldman et al. 1993; De Sousa et al. 2000; Ding et al. 2000).
After anaesthesia with intraperitoneal sodium pentobarbital (50 mg/kg), the animals were killed; body weight (BW) and soleus weight (Sol) were evaluated. The Sol/BW ratio was used as the index of muscle atrophy. Muscles were immediately frozen in liquid nitrogen and stored at −80 °C. Left ventricle weight (LVW) and right ventricle weight (RVW) normalized by BW (LVW/BW and RVW/BW, respectively) were used as indexes of ventricular hypertrophy. This experiment was approved by Ethics Committee of Instituto de Biociências, UNESP, Botucatu, SP, Brazil.
Histochemical analysis
Frozen soleus mid-belly regions were mounted vertically on a cryostat chuck in tissue-freezing medium (Jung, Nussloch, Germany). Transverse cryosections approximately 10 µm thick were cut in a cryostat cooled to −20 °C. Sections were stained histochemically for myofibrillar ATPase after acid preincubation at pH 4.32 and 4.5 (Brooke & Kaiser 1970), and fibres were classified as types I, Ic/IIc and IIa. Fibre diameters were measured using a compound microscope attached to a computerized imaging analysis system (Qwin, Leica, Nussloch, Germany). At least 400 fibres from each muscle were measured using the smallest diameter method (Dubowitz 1985), and their frequency was expressed as number of fibres per type against total number of fibres measured.
Electrophoretic separation of MHC
MHC isoform analysis was performed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Six to 10 serial cross sections (12 µm thick) were placed in 250 μl of a solution containing 10% (wt/vol) glycerol, 5% (v/v) 2-mercaptoethanol, 2.3% (wt/vol) SDS and 0.9% (wt/vol) Tris–HCl (pH 6.8) for 10 min at 60 °C. Small amounts of the extracts (8 µl) were loaded onto a 7–10% SDS-PAGE separating gel with a 4% stacking gel, run overnight (19–21 h) at 120 V and stained with Coomassie blue. MHC isoforms were identified according to molecular mass, and their relative percentages were quantified by densitometry. Two MHC isoforms (MHC1 and MHC2a) were separated on the basis of their relative mobility in rat soleus muscle.
Statistical methods
Data are expressed as mean ± SD. Anatomical data were compared using anova; the Tukey multiple comparison test was used to localize differences when appropriate. Comparisons of skeletal muscle fibre frequency and diameter and MHC distribution percentage between control and AS groups were made by repeated measurement analysis (multivariate analysis − mean profile). Differences were considered to be significant when P < 0.05.
Results
Clinical and anatomical data
Criteria for heart failure were based on previous studies in which animals with evidence of heart failure had findings that included laboured respiration, left atrial thrombi, pleural and pericardial effusions, congested liver and right ventricular hypertrophy (Feldman et al. 1993; Cicogna et al. 1999; Ding et al. 2000). In this study, all animals with heart failure had laboured respiration, pleural and/or pericardial effusion and right ventricle hypertrophy (RVW/BW > 0.80). None of the aortic banded animals studied at 12 or 18 weeks after surgery exhibited any of these pathological features.
Table 1 summarizes the anatomical data. There was no significant difference in BW and Sol weights between AS and C groups. Sol/BW was similar except 18 weeks after surgery, when the AS group was larger than the C group. LVW and LVW/BW were always greater in AS groups. RVW and RVW/BW were larger in the AS group only during heart failure.
Table 1.
