Abstract
Classic studies of tuberculosis (TB) revealed morphologic evidence of considerable heterogeneity of macrophages (MØs), but the functional significance of this heterogeneity remains unknown. We have used newly available specific antibodies for selected membrane and secretory molecules to examine the phenotype of MØs in situ in a range of South African patients with TB, compared with sarcoidosis. Patients were human immunodeficiency virus-negative adults and children, and the examined biopsy specimens included lung and lymph nodes. Mature pulmonary MØs (alveolar, interstitial, epithelioid and multinucleated giant cells) selectively expressed scavenger receptor type A and a novel carboxypeptidase-like antigen called carboxypeptidase-related vitellogenin-like MØ molecule (CPVL). CPVL did not display enhanced expression in sarcoidosis, vs. TB patients, as observed with angiotensin-converting enzyme (ACE), a related molecule. Immunocytochemical studies with surfactant proteins (SP)-A and -D showed that type II alveolar cells expressed these collectins, as did MØs, possibly after binding of secreted proteins. Studies with an antibody specific for the C-terminus of fractalkine, a tethered CX3C chemokine, confirmed synthesis of this molecule by bronchiolar epithelial cells and occasional endothelial cells. These studies provide new marker antigens and extend previous studies on MØ differentiation, activation and local interactions in chronic human granulomatous inflammation in the lung.
Keywords: antigens, CPVL, fractalkine, macrophages, sarcoidosis, scavenger receptor, tuberculosis
Macrophages (MØs) play a central role in chronic inflammation that is associated with infections, such as tuberculosis (TB) (Dannenberg & Rook 1994) and similar granulomatous diseases of unknown origin, such as sarcoidosis (Siltzbach 1976). Much has been learned, concerning the activation of MØs in cellular immunity, mostly from initial studies in vitro, (Schreiber & Hamilton 2002), subsequently confirmed by analysis of mycobacterial infections utilizing null mutations in mice (Dalton et al. 1993) and inborn errors in humans (Casanova & Abel 2002). Yet, we still know very little about the differentiation, heterogeneity and functions of MØs in situ, and of their interactions with other cells in a particular local microenvironment including the lung (Gordon & Hughes 1997), a prime target of these diseases.
Histological studies of human TB, mainly in the pre-antibiotic era, revealed striking morphologic heterogeneity among pulmonary alveolar MØs, epithelioid cells and multinucleated (Langhans'-type) giant cells (Dannenberg & Rook 1994). Experimental analysis of mycobacterial infection in the rabbit, (Dannenberg & Rook 1994), guinea pig (Adams 1976) and the mouse (Orme & Collins 1994) mimicked some, but not all, features of human infection (Spector 1969). Subsequent work on the cell biology of host–mycobacterial interactions has brought insights into pathogen entry (Schlesinger 1997), phagosome pathophysiology (Clemens & Horwitz 1995) and evasion of antimicrobial mechanisms (Russell 2001). Recent completion of the genome of selected Mycobacterium tuberculosis strains has focused attention on microbial factors responsible for virulence (Glickman & Jacobs Jr. 2001).
TB remains a major cause of morbidity and death, especially in sub-Saharan Africa, where the increasing incidence of acquired immune deficiency syndrome has produced an explosive epidemic of both diseases. We have, therefore, undertaken the study of MØs in tissue obtained from patients with florid disease, TB and, for comparison, sarcoidosis, but free of human immunodeficiency virus (HIV). We have selected new antigenic markers of MØs, to learn more about the natural history of MØs in lungs and lymph nodes obtained from adults and children. The type A scavenger receptor (SR-A) (Krieger & Hertz 1994) is a pattern recognition membrane molecule and a candidate receptor for non-opsonic uptake of mycobacteria by MØs (Ernst 1998), along with mannosyl (Schlesinger 1997), complement receptors (Hirsch et al. 1994) and CD1 (Porcelli et al. 1998). It also plays a role in modulating effector responses, such as tumour necrosis factor-α production, by immunologically primed MØs (Haworth et al. 1997), together with other receptors, such as CD14 (Heldwein & Fenton 2002) and Toll-like receptors (Stenger & Modlin 2002), in experimental mycobacterial infection.
A second antigen chosen for analysis is a newly identified carboxypeptidase-related vitellogenin-like MØ molecule (CPVL), selectively expressed by MØs in vitro (Mahoney et al. 2001). CPVL resembles a known marker enzyme, angiotensin-converting enzyme (ACE), which is upregulated by chronic granulomatous diseases, especially sarcoidosis (Silverstein et al. 1979), and which is potentially significant in modulating the activity of inflammatory mediators.
In order to learn more about local interactions in the lung, we also selected marker molecules expressed by epithelial cells and possibly important in MØ responses. Surfactant proteins (SP)-A and -D are produced by type II alveolar cells and are candidate opsonins for clearance of micro-organisms, including mycobacteria, (Schlesinger 1997; Holmskov 2000), by MØs; fractalkine (CX3CL1) is a unique membrane-anchored chemokine for mononuclear cell recruitment and adhesion (Bazan et al. 1997). Recent studies have utilized a specific polyclonal antibody directed against an intracellular, cytoplasmic peptide, C-terminal fractalkine (C-Fk), to detect synthesis of the transmembrane forms by epithelial cells in gut and skin (Lucas et al. 2001).
In this paper, we report on the expression of the above antigens in situ, in relation to MØ specificity and selected aspects of disease.
Materials and methods
Patient details
Human tissues were obtained from the Department of Anatomical Pathology, Tygerberg Hospital, Cape Town, South Africa. Control uninfected lung was obtained from a cancer patient following a lobectomy. The investigated sections originate from an area distant from the tumour and free of disease. Lung sections from ten adult TB patients were obtained during surgery that resulted from haemoptysis. Only 60% of these patients had received pre-operative treatment. Nine adult sarcoidosis patients were used for comparison with TB as a chronic granulomatous disease of unknown aetiology. Sarcoidosis patients were untreated before their open lung biopsy. Lung sections from three child TB patients were analysed, together with lymph nodes from seven M. tuberculosis infected children. Paediatric lung biopsies were performed as a result of respiratory complications of disease subsequently diagnosed as TB. Lymph node biopsies were used in the diagnosis of TB. Patient details are outlined in Table 1. All patients were HIV negative; the TB patients were all culture positive for drug-sensitive M. tuberculosis. Approval for this study was obtained from the Ethical Review Committee, University of Stellenbosch (Stellenbosch, South Africa).
Table 1.
Patient details
| Patient | Age (years) | Sex | Tissue | Time (years) since previous episode | Duration of pre-operative treatment (months) |
|---|---|---|---|---|---|
| A 1–7 | 42 ± 14 | F | L (TB) | Np-24 | 0–12 |
| A8–10 | 40 ± 6 | M | L (TB) | Np | 0–4 |
| A11–14 | 36 ± 10 | F | L (S) | 0 | 0 |
| A15–19 | 35 ± 8 | M | L (S) | 0 | 0 |
| C1–2 | 3 and 6 | F | L (TB) | 2 and Np | 6 |
| C3 | 7 | M | L (TB) | 5 | 2 |
| C4–5 | 2 and 8 | F | LN (TB) | Np | 4–5 days |
| C6–10 | 4 ± 2 | M | LN (TB) | Np | 0–20 days |
Pre-operative treatment included rifampicin, isoniazid and pyrazinamide. Age is mean ± SEM. C, child; A, adult; F, female; M, male; L, lung; LN, lymph node; TB, tuberculosis; S, sarcoid; Np, no previous episode.
