Abstract
Pancuronium bromide (PB) is used in neonates and pregnant women to induce limp, flaccid paralysis in order to allow mechanical ventilation during intensive care. Such non-depolarizing neuromuscular blocking drugs are administered to 0.1% of all human births in the UK. In this study, we examined PB effects on skeletal development in chick embryos. PB treatment produced skeletal deformities associated with significant reduction in longitudinal growth of all appendicular elements. This was associated with greater cartilage to bone ratios, indicating a preferential reduction in osteogenesis. PB also increased the incidence of knee joint flexion and tibiotarsal joint hyperextension. In addition to limb, spinal and craniofacial deformities, flaccid immobility appears to convert the normal geometric pattern of weight gain to a simple arithmetic accretion. This novel study highlights the potentially harmful effects of pharmacologically induced flaccid immobility on chick embryonic skeletal development. Whilst in ovo avian development clearly differs from human, our findings may have implications for the fetus, premature and term neonate receiving such non-depolarizing neuromuscular blocking drugs.
Keywords: bone, cartilage, chick embryo, limb, neuromuscular blocking drugs, paralysis
Non-depolarizing neuromuscular drugs, such as pancuronium bromide (PB) and several of its functional analogues, namely rocuronium, vecuronium, pipecuronium and rapacuronium bromides are widely administered to adults, pregnant women and neonatal infants (Atherton & Hunter 1999). The administration of these muscle relaxants is commonly used in order to induce the immobilization necessary to reduce resistance to mechanical ventilation during respiratory distress. These drugs function by combining with postjunctional acetylcholine (ACh) receptors and competitively antagonize the endplate transmitter action of ACh (Rang et al. 1995). Treatment therefore produces a state of limp, flaccid paralysis with muscle relaxation within 2 min of intravenous injection, and because PB is readily metabolized by acetylcholinesterase hydrolysis, its direct actions at neuromuscular junctions are considered completely reversible (Bowman & Rand 1980; Crossland 1980). This favourable pattern of pharmacological characteristics has led to the broad clinical use of PB.
However, the importance of embryonic movement during skeletal development and its role as a major determinant of bone mass, architecture and functional strength is well recognized (Fell & Canti 1934; Frost & Schonau 2000; Rauch & Schoenau 2001). These studies have shown that nutrition and preprogrammed growth are necessary but not sufficient for normal bone growth and development. An interest in the mechanisms of skeletal deformity as well as a desire to determine the factors regulating musculoskeletal development stimulated a search for appropriate animal models capable of shedding light on such phenomena. Initial models utilized d-tubocurarine (d-TC) as an immobilizing agent and showed that d-TC infusion of chick embryos for 1–2 days at mid-gestation (Hamburger & Hamilton 1992) produced dose-dependent multiple contractures that resembled arthrogryposis multiplex congenita (AMC) (Drachman & Coulombre 1962). Such treatment also caused reduced birth weights and produced multiple positional deformities of the extremities (fetal akinesia deformation sequence) (Moessinger 1983).
In humans, neonatal contractures with micrognathia and neck webbing have been described after 11-day maternal treatment for tetanus with d-TC administered between 10 and 12 weeks gestation (Jago 1970). Although PB-like drugs are more commonly used in neonates suffering from respiratory distress, their effects on the developing musculoskeletal system in this context are very difficult to assess. An association between the mechanism of action of such drugs and the acquisition of neonatal contractures has nonetheless already been made (Jago 1970; Vincent et al. 1995; Riemersma et al. 1996; Jacobson et al. 1999; Godfrey & Barker 2000; Brownlow et al. 2001; Godfrey et al. 2001). Despite this, the possibility that PB administration may exert side effects on the developing skeleton remains unaddressed.
We have previously found that PB treatment rapidly induces a state of flaccid paralysis in the developing embryonic chick (Osborne et al. 2002). By administering PB in ovo, it has been possible to study the role of movement on limb development. This study is the first to report that the PB treatment induces a significant range of fixed, and diverse, positional deformities. Prolonged administration with PB also resulted in decreased body weight, accompanied by profound changes in skeletal growth. In addition to limb, spinal and craniofacial deformities, such treatment during the second half of gestation appears to convert a normal geometric pattern of weight gain to a simple arithmetic accretion. Analysis of the bones reveals significant reductions in limb length with an increase in cartilage to bone ratio indicating that flaccid paralysis also selectively provokes aberrations in the normal process of endochondral ossification. Finally, it is tempting to speculate that the skeletal phenotype induced by PB treatment in ovo represents a longer term consequence of isolating embryos from the sequelae of normal mobility during musculoskeletal development, and if parallels can be drawn between chick and human development then it raises questions regarding the prolonged use of such drugs in preterm neonates.
