Abstract
The function of the small-Mr Ras-like GTPase Rap1 remains largely unknown, but this protein has been demonstrated to regulate cortical actin-based morphologic changes in Dictyostelium and the oxidative burst in mammalian neutrophils. To test whether Rap1 regulates phagocytosis, we biochemically analyzed cell lines that conditionally and modestly overexpressed wild-type [Rap1 WT(+)], constitutively active [Rap1 G12T(+)], and dominant negative [Rap1 S17N(+)] forms of D. discoideum Rap1. The rates of phagocytosis of bacteria and latex beads were significantly higher in Rap1 WT(+) and Rap1 G12T(+) cells and were reduced in Rap1 S17N(+) cells. The addition of inhibitors of protein kinase A, protein kinase G, protein tyrosine kinase, or phosphatidylinositide 3-kinase did not affect phagocytosis rates in wild-type cells. In contrast, the addition of U73122 (a phospholipase C inhibitor), calphostin C (a protein kinase C inhibitor), and BAPTA-AM (an intracellular Ca2+ chelator) reduced phagocytosis rates by 90, 50, and 65%, respectively, suggesting both arms of the phospholipase C signaling pathways played a role in this process. Other protein kinase C–specific inhibitors, such as chelerythrine and bisindolylmaleimide I, did not reduce phagocytosis rates in control cells, suggesting calphostin C was affecting phagocytosis by interfering with a protein containing a diacylglycerol-binding domain. The addition of calphostin C did not reduce phagocytosis rates in Rap1 G12T(+) cells, suggesting that the putative diacylglycerol-binding protein acted upstream in a signaling pathway with Rap1. Surprisingly, macropinocytosis was significantly reduced in Rap1 WT(+) and Rap1 G12T(+) cells compared with control cells. Together our results suggest that Rap1 and Ca2+ may act together to coordinate important early events regulating phagocytosis.
INTRODUCTION
Rap1 (Krev-1/smg p21) is a small-molecular-weight GTP-binding protein that belongs to the Ras-like superfamily of GTPases. To date, there have been four Rap-like proteins identified in humans: Rap1A, Rap1B, Rap2A, and Rap2B (Pizon et al., 1988a, 1988b; Farrel et al., 1990; Ohmstede et al., 1990), of which all share ∼50% amino acid sequence identity with the p21 Ras oncoprotein. Rap1 was originally identified based on its ability to suppress a transformed phenotype in Ki-Ras-transformed NIH3T3 cells (Kitayama et al., 1989), and, consequently, a number of studies have focused on the potential of Rap1 to act antagonistically toward Ras. It has been suggested that Rap1 can antagonize Ras-induced transformation by competitively binding to common effector proteins like Ras-GAP (Frech et al., 1990; Cook et al., 1993). However, there are also conflicting data that suggest Rap1 can act in a similar manner to Ras and activate downstream signaling proteins such as B-Raf protein kinase (Ohtsuka et al., 1996). It is not clear whether Rap1 acts in a signaling pathway parallel to or independent from Ras, or whether the ability of Rap1 to antagonize or complement Ras-induced transformation is an indirect effect of Rap1 function.
In addition to its ability to modulate Ras-induced transformation, other roles for Rap1 have been proposed. For instance, Rap1 associates with cytochrome B558 in a phosphorylation-dependent manner (Bokoch et al., 1991), and Rap1 may modulate the oxidative burst in phagocytic cells (Maly et al., 1994). Also, a Rap1 homologue exists in yeast (RSR/BUD1) that is required for bud site localization, indicating a potential role for Rap1 in regulating actin cytoskeleton rearrangements (Bender and Pringle, 1989; Chant and Herskowitz, 1991). Furthermore, it has been shown that Rap1 is a substrate for protein kinase A (PKA) (Hoshijima et al., 1988), and in vitro data suggest that Rap1 can enhance the activity of protein kinase C (PKC), indicating that Rap1 may play a role in an intracellular signaling pathway leading to PKC activation (Labadia et al., 1993). Finally, it has been demonstrated that Rap1a and Rap1b associate with endocytic and phagocytic compartments in mammalian cells, implicating Rap1 in the regulation of endocytic processes, whereas Rap2a associates with the Golgi (Pizon et al., 1994). Thus, the proposed functions of this protein, although quite diverse, suggest that Rap1 may regulate endocytosis and/or phagocytosis.
A Dictyostelium cDNA, designated DdRap1, has been isolated that encodes a protein that is 76% identical in amino acid sequence to human Rap1A (Robbins et al., 1990). Dictyostelium discoideum, which behaves similarly to neutrophils in terms of phagocytosis and chemotactic motility, is amenable to molecular genetic manipulations and biochemical studies, making it an excellent system in which to investigate the function of small G proteins. Moreover, the endo-lysosomal system (Cardelli, 1993) and regulation of the actin cytoskeleton of D. discoideum have been well characterized and shown to be regulated by Ras-like proteins and Ras protein effectors (see below), thus enabling us to more readily test the role of Rap1 in regulating pinocytosis, phagocytosis, and the actin cytoskeleton.
Pinocytosis rates are high in axenic strains of Dictyostelium, and at least two routes of entry have been postulated to exist. Earlier studies indicated an important role for micropinocytosis in fluid internalization (Rodriguez-Paris et al., 1993) that may be clathrin mediated (O’Halloran and Anderson, 1992; Ruscetti et al., 1994). In more recent studies, evidence has been presented suggesting that the majority of the fluid phase is internalized by macropinocytosis (Hacker et al., 1997), and that this process is regulated by the phosphatidylinositide 3-kinases (PI 3-kinases) DdPIK1 and DdPIK2 (Buczynski et al., 1997b), actin (Temesvari et al., 1996c; Hacker et al., 1997), coronin (Maniak et al., 1995), and RacC (Seastone et al., 1998). Other proteins have also been implicated in regulating pinocytosis, including the proton pump (Temesvari et al., 1996b), myosin IA and IB (Novak et al., 1995; Jung et al., 1996; Temesvari et al., 1996a), and RabD (Bush et al., 1996), although it is not known whether these proteins preferentially regulate macro- or micropinocytosis. Micropinosomes (and most likely vesicles derived from macropinosomes) fuse to form acidic lysosomes, followed by the delivery of the fluid phase contents to larger, less-acidic postlysosomes (Aubry et al., 1993; Padh et al., 1993). All of the fluid phase appears to be released from cells via postlysosomes 2–3 h after internalization; no major early endosomal fluid phase recycling compartment has been demonstrated. A number of proteins have been identified recently that regulate the lysosome to postlysosome transport step, including DdPIK1 and DdPIK2 (Buczynski et al., 1997b), RabD (Bush et al., 1996), actin (Temesvari et al., 1996c; Jenne et al., 1998), Rab7 (Buczynski et al., 1997a), and vacuolin B (Jenne et al., 1998).
Particulate matter, including latex beads, bacteria, and yeast, are also readily internalized by Dictyostelium by a process that morphologically appears to resemble the zipper model for internalization (Maniak et al., 1995). A variety of proteins have been described that regulate phagocytosis, including actin (Maniak et al., 1995; Temesvari et al., 1996c), Gβ (Wu et al., 1995; Peracino et al., 1998), coronin (Maniak et al., 1995), myosin Is (Jung et al., 1996), ABP-120 (Cox et al., 1996), Rab7 (Buczynski et al., 1997a), talin (Niewohner et al., 1997), and the novel Rho protein RacC (Seastone et al., 1998), although relatively little is known about the signal transduction pathway that regulates the formation of the phagocytic cup and directs the internalization of particles. However, it has been proposed that macropinocytosis and phagocytosis are functionally identical processes (Hacker et al., 1997).
We have previously reported that cells conditionally overexpressing wild-type Rap1 [Rap1 WT(+) cells] or constitutively activated Rap1 [Rap1 G12T(+) cells] were flatter and contained more actin-rich membrane ruffles than control cells (Rebstein et al., 1993; Rebstein et al., 1997), whereas cells expressing dominant negative Rap1 [Rap1 S17N(+) cells] were more polar in shape. These phenomena were accompanied by alterations in the cortical actin cytoskeleton [formation of lamellopodia for Rap1 WT(+) and Rap1 G12T(+)], although the biological consequences of these morphological changes remains to be determined.