Anatomical data
| Experimental periods (weeks) | |||
|---|---|---|---|
| 12 (n = 7) | 18 (n = 7) | 28 (n = 6) | |
| Body weight (g) | |||
| Control group | 425 ± 34a | 494 ± 37a | 629 ± 88b |
| Aortic stenosis group | 434 ± 53a | 504 ± 58b | 589 ± 80c |
| Left ventricle weight (g) | |||
| Control group | 0.80 ± 0.09a | 0.87 ± 0.06a | 1.05 ± 0.09b |
| Aortic stenosis group | 1.32 ± 0.15a* | 1.42 ± 0.17a* | 1.72 ± 0.12b* |
| Right ventricle weight (g) | |||
| Control group | 0.26 ± 0.03a | 0.29 ± 0.04a | 0.29 ± 0.04a |
| Aortic stenosis group | 0.29 ± 0.07a | 0.30 ± 0.06a | 0.60 ± 0.05b* |
| Left ventricle weight/body weight (mg/g) | |||
| Control group | 1.89 ± 0.15a | 1.77 ± 0.09a | 1.68 ± 0.12a |
| Aortic stenosis group | 3.07 ± 0.39a* | 2.83 ± 0.29a* | 2.99 ± 0.50a* |
| Right ventricle weight/body weight (mg/g) | |||
| Control group | 0.61 ± 0.10b | 0.58 ± 0.05a,b | 0.46 ± 0.03a |
| Aortic stenosis group | 0.66 ± 0.11a | 0.61 ± 0.11a | 1.03 ± 0.16b* |
| Soleus weight (mg) | |||
| Control group | 208 ± 20a | 247 ± 19a | 303 ± 36b |
| Aortic stenosis group | 209 ± 26a | 278 ± 49b | 282 ± 23b |
| Soleus weight/body weight (mg/g) | |||
| Control group | 0.49 ± 0.02a | 0.50 ± 0.04a | 0.49 ± 0.09a |
| Aortic stenosis group | 0.48 ± 0.03a | 0.55 ± 0.05b* | 0.48 ± 0.05a |
n, number of animals. Values are mean ± SD. Experimental periods that do not share a common superscript letter are statistically different (P < 0.05).
P < 0.05 vs. control.
At 28 weeks, group C (C28) presented BW, LVW and Sol weight greater than those of the other two C groups (C12 and C18). BW was different between all AS groups. The heart failure group (AS28) presented higher LVW, RVW and RVW/BW than the other AS groups (AS12 and AS18). RVW/BW was higher in C12 than C28 and similar between C12 and C18. RVW, LVW/BW and Sol/BW were equal in all three C groups. Sol/BW was higher in AS18 than that in AS12 and AS28. LVW/BW was similar in all three AS groups.
Morphometric analysis and fibre type frequency
Table 2 summarizes morphometric analysis and fibre type frequency data. Fibre cross-section frequencies were similar between the AS and C group 12 weeks after surgery. There were decreased type I and increased type IIa fibre frequencies in the AS groups, compared with their corresponding C groups 18 and 28 weeks after surgery. There were no significant changes in fibre cross-section diameter between AS and C groups throughout the experiment, except that type IIa showed a decrease compared with its corresponding C group 28 weeks after surgery.
Table 2.
Characteristics of fibres from soleus muscle
| Frequency (%) | Fibre diameter (µm) | ||||
|---|---|---|---|---|---|
| Experimental periods (week) | Fibre type | Control | Aortic stenosis | Control | Aortic stenosis |
| 12 | I | 68.5 ± 4.6 | 69.0 ± 5.2 | 51.8 ± 5.7 | 48.5 ± 3.7 |
| Ic/Ilc | 3.0 ± 1.9 | 2.6 ± 1.5 | 49.0 ± 6.4 | 41.7 ± 8.6 | |
| Ila | 28.6 ± 5.1 | 28.5 ± 6.2 | 48.8 ± 5.4 | 46.6 ± 3.4 | |
| 18 | I | 80.5 ± 5.0 | 71.8 ± 8.2* | 49.0 ± 2.7 | 48.9 ± 2.8 |
| Ic/Ilc | 3.1 ± 1.8 | 2.1 ± 1.9 | 44.2 ± 9.2 | 42.3 ± 7.1 | |
| Ila | 16.4 ± 5.5 | 26.1 ± 8.6* | 48.2 ± 2.5 | 47.4 ± 3.6 | |
| 28 | I | 79.0 ± 7.9 | 66.0 ± 6.3* | 54.3 ± 1.6 | 51.1 ± 3.1 |
| Ic/Ilc | 3.1 ± 2.6 | 1.2 ± 1.5 | 48.9 ± 3.2 | 38.4 ± 14.0 | |
| Ila | 17.9 ± 7.5 | 32.8 ± 6.7* | 56.8 ± 4.1 | 51.3 ± 2.7* | |
Values are mean ± SD.