Materials and methods
Induction of flaccid paralysis
Fertilized white leghorn eggs were ‘windowed’ according to standard protocols (Belecky-Adams et al. 1996). Embryonic chicks at stage 36 (Hamburger & Hamilton 1992) were treated with 100 µl of a sterile-filtered solution of PB at 8 mg/ml in Tyrode's solution (TS, 0.8% NaCl, 0.02% KCl, 0.005% NaH2 PO4.H2O, 0.1% glucose and 0.1% NaHCO3 at pH 7.4) on the first day of treatment and at 5 mg/ml daily thereafter. Each daily 100 µl dose of PB was aseptically injected directly onto the chorioallantoic membrane. Three groups of chicks (n = 9 in each group) received daily treatment with PB; group 1 at stage 36, group 2 from stage 36 to 39 and group 3 from stage 36 to 43. Another three groups of chicks received TS, as a control, at the same times. One day after the final treatment (at stages 37, 40 and 44, respectively), chicks were killed by Schedule 1 procedure conforming to the Animal Scientific Procedures act 1986. This dosing regime examines the effects of 1, 4 or 7 days of immobilization commencing at day 10, during the 21-day gestation period of embryonic chicks.
Assessment of embryonic skeletal deformities
After the removal of skin and viscera, chicks were fixed in 96% ethanol for 3 days, weighed and the degree of skeletal deformities assessed; these included, beak, spine and limb appearances. The upper beak in a normal chick embryo protrudes slightly further than the lower beak (0 score). If the lower beak protruded further then the upper beak it was given 1–3 score depending on severity. Spinal deformities were also assessed. Rotational neck deformity (torticollis) was graded from 0 (normal phenotype) to 2 (severe neck curvature). Neck extension was ascribed when the angle between the upper half of the neck and the lower beak was more then 45°. Flexion was therefore less then 45°. Kyphosis is an abnormal anterior curvature of the thoracic or lumbar spine. The presence of a hump in the mid-thoracic spine was given a score of 1 and a normal smooth thoracic curve a score of 0. The tibiotarsal joint is normally held in flexion with a joint angle less than 45°; thus joint angles of greater values were defined as extended. The digits were examined for flexion at the metatarsophalangeal (MTP) or interphalangeal joints. If flexion was seen at any of these joints, the digit was considered to be flexed. Flexed and extended joints of each lower limb were recorded.
Assessment of cartilage and bone formation
After ethanol fixation, embryos were placed in acetone for 2 days to remove fat. Embryos were then rinsed in 96% ethanol for 1–2 h prior to staining in 0.015% Alcian blue, 0.005% Alizarin red in 70% ethanol, 20% acetic acid and 10% dH2O at 37 °C for up to 4 h. After rinsing in 96% ethanol for 1 h and washing under running tap water for another hour, muscle was cleared in an aqueous solution of 1% potassium hydroxide until the skeletons were visible. Stained embryos were then de-stained in a graded sequence of glycerol/potassium hydroxide starting with 20% glycerol/0.8% KOH, then 50% glycerol/0.5% KOH and finishing with 80% glycerol/0.2% KOH. Embryos were stored in 100% glycerol.
Image analysis
Images of each chick limb, orientated in the medial–lateral plane, were acquired using the KS300 Imaging System and a JVC 3CCD colour video. The following skeletal dimensions were determined for each element: (i) length of the femur (F), tibiotarsus (T) and tarsometatarsus (TM) defined as the distance between the proximal and distal articular surfaces; (ii) mid-diaphyseal breadth (F, T, TM); (iii) maximum breadth of proximal and distal epiphyses of stifle (knee, S), tibiotarsal (TT) and MTP joints. The area of bone was defined as those regions encompassed by staining with Alizarin red in femoral, tibiotarsal and metatarsal elements. This area was quantified automatically following image digitization, while manual measurements were made to determine the area of cartilage in proximal and distal articular joint regions of the stifle (knee), tibiotarsal and metatarsal phalangeal joints. All measurements were made on limbs from at least nine chicks in each treatment group.
Assessing the reversibility of flaccid paralysis
Twenty embryonic chicks at stage 36 (day 10) were treated with 100 µl of PB at 8 mg/ml in TS; three subsequent doses of 5 mg/ml were given daily until stage 39 (day 14). A control group of chicks (n = 5) were given TS alone. Thereafter, chicks were incubated without further intervention until stage 44 (day 18). At this time, they were removed from the egg, monitored for evidence of movement and killed by Schedule 1 procedure, and any limitation in the passive range of motions in the limbs was assessed.
Statistical assessment
Statistical analysis was performed using the Student's t-test. Significance of differences were attributed P < 0.05, P < 0.01 and P < 0.001.