Immunohistochemical analysis
Immunohistochemical analysis with a panel of antibodies was performed using fixed, paraffin-embedded sections. The antibodies included anti-SR-A, anti-CPVL, anti-ACE, anti-SP-A, anti-SP-D and anti-C-Fk. The antibody details, sources and corresponding secondary antibodies are described in Table 2. Consecutive sections of the paraffin-embedded tissue were cut as 5 µm sections and fixed to slides precoated with aminopropyltriethoxysilane (5 µg/ml) (Sigma-Aldrich, St. Louis, MO, USA). Sections were deparaffinized in xylene and rehydrated through graded ethanols. After rinsing sections in water, endogenous peroxidase activity was blocked by exposing sections to 0.3% hydrogen peroxide/methanol solution. Antigen retrieval for anti-CD68 (Micklem et al. 1989) was performed by treating the sections with trypsin (1 mg/ml) (Sigma-Aldrich) for 10 min. Antigen retrieval for all other antibodies was performed by microwave for four 5-min periods and two 5-min periods for diseased and normal tissue, respectively, at 700 W in 10 mM citrate buffer (pH 6.0). The sections were left in the hot citrate buffer for 15 min and then washed in distilled water for 5 min. After 10-min blocking at room temperature with 5% normal sheep serum (Sigma-Aldrich) in phosphate-buffered saline (PBS) for anti-SR-A and anti-CD68, 5% normal rabbit serum (Sigma-Aldrich) for anti-ACE and 5% normal donkey serum (Sigma-Aldrich) for anti-CPVL, anti-SP-A, anti-SP-D and anti-C-Fk, sections were incubated with appropriate polyclonal antibodies overnight at 4 °C for diseased tissue or 1 h at room temperature for normal tissue. The antibodies were diluted in 5% serum in PBS to optimal concentrations determined by preliminary titrations. After the slides were placed in three 5-min washes in PBS, the sections were incubated with secondary antibody (Table 2) at 4 °C for 1 hour, washed again for three 5-min intervals and then incubated with avidin conjugated to horseradish peroxidase (DAKO, Glostrup, Denmark) for 30 min. Sections were washed again in PBS before incubation with DAB (Vector Laboratories, Burlingame, CA, USA). Sections were finally counterstained with Meyer's haematoxylin (Sigma-Aldrich) and mounted with aqueous Faramount (DAKO). As a negative control, primary antibody was omitted or neutralized with the appropriate immunizing peptide. In the case of CPVL, this was performed according to Mahoney et al. (2001). Specificity of the CPVL reagent was confirmed by staining of transfected Chinese hamster ovary (CHO) cells (unpublished observation). Anti-C-Fk activity was removed as described by Lucas et al. (2001).
Table 2.
Antibodies used in this study
| Reagent | Control reagent | Source of primary antibody | Secondary antibody | Source of secondary antibody | Reference |
|---|---|---|---|---|---|
| Mouse anti-human SR-A (polyclonal) | Mouse IgG1 | P. Gough (University of Oxford) | Sheep anti-mouse Ig | Boehringer-Mannheim Roche | (Gough et al. 1999) |
| Rabbit anti-human CPVL | Pre-absorption with ×10 molar | J. Mahoney (University of Oxford) | Donkey anti-rabbit F(ab′)2 fragment | Jackson Immuno-research Laboratory | (Mahoney et al. 2001) |
| excess immunizing peptide | |||||
| Chicken anti-human ACE | E. Sturrock (UCT) | Rabbit anti-chicken Ig | Sigma-Aldrich | (Brice et al. 1995) | |
| Rabbit anti-human SP-A | K. Reid (University of Oxford) | Donkey anti-rabbit F(ab′)2 fragment | Jackson Immuno-research Laboratory | (Holmskov 2000) | |
| Rabbit anti-human SP-D | K. Reid (University of Oxford) | Donkey anti-rabbit F(ab′)2 fragment | Jackson Immuno-research Laboratory | (Holmskov 2000) | |
| Rabbit anti-human C-Fk | Pre-absorption with ×10 molar | A. Lucas (University of Oxford) | Donkey anti-rabbit F(ab′)2 fragment | Jackson Immuno-research Laboratory | (Lucas et al. 2001) |
| excess immunizing peptide | |||||
| Mouse anti-human CD68 (mAb) | Mouse IgG1 | DAKO | Sheep anti-mouse Ig | Boehringer-Mannheim Roche | (Micklem et al. 1989) |
Polyclonal first antibodies were affinity purified. Primary and secondary antibodies were used at optimal concentrations that were determined for diseased and normal tissue by preliminary titration.
Ig, immunoglobulin; SR-A, scavenger receptor A; CPVL, carboxypeptidase-related vitellogenin-like macrophage molecule; ACE, angiotensin-converting enzyme, SP-A, surfactant protein A; SP-D, surfactant protein D; C-Fk, C-terminal fractalkine; mAb, monoclonal antibody.
Image capture
A Zeiss microscope (Axioskop 2), fitted with a video camera, was used to capture the images, which were saved by Axiovision (Zeiss, Germany).
Assessment of sections
The immunohistochemistry technique employed in this study is not quantitative. However, the immunoreactivity of each section, indicated by the brown colour reaction, was graded as: – (no positivity), + (weak positivity), ++ (moderate positivity) and +++ (strong positivity) for each antigen (SR-A, CPVL, ACE, SP-A, -D and C-Fk) in every patient's specimen. Sections were scored independently by three different observers to ensure reproducibility.
Results
Histological analysis of patient granulomas
The ten adult TB patients (A1–10; Table 1) presented with florid granulomas that were either necrotic (containing caseous necrosis) or non-necrotic. Granulomas were associated with giant cells at both stages of development. Ziehl–Neelsen staining of these adult TB lung sections revealed low levels of acid-fast bacilli within the regions of caseous necrosis (not shown), despite the fact that most patients had received multidrug therapy prior to surgery. The nine adult sarcoidosis patients (A11–19; Table 1) presented with non-necrotic pulmonary granulomas with interspersed giant cells. Lung sections from these patients were negative for acid-fast bacilli upon Ziehl–Neelsen staining. The children's lung (C1–3; Table 1) and lymph node tissue (C4–10; Table 1) presented with non-necrotic and necrotic granulomas that were associated with giant cells. Ziehl–Neelsen staining revealed the presence of acid-fast bacilli (not shown), although all these patients had received multidrug therapy prior to surgery (C1–10; Table 1). Granulomas from all patients, irrespective of their age, the disease or the tissue, contained epithelioid MØs. These large MØs could be distinguished from interstitial mature MØs that occupied areas between the blood vessels and the alveolar spaces.
General assessment of staining pattern
Overall evaluation of the relative staining of the tissue for each antigen was done at low-power (×100) magnification, and the results are summarized in Tables 3 and 4. Table 3 compares the expression of, mainly, MØ antigens in different tissues (see below for detailed cellular specificity). SR-A was expressed at moderately to strongly positive levels in child TB lung and lymph node, and adult TB lungs. Lung sections from sarcoidosis patients demonstrated a wider, generally weaker range of signal. CPVL expression in child TB lung sections was intense in all sections, compared to adult TB and sarcoid lung, and child TB lymph node, where expression varied more widely. ACE was absent in 20% of adult TB patients, whereas the remaining adult patients demonstrated weak to moderate immunoreactivity.