P < 0.05 vs. control.
MHC electrophoretic pattern
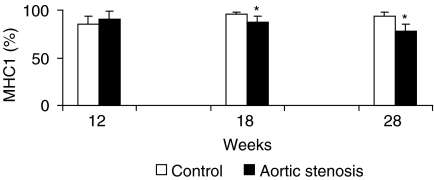
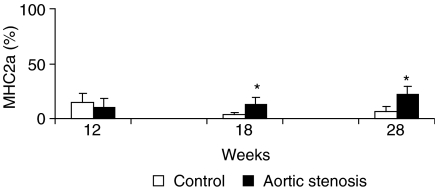
Figures 1 and 2 show soleus muscle MHC percentage distributions. MHC1 and MHC2a expressions were similar between AS and C groups 12 weeks after surgery (AS = 90.1 ± 8.8%vs. C = 85.5 ± 8.4% and AS = 9.9 ± 8.8%vs. C = 14.5 ± 8.4%, respectively). Eighteen weeks after surgery, MHC1 decreased (AS = 87.2 ± 6.1%vs. C = 96.3 ± 2.1%, P < 0.05) and MHC2a increased (AS = 12.8 ± 6.2%vs. C = 3.7 ± 2.1%, P < 0.05) in the AS group compared to the C group. Twenty-eight weeks after surgery, the AS group also presented a shift towards the fast isoform compared with its corresponding C group; MHC1 decreased (AS = 77.9 ± 7.3%vs. C = 93.5 ± 4.3%, P < 0.05), whereas MHC2a increased (AS = 22.1 ± 7.3%vs. C = 6.5 ± 4.3%, P < 0.05).
Figure 1.
Percentage distribution of myosin heavy chain-1 (MHC1) in the soleus muscle of control and aortic stenosis groups (*P < 0.05 vs. control group).
Figure 2.
Percentage distribution of myosin heavy chain-2a (MHC2a) in the soleus muscle of control and aortic stenosis groups (* P < 0.05 vs. control group).
Discussion
The purpose of this investigation was to determine whether morphological and biochemical changes in skeletal muscle are present during transition from cardiac hypertrophy to heart failure induced by AS.
The most important finding in this study was an increase in MHC2a and type IIa fibres and a decrease in MHC1 and type I fibres in the soleus muscle during late cardiac hypertrophy without heart failure (AS18). This diverges from the results of Coirault et al. (1999), who did not observe changes in soleus MHC composition during the early stages of pressure cardiac overload induced by subtotal constriction of the suprarenal abdominal aorta in rabbits. Vescovo et al. (1998a), using an alkaloid to induce severe hypertension, right ventricular hypertrophy and heart failure in rats, also reported that right ventricular hypertrophy is not accompanied by changes in soleus MHC composition. These disparities may be because of the different models used. The data obtained in our model, chronic left ventricular pressure overload, indicate that skeletal muscle myopathy occurs early in the course of this cardiopathy because of biochemical changes and not on account of muscle atrophy.
The biochemical changes found during left ventricular hypertrophy were also observed during heart failure. In addition, we found type IIa fibre atrophy in animals with this syndrome, even though we did not detect loss of soleus muscle bulk, indicated by Sol/BW. We can speculate that this may be because of fibrosis, as detected by Filippatos et al. (2003) in skeletal muscle of heart failure patients. Skeletal muscle myopathy, which is accompanied by alterations in MHC pattern that reflect changes in fibre types and also by atrophy, is a well-described phenomenon during heart failure (Sullivan et al. 1990; Drexler et al. 1992; Simonini et al. 1996; Vescovo et al. 1998a; De Sousa et al. 2000; Spangenburg et al. 2002).