Results
PB-induced flaccid paralysis diminishes embryo survival and body mass
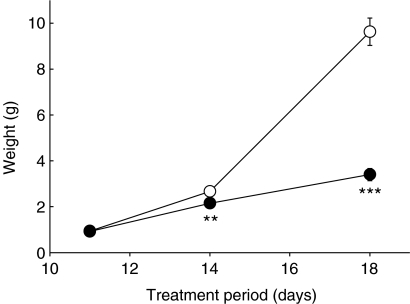
Daily PB administration from stage 36 (day 10) resulted in 100, 66 and 31% embryo survival at stage 37 (day 11), stage 40 (day 14) and stage 44 (day 18), respectively. Chick weights were not significantly affected after a single day of PB treatment. However, 3 days of treatment reduced weights by 24% (P < 0.01) and by 63% (P < 0.001) after 7 days compared to controls (Figure 1). This restricted growth in PB-treated chicks was associated with substantial preservation of the yolk (not shown) that is likely to be surplus to the requirements in an immobile chick embryo or to represent a fraction rendered inaccessible by such paralysis.
Figure 1.
Embryonic chick weights after flaccid immobilization. Measurement of weights (g) of embryonic chicks at stages 37,40 and 44 after 1, 3 and 7 days of treatment, respectively, with Tyrode's solution (○) or pancuronium bromide to induce flaccid paralysis (•). Each point represents the mean ± standard error of mean for nine chicks. ** denotes P < 0.01 and *** denotes P < 0.001, as assessed by the Student's t-test.
PB treatment induces skeletal abnormalities in embryonic chicks
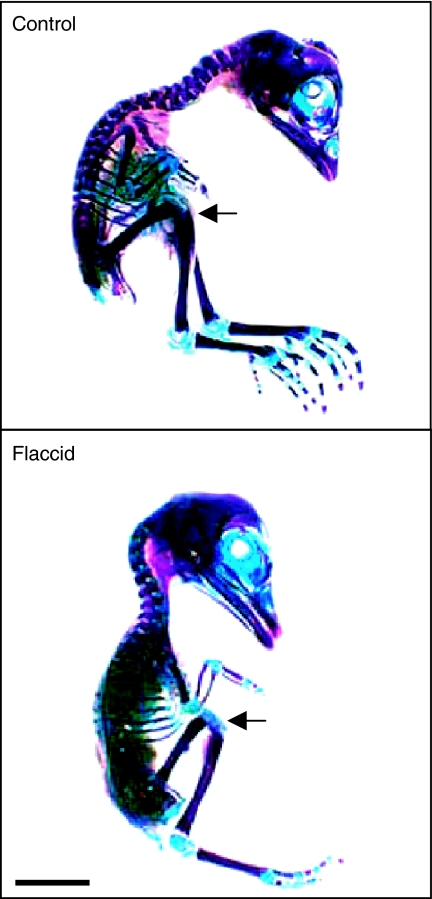
To determine whether PB-induced growth restriction was associated with any specific pattern of deformity, a systematic blind evaluation of macroscopic characteristics was performed (Table 1). Jaw prognathism was neither present in control chicks at any developmental stage nor in immobilized chicks after 1 day of treatment. All PB-treated chicks exhibited prognathism after prolonged immobilization, and the severity of lower jaw protrusion increased with increasing duration of PB-induced immobility (Figure 2 and Table 1). At day 11 (stage 37), a large proportion of control and PB-treated chicks exhibited features of torticollis. This phenotype therefore appears to be related to some normal pattern of muscular development at this stage. By day 14 (stage 40), the severity of torticollis had diminished in controls; however, with PB treatment, its severity was more pronounced and its incidence had increased to 88%, and by day 18, all PB-treated chicks exhibited torticollis. Kyphosis was not seen in any chicks. Head position was unaffected by PB; all chicks expressed a flexed phenotype during development (Table 1).
Table 1.
Effect of PB-induced flaccid immobilization on the percentage incidence of the skeletal deformities, jaw prognathism, torticollis and kyphosis compared to the equivalent stage-matched control chick
| Jaw prognathism (score) | Torticollis (score) | Kyphosis (score) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period of treatment (stages) | Treatment | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 0 | 1 | 2 |
| 36 and 37 | Control (TS) | 100 | 0 | 0 | 0 | 34 | 22 | 44 | 100 | 0 | 0 |
| Immobilized (PB) | 100 | 0 | 0 | 0 | 56 | 33 | 11 | 100 | 0 | 0 | |
| 36–40 | Control (TS) | 100 | 0 | 0 | 0 | 38 | 62 | 0 | 87 | 13 | 0 |
| Immobilized (PB) | 22 | 67 | 11 | 0 | 11 | 44 | 44 | 100 | 0 | 0 | |
| 36–44 | Control (TS) | 100 | 0 | 0 | 0 | 57 | 29 | 14 | 100 | 0 | 0 |
| Immobilized (PB) | 11 | 22 | 56 | 11 | 0 | 67 | 33 | 46 | 54 | 0 | |
pancuronium bromide
Tyrode's solution.
Figure 2.