Therefore, to better define the biochemical function of Rap1, we have initiated studies to analyze cell lines that conditionally overexpress modest amounts of wild-type and mutant forms of Rap1 to measure changes in rates of pinocytosis, phagocytosis, and exocytosis, processes that have been demonstrated to be regulated by small G proteins in other systems. Our results reported here reveal that Rap1 positively regulates phagocytosis and negatively regulates macropinocytosis. We present a model proposing that Rap1 may function at the plasma membrane as part of a signal transduction pathway downstream of phospholipase C activity to regulate particle internalization.
MATERIALS AND METHODS
Cell Cultures
All D. discoideum strains (Ax2, wild-type, and cell lines expressing Rap1 proteins) were grown axenically in HL5 medium (1% oxoid proteose peptone, 1% glucose, 0.5% yeast extract [Difco, Detroit, MI], 2.4 mM Na2HPO4, 8.8 mM KH2PO4, pH 6.5) in 175-cm2 tissue culture flasks (Sarstedt, Newton, NC) at 20°C. The Rap1 WT(+), Rap1 G12T(+), and Rap1 S17N(+) cell lines were generated as described (Rebstein et al., 1997). Transformed cell lines were maintained under G418 selection (10 μg/ml) in the presence of 1 mM folate. The removal of folate activates the discoidin I promoter that drives expression of the Rap1 proteins. For the experiments described here, tissue culture flasks were seeded with 1 × 106 cells and harvested 2 d later. After performing Western blot analysis using anti-Rap1 antibodies, the subsequent blot and Coomassie Blue–stained gel were digitized using the AlphaImager 2000 gel documentation system (Alpha Innotech, San Leandro, CA; version 4.0). After making the assumption that expression of exogenous Rap1 did not influence the levels of endogenous Rap1, the levels of the various exogenous forms of Rap1 were normalized to total protein. On average, exogenously expressed Rap1 WT, Rap1 G12T, and Rap1 S17N accumulated to levels 1.5-, 3-, and 7.5-fold over the level of endogenous Rap1, respectively, and phenotypic changes correlated temporally with changes in the levels of Rap1. Interestingly, when cells were grown in shaking flasks, the levels of expressed Rap1 proteins were 10- to 20-fold higher than endogenous levels of Rap1 (Rebstein et al., 1993, 1997).
Measurement of Fluid Phase Pinocytosis and Exocytosis
Cells were harvested from tissue culture flasks by shaking, collected by centrifugation (500 × g for 5 min), and resuspended at a concentration of 3 × 106 cells/ml in fresh HL5 medium supplemented with 2 mg/ml FITC-dextran (Mr 70,000; Sigma, St. Louis, MO). Samples of cells were recovered at various times during the 3-h pulse and washed, and the accumulated intracellular fluorescence was measured using a spectrofluorometer (excitation, 492 nm; emission, 525 nm). In some instances (for exocytosis of fluid phase after a 180-min loading), cells were removed from FITC-dextran–containing medium, washed, and placed in fresh HL5 medium. Samples of cells were taken at the indicated times, and fluid phase exocytosis was determined by measuring the amount of FITC-dextran remaining inside cells. Fluorescence measurements were normalized to total protein load to account for differences in cell size between various strains.
Measurement of Latex Bead and Bacterial Phagocytosis
Phagocytosis rates using fluorescent latex beads (1-μm crimson beads; Molecular Probes, Eugene, OR) and FITC-labeled Escherichia coli were also determined using a spectrofluorimeter (excitation, 625 nm; emission, 645 nm) as described (Buczynski et al., 1997b). Fluorescence measurements were normalized to total protein to account for any differences in cell size between various strains.
Radiolabel Pulse–Chase Analysis and Immunoprecipitation
Radiolabel pulse–chase analyses and immunoprecipitation of the lysosomal enzyme α-mannosidase were performed as previously described (Mierendorf et al., 1985) using specific monoclonal antibodies (Mierendorf and Dimond, 1983). Immunoprecipitates were subjected to SDS-PAGE followed by fluorography.
RESULTS
Evidence that Rap1 Regulates Phagocytosis of Latex Beads and Bacteria
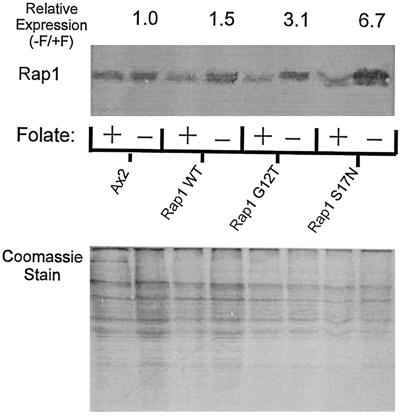
We have described in a previous publication the characterization of stable cell lines that conditionally overexpress Rap1 WT and mutant proteins (Rebstein et al., 1993, 1997). Under our conditions of growth of cells in plastic tissue culture flasks, the removal of folate (to induce expression of these proteins under control of the discoidin promoter) results 48 h later in reproducible expression levels for exogenous Rap1 proteins ranging from 1.5 to 7.5 higher than levels for endogenous Rap1 (Figure 1). These numbers represent the average observed for the phagocytosis experiments described below (see MATERIALS AND METHODS for details concerning the derivation of these numbers). This relatively modest overexpression minimizes the possibility that the mutant Rap1 proteins will nonspecifically interact with other small G protein–mediated signal transduction pathways.
Figure 1.
Expression pattern of Rap1 mutants. Top, Western blot analysis of cells that were grown in the absence of folate for 48 h to induce expression of various mutant forms of Rap1 under control of the discoidin I promoter. Blots were probed with a polyclonal antibody specific for Rap1. Relative expression levels were calculated as described (see MATERIALS and METHODS). Rap1 WT, Rap1 G12T, and Rap1 S17N were expressed at levels 1.5, 3.1, and 6.7 times that of the folate-repressed control, respectively. Bottom, Coomassie Blue–stained gel of total protein load for each of the Western blot samples shown above that was used in calculating relative expression levels.
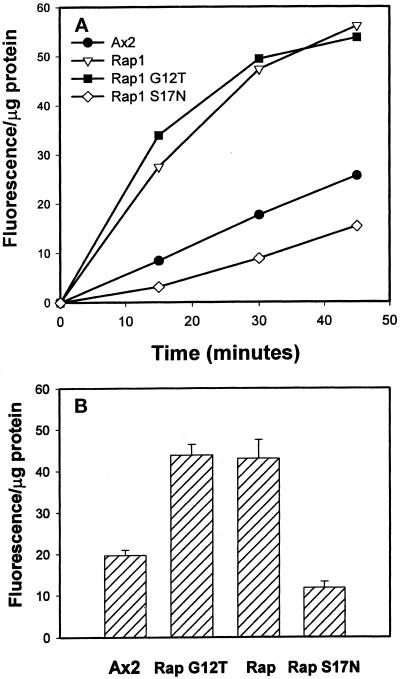
To test whether overexpression of Rap1 proteins altered phagocytosis, we first measured the ability of the control and Rap1-expressing cell lines to internalize 1-μm fluorescent latex beads 48 h after the removal of folate from growth media. Phagocytosis assays were performed using cells recovered from tissue culture flasks and placed in flasks shaking in suspension; under these conditions (the standard phagocytosis assay conditions for Dictyostelium) all cell lines appear spherical in shape, as determined by phase-constrast microscopy. We normalized bead internalization for each time point to total cellular protein to correct for potential differences in cell size, although FACS analysis indicated the average diameters of all the strains of cells were very close. As indicated in Figure 2A (a representative experiment), when compared with parental Ax2 cells, the rates of phagocytosis were increased more than two-fold for Rap1 WT(+) and Rap1 G12T(+) cells, whereas in contrast, the rate of phagocytosis was reduced by 50% in Rap1 S17N(+) cells. Figure 2B summarizes the data of multiple experiments and indicates that these observed differences in phagocytosis rates were significant.
Figure 2.
