Abstract
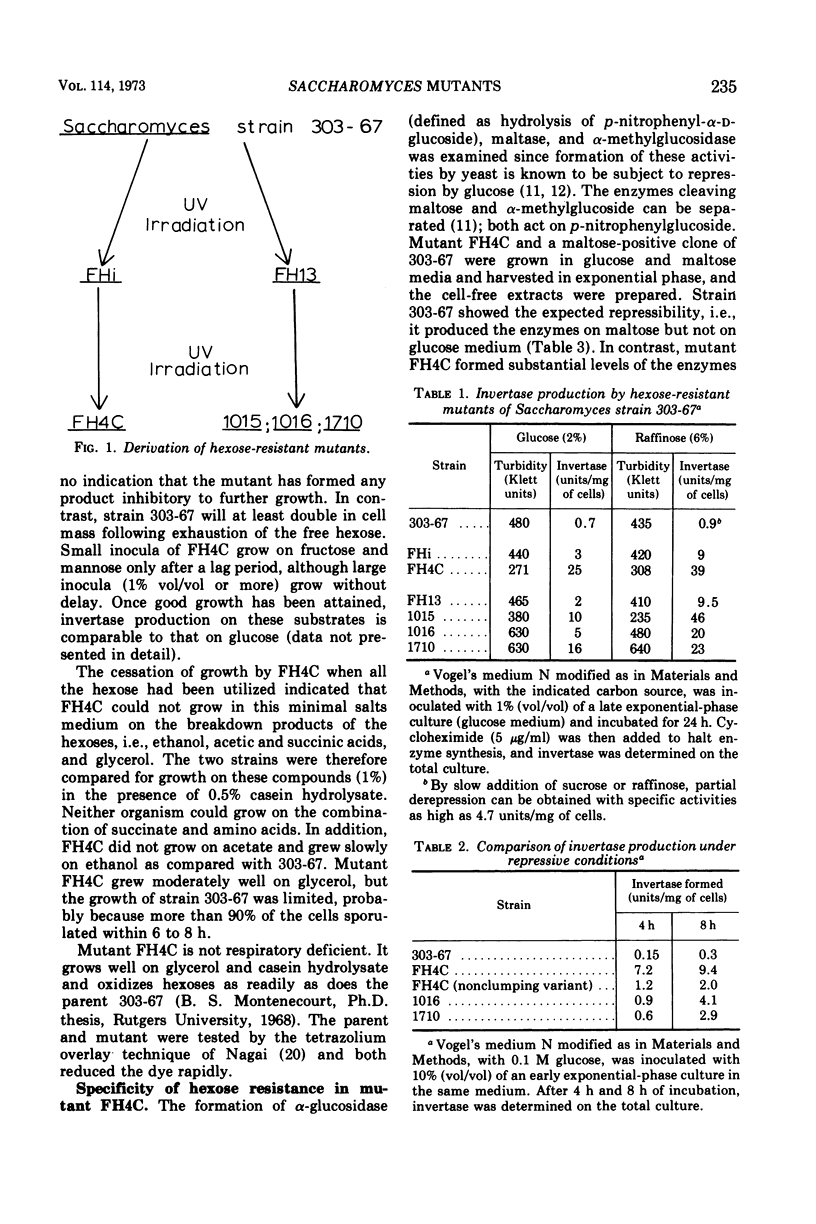
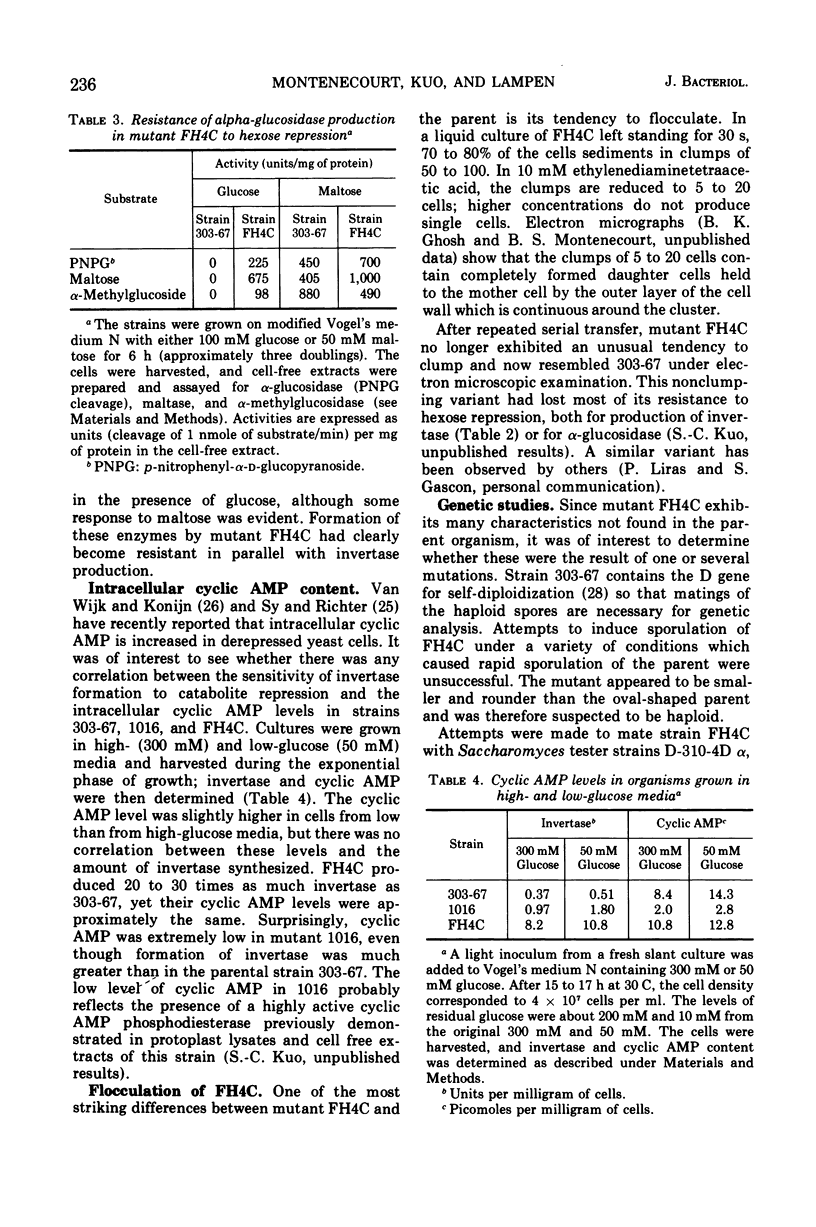
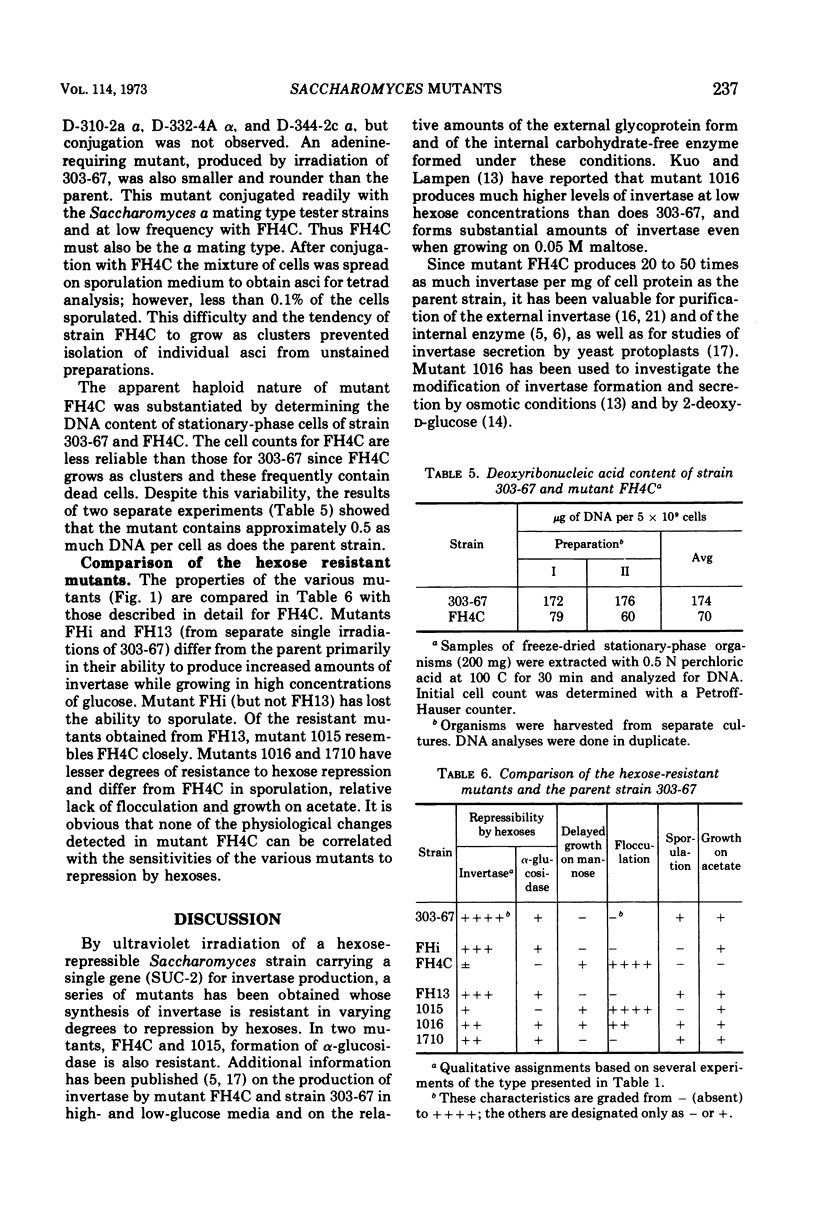
Production of invertase by many strains of yeast is repressed in the presence of hexoses. This phenomenon interferes with studies on the secretion of invertase and with the preparation of large quantities of the enzyme for examination of its chemical and physical characteristics. Saccharomyces strain 303-67, a diploid carrying the single gene SUC-2 for (hexose repressible) invertase production, was subjected to ultraviolet irradiation. No single-step mutations to high level resistance were detected. By a two-step irradiation process mutants were obtained with differing degrees of resistance. The biochemical and genetic characteristics of these mutants are summarized with particular emphasis on FH4C (the most resistant). Although the steady state level of cyclic 3′, 5′-adenosine monophosphate (cyclic AMP) was usually slightly higher in cells grown in low- rather than in high-glucose media, the level of cyclic AMP was not correlated with the sensitivity of invertase synthesis to glucose repression. In mutant FH4C, 1 to 2% of the total cell protein is present as invertase; synthesis of alpha-glucosidase is also resistant to repression by hexoses. This mutant does not sporulate and is probably a haploid of a-mating type with low frequency of