Macroscopic appearance of flaccidly immobilized chicks. Macroscopic appearance of stage 44 chicks, Alcian blue- and Alizarin red-stained for cartilage and bone, respectively. Control chicks were treated with Tyrode's solution and flaccid chicks were treated with pancuronium bromide to induce flaccid paralysis from stage 36. Arrows indicate the position of the stifle joint. Scale bar is 1 cm.
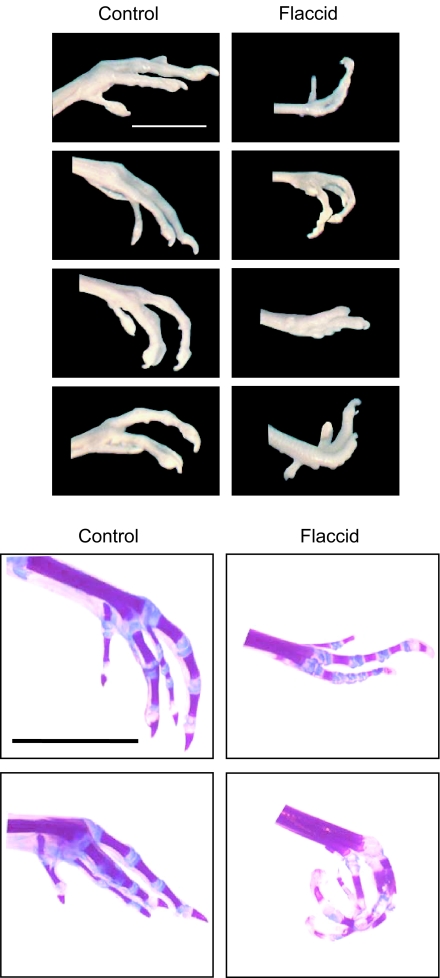
PB-treated chicks showed marked flexion of the stifle (knee) and increased incidence of tibiotarsal joint hyperextension (Figure 2), which were proportional to the duration of paralysis. At all stages, the majority of control chicks exhibited a neutral foot position (neither extended nor flexed). Within a single day of PB treatment (day 11), the majority of chicks showed greater foot extension (Table 2). At day 14, this PB-induced extension was still apparent in 60% of treated chicks. However, after 7 days of PB treatment (day 18), the foot positions present in the flaccidly immobilized chicks showed heterogeneity of phenotype, with equal numbers of feet in extreme flexed and extreme extended positions (Figure 3 and Table 2).
Table 2.
Effect of PB-induced flaccid immobilization on the percentage incidence of distinct foot positions.
| Foot position (% total) | ||||
|---|---|---|---|---|
| Period of treatment | Treatment | Hyperextended (stages) | Neutral | Hyperflexed |
| 36 and 37 | Control (TS) | 24 | 76 | 0 |
| Immobilized (PB) | 59 | 22 | 19 | |
| 36–40 | Control (TS) | 0 | 96 | 4 |
| Immobilized (PB) | 63 | 26 | 11 | |
| 36–44 | Control (TS) | 14 | 82 | 4 |
| Immobilized (PB) | 41 | 22 | 37 | |
pancuronium bromide
Tyrode's solution.
Figure 3.
Range of positional deformities in flaccidly immobilized chicks. Distal hind limbs from stage 44 chicks are shown before and after Alcian blue/Alizarin red staining. Chicks were treated with Tyrode's solution (control) or with pancuronium bromide (flaccid) from stage 36. Scale bar is 1 cm.
Flaccid paralysis produces specific changes in skeletal development
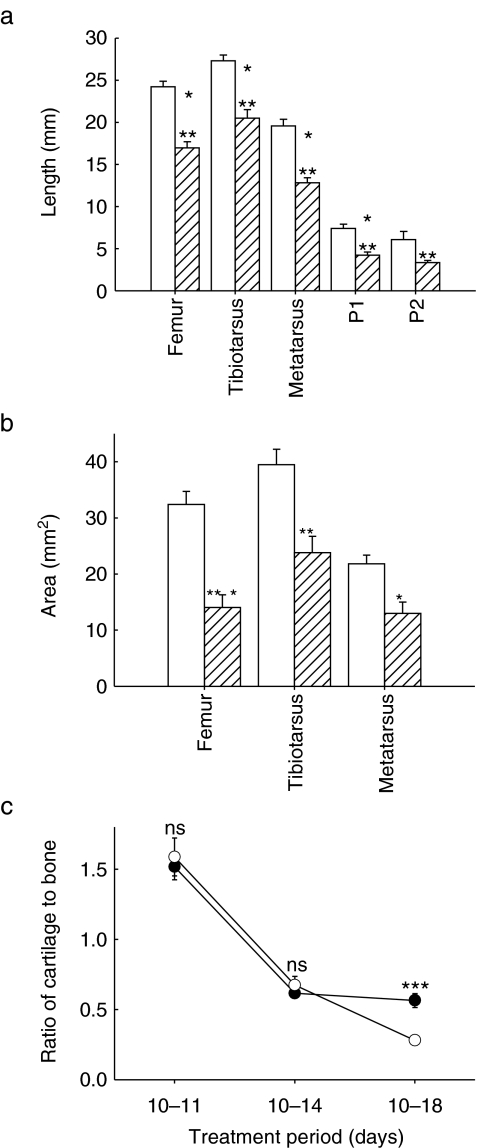
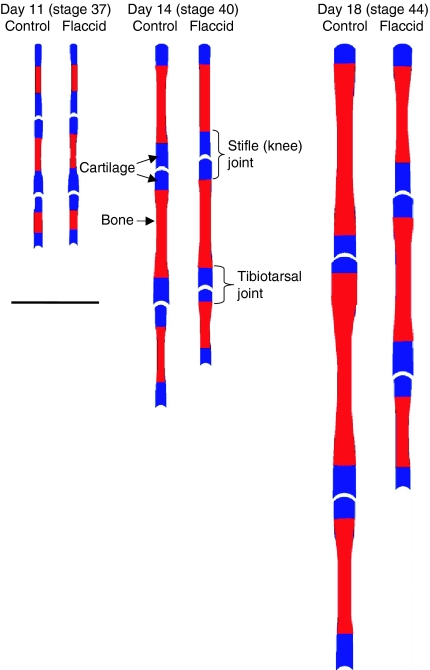
It is evident that the range of positional deformity in PB-treated chick limbs appears to be greatest at the extremities, and therefore we have examined whether this is related to specific changes in longitudinal or radial element growth or cartilage and bone area. Direct measurements showed that PB treatment significantly reduced the length of developing femoral, tibiotarsal, metatarsal and phalangeal elements (Figure 4a). This immobilization-induced diminution of longitudinal growth was not associated with any significant effect on epiphyseal or diaphyseal widths. In contrast, there was a significant reduction in element length, with the greatest reduction (45%) evident in the most distal elements.
Figure 4.