Rap1 regulates the rate of phagocytosis of latex beads. The rate of phagocytosis was measured by incubating cells with 1-μm fluorescent latex beads, collecting the cells at various times, lysing them, and measuring intracellular fluorescence using a spectrofluorimeter. The fluorescence of the samples was normalized to total protein to account for differences in cell size among the strains; this value is reported as fluorescence per microgram of protein. (A) One phagocytosis assay that was performed, showing the rates of phagocytosis among the various Rap1-overexpressing cell lines were nearly linear for 30 min. (B) Bar graph showing the average fluorescence per microgram of protein for each of the strains at the 30-min time point ± SD. Statistics: Ax2, 19.7 ± 3.7 (n = 8); Rap1 WT(+), 43.7 ± 7.3 (n = 8); Rap1 G12T(+), 43.0 ± 12.9 (n = 8); Rap1 S17N(+), 11.8 ± 2.63 (n = 3). Student’s two-tailed t tests were performed using the statistical program Instat (version 1.12a; IBM, White Plains, NY).
To determine whether the increase in phagocytosis observed in strains overexpressing constitutively active Rap1 or wild-type Rap1 was a phenomenon specific only to latex beads, we also measured the rates of uptake of fluorescently labeled E. coli, one of the natural food sources for this organism. Fluorometric measurements (see the legend of Figure 3A) indicated that Rap1 WT(+) and Rap1 G12T(+) cells internalized bacteria at two to three times the rate of the parental Ax2 strain on a per protein basis. Therefore, we conclude that the increased phagocytic rates observed for cell lines overexpressing Rap1 WT and constitutively activated Rap1 are physiologically relevant.
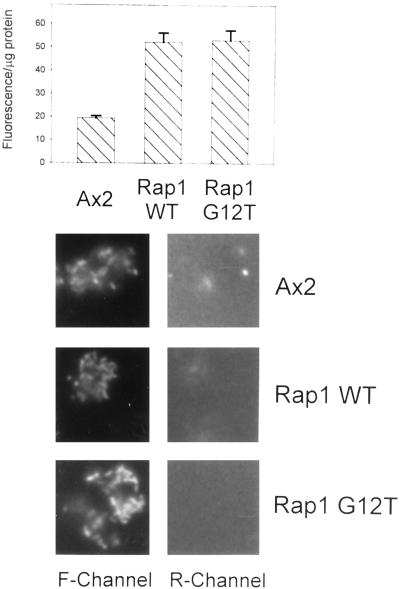
Figure 3.
Rap1 regulates the rate of phagocytosis of FITC-E. coli. Top, Bar graph showing bacterial uptake and the average fluorescence per microgram of protein for each of the strains at the 30-min time point ± SD. Phagocytosis assays were performed as in Figure 2, except FITC-labeled E. coli was used instead of fluorescent latex beads. Bottom, Cells were incubated with FITC-labeled E. coli for 10 min, washed, and placed on a coverslip. After adding ethidium bromide (5 μg/ml) to the medium, the coverslips were mounted and viewed using either the green (F-Channel) or red (R-Channel) of a fluorescence microscope. Under these experimental conditions for all the strains examined, FITC-E. coli was readily internalized by the cells (see F-channel), but <5% of the bacteria stuck to the outside of the cell (see R-channel).
The following experiments were performed to demonstrate that bacteria were actually being internalized at a higher rate into Rap1 WT(+) and Rap1 G12T(+) cells as opposed to sticking more avidly to the outside of these cells. Cells were allowed to internalize FITC-labeled E. coli for 10 min, were washed, and then were spotted on coverslips for 5 min. Ethidium bromide was added to a final concentration of 5 μg/ml, and coverslips were mounted on slides and viewed by fluorescent microscopy. Figure 3, bottom, shows photographs of control, Rap1 WT(+), and Rap1 G12T(+) cells, respectively, viewed using the fluorescein (F-channel) or rhodamine (R-channel) filter configuration. Only bacteria outside of cells that have absorbed ethidium bromide will fluoresce in the red channel. As seen in Figure 3, <5% of the bacteria remained outside of cells for all strains at the termination of the phagocytosis assay. As an alternative approach, we performed phagocytosis assays in the presence of the fluorescent fluid phase marker lucifer yellow. We observed that >90% of the particles associated with cells at the completion of the assay were ringed with lucifer yellow, indicating that they resided on the inside of cells (our unpublished results).
Evidence That Phospholipase C Activity Is Important in Regulating Phagocytosis in Wild-Type Cells
The studies described thus far suggest that Rap1 may act as part of a signal transduction pathway that regulates phagocytosis in Dictyostelium. This internalization pathway remains poorly described biochemically, and thus far only a few proteins have been identified that appear to play an important role in particle internalization in Dictyostelium. Included in this list are actin (Maniak et al., 1995; Temesvari et al., 1996c), coronin (Maniak et al., 1995), talin (Niewohner et al., 1997), ABP-120 (Cox et al., 1996), myosin IB (Jung et al., 1996), the G protein β subunit Gβ (Wu et al., 1995; Peracino et al., 1998), and RacC (Seastone et al., 1998). How and whether Rap1 interacts with these and other proteins, and how these proteins combine to regulate temporal and spacial changes in the actin cytoskeleton and internalization of particles, remain to be defined. Therefore, we initiated a pharmacological approach to identify additional key enzymes and proteins that may regulate phagocytosis and to determine where in the phagocytosis pathway Rap1 might function. Many of the enzymes targeted for these drug studies have previously been shown to regulate phagocytosis in mammalian cells. For instance, PKC, PI 3-kinases, and protein tyrosine kinases (PTK) have been implicated in regulating the internalization of particles in macrophages and neutrophils (Allen and Aderem, 1995; Hutchinson et al., 1995; Araki et al., 1996). However, the addition of specific PKC inhibitors (up to 10 μM bisindolymaleimide and 10 μM chelethrine), PI 3-kinase inhibitors (up to 10 μM wortmannin and 50 μM LY294002), and PTK inhibitors (up to 500 μM various tyrphostins) to cultures of wild-type Ax2 cells did not significantly affect the rate of phagocytosis in Dictyostelium (Table 1). The addition of protein kinase G (PKG) inhibitors (H8) and PKA inhibitors (H89) or activators (Br2-cAMP) were also without effect (Table 1). All of these drugs were ineffective regardless of the preincubation times with cells before the addition of particles (also see the footnote to Table 1 for more details).
Table 1.
Summary of drug effects on phagocytosis
| Drug (IC50, μM) | Target | Concentration (μM) | % of control |
|---|---|---|---|
| U73122* (1.0) | PLC | 6 | 33.5 ± 13.1 |
| U73122* | PLC | 12 | 10.5 ± 4.5 |
| U73343 | PLC | 6 | 107.1 |
| Calphostin C* (0.05) | PKC | 1 | 51.2 ± 7.9 |
| Calmidazolium* (0.01)a | Ca2+ | 5 | 164.0 ± 5.6 |
| BAPTA-AM*b | Ca2+ | 300 | 34.8 ± 4.9 |
| Chelerythrine (0.66) | PKC | 10 | 111.4 ± 17.2 |
| Bisindolylmal. (0.01) | PKC | 10 | 97.7 ± 5.4 |
| Tyrphostin46 (10)3c | PTK | 500 | 103.7 ± 4.0 |
| H8 (0.48) | PKG | 50 | 102.0 ± 9.3 |
| H7 (3/6) | PKA/PKC | 50 | 94.5 ± 5.9 |
| H89 (0.05)d | PKA | 10 | 89.1 ± 6.4 |
| cAMP (1) | PKA | 1000 | 99.0 ± 1.0 |
| Wortmannin (0.005)e | PI 3K | 2 | 133.9 ± 15.8 |
The asterisks denote those drugs that had a significant effect on phagocytosis (Student’s unpaired two-tail t test; p < 0.05). All drugs except tyrphostins were added to cells 15–30 min before the addition of latex bead particles. Cells were incubated with tryphostins for 17 hours. The IC50 is the reported concentration of the drug that inhibits the activity of the enzyme the drug is most specific for. The last column represents the relative rate of phagocytosis of cultures treated with the indicated drugs as a percent of the control rate set to 100%.
Calmidazolium is a calmodulin antagonist.
BAPTA-AM is a membrane-permeable intracellular Ca2+ chelator.
Genistein (IC50 2.6), tyrphostin 25 (IC50, 3), and tyrphostin 51 (IC50, 1) were also without effect.
A knockout of the single gene encoding protein kinase A was also normal in phagocytosis.
LY294002 at concentrations up to 100 μM was also without effect.
