Abstract
The central dogma of radiation biology, that biological effects of ionizing radiation are a direct consequence of DNA damage occurring in irradiated cells, has been challenged by observations that genetic/epigenetic changes occur in unexposed “bystander cells” neighboring directly-hit cells, due to cell-to-cell communication or soluble factors released by irradiated cells. To date, the vast majority of these effects are described in cell-culture systems, while in vivo validation and assessment of biological consequences within an organism remain uncertain. Here, we describe the neonatal mouse cerebellum as an accurate in vivo model to detect, quantify, and mechanistically dissect radiation-bystander responses. DNA double-strand breaks and apoptotic cell death were induced in bystander cerebellum in vivo. Accompanying these genetic events, we report bystander-related tumor induction in cerebellum of radiosensitive Patched-1 (Ptch1) heterozygous mice after x-ray exposure of the remainder of the body. We further show that genetic damage is a critical component of in vivo oncogenic bystander responses, and provide evidence supporting the role of gap-junctional intercellular communication (GJIC) in transmission of bystander signals in the central nervous system (CNS). These results represent the first proof-of-principle that bystander effects are factual in vivo events with carcinogenic potential, and implicate the need for re-evaluation of approaches currently used to estimate radiation-associated health risks.
Keywords: cancer risk, DNA damage, in vivo, medulloblastoma, radiation
Ionizing radiation is a well known genotoxic agent and human carcinogen that causes different short- and long-term effects (1, 2). A longstanding paradigm for biological radiation effects has been that radiation traversal through the cell nucleus is required for genetic damage and biological responses. In the last two decades this view has been challenged by reports of radiation effects in unirradiated cells neighboring or cocultured with exposed cells (3–5). Bystander effects have been shown in single-cell systems and in more complex three-dimensional human tissue models (6, 7) for endpoints like sister chromatid exchanges (3), mutations (8), chromosome aberrations (9), cell transformation (10). In vivo, the mouse spleen was a target of radiation-bystander DNA damage (11).
Germ-line heterozygous inactivation of PTCH, the Sonic Hedgehog (SHH) receptor and negative regulator of the pathway, predisposes to medulloblastoma, a childhood brain tumor originating from neural granule cell progenitors (GCPs; ref. 12), and to tumors in other tissues, including skin (13). Similarly, Ptch1 mutant mice develop cerebellar tumors resembling human medulloblastoma (14). Neonatal irradiation greatly accelerates medulloblastoma, and promotes basal cell carcinoma (BCC) precursor lesions to progress to large infiltrative BCC (15).
To prevent early mortality for medulloblastoma and improve characterization of the skin phenotype in irradiated Ptch1+/− mice, we have conducted experiments using expressly-designed lead shields for protecting mouse heads. Unpredictably, we observed marked enhancement of medulloblastoma in mice irradiated with shielded brains. We therefore analyzed in shielded cerebella induction of DNA double-strand breaks (DSBs) measured by γ-H2AX focus formation in situ (16), and fractions of apoptotic cells. We found marked differences in magnitude and dynamics of these endpoints in shielded relative to exposed cerebellum, showing that bystander-related damage is induced in remote unirradiated tissues, and can initiate tumorigenesis in radiosensitive Ptch1+/− neural precursors. We explored the mechanisms of this phenomenon and report significant modulation of initial GCP damage by 12-O-tetradecanoylphorbol-13-acetate (TPA), a potent protein kinase C activator and cell-to-cell communication inhibitor (17), suggesting a mechanism mediated by gap-junctional intercellular communication (GJIC) for transmission of bystander damage to shielded cerebellum. These findings are the first demonstration that bystander radiation responses can initiate tumorigenesis in unexposed tissues in vivo.
Results
High Medulloblastoma Incidence in Shielded Cerebellum of Ptch1+/− Mice.
Ptch1+/− mice (18) are highly responsive to radiation damage in neonatal cerebellum, and develop high medulloblastoma incidence (up to 80%) after irradiation in early postnatal age (14). We irradiated progenies of Ptch1+/− and wild-type (WT) mice at postnatal day 2 (P2) with 3 Gy of X rays. Mice were whole-body (WB) exposed, or irradiated with individual cylindrical lead shields providing protection of heads (SH groups; Fig. 1 A and C). Clear demarcation between exposed and shielded regions was evident at P10 due to hair-growth delay in exposed skin (Fig. 1B). Shielding was checked by dosimetry and Monte Carlo simulation. The dose due to primary photons beneath the shields was <0.26%. Shielded tissues, however, receive scattered radiation due to x-ray deflection through irradiated tissues. A conservative value of the dose (attenuated + scattered) of 1.2% of the total dose to shielded tissues was estimated. Thus, another cohort of mice was WB exposed to 0.036 Gy as internal control. Two additional cohorts were sham irradiated, or left untreated (CN).