Altered skeletal development induced by flaccid immobilization. (a) Effect of flaccid immobilization between stages 36 and 44 on length of femur, tibiotarsus, metatarsus, first (P1) and second (P2) phalanges ( ) and control embryonic chicks (□). (b) Effect of flaccid immobilization on relative area of Alizarin red-stained bone in femur, tibiotarsus and metatarsus. Data are expressed as mean ± standard error of mean for at least nine chicks. □, control chicks;
) and control embryonic chicks (□). (b) Effect of flaccid immobilization on relative area of Alizarin red-stained bone in femur, tibiotarsus and metatarsus. Data are expressed as mean ± standard error of mean for at least nine chicks. □, control chicks;  , flaccidly immobilized chicks. (c) Effect of flaccid immobilization on the ratio of cartilage to bone in tibiotarsus, metatarsus and femur. Data are expressed as the mean ± standard error of mean for all limbs pooled. □, control chicks;
, flaccidly immobilized chicks. (c) Effect of flaccid immobilization on the ratio of cartilage to bone in tibiotarsus, metatarsus and femur. Data are expressed as the mean ± standard error of mean for all limbs pooled. □, control chicks;  , flaccidly immobilized chicks. Statistical analysis was performed using the Student's t-test where * denotes P < 0.05, ** denotes P < 0.01 and *** denotes P < 0.001.
, flaccidly immobilized chicks. Statistical analysis was performed using the Student's t-test where * denotes P < 0.05, ** denotes P < 0.01 and *** denotes P < 0.001.
Whole mount staining of embryos (stage 44) clearly differentiated the regions of cartilage and developing bone (Figure 2). Examination of bone and cartilage showed a significant reduction in bone area in hind limbs. This was greatest in the femur where bone area was reduced by 57% (P < 0.001) by day 18 (Figure 4b). By contrast, the area of cartilage in proximal and distal epiphyseal compartments of individual elements remained unaffected (not shown). By day 18, there were dramatic reductions in the cartilage to bone area ratio (Figure 4c) in all limb elements (P < 0.001). These results show that the cartilage to bone ratios in the femur, tibiotarsus and metatarsus diminish during development in normal limbs by a process that is likely to modify rates of endochondral ossification and that this is dramatically restricted by treatment with PB. Measurement of hind limb element length, width, breadth, bone and cartilage area (Fig. 5) show increasingly pronounced changes with progressively longer immobilization.
Figure 5.
Effects of pancuronium bromide (PB)-induced flaccid immobilization on skeletal development. Effects of flaccid (PB) paralysis (from stage 36) on element length, epiphyseal and diaphyseal breadth and proportional distribution of bone (red) and cartilage (blue) in developing limbs at stages 37, 40 and 44, compared to control chicks. Scale bar is 1 cm.