In contrast to the above results, the addition of the phospholipase C (PLC) inhibitor U73122 significantly reduced phagocytosis in a dose-dependent manner (Table 1). In fact, at the highest concentration of 12 μM, the drug reduced the rate of phagocytosis by >90%. As a negative control, we added the closely related but much less effective PLC-inactivating drug U73343 and detected no significant reduction of phagocytosis at concentrations up to 6 μM, suggesting that U73122 was acting by specifically inhibiting PLC activity.
To determine which of the products of PLC activity, diacylglycerol (DAG) or inositol triphosphate (IP3), was more important in regulating phagocytosis, we treated cells with calphostin C (this drug binds to DAG binding domains of proteins including PKC; Kobayashi et al., 1989); and with bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) (to chelate intracellular Ca2+ released by IP3; Dieter et al., 1993). Table 1 indicates that the addition of either calphostin C (final concentration, 1.0 μM) or BAPTA-AM (final concentration, 0.3 mM) decreased the rate of phagocytosis by 50 and 65%, respectively. Furthermore, the addition of calmidazolium (final concentration, 5 μM), previously demonstrated to increase intracellular levels of Ca2+ in Dictyostelium by facilitating release from IP3-sensitive stores (Schlatterer and Schaloske, 1996), significantly stimulated the rate of phagocytosis by >50%. Together, these results suggest that both PLC products DAG and Ca2+ play an important role in regulating phagocytosis, and that calphostin C may act by inhibiting the function of a DAG-binding and/or activated protein that does not have PKC activity, because other specific PKC inhibitors were ineffective (Table 1).
Evidence that Rap1 Functions Downstream of a DAG-activated Protein to Regulate Phagocytosis
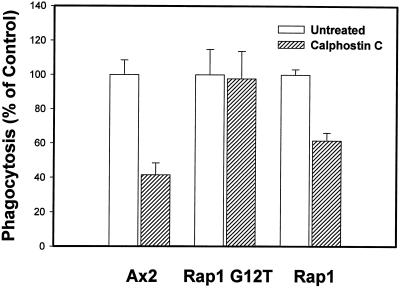
Given its apparent role in phagocytosis, Rap1 could function in one or both of the pathways regulated by the products of PLC activity. To begin to address this, we measured the rate of phagocytosis in control cells, Rap1 WT(+) cells, and Rap1 G12T(+) cells treated with calphostin C. As indicated in Figure 4, phagocytosis rates were decreased in calphostin-treated Ax2 and Rap1 WT(+) cells by 60 and 40%, respectively. In contrast, drug-treated cells overexpressing the constitutively active form of Rap1 showed no significant decrease in the rate of particle internalization. This result suggests that the calphostin C target, perhaps a DAG-binding, non-PKC protein (see above), acts upstream of Rap1, which would place Rap1 activity in the DAG branch of the PLC pathway. It also suggests that the effect of calphostin C is not due to a nonspecific inhibition of general metabolic processes.
Figure 4.
Rap1 functions downstream of a calphostin C target protein to regulate phagocytosis. Cells were pretreated with calphostin C (0.75 μM) for 20 min, fluorescent latex beads were added to the cells, and phagocytosis rates were determined as described in MATERIALS AND METHODS. The rate (fluorescence per microgram of protein) of the untreated control for each strain at the 30-min time point was normalized to a phagocytic value of 100, and the rate for the respective drug-treated strain was normalized accordingly. Values are reported as the ratio of (phagocytic index for the drug-treated cells at 30 min)/(phagocytic index for the untreated culture at 30 min) ± SD for each strain. Statistical analysis was performed as described in Figure 1 to ensure that the reported decreases were significant. Control Ax2 cells, treated with calphostin C, displayed a 60% inhibition in bead uptake, whereas Rap1 WT(+) cells displayed a 40% inhibition in bead uptake. In contrast, phagocytosis was unaffected in Rap1 G12T(+) cells treated with the drug, suggesting that a calphostin C–sensitive protein acts upstream of Rap1 in a signal transduction pathway that regulates phagocytosis.
Rap1 Overexpression Negatively Regulates Macropinocytosis
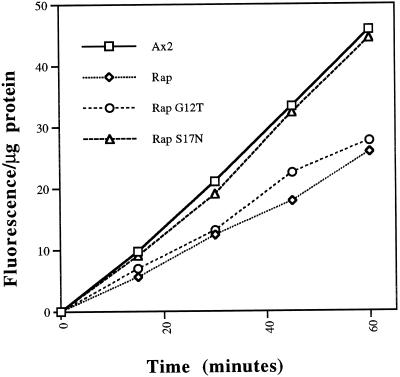
It has been suggested that macropinocytosis and phagocytosis in Dictyostelium may be biochemically comparable and perhaps identical processes (Hacker et al., 1997). There are at least two pinocytic routes of fluid phase entry that have been described for Dictyostelium. The macropinosomal route of entry, recently described by Hacker et al., (1997), probably accounts for >50% of the total internalization of fluid, whereas the micropinosomal pathway (likely clathrin mediated) apparently accounts for the rest (O’Halloran and Anderson, 1992; Ruscetti et al., 1994; Rupper and Cardelli, unpublished observations). Macropinocytosis results in the formation of relatively large vesicles, up to 3 μm in diameter, that can be visualized by phase-contrast microscopy. Based on the data presented above describing an increase in phagocytosis for Rap1 WT(+) and Rap1 G12T(+) cells, we predicted that the rate of macropinocytosis (and thus pinocytosis overall) might also be higher in these, compared with control cells. Therefore, we measured pinocytosis rates in the cell lines overexpressing Rap1 WT and mutant Rap1 proteins by analyzing the rate of internalization of the fluid phase marker FITC-dextran. The measurements are presented as fluorescence units internalized per microgram of cellular protein to correct for any differences in cell size between the various strains. Figure 5 indicates that the pinocytosis rates in Rap1 WT(+) and Rap1 G12T(+) cells were 40% lower than those observed for control cell-lines. Control cells internalized 19.9 ± 3.7 (SD) fluorescence units/30 min per μg of protein, whereas Rap1 WT(+) and Rap1 G12T(+) cells internalized 12.2 ± 0.6 and 12.0 ± 3.7 units/30 min per μg of protein, respectively. In contrast, Rap1 S17N(+) cells showed no significant change in the pinocytic rate when compared with parental Ax2 cells (Fig. 5).
Figure 5.
Rap1 negatively regulates fluid phase pinocytosis. Cells were incubated with 2 mg/ml FD, and at the indicated times, 1-ml samples were harvested, washed, and lysed, and fluorescence of the cells was analyzed as described in MATERIALS AND METHODS. Fluorescence was normalized to total protein per sample to account for potential differences in cell size among strains. Depicted here is a representative experiment of three separate trials that were performed. Rap1 WT(+) and Rap1 G12T(+) cells internalized FD at 60% the rate of control Ax2 cells. In contrast, Rap1 S17N(+) cells showed no inhibition in fluid phase uptake compared with control Ax2 cells. Student’s two-tailed t test was performed on the fluorescence data from both the 30- and 45-min time points (when the rate of uptake is linear) to ensure that the reported decreases were significant (see Figure 1 legend). The values below are reported as the average fluorescence per microgram of protein ± SD for the 30-min time point. Statistics: Ax2, 19.9 ± 3.7 (n = 4); Rap1 WT(+) 12.2 ± 0.6 (n = 3); Rap1 G12T(+), 12.0 ± 3.7 (n = 4); Rap S17N(+), 17.6 ± 5.5 (n = 3).