The causes of the changes in skeletal muscle MHC during heart failure are still unknown. Cytokine activation and loss of anabolic function (McMurray et al. 1991), ergo-metaboloreceptor dysfunction (Coats 1996), changes in blood flow (Wilson et al. 1984), reduction in neuromuscular activity (Talmadge 2000) and skeletal muscle apoptosis (Vescovo et al. 1998a), however, may be the probable causes. This last point has undergone considerable debate. According to Vescovo et al. (1998b), endothelial apoptosis, even without changes in skeletal muscle blood flow, could alter myofibre nutrition and induce relative ischaemia to which muscle fibres adapt by shifting towards the fast myosin isoforms; also, apoptosis and atrophy may be closely linked once myonuclei undergo apoptosis in heart failure together with endothelial cells (Allen et al. 1997; Vescovo et al. 1998b; Dalla Libera et al. 1999). In fact, Vescovo et al. (2000) showed an inverse correlation between the number of apoptotic myonuclei and the degree of muscle atrophy.
The mechanisms involved in skeletal muscle adaptations that occurred in rats with 18 weeks of AS and without signs of heart failure need to be determined. Observation has, however, indicated that skeletal muscle alterations in heart failure are, after all, consequences of impaired cardiac function. The degree and duration of cardiac dysfunction may influence structural and biochemical damage to the skeletal muscle (Coirault et al. 1999). Bregagnollo et al. (2003) demonstrated that after 6 weeks of AS, animals consistently presented a significant increase in left ventricle end diastolic pressure; the authors also showed that after 21 weeks of AS, rats without signs of heart failure also presented significant systolic dysfunction, evaluated by haemodynamic and echocardiograph methods. Therefore, although the animals did not present heart failure, they showed important signs of left ventricle dysfunction. These alterations were probably also present in our animals after 18 weeks of AS and may have induced the changes in muscle fibre phenotype observed in this experiment.
In summary, aortic banded animals develop a myopathy in the soleus muscle with decreases in both MHC1 and type I fibres along with increases in both MHC2a and type IIa fibres during both late cardiac hypertrophy and heart failure. Type IIa fibre atrophy occurs only during heart failure. If analogous phenomena occur in a clinical setting with cardiac hypertrophy and subsequent failure, attention should be given to the fact that skeletal muscle phenotype changes occur prior to overt heart failure symptoms.
Acknowledgments
We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support and V. Marcos de Souza and S. Cruz Michelin for technical assistance. This work constitutes part of MSc Thesis presented to Universidade Estadual de Campinas, UNICAMP in 2003, by R.F.C.
References
- Allen DL, Linderman K, Roy RR, et al. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am. J. Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- Bregagnollo EA, Okoshi K, Padovani CR, Okoshi MP, Cicogna AC. Effects of chronic angiotensin-converting enzyme inhibition on morphology and function of the hypertrophy left ventricle of rats with persistent pressure overload. Arq. Bras. Cardiol. doi: 10.1590/s0066-782x2005000300006. in press. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J. Histochem. Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Chati Z, Zannad F, Michel C, et al. Skeletal muscle phosphate metabolism abnormalities in volume-overload experimental heart failure. Am. J. Physiol. 1994;267:H2186–H2192. doi: 10.1152/ajpheart.1994.267.6.H2186. [DOI] [PubMed] [Google Scholar]
- Cicogna AC, Robinson KG, Conrad CH, et al. Direct effects of colchicine on myocardial function. Studies in hypertrophied and failing spontaneously hypertensive rats. Hypertension. 1999;33:60–65. doi: 10.1161/01.hyp.33.1.60. [DOI] [PubMed] [Google Scholar]
- Coats AJS. The “muscle hypothesis” of chronic heart failure. J. Mol. Cell. Cardiol. 1996;28:2255–2262. doi: 10.1006/jmcc.1996.0218. [DOI] [PubMed] [Google Scholar]