Table 3.
SR-A-, CPVL- and ACE-staining intensities in tuberculosis and sarcoid tissue
| SR-A (%) | CPVL (%) | ACE(%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue type (number analysed) | – | + | ++ | +++ | – | + | ++ | +++ | – | + | ++ | +++ |
| Adult TB lung (10) | 90 | 10 | 40 | 50 | 10 | 20 | 50 | 20 | ||||
| Adult sarcoid lung (9) | 22 | 45 | 33 | 11 | 11 | 67 | 11 | 33 | 67 | |||
| Child TB lung (3) | 100 | 100 | 100 | |||||||||
| Child TB lymph node(7) | 29 | 71 | 28.5 | 28.5 | 43 | 67 | 33 | |||||
The number of sections demonstrating a specific signal intensity is presented as a percentage of the total number of cases of each type.
SR-A, scavenger receptor A; CPVL, carboxypeptidase-related vitellogenin-like macrophage molecule; ACE, angiotensin-converting enzyme; TB, tuberculosis; –, no positivity; +, weak positivity; ++, moderate positivity; +++, strong positivity.
Table 4.
SP-A-, SP-D- and C-Fk-staining intensities in tuberculosis and sarcoid tissue
| SP-A (%) | SP-D (%) | C-Fk(%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue type (number analysed) | – | + | ++ | +++ | – | + | ++ | +++ | – | + | ++ | +++ |
| Adult TB lung (10) | 10 | 60 | 30 | 40 | 60 | 20 | 80 | |||||
| Adult sarcoid lung (9) | 67 | 33 | 44 | 56 | 56 | 11 | 33 | |||||
| Child TB lung (3) | 67 | 33 | 33 | 67 | 100 | |||||||
| Child TB lymph node (7) | 100 | 100 | 86 | 14 | ||||||||
The number of sections demonstrating a specific signal intensity is presented as a percentage of the total number of cases of each type. SP-A, surfactant protein A; SP-D, surfactant protein D; C-Fk, C-terminal fractalkine; TB, tuberculosis; –, no positivity; +, weak positivity; ++, moderate positivity; +++, strong positivity.
Child TB lung and lymph node demonstrated moderate and moderate to strong ACE signal, respectively. ACE expression in most of the lung sections from sarcoidosis patients was moderate to strong. In summary, the main difference between TB and sarcoidosis in adult lung was the higher level of ACE expression in sarcoidosis, whereas SR-A and CPVL were comparable or slightly reduced. Specimens from children with TB stained more strongly for all three markers than from adults, approaching the high level of staining in sarcoid lung in the case of ACE. Whilst the number of child TB lung specimens was small compared with others, staining was more homogeneous than in diseased lymph nodes.
Table 4 summarizes staining intensities for markers predominantly expressed by epithelial cells. All patients expressed moderate to strong SP-A and -D, with no obvious difference associated with disease, age or tissue. By contrast, whilst C-Fk expression was strongly positive in TB lung (late adult and child), it was absent to minimal in lymph nodes from children with TB, consistent with a tissue-specific source (see below). C-Fk expression in adult sarcoid lung was also more heterogeneous (33% strongly positive and 56% negative) than in adult TB lung, consistent with disease-related differences.
Cellular expression of different antigens
The above analysis showed consistent results among patients with the markers investigated. The specificity of the cell types expressing each antigen will now be described and illustrated in detail. The results are summarized in Tables 5 and 6.
Table 5.
Cell localization of SR-A, CPVL and ACE in tuberculosis and sarcoid tissue
| Cell type | SR-A | CPVL | ACE |
|---|---|---|---|
| Monocytes | – | – | – |
| Alveolar macrophages | + | + | +* |
| Epithelioid macrophages | + | + | + |
| Interstitial macrophages | + | + (TB)† | – |
| Multinucleated giant cells | +/– | +/– | + |
| Epithelium | – | – | + |
| Endothelium | – | – | – |
| Type II pneumocytes | – | – | +/– |
SR-A, scavenger receptor A; CPVL, carboxypeptidase-related vitellogenin-like macrophage molecule; ACE, angiotensin-converting enzyme; TB, tuberculosis;+, immunoreactive cells; –, immuno-unreactive cells; +/–, immunoreactive and immuno-unreactive cells within a section
Signal weaker in TB cases compared to sarcoidosis sections, although highly variable between patients.
No signal in adult sarcoid lung tissue
Table 6.
Cell localization of SP-A, SP-D and C-Fk in tuberculosis and sarcoid tissue
| Cell type | SP-A | SP-D | C-Fk |
|---|---|---|---|
| Monocytes | + | – | – |
| Alveolar macrophages | + | + | – |
| Epithelioid macrophages | + (TB child lung LN; sarcoid)* | + (TB LN; sarcoid)‡ | – |
| Interstitial macrophages | + | – | – |
| Multinucleated giant cell | + (Sarcoid)† | (TB LN; sarcoid)§ | – |
| Epithelium | + | – | + |
| Endothelium | – | – | + |
| Type II pneumocytes | + | + | +/– |
SP-A, surfactant protein A; SP-D, surfactant protein D; C-Fk, C-terminal fractalkine; TB, tuberculosis; + immunoreactive cells; –, immuno-unreactive cells; +/-, immunoreactive and immuno-unreactive cells within a section; LN, lymph node.
No signal in adult TB lung tissue.
No signal in adult and child TB tissue.
No signal in adult and child TB lung tissue.
No signal in adult and child TB lung tissue.
Comparison of SR-A and CD68 expression
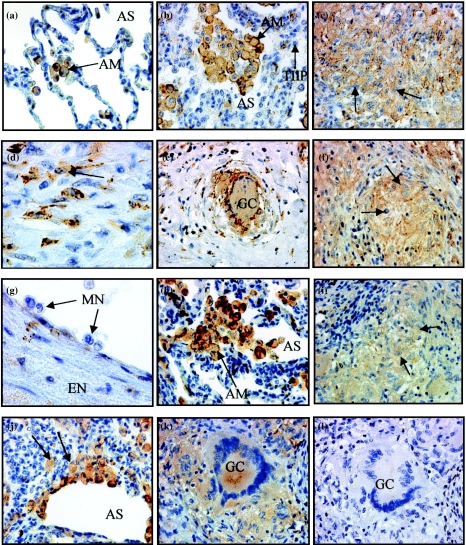
SR-A expression in normal lung sections was restricted to alveolar MØs (Gough et al. 1999) (Figure 1a). In ten adult TB and nine sarcoid lung sections, SR-A expression localized to mature MØs, where it demonstrated notable cell-surface staining (Figure 1b, h). The immunoreactive subpopulations in diseased lung sections included alveolar (Figure 1a, b) epithelioid (Figure 1c, i; TB and sarcoidosis, respectively) and interstitial MØs (Figure 1d, j; TB and sarcoidosis, respectively) (Table 5). Epithelioid MØs in child TB lymph nodes also demonstrated SR-A immunoreactivity (Figure 1f). The signal intensity of alveolar MØs (Figure 1b, h) was stronger than that in epithelioid (Figure 1c, f, i) and interstitial MØs (Figure 1d, j), suggesting that distinct MØ populations may differ in their role in the immune response or possess different functions within lung tissue. Giant cells demonstrated SR-A immunoreactivity in tissues from both diseases (Figure 1e, k), whereas type II pneumocytes (Figure 1b), endothelium (Figure 1g) and epithelium were negative for SR-A (Table 5). Monocytes within blood vessels of diseased tissues were also negative for SR-A (Figure 1g), consistent with other studies (Gough et al. 1999). These results show that SR-A co-localized to the same cells in TB and non-TB sections. When the primary antibody was omitted (Figure 1l) or replaced by an isotype-matched control, immunoglobulin G1 (IgG1) (not shown), no signal was evident.