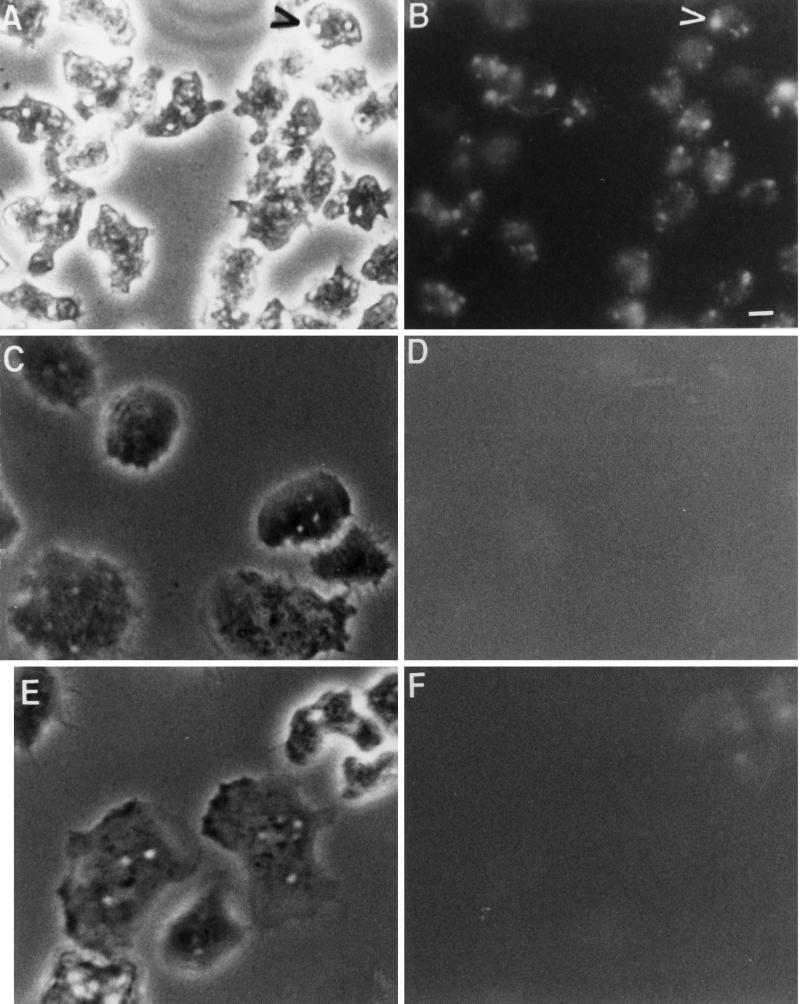
To determine whether the decrease in pinocytosis was due at least in part to a decrease in macropinocytosis, control, Rap1 WT(+), and Rap1 G12T(+) cells attached to coverslips were incubated in growth medium for 5 min with the fluid phase marker lucifer yellow. After this pulse period, cells were quickly washed and viewed using a fluorescence microscope. Figure 6, A and B, indicates that >90% of the control cells contained at least one and usually contained three or four fluorescent vesicles that were at least 1–2 μm in diameter; given the short time for the pulse period, we hypothesize that these vesicles represent macropinosomes and not lysosomes or postlysosomes. In contrast, <30% of the Rap1 WT(+) (Figure 6, C and D) and Rap1 G12T(+) (Figure 6, E and F) cells contained large fluorescent vesicles, suggesting that the 40% reduction in pinocytosis rates observed for cells expressing Rap1 may be primarily due to an inhibition of macropinocytosis; however, this experimental approach does not address whether Rap1 regulates micropinocytosis or the relative contribution of micropinocytosis to total fluid phase pinocytosis.
Figure 6.
Rap1 negatively regulates fluid phase macropinocytosis. Control Ax2 cells (A and B), Rap1 WT(+) cells (C and D), and Rap1 G12T(+) cells (E and F) were shaken from 175-cm2 tissue culture flasks, collected by centrifugation, placed on coverslips, and incubated with lucifer yellow for 5 min. Cells were washed in fresh media and prepared for phase contrast (A, C, and E) or fluorescence (B, D, and E) microscopy. Control Ax2 cells internalized lucifer yellow into large vesicles (up to 5 μm in size) that represent macropinosomes (B; see arrowhead). Individual control Ax2 cells contained, on average, three to five macropinosomes containing lucifer yellow after a 5-min pulse. No macropinosomes were observed in Rap1 WT(+) or Rap1 G12T(+) cell lines (D and F, respectively). Bar, 5 μm.
Rap1 Overexpression Does Not Affect Steps in the Late Endosomal Pathway or Steps in the Secretory Pathway Regulating the Processing, Sorting, and Secretion of Lysosomal Hydrolases
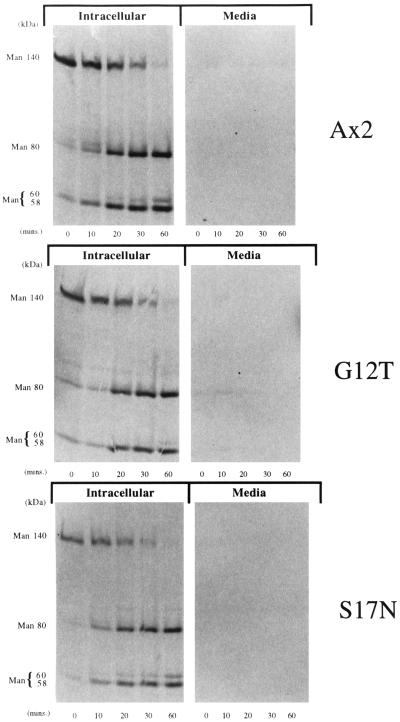
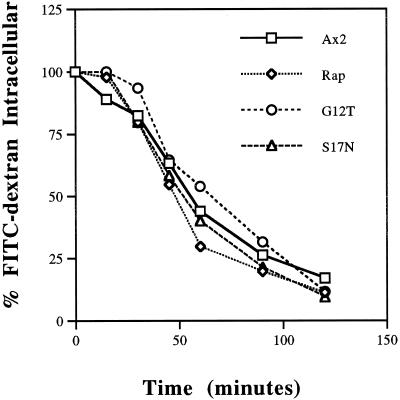
Our results thus far suggest that Rap1 acts at internalization steps in the pinosomal and phagosomal pathway, perhaps by regulating the organization of the actin cytoskeleton (Rebstein et al., 1997). The actin cytoskeleton has been demonstrated recently to be important in regulating postinternalization vesicle trafficking steps. For instance, treatment of cells with cytochalasin A to disrupt the actin cytoskeleton blocks efflux of fluid phase from an acidic endosomal compartment (Temesvari et al., 1996c, Jenne et al., 1998). Furthermore, primordial endosomal compartments (postlysosomes) are ringed by F-actin (Rauchenberger et al., 1997). Therefore, to determine whether Rap1 regulated lysosomal protein targeting and secretory pathways, we performed radiolabel pulse–chase experiments to measure the processing and sorting of the lysosomal hydrolase α-mannosidase. Figure 7 indicates that the rate of processing of the α-mannosidase 140-kDa precursor to the intermediate (80 kDa) and mature forms (58 and 60 kDa) was identical for control cells and cells expressing Rap1 G12T and Rap1 S17N, suggesting the enzyme is appropriately targeted to lysosomes. Furthermore, missorting and oversecretion of the newly synthesized α-mannosidase precursor was negligible in all strains examined (Figure 7). Processing and sorting were also normal for Rap1 WT(+) cells. Finally the efflux rates of preinternalized fluid phase (Figure 8) was similar for all strains. These results are consistent with Rap1 playing a direct role in regulating phagocytosis as opposed to simply affecting the actin cytoskeleton in a way that indirectly modulates phagocytosis and endosomal flux.
Figure 7.
Rap1 does not play a role in the processing or targeting of α-mannosidase to lysosomes. Growing cells were pulsed with [35S]methionine in HL5 medium for 20 min and chased in unlabeled medium for the times indicated. After centrifugation, both the cell pellet and the extracellular medium were incubated with a monoclonal antibody specific for α-mannosidase (Mierendorf et al., 1983). The immunoprecipitated proteins were separated by SDS-PAGE, and autoradiography was used to visualize the labeled proteins. None of the Rap1 overexpressing cell lines examined displayed a defect in the processing kinetics, targeting, or secretion of α-mannosidase.
Figure 8.
Rap1 does not play a role in the exocytosis of fluid phase. Growing cells were incubated with FITC-dextran (2 mg/ml) for 3 h to load the endo-lysosomal system to steady-state levels. Cells were washed, resuspended in fresh media, and incubated for an additional 2 h, and at various times, 1-ml samples were harvested. Fluorescence associated with the cells was calculated as described in MATERIALS AND METHODS. Compared with control Ax2 cells, none of the Rap1 overexpressing cell lines examined showed a significant change in the rate of exocytosis of fluid phase.