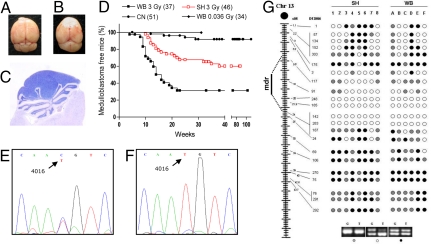
Fig. 1.
Irradiation set up for shielded irradiation. (A) Neonatal Ptch1+/− and Ptch1+/+ mice placed in polystyrene boxes were irradiated with heads and upper body shielded by individual custom-built lead cylinders. (B) Demarcation between exposed and shielded regions at P10 due to hair-growth delay in exposed skin. (C) Characteristics of the lead shields.
Ptch1+/− mice were placed on a lifetime study and monitored for tumor development. Congruent with previous reports (14), WB-irradiated animals developed cerebellar tumors (Fig. 2 A–C). A high percentage of mice (62%) died of aggressive disease by 23 weeks, with median survival of 14 weeks (P < 0.0001 vs. CN, log rank test; Fig. 2D). Significantly, we also observed a remarkably increased medulloblastoma rate (39%) in SH-irradiated Ptch1+/− mice. Although this tumor response was lower than in WB-exposed animals (P = 0.0011), SH mice developed significantly more tumors with highly reduced latency relative to CN mice (P = 0.0003; Fig. 2D), showing that partial-body irradiation promotes Ptch1-driven tumorigenesis in shielded cerebellum. Mice WB exposed to the estimated scatter dose (0.036 Gy) were, at the time this manuscript was written, well into the age (i.e., 31 weeks) when radiation tumorigenic effects become manifest in cerebellum. Yet, they had not shown signs of disease above background level (Fig. 2D).
Fig. 2.
Medulloblastoma development in Ptch1+/− mice. (A) Normal brain. (B) Macroscopic aspect of cerebellar tumors developing in the posterior fossa. (C) Histology of medulloblastoma, composed of tightly packed, small round cells with minimal surrounding cytoplasm. (D) Kaplan–Meier kinetic analysis of medulloblastoma in whole-body irradiated (WB), shield-irradiated (SH), and control Ptch1+/− mice (CN). Included is the tumor-free survival at 31 weeks of mice that were WB irradiated with a dose of 0.036 Gy (WB-0.036), equivalent to the scatter dose to SH cerebellum. (E and F) Representative electropherogram of genomic and tumor DNAs showing retention of both Ptch1 alleles in normal tissue (E) and loss of WT Ptch1 allele in medulloblastomas (F). (G) Analysis of chr-13 LOH in tumors from WB and SH Ptch1+/− mice. The distance of microsatellite markers (D13Mit) from the centromere is given in cM. Closed circles indicate no LOH; open circles denote LOH; gray circles indicate not informative (NI) markers; mdr: minimum deleted region.
The SH-irradiated Ptch1+/− mouse model is a highly reproducible system, because the tumor results described here are consistent with data from two independent experiments involving a total of 85 mice, in which we used a simplified shielding method [supporting information (SI) Fig. S1].
Ptch1 Loss of Heterozygosity (LOH) in Tumors from WB or SH Irradiated Mice.
Biallelic Ptch1 loss represents the major pathway to medulloblastoma development in Ptch1+/− mice (14, 19). We analyzed tumors from irradiated and shielded mice for Ptch1 allelic imbalance by exploiting a T/C polymorphism at position 4016 of Ptch1, that discriminates the 129Sv-derived mutant from the CD1-derived WT allele (14). We show that, similar to WB-irradiated Ptch1+/− mice, medulloblastomas from SH–exposed mice show loss of the WT-CD1 allele (Fig. 2 E and F).
To assess LOH mechanisms, panels of medulloblastomas from SH or WB mice were analyzed using a minimum of 22 informative microsatellite markers spanning mouse chr 13 (Fig. 2G). None of the tumors had loss of the entire chr 13, because we never found reduction to homozigosity of all markers analyzed. Instead, all medulloblastomas had a chr-13 interstitial region in which several contiguous loci showed LOH. Overall, the pattern lacked highly stringent breakpoint clustering, supporting a genetic mechanism based on loss of a critical gene within the chr-13 minimal deleted region where we localized Ptch1 according to the Mouse Chromosome 13 Linkage Map (http://www.informatics.jax.org/). Hence, our results define very similar LOH patterns in tumors from SH- and WB-exposed mice (Fig. 2G).
Induction of DSBs in Irradiated and Bystander EGL.