Figure 1.
Scavenger receptor A (SR-A) expression in normal lung, tuberculosis (TB) and sarcoidosis tissue. Immunohistochemistry was performed on paraffin-embedded adult TB lung, child TB lymph node and adult sarcoid lung. Sections were stained with anti-SR-A antibody, followed by streptavidin-biotin immunoperoxidase. The immunoreaction product is brown, and sections were counterstained with Meyer's haematoxylin. (a) SR-A+ alveolar macrophages (MØs) in normal lung. SR-A+ alveolar (b) and epithelioid (c) MØs on the edge of a granuloma, interstitial MØs (d) in TB adult lung and positive epithelioid MØs (f) on the edge of a granuloma in a child TB lymph node. SR-A– type II pneumocytes (b) in adult TB lung are shown adjacent to positive alveolar MØs and SR-A– monocytes in adult TB lung (g) Alveolar MØs (h) in adult sarcoidosis lung are SR-A+. Epithelioid (i) and interstitial (j) MØs and giant cells (k) from the edge of a granuloma in sarcoidosis lung are SR-A+. Sections adjacent to those in (a)–(k) were negative when anti-SR-A was omitted (l) Magnification, ×400 [(a)–(c), (e) –(f), (h)–(l)] and ×1000 [(d), (g)]. AM, alveolar MØ; AS, alveolar space; EN, endothelium; GC, giant cell; MN, monocytes; TIIP, type II pneumocytes.
Unlike SR-A which demonstrated pronounced surface as well as intracellular staining of MØs, CD68 expression was mainly intracellular (not shown). Although the function of CD68 is unknown, it serves as a well-accepted pan-MØ marker. SR-A was, therefore, compared with CD68 as a possible alternative MØ marker. Similarly to SR-A, CD68 immunoreactive cells included MØs (alveolar, interstitial and epithelioid) and giant cells in lung and lymph node sections from TB and sarcoidosis patients (not shown). Unlike SR-A labelling, CD68 immunoreactivity of interstitial and epithelioid MØs, as well as of giant cells, was as strong as that of alveolar MØs. Epithelium, endothelium and type II pneumocytes did not display any CD68 signal (not shown). CD68 expression was, therefore, restricted to MØ-related cells in all sections investigated, similar to SR-A.
Analysis of CPVL expression
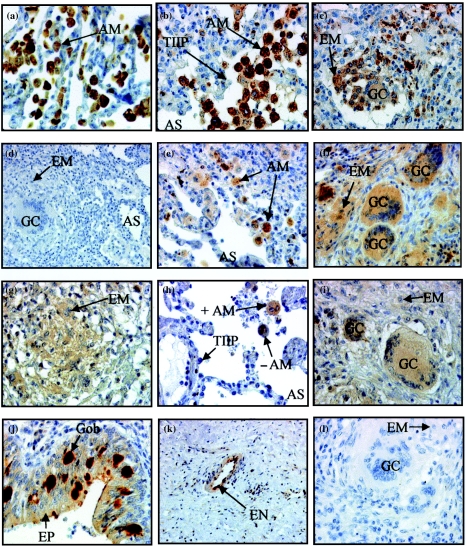
CPVL expression in normal lung was exclusively localized to alveolar MØs (Figure 2a). Specific CPVL immunoreactivity in TB lung made the alveolar (Figure 2b), epithelioid MØs and giant cells (Figure 2c) particularly easy to identify. Expression appeared to be intracellular (cytoplasmic) (Figure 2a, b, c) and was associated with granulomas (not shown). Among different subpopulations, epithelioid MØs and giant cells exhibited lower signal intensity than alveolar MØs, while monocyte immunoreactivity was absent (not shown). Apart from the absence of signal in interstitial MØs, similar cell localization was evident in sarcoid lung. Owing to the restricted cell localization of this antigen (Table 5), CPVL appears to be an excellent in situ marker for cells of the MØ lineage in this tissue. Epithelial cells, endothelial cells and type II pneumocytes did not express CPVL (Table 5). Despite identical cell localization patterns in adult and child TB lung, the signal intensity in the adult lung was weaker than in child lung (Table 3). CPVL signal was eliminated after neutralization of anti-CPVL with the specific peptide, against which the polyclonal antibodies had been raised (Figure 2d), demonstrating both the presence of the antigen and the specificity of the antibody.
Figure 2.
Expression of carboxypeptidase-related vitellogenin-like macrophage molecule (CPVL) and angiotensin-converting enzyme (ACE) in normal lung, tuberculosis (TB) and sarcoidosis tissue. (a) CPVL+ alveolar macrophages (MØs) in normal adult lung. Alveolar MØs (b) and giant cells and epithelioid MØs (c) surrounding a granuloma in adult TB lung were also positive. Neutralizing anti-CPVL with its peptide prevents signal detection (d) ACE+ alveolar MØs (e), epithelioid MØs and giant cells (f) in sarcoidosis lung. ACE+ epithelioid MØs (g), ACE-positive (+) and -negative (–) alveolar MØs (h) and giant cells (i) in adult TB lung. ACE+ epithelial and goblet cells (j) and endothelial cells (k) in child TB lung. Sections adjacent to those in (e)–(k) were negative when anti-ACE was omitted (l) Magnification, ×400 [(a)–(c), (e)–(j), (l)] and ×200 [(d), (k)]. AM, alveolar MØ; AS, alveolar space; EM, epithelioid MØ; EN, endothelium; EP, epithelium; GC, giant cell; Gob, goblet cell; MN, monocytes; TIIP, type II pneumocytes.
Comparison of CPVL with ACE expression
Because CPVL and ACE share some features of carboxypeptidases, we compared their expression in detail. ACE expression by epithelioid MØs and giant cells in sarcoid lung has been reported previously (Silverstein et al. 1979; Brice et al. 1995). Under the conditions used here, we have confirmed that alveolar MØs (Figure 2e), epithelioid MØs (Figure 2f) and giant cells (Figure 2f) as well as epithelial cells (Table 5) express ACE in adult sarcoidosis patient lung sections. Furthermore, specific and intense staining of goblet cells was observed in a few patients (Figure 2j). We also observed that for a given patient, some type II pneumocytes were positive, whilst others were negative (Table 5). ACE signal intensity in TB tissue was notably lower than that observed in sarcoid lung (Table 3). However, ACE expression in TB tissue localized to the same cells, as in sarcoidosis, namely epithelioid MØs (Figure 2g), some alveolar MØs, although the majority of alveolar MØs are clearly negative (Figure 2h), giant cells (Figure 2i), epithelial and goblet cells (Figure 2j) and endothelial cells (Figure 2k) (Table 5). ACE signal was not detected in normal lung sections (not shown). This agrees well with results of Inoue et al. (1987), who did not detect ACE in alveolar MØs isolated from bronchiolar lavage of healthy volunteers. Staining was absent when the anti-ACE antibody was omitted (Figure 2l). In summary, although there was some overlap between CPVL and ACE staining, CPVL was restricted to cells of the MØ lineage and was not characteristic of sarcoidosis.