DISCUSSION
In this report, we present experimental evidence suggesting that in Dictyostelium Rap1 positively regulates phagocytosis and negatively regulates macropinocytosis. This study represents, to our knowledge, the first demonstration of a Ras-like family member playing an important role in regulating phagocytosis, although Rho family members have been demonstrated to regulate this process, and Ras has been demonstrated to regulate pinocytosis. The biochemical function of Rap1 appeared limited to early internalization steps in phagocytosis and pinocytosis, because the efflux of fluid phase and the transport, processing, and targeting of lysosomal hydrolases were unaffected by overexpression of wild-type and mutant forms of Rap1. Finally, PLC activity appeared to play a critical role in phagocytosis in wild-type Ax2 cells, and Rap1 apparently acted downstream of PLC in the DAG-activated arm of the pathway.
Cell lines overexpressing WT Rap1 or constitutively activated Rap1 phagocytosed beads two to three times faster than control Ax2 on a per protein basis, whereas cell lines overexpressing a dominant negative form of Rap1 (Rap1 S17N) phagocytosed beads at one-half the rate of Ax2. This result suggests that this GTPase regulates an important step in the signal transduction pathway that regulates internalization of particles that enter the phagosomal pathway. A similar result was obtained when bacteria were used instead of latex beads, suggesting this was a physiologically relevant observation. Because the overexpression of the Rap1 proteins was conditional and modest, we conclude the phenotypic changes observed are not the result of an indirect and/or nonspecific effect of Rap1 expression on other GTPase-regulated pathways. It is interesting that overexpression of Rap1 WT was as effective as Rap1 G12T in stimulating phagocytosis, suggesting Rap1 is normally rate limiting in regulating this process and that the majority of the Rap1 wild-type protein is found in the GTP bound state.
To our knowledge, this is the first report demonstrating a role for Rap1 in regulating phagocytosis, although others have demonstrated that Rap1 may play a role in regulating the oxidative burst in neutrophils during internalization of bacteria (Maly et al., 1994). The proposed role for Rap1 in neutrophils is similar to that in Dictyostelium, namely to initiate the association of proteins that, in neutrophils, can regulate the oxidative burst or, in Dictyostelium, perhaps the internalization of particles.
Little is known about the biochemical mechanism(s) that regulate particle internalization in Dictyostelium. We report here that the addition of inhibitors of PI 3-kinases PKA, PKG, PKC, and PTK had no significant negative effect on phagocytosis in wild-type Ax2 cultures; similar results have recently been published for some of these agents (Peracino et al., 1998). These negative results were observed regardless of the preincubation times and despite using concentrations of drugs orders of magnitude higher then their reported IC50 values. Furthermore, Western blot analysis indicated that treatment of cells with tyrphostins 25 and 46 reduced the level of actin tyrosine phosphorylation by >95%, suggesting this drug was effective in inhibiting PTK activity. However, these data do not address the possibility of drug-resistant enzymes, belonging to the classes listed above, that may play a role in regulating phagocytosis.
In macrophages, PTKs play an important role in regulating phagocytosis. Opsonized bacteria that bind to Fcγ receptors trigger tyrosine phosphorylation of the cytoplasmic tail of the receptor protein that results in the binding of proteins containing SH2 domains (Crowley et al., 1997), and this initiates a signaling pathway resulting in changes in the actin cytoskeleton and the internalization of particles. This pathway has not been demonstrated to exist in Dictyostelium (no PTK receptors have been discovered), and our results using PTK inhibitors suggest that this pathway would most likely play only a minor role in regulating phagocytosis if it existed. It has also been demonstrated that the PTK pathway involves the activation of PI 3-kinases that, in macrophages, have been demonstrated to play a role in the final closure of membranes to form the internalized phagosome (Araki et al., 1996). The results of biochemical and genetic studies suggest that the Dictyostelium PI 3-kinases DdPIK1 and DdPIK2 regulate macropinocytosis (Rupper and Cardelli, unpublished results) but do not appear to play a major role in regulating phagocytosis (Buczynski et al., 1997b), further suggesting that the biochemical mechanisms regulating phagocytosis in Dictyostelium may be distinct from those regulating phagocytosis in macrophages.
Instead, it appears that in Dictyostelium and perhaps in other cells, heterotrimeric G proteins may play an essential role in regulating phagocytosis and phagosomal maturation. In support of this, Dictyostelium Gβ(−) cells internalize particles at only 20–25% of the rate observed for control cells (Peracino et al., 1998), and Gβ subunits have been localized to the phagosomal membranes in mammalian cells (Desjardins et al., 1994), where they may regulate phagosome-lysosome fusion in mammalian cells (Beron et al., 1995). It has also been recently demonstrated that in a variety of cells the Gβγ subunits, in addition to the Gα subunits, can initiate signaling pathways by binding to specific enzymes such as PLC (Yan and Gautam, 1997) and regulating their activity (Zhang et al., 1996). Interestingly, the PLC inhibitor U73122 repressed phagocytosis in Dictyostelium by >90%, whereas the chemically related but inactive analogue U73322 was ineffective, demonstrating the specificity of action of U73122.
Additional experiments reported here support a role for PLC activity in regulating phagocytosis in Dictyostelium. In mammalian cells, PLC activity results in the generation of DAG and increases in cytosolic Ca2+ via IP3 action on internal stores. Not surprisingly then, phagocytosis was also negatively affected by the addition of calphostin C, which competes with DAG for DAG-binding protein domains, and other drugs that altered intracellular calcium levels. Calphostin C is a PKC inhibitor, but other specific PKC inhibitors that interact with the active site of the enzyme did not affect phagocytosis, suggesting that a non-PKC DAG-binding protein was involved. While this manuscript was under review, the Bozzaro group reported in agreement with our results that Ca2+ and PLC were involved in regulating phagocytosis (Peracino et al., 1998).
Phagocytosis in Rap1 G12T(+) cells, but not Rap1 WT(+) cells, was insensitive to the presence of calphostin C, suggesting that the calphostin C target acts upstream of Rap1 to regulate phagocytosis. The molecular nature of this target remains to be defined, but it has already been demonstrated that some GTPase guanine exchange factors contain DAG-binding domains (Cerione and Zheng, 1996). This would be consistent with the calphostin C target being a Rap1 GEF that would not be required in cells overexpressing the constitutively active Rap1 G12T protein. We also propose that, in addition to the DAG pathway, the Ca2+-mediated pathway, perhaps acting in parallel with Rap1, positively regulates phagocytosis. Interestingly, it has recently been reported that Ca2+ plays a role in regulating Rap1 activity in human platelets (Franke et al., 1997). The source for the increase in cytoplasmic Ca2+ (extracellular or intracellular stores) remains to be determined, although the proposed mechanism of action of calmidazolium, demonstrated here to stimulate phagocytosis, involves the induced release of Ca2+ from internal stores, which triggers the influx of extracellular Ca2+ (Schlatterer and Schaloske, 1996).
Members of the Rho family of GTPases, including Cdc42, Rac, and Rho, have been implicated in regulating phagocytosis in mammalian cells (Adam et al., 1996; Cox et al., 1997; Hackam et al., 1997). We have also recently observed that a novel Dictyostelium Rho protein, RacC, positively regulates phagocytosis (Seastone et al., 1998) and we speculate that Rap1 could act as an upstream activator of RacC and perhaps other small G proteins to regulate phagocytosis. G protein–coupled signal cascades have also been proposed to function in fibroblasts and macrophages to regulate actin cytoskeletal and gene expression changes. Figure 9 represents one speculative and testable model to account for our observations.
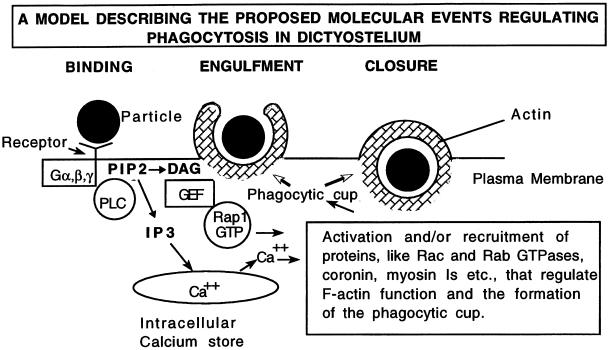
Figure 9.