conjugation and poor viability of conjugants. Mutants 1016 and 1710 are substantially resistant to hexose repression and still sporulate well. They may be useful for genetic analysis of hexose resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES A. Invertase formation in Saccharomyces fragilis. J Gen Microbiol. 1956 Feb;14(1):109–121. doi: 10.1099/00221287-14-1-109. [DOI] [PubMed] [Google Scholar]
- DAVIES R. Enzyme formation in Saccharomyces fragilis. I. Invertase and raffinase. Biochem J. 1953 Oct;55(3):484–497. doi: 10.1042/bj0550484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODYK F., ROTHSTEIN A. FACTORS INFLUENCING THE APPEARANCE OF INVERTASE IN SACCHAROMYCES CEREVISIAE. Arch Biochem Biophys. 1964 Mar;104:478–486. doi: 10.1016/0003-9861(64)90492-8. [DOI] [PubMed] [Google Scholar]
- Gascón S., Lampen J. O. Purification of the internal invertase of yeast. J Biol Chem. 1968 Apr 10;243(7):1567–1572. [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Gascón S., Ottolenghi P. Invertase isozymes and their localization in yeast. C R Trav Lab Carlsberg. 1967;36(5):85–93. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU A. S., EPSTEIN R., HALVORSON H. O., BOCK R. M. Yeast beta-glucosidase: comparison of the physical-chemical properties of purified constitutive and inducible enzyme. Arch Biochem Biophys. 1960 Dec;91:210–218. doi: 10.1016/0003-9861(60)90492-6. [DOI] [PubMed] [Google Scholar]