Analysis of SP-A and SP-D expression
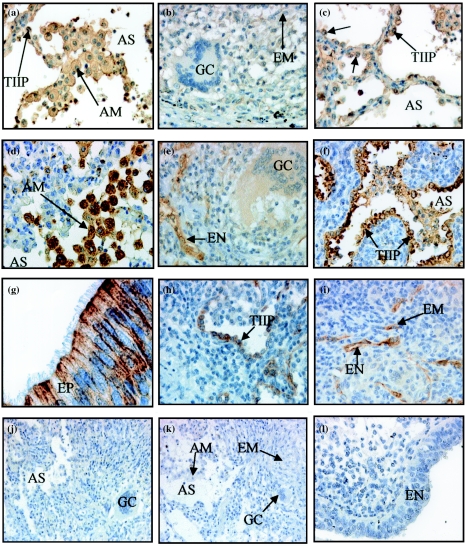
Our studies of the expression of two of the surfactant proteins, SP-A and -D, in TB, sarcoidosis and normal lung tissue have confirmed SP-A and -D expression in normal lung (Holmskov 2000; results not shown), where SP-A appears to ‘coat’ the cells of the alveolar space, whereas SP-D was more localized to type II cells and MØs. This agrees well with the fact that SP-A is a major component of surfactant (Holmskov 2000). SP-D expression on these MØs in the normal tissue was extremely weak (not shown). In contrast, strong SP-A and -D immunoreactivity was evident in adult lung tissue from TB and sarcoidosis patients as well as lung and lymph nodes from child TB patients (Table 4). As expected, SP-A signal localized to type II pneumocytes (Figure 3a, c) but was also observed on alveolar MØs in TB tissue (Figure 3a) (Table 6). In addition, epithelioid MØs and giant cells were positive for SP-A in sarcoid lung (Table 6) but were not immunoreactive in adult TB lung tissue (Figure 3b). Monocytes in TB and sarcoidosis tissue appeared to be immunoreactive (not shown). This signal may reflect the function of these cells in the metabolism of the surfactant proteins. Respiratory epithelium in both diseases was also positive for SP-A (data not shown). SP-A signal was ubiquitous in the tissues and the antibody appeared to be very ‘sticky’. Signal was also associated with cells of granulomas. In addition to the immunoreactive cells of TB lung tissue, child lymph node tissue also demonstrated immunoreactive epithelioid MØs (Table 6). Because type II pneumocytes are a primary source of SP-A, it is possible that the high SP-A signal intensity in lymph nodes reflects receptor bound or internalized SP-A (Holmskov 2000). When primary antibody was omitted, there was no staining (Figure 3j).
Figure 3.
Expression of surfactant protein A (SP-A), surfactant protein D (SP-D) and C-terminal fractalkine (C-Fk) in adult tuberculosis (TB) lung. SP-A+ alveolar macrophages (MØs) (a) and type II pneumocytes [(a), (c) ] and SP-A– giant cell (b) in adult TB lung. SP-D+ alveolar MØs (d), type II pneumocytes (f) and SP-D– giant cell (e) in adult TB lung. C-Fk-positive-ciliated epithelium (g) is shown with strong apical and lateral surface staining. Selected C-Fk-positive type II pneumocytes (h), endothelium and an epithelioid MØ (i) in peripheral areas of a granuloma in adult TB lung. Sections adjacent to those in [(a)–(i)] were negative when anti-SP-A (j) or anti-SP-D (k) was omitted and when anti-C-Fk (l) was neutralized using specific peptide. Magnification, ×400 [(a)–(i), (l)] and ×200 [(j)–(k)]. AM, alveolar MØ; AS, alveolar space; EM, epithelioid MØ; EN, endothelium; EP, epithelium; GC, giant cell; MN, monocytes; TIIP, type II pneumocytes.
SP-D immunoreactivity was strong in alveolar type II pneumocytes in both child and adult TB lung tissue (Figure 3f) and sarcoid patients' lungs (Table 6). Within child and adult lung tissue, alveolar MØs were also associated with SP-D signal (Figure 3d). The immunoreactivity within granulomas varied between patients, and within a section from a single patient, some granulomas were positive for SP-D, whilst others were negative. Granuloma SP-D expression within a section varied between 50 and 100%, depending on the patient, but was unrelated to whether granulomas were necrotic or non-necrotic. Interstitial MØs, bronchiolar epithelial and endothelial cells from TB patients did not label with SP-D antibody in the lung (Table 6). However, epithelioid MØs in TB lymph node sections and sarcoidosis lung were SP-D positive (Table 6). Giant cells from child and adult lung samples were poorly immunoreactive in TB lung sections (Figure 3e), although they were positive in sarcoid tissue and TB lymph node sections (Table 4). These differences were consistent between patients. When primary antibody was omitted, there was no labeling (Figure 3k).
The presence of SP-A and -D on certain MØ subpopulations, but absence on others (Table 6), is consistent with the observations (Holmskov 2000; Lu et al. 2002) that various MØ subpopulations express receptors for these surfactant proteins. Our results suggest that SP-A and -D are markers for both epithelial cells and MØs, although these cells may differ in their ability to synthesize these proteins.
Analysis of C-Fk expression
From our investigation of C-Fk expression in diseased lung tissue, we found C-Fk expression to be prominent in bronchiolar epithelial cells of both TB (Figure 3g) and sarcoid patients (Table 6). However, expression was also detected in healthy lung tissue (not shown), where it localized to epithelial cells. Selected type II pneumocytes (Figure 3h), endothelial cells and occasional epithelioid MØs (Figure 3i) demonstrated transmembrane C-Fk expression in adult TB lung. These positive type II pneumocytes, endothelial cells and epithelioid MØs were in peripheral, inflamed areas surrounding granulomas that were both necrotic and non-necrotic. The majority of type II pneumocytes were, however, negative, especially those lining the alveolar spaces where inflammation and granuloma development were absent (Table 6). In 44% of sarcoidosis patients, C-Fk immunoreactivity localized to the same cells as in TB child and adult lung sections, although 11% of patients demonstrated a weak signal, compared to the strong signal in 33% of patients (Table 4). However, the majority of sarcoid lung sections displayed no C-Fk signal, a scenario that was evident in most child TB lymph nodes (Table 4.) The C-Fk immunoreactivity in endothelium of one TB lymph node section was weak (Table 4). Although rare epithelioid MØs were positive, all MØ populations, as well as giant cells, were negative in diseased conditions of both child lung and lymph node sections (Table 6). When primary antibody was omitted, or neutralized with its specific peptide, there was no labeling (Figure 3l) indicating the specificity of the antibody as well as the presence of the antigen. We may, therefore, conclude that C-Fk is constitutively expressed in lung epithelium, as evident in normal lung sections, although its expression appears to be upregulated during M. tuberculosis infection. C-Fk expression appears to be tissue- and disease-dependent.