Current working model to explain the signal transduction pathway regulating phagocytosis in Dictyostelium. Binding of particles or bacteria to a heterotrimeric G protein–coupled receptor sets off a signal transduction cascade resulting in the activation of phospholipase C and cleavage of PIP2 to DAG and IP3. The pathway bifurcates and IP3 causes the release of Ca2+ from intracellular stores. Meanwhile, DAG binds to a guanine exchange factor, which, in turn, activates Rap1. Together, both these pathways lead to the recruitment of other proteins that regulate the formation of the phagocytic cup.
Compared with control cells, the rates of pinocytosis were 40% lower for Rap1 WT(+) and Rap1 G12T(+) cells, and most of this decrease appeared to be accounted for by a major inhibition of macropinocytosis. The decrease in macropinocytosis is intriguing because Rap1 WT(+) and Rap1 G12T(+) cells, when attached to cell surfaces, display prominent membrane ruffles (Rebstein et al., 1997), formations that, in other systems, have been suggested to be important in stimulating macropinocytosis (Araki et al., 1996). However, it has been recently reported that Ras expression stimulates pinocytosis independent of its effect on the formation of lamellipodia (Li et al., 1997), suggesting that prominent changes in the morphology of the plasma membrane do not necessarily indicate an involvement in internalization processes such as macropinocytosis.
The data presented here also support the hypothesis that the processes of macropinocytosis and phagocytosis, although related in terms of shared components such as actin, may use different proteins that independently regulate each process. Additional published evidence supports this hypothesis. For instance, genetic and biochemical approaches have indicated that the proteins DdPIK1 and DdPIK2, related to PI 3-kinases in amino acid sequence, are required for macropinocytosis but are not involved in regulating phagocytosis (Buczynski et al., 1997b). In addition, we have recently found that overexpression of RacC, a novel Rho-like GTPase, stimulates phagocytosis and decreases macropinocytosis (Seastone et al., 1998). Finally, the Gβ subunit of the heterotrimeric G protein regulates phagocytosis (Peracino et al., 1998) and not macropinocytosis (our unpublished observations).
The Rap1-mediated effects on internalization of fluid and particles appear limited to early steps of the endosomal and phagosomal pathways, because the efflux of fluid phase, and the synthesis, proteolytic processing, and delivery of mature lysosomal hydrolases to lysosomes were not altered in any of the mutant cell lines we examined. This supports our hypothesis that Rap1 functions are specific and therefore limited in scope. Consistent with the localization of Rap1 to the plasma membrane, these data suggest that the effects exerted by Rap1 are limited to the internalization arm of the phagosomal pathway.
In summary, our results support an important role for the small Mr GTPase Rap1 and both arms of the PLC pathway (DAG and IP3) in regulating phagocytosis in Dictyostelium. The goal of future investigations will be to provide an insight into the biochemical mechanisms that coordinate changes in PLC activity and Rap1 function with actin cytoskeleton changes and activation of other effector proteins (including possibly other G proteins) to regulate formation of the phagocytic cup.
ACKNOWLEDGMENTS
We acknowledge the support from the Feist-Weiller Cancer Center and the Center for Excellence in Arthritis and Rheumatology. We also thank members of the Cardelli laboratory for critical reading of the manuscript. This work was supported by grants from the National Science Foundation (DCB9104576) and National Institutes of Health (DK 3923205) to J.A.C.
REFERENCES
- Adam T, Giry M, Boquet P, Sansonetti P. Rho-dependent membrane folding causes Shigella entry into epithelial cells. EMBO J. 1996;15:3315–3321. [PMC free article] [PubMed] [Google Scholar]
- Allen LH, Aderem A. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J Exp Med. 1995;182:829–840. doi: 10.1084/jem.182.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry L, Klein G, Martiel JL, Satre M. Kinetics of endosomal pH evolution in Dictyostelium discoideum amoebae. Study by fluorescence spectroscopy. J Cell Sci. 1993;105:861–866. doi: 10.1242/jcs.105.3.861. [DOI] [PubMed] [Google Scholar]
- Bender A, Pringle JR. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc Natl Acad Sci USA. 1989;86:9976–9980. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beron W, Colombo MI, Mayorga LS, Stahl PD. In vitro reconstitution of phagosome-endosome fusion: evidence for regulation by heterotrimeric GTPases. Arch Biochem Biophys. 1995;317:337–342. doi: 10.1006/abbi.1995.1172. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Quilliam LA, Bohl BP, Jesaitis AJ, Quinn MT. Inhibition of Rap1A binding to cytochrome b558 of NADPH oxidase by phosphorylation of Rap1A. Science. 1991;254:1794–1796. doi: 10.1126/science.1763330. [DOI] [PubMed] [Google Scholar]
- Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli J. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol Biol Cell. 1997a;8:1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski G, Grove B, Nomura A, Kleve M, Bush J, Firtel RA, Cardelli J. Inactivation of two Dictyostelium discoideum genes, DdPIK1 and DdPIK2, encoding proteins related to mammalian phosphatidylinositide 3-kinases, results in defects in endocytosis, lysosome to postlysosome transport, and actin cytoskeleton organization. J Cell Biol. 1997b;136:1271–1286. doi: 10.1083/jcb.136.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush J, Temesvari L, Rodriguez-Paris J, Buczynski G, Cardelli J. A role for a Rab4-like GTPase in endocytosis and in regulation of contractile vacuole structure and function in Dictyostelium discoideum. Mol Biol Cell. 1996;7:1623–1638. doi: 10.1091/mbc.7.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli JA. Regulation of lysosomal trafficking and function during growth and development of Dictyostelium discoideum. In: Storie B, Murphy R, editors. Advances in Cell and Molecular Biology of Membranes. Greenwich, CT: JAI Press; 1993. pp. 341–390. [Google Scholar]
- Cerione RA, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol. 1996;8:216–222. doi: 10.1016/s0955-0674(96)80068-8. [DOI] [PubMed] [Google Scholar]
- Chant J, Herskowitz I. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell. 1991;65:1203–1212. doi: 10.1016/0092-8674(91)90015-q. [DOI] [PubMed] [Google Scholar]
- Cook SJ, Rubinfeld B, Albert I, McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993;12:3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Wessels D, Soll DR, Hartwig J, Condeelis J. Re-expression of ABP-120 rescues cytoskeletal, motility, and phagocytosis defects of ABP-120-Dictyostelium mutants. Mol Biol Cell. 1996;7:803–823. doi: 10.1091/mbc.7.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124:677–688. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter P, Fitzke E, Duyster J. BAPTA induces a decrease of intracellular free calcium and a translocation and inactivation of protein kinase C in macrophages. Biol Chem Hoppe Seyler. 1993;374:171–174. doi: 10.1515/bchm3.1993.374.1-6.171. [DOI] [PubMed] [Google Scholar]
- Farrell FX, Ohmstede CA, Reep BR, Lapetina EG. cDNA sequence of a new ras-related gene (rap2b) isolated from human platelets with sequence homology to rap2. Nucleic Acids Res. 1990;18:4281. doi: 10.1093/nar/18.14.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Akkerman JW, Bos JL. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 1997;16:252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech M, John J, Pizon V, Chardin P, Tavitian A, Clark R, McCormick F, Wittinghofer A. Inhibition of GTPase activating protein stimulation of Ras-p21 GTPase by the Krev-1 gene product. Science. 1990;249:169–171. doi: 10.1126/science.2164710. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Schreiber A, Zhang W, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- Hoshijima M, Kikuchi A, Kawata M, Ohmori T, Hashimoto E, Yamamura H, Takai Y. Phosphorylation by cyclic AMP-dependent protein kinase of a human platelet Mr 22,000 GTP-binding protein (smg p21) having the same putative effector domain as the ras gene products. Biochem Biophys Res Commun. 1988;157:851–860. doi: 10.1016/s0006-291x(88)80953-7. [DOI] [PubMed] [Google Scholar]