- Khan N. A., Eaton N. R. Genetic control of maltase formation in yeast. I. Strains producing high and low basal levels of enzyme. Mol Gen Genet. 1971;112(4):317–322. doi: 10.1007/BF00334433. [DOI] [PubMed] [Google Scholar]
- Khan N. A., Eaton N. R. Purification and characterization of maltase and alpha-methyl glucosidase from yeast. Biochim Biophys Acta. 1967 Sep 12;146(1):173–180. doi: 10.1016/0005-2744(67)90084-8. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Inhibition by 2-deoxy-D-glucose of synthesis of glycoprotein enzymes by protoplasts of Saccharomyces: relation to inhibition of sugar uptake and metabolism. J Bacteriol. 1972 Aug;111(2):419–429. doi: 10.1128/jb.111.2.419-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Osmotic regulation of invertase formation and secretion by protoplasts of Saccharomyces. J Bacteriol. 1971 Apr;106(1):183–191. doi: 10.1128/jb.106.1.183-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. External enzymes of yeast: their nature and formation. Antonie Van Leeuwenhoek. 1968;34(1):1–18. doi: 10.1007/BF02046409. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. A gene affecting the repression of invertase and trehalase in Neurospora. Arch Biochem Biophys. 1962 Mar;96:468–474. doi: 10.1016/0003-9861(62)90322-3. [DOI] [PubMed] [Google Scholar]