- Coirault C, Langeron O, Lambert F, et al. Impaired skeletal muscle performance in the early stage of cardiac pressure overload in rabbits: beneficial effects of angiotensin-converting enzyme inhibition. J. Pharmacol. Exp. Ther. 1999;291:70–75. [PubMed] [Google Scholar]
- Dalla Libera L, Zennaro R, Sandri M, Ambrosio GB, Vescovo G. Apoptosis and atrophy in rat slow skeletal muscle in chronic heart failure. Am. J. Physiol. 1999;277:C982–C986. doi: 10.1152/ajpcell.1999.277.5.C982. [DOI] [PubMed] [Google Scholar]
- De Sousa E, Veksler V, Bigard X, Mateo P, Ventura-Clapier R. Heart failure affects mitochondrial but not myofibrillar intrinsic properties of skeletal muscle. Circulation. 2000;102:1847–1853. doi: 10.1161/01.cir.102.15.1847. [DOI] [PubMed] [Google Scholar]
- Ding B, Price RL, Goldsmith EC, et al. Left ventricular hypertrophy in ascending aortic stenosis mice. Circulation. 2000;101:2845–2862. doi: 10.1161/01.cir.101.24.2854. [DOI] [PubMed] [Google Scholar]
- Drexler H, Reide U, Münzel T, König H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Muscle Biopsy: a Practical Approach. 2. London: Baillière Tindall; 1985. [Google Scholar]
- Feldman AM, Wienberg EO, Ray PE, Lorell BH. Selective changes in cardiac gene expression during compensated hypertrophy and transition to cardiac decompensation in rats with chronic aortic banding. Circ. Res. 1993;73:184–192. doi: 10.1161/01.res.73.1.184. [DOI] [PubMed] [Google Scholar]
- Filippatos GS, Kanatselos C, Manolatos DD, et al. Studies on apoptosis and fibrosis in skeletal musculature: a comparison of heart failure patients with and without cardiac cachexia. Int. J. Cardiol. 2003;90:107–113. doi: 10.1016/s0167-5273(02)00535-1. [DOI] [PubMed] [Google Scholar]
- Levy LB, Avkiran M, Ferrari R, Hearse DJ. Impaired skeletal muscle fatigue resistance in rats with pressure overload-induced left ventricular hypertrophy. J. Mol. Cell. Cardiol. 1996;28:183–195. doi: 10.1006/jmcc.1996.0018. [DOI] [PubMed] [Google Scholar]
- Lipkin D, Jones D, Round J, Pooole-Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int. J. Cardiol. 1988;18:187–195. doi: 10.1016/0167-5273(88)90164-7. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- McMurray J, Abdullah I, Dargie HJ, Shapiro D. Increased concentration of tumor necrosis factor in ‘cachectic’ patients with severe chronic heart failure. Br. Heart J. 1991;66:356–358. doi: 10.1136/hrt.66.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonini A, Massie BM, Long CS, Qi M, Samarel AM. Alterations in skeletal muscle gene expression in rat with chronic congestive heart failure. J. Mol. Cell. Cardiol. 1996;28:1683–1691. doi: 10.1006/jmcc.1996.0158. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Talmadge RJ, Musch TI, Pfeifer PC, McAllister RM, Willians JH. Changes in skeletal muscle myosin heavy chain isoform content during congestive heart failure. Eur. J. Appl. Physiol. 2002;87:182–186. doi: 10.1007/s00421-002-0615-3. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve. 2000;23:661–679. doi: 10.1002/(sici)1097-4598(200005)23:5<661::aid-mus3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Vescovo G, Ceconi C, Bernocchi P, et al. Skeletal muscle myosin heavy chain expression in rats with monocrotaline-induced cardiac hypertrophy and failure. Relation to blood flow and degree of muscle atrophy. Cardiovasc. Res. 1998a;39:233–241. doi: 10.1016/s0008-6363(98)00041-8. [DOI] [PubMed] [Google Scholar]
- Vescovo G, Volterrani M, Zennaro R, et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart. 2000;84:431–437. doi: 10.1136/heart.84.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo G, Zennaro R, Sandri M, et al. Apoptosis of skeletal muscle myofibres and interstitial cells in experimental heart failure. J. Mol. Cell. Cardiol. 1998b;30:2449–2459. doi: 10.1006/jmcc.1998.0807. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation. 1984;69:1079–1087. doi: 10.1161/01.cir.69.6.1079. [DOI] [PubMed] [Google Scholar]