Discussion
Our main findings can be summarized as follows:
SR-A and CPVL are excellent markers for all mature MØs examined in normal and diseased lung, comparable to CD68.
SP-D and -A, products of type II alveolar epithelial cells, also associate variably with MØs, consistent with binding to a collectin receptor and/or synthesis by MØs.
C-Fk is made by bronchiolar epithelium and may be induced to a limited extent on endothelium within the inflammatory response.
The granulomatous inflammatory process is very similar for TB and sarcoidosis, with ACE expression somewhat more selective for sarcoid but less specific to MØ than CPVL in both TB and sarcoidosis.
Expression of each antigen is broadly similar, irrespective of age or anatomic site, except for obvious differences between lung and lymph node in regard to local epithelium. Overall, the results bear on MØ heterogeneity in chronic granulomatous inflammation and highlight potential epithelial–MØ interactions in pulmonary TB and sarcoidosis.
Differentiation of MØ in vivo
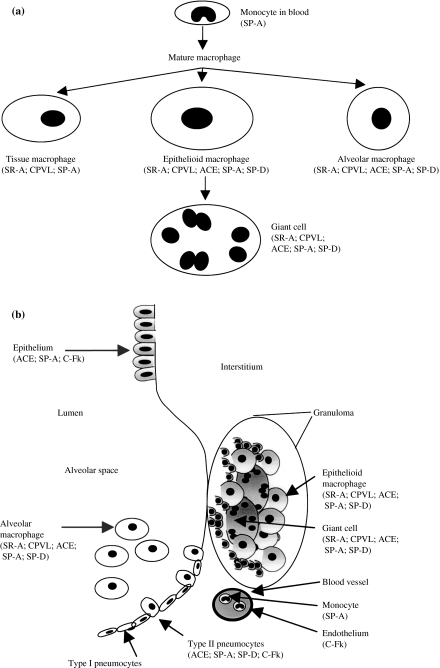
Figure 4a schematically shows the expression of antigen markers by different mature MØs, compared with intravascular monocytes, possibly, already activated by the disease process. Our studies cannot establish whether interstitial tissue MØs are terminally differentiated, or intermediate precursors of epithelioid and multinucleated giant cells. At present, the latter two types of cells, clearly part of the MØ lineage (Dannenberg & Rook 1994; Adams 1976; Spector 1969), cannot be distinguished by expression of various markers. Their phenotype, mechanism of differentiation and possible specialized functions remain obscure. Apart from their distinctive rounded morphology, the alveolar MØ population may be relatively autonomous and self-replicating as well as the products of further recruitment from blood monocytes (Gordon & Hughes 1997). The present markers do not distinguish them from other pulmonary tissue MØs, nor do they distinguish possible differences in cell activation. Previous studies (Keshav et al. 1992) showed that alveolar MØs already represent a stimulated population that may be immunosuppressive (Thepen et al. 1994). The addition of new MØ antigen markers will facilitate future double-labelling studies and identification of the sources of important cytokines, such as interleukin-4 (IL-4) and interferon-γin situ (Fenhalls et al. 2000). Finally, we cannot exclude that the present markers are not also expressed by tissue dendritic cells (DCs); in our own experience, many MØs in lung express supposed DC markers, including major histocompatibility complex class II, CD11c and CD1 (results not shown). Fractalkine has not been detected on DCs in other tissues (A. Lucas, unpublished). SP-A and -D have been reported to be expressed by murine DCs in vitro (Brinker et al. 2003; Brinker et al. 2001). SR-A expression has been reported on cultured human monocyte-derived DCs and on interstitial DCs in the lymph nodes of Rhesus monkeys (Harshyne et al. 2003). However, none of these markers, nor CPVL, have been characterized on authentic DCs in the lung.
Figure 4.
Immunophenotype of macrophages (MØs) in relation to granulomatous diseases in the lung. (a) Putative lineage relationships based on antigen expression by different MØs. (b) Antigen expression by epithelial cells, endothelium and MØs, indicating marker antigens and possible cellular interactions.
SR-A
Present findings with TB and sarcoidosis are consistent with previous reports in other situations. Monocytes lack SR-A, although it is present on many mature MØs in humans (Gough et al. 1999) and mouse (Hughes et al. 1995). Known upregulators of SR-A expression in vitro include colony stimulating factor (CSF-1) (de Villiers et al. 1994) and IL-10 (N. Platt & S. Gordon, unpublished). Clear surface expression of SR-A is consistent with a role in cell adhesion/retention (Fraser et al. 1993) as well as in phagocytosis of microorganisms (Peiser et al. 2000) and apoptotic cells (Platt et al. 1996). Several pattern recognition receptors have been implicated in mycobacterial uptake (Heldwein & Fenton 2002; Schlesinger 1997; Stenger & Modlin 2002), including undefined polyanion-sensitive scavenger-type receptors (Ernst 1998). Further studies are required to establish whether SR-A detected here can contribute to uptake of M. tuberculosis by MØs. A distinct role for SR-A could be in clearance of mycobacterial wall constituents and in protection of the host to lipopolysaccharide-induced septic shock (Haworth et al. 1997) mediated via CD14 and Toll-like receptors (Stenger & Modlin 2002).
CPVL and ACE
The present study is the first to immunolocalize CPVL in situ. The MØ specificity is consistent with the cell-specific expression strategy used to isolate the cDNA by differential display polymerase chain reaction (Mahoney et al. 2001). Previous studies showed mRNA at relatively high levels in immune tissues, as well as in heart and kidney. Further in situ analysis is needed to establish the cellular sources in the latter organs. The comparison with ACE showed differences in cell specificity as well as in disease association (see below). Possible functions for this novel MØ-specific carboxypeptidase-like molecule include antigen processing (expression by DC as well as MØ cannot be excluded by present studies), microbial degradation and modulation of extracellular polypeptide mediators of inflammation after secretion. Although not dependent on the disease process (alveolar MØs in non-infected lung express CPVL), further studies are needed to establish the effects of cytokines and other immunomodulators on CPVL expression.
SP-A and SP-D
These collectins have been implicated in host defence and in surfactant metabolism (Holmskov 2000). Previous studies have shown expression by a range of epithelial cells outside, as well as in lung, and association with MØs (Madsen et al. 2000). The present studies cannot distinguish sites of synthesis from adsorptive uptake of soluble protein or complexes via MØ receptors. Candidate receptors for SP-D on MØ include gp340 (Holmskov 2000), a member of the scavenger receptor cysteine-rich domain-containing family. In our hands, the SP-D antigen expression was more clearly defined than SP-A, but this might depend on the antibody, as well as on secretion and binding to various cell types, including MØ. The availability of newly available knock-out mice may clarify the role of these molecules in mycobacterial infection in vivo (Crouch & Whitsett 2002).
C-Fk
The above dilemma (production vs. secretion) does not apply to this reagent, which clearly implicates the bronchiolar epithelium as the predominant source of the molecule, analogous to epithelial production outside the lung (Lucas et al. 2001; Muehlhoefer et al. 2000). Epithelial expression may be constitutive, or enhanced by local inflammation, unlike endothelial expression which is thought to be inducible (Lucas et al. 2001). The expression pattern on ciliated columnar epithelium (Figure 3g) is very striking on both apical and lateral surfaces. The mechanism and significance of polarized expression of C-Fk will be of great interest in future studies.