Effects of PB-induced flaccid paralysis are not readily reversed in ovo
Clinical use of PB is reliant on the reversibility of its direct actions at neuromuscular junctions. To evaluate the reversibility of its effects on phenotypic embryonic deformity, daily PB doses were administered from stage 36 (day 10) to 39 (day 13), without further treatment thereafter (until stage 44, day 18). This resulted in 30% (six of 20) chick survival with a 30% reduction in weight (data not shown). Surprisingly, none of these PB-treated embryos displayed any overt signs of movement at stage 44 and none of the hind limbs could be flexed or extended upon physical manipulation. Controls displayed normal movement and no limitation in the passive range of hind limb motion. The phenotype of these stage 44 chicks subjected to such brief PB treatment was indistinguishable from that observed in stage-matched embryos that had received prolonged PB administration from stage 36 to 43 (see above), indicating that no obvious recovery from brief immobilization had occurred at these times.
Discussion
We have found that PB administration to chick embryos in ovo results in fixed, but diverse, positional limb deformities. PB also restricts skeletal growth and induces malformations in the axial and appendicular skeleton and craniofacial deformity. Analysis of embryonic limbs reveals significant reductions in skeletal element length that involve selective diminution in bone deposition. Thus, our findings suggest that PB administration may indeed exert side effects on the developing chick skeleton. The diverse range of deformities in the body habitus is clearly a feature of our model and must provide clues to their aetiology.
The range and pattern of these deformities is evident as hyperflexion or hyperextension of similar joints in the distal elements. As the observed patterns of limb growth disturbance in our model are relatively homogenous, we consider it unlikely that differential growth underlies these phenotypes. Furthermore, decamethonium-induced rigid paralysis is known to induce the formation of a single pattern of stereotypic contractures (Hosseini & Hogg 1991a, b; Osborne et al. 2002). In contrast, PB induces phenotypic plasticity, suggesting that while decamethonium tonically drives particular deformities, PB promotes a range of ‘muscularly unlimited’ phenotypes.
These studies highlight the possibility that PB-induced flaccid paralysis isolates embryos from epigenetic (extrinsic) stimuli and allows intrinsic mechanisms to dominate development. Therefore, epigenetic mechanical influences may not only condition mobility, but when removed by flaccid immobility, either act to isolate the musculoskeletal system or can prolong such immobility at later times. It is debatable whether we should view these deformities as an appropriate ‘active process’ of functional adaptation or whether they represent a ‘blueprint’ upon which extrinsic mechanical influences can act.
However, it has previously been speculated that flaccid paralysis may produce more systemic alterations that indirectly impact upon the limb; for example, it is possible that paralysis modifies blood flow or nutrient supply (Visschedijk et al. 1985; Deeming et al. 1987; Bertram et al. 1997). It would also be naïve to imply that the avian embryo can be used as a model for the effects of PB on developing human embryos. Although flaccid PB-induced immobility leads to the formation of heterogeneous positional deformities in the developing chick, direct extrapolation of our findings to human fetuses should not be made. If parallels were drawn between chick and human development, it is of interest that the PB-induced chick skeletal phenotype has some correlates with clinical observations in PB-treated preterm neonates. Thus, it may be interpreted that both represent a longer term consequence of isolating preterm embryos from the sequelae of normal mobility during musculoskeletal development.
In this study, PB converts the longitudinal growth of long bones from a geometric to a linear accretion, and this is most drastic at early stages of long bone development. In contrast to the long-term effects of PB treatment on ossification, the area of epiphyseal cartilage remains largely unaffected and suggests that PB-induced failure in cartilage maturation, calcification and later replacement by bone is the reason that PB-treated elements expand at a diminished rate (Tanck et al. 2000). The fact that PB does not significantly affect diaphyseal width indicates that these growth processes are less dependent upon movement. The importance of differentiating between growth in bone width and length has been stressed (Bertram et al. 1997), and our findings suggest that the cellular populations responsible for growth plate-associated increases in length are more sensitive to the effects of flaccid immobility than periosteal osteoblasts. This agrees with studies using decamethonium-induced rigid paralysis, which produced diminished rates of chondrocyte proliferation (and recruitment) in chick growth plates (Germiller & Goldstein 1997) and significant reductions in the growth of clavicular, mandibular and long bones (Hall & Herring 1990). As a result, there is increasing interest in the importance of muscular action for normal bone growth in the new-born and in infancy, with a recognition for a need to think beyond purely nutritional concepts (Rodriguez et al. 1988a; b; Frost & Schonau 2000; Rauch & Schoenau 2001). Efforts to minimize the deleterious effects of immobility on bone mineralization and growth in the preterm infant have resulted in the successful implementation of daily physical exercise for very low-birth-weight infants (Moyer-Mileur et al. 2000).