- Hutchinson MJ, Harrison PT, Floto RA, Allen JM. Fc gamma receptor-mediated phagocytosis requires tyrosine kinase activity and is ligand independent. Eur J Immunol. 1995;25:481–487. doi: 10.1002/eji.1830250226. [DOI] [PubMed] [Google Scholar]
- Jenne N, Rauchenberger R, Hacker U, Kast T, Maniak M. Targeted gene disruption reveals a role for vacuolin B in the late endocytic pathway and exocytosis. J Cell Sci. 1998;111:61–70. doi: 10.1242/jcs.111.1.61. [DOI] [PubMed] [Google Scholar]
- Jung G, Wu X, Hammer JA. Dictyostelium mutants lacking multiple classic myosin I isoforms reveal combinations of shared and distinct functions. J Cell Biol. 1996;133:305–323. doi: 10.1083/jcb.133.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Labadia ME, Bokoch GM, Huang CK. The Rap1A protein enhances protein kinase C activity in vitro. Biochem Biophys Res Commun. 1993;195:1321–1328. doi: 10.1006/bbrc.1993.2188. [DOI] [PubMed] [Google Scholar]
- Li G, D’Souza-Schorey C, Barbieri MA, Cooper JA, Stahl PD. Uncoupling of membrane ruffling and pinocytosis during Ras signal transduction. J Biol Chem. 1997;272:10337–10340. [PubMed] [Google Scholar]
- Maly FE, Quilliam LA, Dorseuil O, Der CJ, Bokoch GM. Activated or dominant inhibitory mutants of Rap1A decrease the oxidative burst of Epstein-Barr virus-transformed human B lymphocytes. J Biol Chem. 1994;269:18743–18746. [PubMed] [Google Scholar]
- Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch G. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell. 1995;83:915–924. doi: 10.1016/0092-8674(95)90207-4. [DOI] [PubMed] [Google Scholar]
- Mierendorf RCJ, Cardelli JA, Dimond RL. Pathways involved in targeting and secretion of a lysosomal enzyme in Dictyostelium discoideum. J Cell Biol. 1985;100:1777–1787. doi: 10.1083/jcb.100.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierendorf RCJ, Dimond RL. Functional heterogeneity of monoclonal antibodies obtained using different screening assays. Anal Biochem. 1983;135:221–229. doi: 10.1016/0003-2697(83)90754-6. [DOI] [PubMed] [Google Scholar]
- Niewohner J, Weber I, Maniak M, Muller-Taubenberger A, Gerisch G. Talin-null cells of Dictyostelium are strongly defective in adhesion to particle and substrate surfaces and slightly impaired in cytokinesis. J Cell Biol. 1997;138:349–361. doi: 10.1083/jcb.138.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak KD, Peterson MD, Reedy MC, Titus MA. Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis. J Cell Biol. 1995;131:1205–1221. doi: 10.1083/jcb.131.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Halloran TJ, Anderson RG. Clathrin heavy chain is required for pinocytosis, the presence of large vacuoles, and development in Dictyostelium. J Cell Biol. 1992;118:1371–1377. doi: 10.1083/jcb.118.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmstede CA, Farrell FX, Reep BR, Clemetson KJ, Lapetina EG. RAP2B: a RAS-related GTP-binding protein from platelets. Proc Natl Acad Sci USA. 1990;87:6527–6531. doi: 10.1073/pnas.87.17.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Shimizu K, Yamamori B, Kuroda S, Takai Y. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J Biol Chem. 1996;271:1258–1261. doi: 10.1074/jbc.271.3.1258. [DOI] [PubMed] [Google Scholar]
- Padh H, Ha J, Lavasa M, Steck TL. A post-lysosomal compartment in Dictyostelium discoideum. J Biol Chem. 1993;268:6742–6747. [PubMed] [Google Scholar]
- Peracino B, Borleis J, Jin T, Westphal M, Schwartz JM, Wu L, Bracco E, Gerisch G, Devreotes P, Bozzaro S. G protein beta subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J Cell Biol. 1998;141:1529–1537. doi: 10.1083/jcb.141.7.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V, Chardin P, Lerosey I, Olofsson B, Tavitian A. Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the “effector” region. Oncogene. 1988a;3:201–204. [PubMed] [Google Scholar]
- Pizon V, Lerosey I, Chardin P, Tavitian A. Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B) Nucleic Acids Res. 1988b;16:7719. doi: 10.1093/nar/16.15.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V, Desjardins M, Bucci C, Parton R, Zerial M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J Cell Sci. 1994;107:1661–1670. doi: 10.1242/jcs.107.6.1661. [DOI] [PubMed] [Google Scholar]
- Rauchenberger R, Hacker U, Murphy J, Niewohner J, Maniak M. Coronin and vacuolin identify consecutive stages of a late actin-coated endocytic compartment in Dictyostelium. Curr Biol. 1997;3:215–218. doi: 10.1016/s0960-9822(97)70093-9. [DOI] [PubMed] [Google Scholar]
- Rebstein PJ, Cardelli J, Weeks G, Spiegelman GB. Mutational analysis of the role of Rap1 in regulating cytoskeletal function in Dictyostelium. Exp Cell Res. 1997;231:276–283. doi: 10.1006/excr.1996.3466. [DOI] [PubMed] [Google Scholar]
- Rebstein PJ, Weeks G, Spiegelman GB. Altered morphology of vegetative amoebae induced by increased expression of the Dictyostelium discoideum ras-related gene rap1. Dev Genet. 1993;14:347–355. doi: 10.1002/dvg.1020140504. [DOI] [PubMed] [Google Scholar]
- Robbins SM, Suttorp VV, Weeks G, Spiegelman GB. A ras-related gene from the lower eukaryote Dictyostelium that is highly conserved relative to the human rap genes. Nucleic Acids Res. 1990;18:5265–5269. doi: 10.1093/nar/18.17.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Paris JM, Nolta KV, Steck TL. Characterization of lysosomes isolated from Dictyostelium discoideum by magnetic fractionation. J Biol Chem. 1993;268:9110–9116. [PubMed] [Google Scholar]
- Ruscetti T, Cardelli JA, Niswonger ML, O’Halloran TJ. Clathrin heavy chain functions in sorting and secretion of lysosomal enzymes in Dictyostelium discoideum. J Cell Biol. 1994;126:343–352. doi: 10.1083/jcb.126.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seastone DJ, Lee E, Bush J, Knecht D, Cardelli J. Overexpression of a novel Rho-family GTPase, RacC, induces unusual actin-based structures and positively affects phagocytosis in Dictyostelium discoideum. Mol Biol Cell. 1998;9:2891–2904. doi: 10.1091/mbc.9.10.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatterer C, Schaloske R. Calmidazolium leads to an increase in the cytosolic Ca2+ concentration in Dictyostelium discoideum by induction of Ca2+ release from intracellular stores and influx of extracellular Ca2+ Biochem J. 1996;313:661–667. doi: 10.1042/bj3130661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temesvari LA, Bush JM, Peterson MD, Novak KD, Titus MA, Cardelli JA. Examination of the endosomal and lysosomal pathways in Dictyostelium discoideum myosin I mutants. J Cell Sci. 1996a;109:663–673. doi: 10.1242/jcs.109.3.663. [DOI] [PubMed] [Google Scholar]
- Temesvari LA, Rodriguez-Paris JM, Bush JM, Zhang L, Cardelli JA. Involvement of the vacuolar proton-translocating ATPase in multiple steps of the endo-lysosomal system and in the contractile vacuole system of Dictyostelium discoideum. J Cell Sci. 1996b;109:1479–1495. doi: 10.1242/jcs.109.6.1479. [DOI] [PubMed] [Google Scholar]
- Temesvari L, Zhang L, Cardelli JA. The role of the actin cytoskeleton in the endolysosomal system of Dictyostelium discoideum. Mol Biol Cell. 1996c;7:451a. [Google Scholar]
- Wu L, Valkema R, Van Haastert PJ, Devreotes PN. The G protein beta subunit is essential for multiple responses to chemoattractants in Dictyostelium. J Cell Biol. 1995;129:1667–1675. doi: 10.1083/jcb.129.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Gautam N. Structural determinants for interaction with three different effectors on the G protein beta subunit. J Biol Chem. 1997;272:2056–2059. doi: 10.1074/jbc.272.4.2056. [DOI] [PubMed] [Google Scholar]
- Zhang S, Coso OA, Collins R, Gutkind JS, Simonds WF. A C-terminal mutant of the G protein beta subunit deficient in the activation of phospholipase C-beta. J Biol Chem. 1996;271:20208–20212. doi: 10.1074/jbc.271.33.20208. [DOI] [PubMed] [Google Scholar]