MØ–epithelial interactions
Figure 4b summarizes expression of various markers by different epithelial cells (bronchiolar epithelium and type II pneumocytes) and different pulmonary MØs in diseased lung. Whilst the importance of local interactions between MØs, lymphocytes and connective tissue cells has long been recognized, it is only relatively recently that the importance of epithelia in innate and acquired immunity has been appreciated (Cunningham & Mahon 2001; Christensen & Trows 1997). Bronchiolar epithelium is a potent source of granulocyte/MØ colony-stimulating factor (GM-CSF) and other cytokines/chemokines as shown by the alveolar proteinosis observed in GM-CSF-null mice (Huffman et al. 1996). The type II pneumocytes are not only sources of surfactant proteins but produce opsonic molecules important in microbial recognition by MØs. In the present context, it has been suggested that they can be directly infected by mycobacteria, although receptors for uptake were not identified (Bermudez & Goodman 1996). The present study has revealed potential interactions between local epithelial cells and different MØs in TB and sarcoidosis, and points to two intriguing routes for such interactions, through ablumenal interactions by contact and diffusion within tissues as well as via the airway. Finally, it is likely that interactions will be reciprocal, including influence of proliferative and secretory activities of epithelial cells by the inflammatory MØs.
Association with disease, age and anatomic site
It is perhaps not surprising that the markers used revealed little selectivity regarding these variables. TB and sarcoidosis are very similar diseases in respect to their histopathology. All TB patients had acid-fast bacilli, whilst none were detected in the sarcoid group. MØs were prominent in granulomatous lesions in both groups and shared several features – epithelioid and giant cell formation and comparable SR-A expression. CPVL did not show the enhanced expression in sarcoidosis, previously noted for ACE expression (Silverstein et al. 1979; Brice et al. 1995), and confirmed, here by, immunocytochemistry, whilst cell specificity was more evident. Although it is possible that CPVL has different functions to ACE, including non-enzymatic lysosomal chaperone properties (Mahoney et al. 2001), it may still share extracellular enzymatic activities. Further studies to compare these molecules will be of interest, not least in identifying sarcoid-specific elements. SP-A and -D expression in TB was not noticeably enhanced above sarcoidosis, whilst cell specificity was more evident. The moderate to strong signal observed in both diseases was not unexpected. Both SP-A and -D demonstrate measurable serum levels in healthy individuals (Kondo et al. 1998; Kuroki et al. 1993), which become elevated for SP-D in TB (Kondo et al. 1998). SP-D levels in sarcoidosis have not been reported. To our knowledge, this is the first example where SP-D expression in situ has been recorded in sarcoidosis. It will be of interest to study C-Fk expression, which demonstrated disease-related differences (Tables 4 and 6) further. To confirm these results, it would be beneficial to compare tissue from untreated TB patients with that from sarcoidosis patients, as the effect of medication on the expression of these antigens is not known.
For the majority of antigens investigated, expression was largely independent of the patients' age. However, child TB lungs displayed higher expression of CPVL and ACE, whilst the other antigens were largely indifferent. The epithelial cell markers (SP-A, -D and C-Fk) did not demonstrate major differences in staining intensity. However, antigens marking MØs and their populations showed a greater heterogeneity in levels of expression, which generally appeared greater in children than in adults. Cell localization of the antigens was largely similar between adult and child TB lung, except for SP-A detection on epithelioid MØs in child lung but absent in adult TB lung.
The antigens investigated appear to be, for the most part, independent of anatomical location, with the obvious exception of C-Fk which was absent in child TB lymph nodes.
In conclusion, the present study has confirmed and extended earlier studies on MØ differentiation, activation and interactions in situ, in two related major disease processes. Further progress in our understanding of the role of MØs in pathogenesis will come from the analysis of additional markers. Of particular value will be the identification of reagents specific for different stages or states of MØs in situ, in order to replicate this process in vitro.
Acknowledgments
This study was supported by the Medical Research Council and The Wellcome Trust (UK) and by the South African Medical Research Council and Glaxo Wellcome (Republic of South Africa). We thank Edward Sturrock, Uffe Holmskov and Kenneth Reid for reagents, Juanita Bezuidenhout for tissue samples and analysis, Willie Pieterse for cutting the sections and Liesel Stevens-Muller and Christine Holt for preparation of the manuscript.
References
- Adams DO. The granulomatous inflammatory response. Am. J. Pathol. 1976;84:164–191. [PMC free article] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, et al. A new class of chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immunity. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice EAW, Friedlander W, Bateman ED, Kirsch RE. Serum angiotensin converting enzyme activity in granulomatous interstitial lung disease, tuberculosis and COPD. Chest. 1995;107:706–710. doi: 10.1378/chest.107.3.706. [DOI] [PubMed] [Google Scholar]
- Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:L232–L241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- Brinker KG, Martin E, Borron P, et al. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L1453–L1463. doi: 10.1152/ajplung.2001.281.6.L1453. [DOI] [PubMed] [Google Scholar]
- Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: The human model. Annu. Rev. Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- Christensen PJ, Trows GB. Role of pulmonary epithelial cells and dendritic cells in regulating pulmonary immune responses. In: Lipscomb MF, Russel SW, Lenfant C, editors. Lung Macrophages and Dendritic Cells in Health and Disease. Vol. 102. New York: Marcel Dekker Inc; 1997. pp. 283–310. Lung Biology in Health and Disease. [Google Scholar]
- Clemens DL, Horwitz MA. Characterization of the Mycobacterum tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch EC, Whitsett JA. Diverse roles of lung collectins in pulmonary innate immunity. In: Ezekowitz RAB, Hoffman JA, editors. Innate Immunity. Totowa, New Jersey, USA: Humana Press; 2002. pp. 205–229. [Google Scholar]
- Cunningham MR, Mahon BR. The immunological role of respiratory tract epithelium. Mod. Asp. Immunobiol. 2001;5:204–209. [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Dannenberg AM, Rook GAW. Pathogenesis of pulmonary tuberculosis; an interplay of tissue-damaging and macrophage-activating immune responses – dual mechanisms that control bacillary multiplication. In: Bloom BR, editor. TuberculosisPathogenesis, Protection and Control. Washington DC: ASM Press; 1994. pp. 459–483. [Google Scholar]