Our results suggest that the immediate neuromuscular action of PB may be coupled with indirect downstream consequences for the developing musculoskeletal system. While the actions of PB at the neuromuscular junction are completely reversible, its prolonged administration may modify embryonic, fetal and neonatal musculoskeletal development. There is evidence indicating a direct association between the use of such drugs and the acquisition of neonatal contractures; the development of contractures in eight premature infants treated with PB (Fanconi et al. 1995); the development of severe tetraparesis and areflexia in 12 adult patients and a 9-month-old girl following prolonged PB treatment (Op de Coul et al. 1985; Bordet et al. 1995); and a large trial on preterm infants, which on the basis of the pulmonary and neurologic effects of PB, could not endorse its routine use (Cools & Offringa 2000). Moreover, it has also been shown that antibodies directed against human fetal Ach receptors (Vincent et al. 1995; Riemersma et al. 1996) act in the same way as PB-like drugs and induce contractures. Dramatic effects on stature and limb deformities are also seen in fetal mice administered with plasma from women bearing children with AMC (Jacobson et al. 1999). Ach receptor mutations produce congenital myasthenia with contractures (Brownlow et al. 2001), supporting the possibility that novel neuromuscular drug design may be capable of selectively targeting maternal or fetal neuromuscular junctions.
So far, we have concentrated on prolonged flaccidity; however, the effects of transient perturbation on the fetal phenotype remain unknown. Application of our findings within the terms recently defined by Barker's hypothesis (Godfrey & Barker 2000) would suggest that transient disturbances in the levels of embryonic motility at some, as yet undefined, stage of skeletal development may have profound long-term implications in the adult. This is supported by studies indicating that a combination of genetic and intrauterine environmental influences during prenatal skeletal development appears to reduce the risk of osteoporosis in adulthood (Godfrey et al. 2001), with mechanical factors vital for promoting normal bone growth and mineralization (Rauch & Schoenau 2001).
In conclusion, our studies are the first to report both stunting and diminished skeletal long bone growth in chick embryos exposed to PB-induced flaccidity, suggesting that immobility produces growth retardation in the context of an abundant, but redundant, food supply: ‘stunting in the face of plenty’. The development of a diverse pattern of PB-induced postural deformities suggests that the induction of flaccid paralysis may also have deleterious side effects that culminate in the irreversible modification in musculoskeletal function.
Acknowledgments
We thank the Arthritis Research Campaign for funding for EK, the Biotechnology and Biological Sciences Research Council for funding for KL and the Wellcome Trust for funding for JL. We also thank Mr R. Emery and A. Wallace for support of DS and Helen Hunt for excellent technical assistance.
References
- Atherton DP, Hunter JM. Clinical pharmacokinetics of the newer neuromuscular blocking drugs. Clin. Pharmacokinet. 1999;36:169–189. doi: 10.2165/00003088-199936030-00001. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams T, Cook B, Adler R. Correlations between terminal mitosis and differentiated fate of retinal precursor cells in vivo and in vitro: analysis with the “window-labeling” technique. Dev. Biol. 1996;178:304–315. doi: 10.1006/dbio.1996.0220. [DOI] [PubMed] [Google Scholar]
- Bertram JE, Greenberg LS, Miyake T, Hall BK. Paralysis and long bone growth in the chick: growth shape trajectories of the pelvic limb. Growth Dev. Aging. 1997;61:51–60. [PubMed] [Google Scholar]
- Bordet F, Contamin B, Berthier JC, Rousson A, Pondarre C. Tetraparesis in an infant after prolonged administration of pancuronium. Ann. Fr. Anesth. Reanim. 1995;14:426–428. doi: 10.1016/s0750-7658(05)80396-x. [DOI] [PubMed] [Google Scholar]
- Bowman W, Rand M. Striated Muscle and Neuromuscular Transmission. Cambridge: Blackwell Scientific Publications; 1980. [Google Scholar]
- Brownlow S, Webster R, et al. Acetylcholine receptor delta subunit mutations underlie a fast-channel myasthenic syndrome and arthrogryposis multiplex congenita. J. Clin. Invest. 2001;108:125–130. doi: 10.1172/JCI12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools F, Offringa M. Neuromuscular paralysis for newborn infants receiving mechanical ventilation. Cochrane Database Syst. Rev. 2000;4:CD002773. doi: 10.1002/14651858.CD002773. [DOI] [PubMed] [Google Scholar]
- Crossland J. Drugs that Block Neuromuscular or Ganglionic Transmission. New York: Churchill Livingstone; 1980. [Google Scholar]
- Deeming DC, Rowlett K, Simkiss K. Physical influences on embryo development. J. Exp. Zool. 1987;1:341–345. [PubMed] [Google Scholar]