- NAGAI S. Induction of the respiration-deficient mutation in yeast by various synthetic dyes. Science. 1959 Oct 30;130(3383):1188–1189. doi: 10.1126/science.130.3383.1188-a. [DOI] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. Purification and properties of yeast invertase. Biochemistry. 1967 Feb;6(2):468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. J., HALVORSON H. O. The components of maltozymase in yeast, and their behavior during deadaptation. J Bacteriol. 1957 Feb;73(2):186–198. doi: 10.1128/jb.73.2.186-198.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON D. D., LAMPEN J. O. Localization of sucrose and maltose fermenting systems in Saccharomyces cerevisiae. Biochim Biophys Acta. 1962 Jan 29;56:303–312. doi: 10.1016/0006-3002(62)90567-x. [DOI] [PubMed] [Google Scholar]
- Sy J., Richter D. Content of cyclic 3',5'-adenosine monophosphate and adenylyl cyclase in yeast at various growth conditions. Biochemistry. 1972 Jul 18;11(15):2788–2791. doi: 10.1021/bi00765a009. [DOI] [PubMed] [Google Scholar]
- Van Wijk R., Konijn T. M. Cyclic 3', 5'-amp in Saccharomyces carlsbergensis under various conditions of catabolite repression. FEBS Lett. 1971 Mar 5;13(3):184–186. doi: 10.1016/0014-5793(71)80231-4. [DOI] [PubMed] [Google Scholar]
- WINGE O., ROBERTS C. A genetic analysis of melibiose and raffinose fermentation. Cr Trav Lab Carlsberg Ser Physiol. 1957;25(18-19):419–459. [PubMed] [Google Scholar]