- Ernst J. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immunity. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenhalls G, Wong A, Bezuindenhout J, van Helden P, Bardin P, Lukey PT. In situ production of gamma interferon, interleukin-4 and tumor necrosis factor alpha mRNA in human lung tuberculosis granulomas. Infect. Immunity. 2000;68:2827–2836. doi: 10.1128/iai.68.5.2827-2836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser IP, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364:343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- Glickman MS, Jacobs WR JR. Microbial pathogenesis of Mycobacterium tuberculosis. Cell. 2001;104:477–485. doi: 10.1016/s0092-8674(01)00236-7. [DOI] [PubMed] [Google Scholar]
- Gordon S, Hughes D. Macrophages and their origins; heterogeneity in relation to tissue microenvironment. In: Lipscomb MF, Russell SW, Lenfant C, editors. Lung Macrophages and Dendritic Cells in Health and Disease. Vol. 102. New York: Marcel Dekker Inc; 1997. pp. 3–31. Lung Biology in Health and Disease. [Google Scholar]
- Gough PJ, Greaves DR, Suzuki H, et al. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1999;19:461–471. doi: 10.1161/01.atv.19.3.461. [DOI] [PubMed] [Google Scholar]
- Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J. Immunol. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- Haworth R, Platt N, Keshav S, et al. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J. Exp. Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldwein KA, Fenton MJ. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 2002;4:937–944. doi: 10.1016/s1286-4579(02)01611-8. [DOI] [PubMed] [Google Scholar]
- Hirsch CS, Ellner JJ, Russell DG, Rich EA. Complement receptor-mediated uptake and tumor necrosis factor α-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J. Immunol. 1994;152:743–753. [PubMed] [Google Scholar]
- Holmskov U. Acta Pathologica, Microbiologica, et Immunologica Scandinavica. (100) Vol. 108. Copenhagen: Munksgaard; 2000. Collectins and collectin receptors in innate immunity; pp. 1–59. [PubMed] [Google Scholar]
- Huffman AJ, Hull WM, Dranoff G, Mulligan RC, Whitsett JA. Pulmonary epithelial cell expression of GM-CSF corrects tshe alveolar proteinosis in GM-CSF-deficient mice. J. Clin. Invest. 1996;97:649–655. doi: 10.1172/JCI118461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DA, Fraser IP, Gordon S. Murine macrophage scavenger receptor: in vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur. J. Immunol. 1995;25:466–473. doi: 10.1002/eji.1830250224. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Hashimoto A, Takada Y, Nishimura K, Hiwada K, Kokubu T. Angiotensin converting enzyme in sarcoidosis and in silicosis. Clin. Exp. Hypertens. 1987;9:481–485. doi: 10.3109/10641968709164217. [DOI] [PubMed] [Google Scholar]
- Keshav S, Stein ML, Chung L-P, Gordon S. Cytokine gene expression in situ. differential expression of lysozyme, Il-1, TNF and Il-6 mRNA in murine liver during BCG infection. Proceedings of the 5th Leiden Conference on mononuclear phagocytes. In: van Furth R, van Furth R, editors. Mononuclear PhagocytesBiology of Monocytes and Macrophages. Dordrecht-Boston-London: Kluwer publishers; 1992. pp. 366–374. [Google Scholar]
- Kondo A, Oketani N, Maruyama M, et al. Signifance of serum surfactant protein-D (SP-D) level in patients with pulmonary tuberculosis. Kekkaku. 1998;73:585–590. [PubMed] [Google Scholar]
- Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors. macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu. Rev. Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Tsutahara S, Shijubo N, et al. Elevated levels of lung surfactant protein A in sera from patients with idiopathic pulmonary fibrosis and pulmonary alveolar proteinosis. Am. Rev. Respir. Dis. 1993;147:723–729. doi: 10.1164/ajrccm/147.3.723. [DOI] [PubMed] [Google Scholar]
- Lu J, Teh C, Kishore U, Reid KB. Collectins and ficolins: sugar pattern recognition molecules of the mammal innate immune system. Biochim. Biophys. Acta. 2002;1572:387–400. doi: 10.1016/s0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Lucas AD, Chadwick N, Warren BF, et al. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am. J. Path. 2001;158:855–866. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen J, Kliem A, Tornøe I, Skjødt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J. Immunol. 2000;164:5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- Mahoney JA, Ntolosi B, da Silva RP, Gordon S, McKnight AJ. Cloning and characterization of CPVL, a novel serine carboxypeptidase from human macrophages. Genomics. 2001;72:243–251. doi: 10.1006/geno.2000.6484. [DOI] [PubMed] [Google Scholar]
- Micklem K, Rigney E, Cordell J, et al. A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. Br. J. Haematol. 1989;73:6–11. doi: 10.1111/j.1365-2141.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Muehlhoefer A, Saubermann LJ, Gu X, et al. Fraktalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J. Immunol. 2000;164:3368–3376. doi: 10.4049/jimmunol.164.6.3368. [DOI] [PubMed] [Google Scholar]
- Orme IM, Collins FM. Mouse model of tuberculosis. In: Bloom BR, Bloom BR, editors. Tuberculosis, Pathogenesis, Protection and Control. Washington, DC: ASAM Press; 1994. pp. 113–134. [Google Scholar]
- Peiser L, Gough PJ, Kodama T, Gordon S. Macrophage class A scavenger receptor-mediated phagocytosis of E. Coli: Role of cell heterogeneity, microbial strain and culture conditions in vitro. Infect. Immunity. 2000;68(4):1953–1963. doi: 10.1128/iai.68.4.1953-1963.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the Class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli SA, Segelte BW, Sugita M, Wilson IA, Brenner MB. The CD1 family of lipid antigen-presenting molecules. Immunol. Today. 1998;19:362–368. doi: 10.1016/s0167-5699(98)01289-4. [DOI] [PubMed] [Google Scholar]
- Russell DG. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. Mol. Cell Biol. 2001;2:569–577. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- Schlesinger LS. The role of mononuclear phagocytes in tuberculosis. In: Lipscomb MF, Russell SW, Lenfant C, editors. Lung Macrophages and Dendritic Cells in Health and Disease. Vol. 102. New York: Marcel Dekker Inc; 1997. pp. 437–480. Lung Biology in Health and Disease. [Google Scholar]
- Schreiber RD, Hamilton TA. Molecular basis of macrophage activation from gene expression to phenotypic diversity. In: Burke B, Lewis CE, editors. The Macrophage. 2. New York: Oxford University Press; 2002. pp. 73–102. [Google Scholar]
- Siltzbach LE. Seventh International Conference on Sarcoidosis and Other Granulomatous Disorders. Ann. N. Y. Acad. Sci. 1976;(1):751–278. doi: 10.1111/j.1749-6632.1976.tb47009.x. [DOI] [PubMed] [Google Scholar]
- Silverstein E, Pertschuk LP, Friedland J. Immunofluorescent localization of angiotensin converting enzyme in epithelioid and giant cells in sarcoidosis granulomas. Proc. Natl. Acad. Sci. U. S. A. 1979;76:6646–6648. doi: 10.1073/pnas.76.12.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector WG. The granulomatous inflammatory exudate. Int. Rev. Exp. Pathol. 1969;8:1–55. [PubMed] [Google Scholar]
- Stenger S, Modlin RL. Control of Mycobacterium tuberculosis through mammalian Toll-like receptors. Curr. Opin. Immunol. 2002;14:452–457. doi: 10.1016/s0952-7915(02)00355-2. [DOI] [PubMed] [Google Scholar]
- Thepen T, Kraal G, Holt PG. The role of alveolar macrophages in regulation of lung inflammation. Ann. N Y Acad. Sci. 1994;725:200–206. doi: 10.1111/j.1749-6632.1994.tb39802.x. [DOI] [PubMed] [Google Scholar]
- de Villiers W, Fraser IP, Hughes DA, Doyle AG, Gordon S. M-CSF selectively enhances macrophage scavenger receptor expression and function. J. Exp. Med. 1994;180:705–709. doi: 10.1084/jem.180.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]