- Drachman D, Coulombre A. Experimental clubfoot and arthrogryposis multiplex congenita. Lancet. 1962;11:523–526. doi: 10.1016/s0140-6736(62)90399-9. [DOI] [PubMed] [Google Scholar]
- Fanconi S, Ensner S, Knecht B. Effects of paralysis with pancuronium bromide on joint mobility in premature infants. J. Pediatr. 1995;127:134–136. doi: 10.1016/s0022-3476(95)70274-1. [DOI] [PubMed] [Google Scholar]
- Fell HB, Canti RG. Experiments on the development in vitro of the avian knee joint. Proc. R. Soc. 1934;B1176:316–351. [Google Scholar]
- Frost HM, Schonau E. The “muscle-bone unit” in children and adolescents: a 2000 overview. J. Pediatr. Endocrinol. Metab. 2000;13:571–590. doi: 10.1515/jpem.2000.13.6.571. [DOI] [PubMed] [Google Scholar]
- Germiller JA, Goldstein SA. Structure and function of embryonic growth plate in the absence of functioning skeletal muscle. J. Orthop Res. 1997;15:362–370. doi: 10.1002/jor.1100150308. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000;71:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- Godfrey K, Walker-Bone K, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J. Bone Miner. Res. 2001;16:1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J. Morphol. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo 1951. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hosseini A, Hogg DA. The effects of paralysis on skeletal development in the chick embryo. II. Effects on histogenesis of the tibia. J. Anat. 1991a;177:169–178. [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Hogg DA. The effects of paralysis on skeletal development in the chick embryo. I. General effects. J. Anat. 1991b;177:159–168. [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Polizzi A, Morriss-Kay G, Vincent A. Plasma from human mothers of fetuses with severe arthrogryposis multiplex congenita causes deformities in mice. J. Clin. Invest. 1999;103:1031–1038. doi: 10.1172/JCI5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jago RH. Arthrogryposis following treatment of maternal tetanus with muscle relaxants. Arch. Dis. Child. 1970;45:277–279. doi: 10.1136/adc.45.240.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessinger AC. Fetal akinesia deformation sequence: an animal model. Pediatrics. 1983;72:857–863. [PubMed] [Google Scholar]
- Moyer-Mileur LJ, Brunstetter V, McNaught TP, Gill G, Chan GM. Daily physical activity program increases bone mineralization and growth in preterm very low birth weight infants. Pediatrics. 2000;106:1088–1092. doi: 10.1542/peds.106.5.1088. [DOI] [PubMed] [Google Scholar]
- Op de Coul AA, Lambregts PC, Koeman J, van Puyenbroek MJ, Ter Laak HJ, Gabreels-Festen AA. Neuromuscular complications in patients given Pavulon (pancuronium bromide) during artificial ventilation. Clin. Neurol. Neurosurg. 1985;87:17–22. doi: 10.1016/0303-8467(85)90060-5. [DOI] [PubMed] [Google Scholar]
- Osborne AC, Lamb KJ, Lewthwaite JC, Dowthwaite GP, Pitsillides AA. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J. Musculoskelet. Neuron Interact. 2002;5:448–456. [PubMed] [Google Scholar]
- Rang H, Dale M, Ritler J. Local Anaesthetics and Other Drugs that Affect Excitable Membranes. New York: Churchill Livingstone; 1995. [Google Scholar]
- Rauch F, Schoenau E. The developing bone: slave or master of its cells and molecules? Pediatr. Res. 2001;50:309–314. doi: 10.1203/00006450-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Riemersma S, Vincent A, Beeson D, et al. Association of arthrogryposis multiplex congenita with maternal antibodies inhibiting fetal acetylcholine receptor function. J. Clin. Invest. 1996;98:2358–2363. doi: 10.1172/JCI119048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JI, Garcia-Alix A, Palacios J, Paniagua R. Changes in the long bones due to fetal immobility caused by neuromuscular disease. A radiographic and histological study. J. Bone Joint Surg. Am. 1988a;70:1052–1060. [PubMed] [Google Scholar]
- Rodriguez JI, Palacios J, Garcia-Alix A, Pastor I, Paniagua R. Effects of immobilization on fetal bone development. A morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif. Tissue Int. 1988b;43:335–339. doi: 10.1007/BF02553275. [DOI] [PubMed] [Google Scholar]
- Tanck E, Blankevoort L, Haaijman A, Burger EH, Huiskes R. Influence of muscular activity on local mineralization patterns in metatarsals of the embryonic mouse. J. Orthop. Res. 2000;18:613–619. doi: 10.1002/jor.1100180414. [DOI] [PubMed] [Google Scholar]
- Vincent A, Newland C, Brueton L, et al. Arthrogryposis multiplex congenita with maternal autoantibodies specific for a fetal antigen. Lancet. 1995;346:24–25. doi: 10.1016/s0140-6736(95)92652-6. [DOI] [PubMed] [Google Scholar]
- Visschedijk AH, Tazawa H, Piiper J. Variability of shell conductance and gas exchange of chicken eggs. Respir. Physiol. 1985;59:339–345. doi: 10.1016/0034-5687(85)90137-9. [DOI] [PubMed] [Google Scholar]