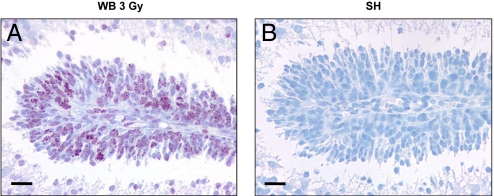
Neural precursor cells are highly radiation sensitive, both in vitro and in vivo (20). We characterized early responses to DNA damage in GCPs post-3Gy irradiation. DSBs were analyzed in irradiated and shielded cerebellum by detection of γ-H2AX foci that are formed at chromosomal sites of DNA DSBs by phosphorylation of histone H2AX on ser 139, and are evident in the nucleus soon after irradiation using specific antibodies (16). At P2, proliferating GCPs cluster over the surface of the developing cerebellum to form the external granule layer (EGL; see Fig. 4 A and I). Intense γ-H2AX staining, involving >85% of GCPs, was present in the EGL at 0.5 h post-3Gy WB irradiation (Fig. 3A). In contrast, no γ-H2AX staining was detected in cerebellum of lead-shielded mice (Fig. 3B). γ-H2AX staining declined in irradiated EGL at later times (3, 6, and 18 h: 5.2, 2.9, and 2.5%), while remaining very low or undetectable in shielded EGL (0.05, 0.15, and 0%).
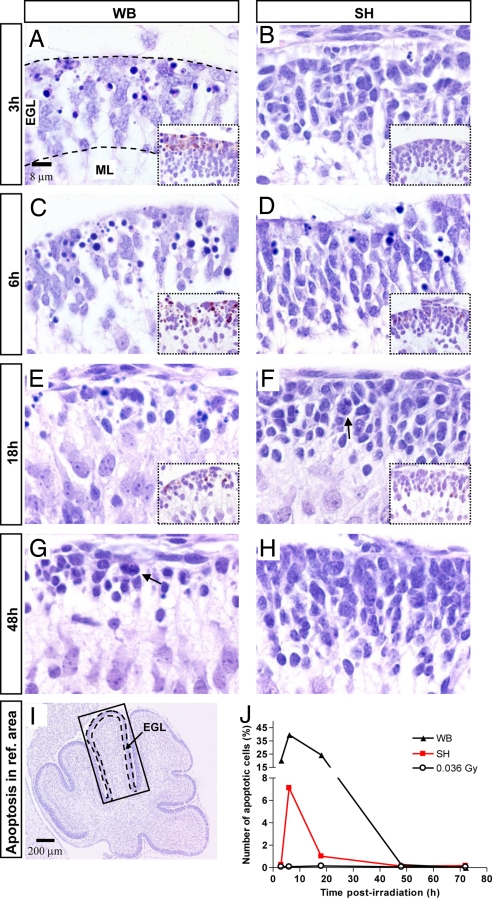
Fig. 4.
Levels and kinetics of apoptosis in exposed and shielded P2 mouse cerebellum. (A–J) Quantification of apoptosis in the anterodorsal cardinal lobe (boxed in I). (A–D Apoptosis at 3 and 6 h postirradiation in the EGL of WB- relative to SH-exposed mice. (A–F, Insets) Immunohistochemical analysis of caspase-3 activation. (E and G) Thinning of the EGL in WB-exposed and to lower degree in SH-exposed mice (F). Cell repopulation with mitotic figures in WB-exposed mice at 48 h (G). Compensatory hyperplasia at 48 h in SH-exposed EGL (F and H). Black dashes in A and I delineate the EGL. (J) Percentage of apoptotic cells as a function of time postirradiation in WB- and SH-exposed mice.
Fig. 3.
γ-H2AX immunohistochemical staining of the EGL of P2 cerebellum after irradiation. (A) A large fraction (> 85%) of GCPs exhibited numerous nuclear foci of γ-H2AX at 0.5 h post-3Gy whole-body (WB) irradiation. (B) Total lack of γ-H2AX staining in the EGL of shielded mice (SH) at 0.5 h postirradiation. (Scale bars, 20 μm.)
Apoptosis in Irradiated and Bystander EGL.
We examined apoptosis levels in irradiated and shielded EGL of the neonatal cerebellum by counting apoptotic nuclei in the anterodorsal cardinal lobe (Fig. 4I) at different times postirradiation (Fig. 4 A–H and J). The extent and kinetics of apoptosis differed in exposed and bystander cerebellum, with substantial levels (20.3%) evident in the EGL of WB-exposed mice at 3 h postirradiation (Fig. 4 A and J), compared with only incidental apoptosis (0.25%) in SH mice (Fig. 4 B and J). At 6 h, however, maximal apoptosis was present in the EGL of WB- (40%) and SH-exposed mice (7.1%) (Fig. 4 C, D, and J). Analysis of caspase-3 activation was consistent with apoptotic cell counts (Fig. 4 A–F). Increases in apoptosis were followed by marked decrease in cellularity and thinning of the EGL (Fig. 4 E and F). A sharp decrease to 1% of apoptotic cells was observed in the EGL of SH mice at 18 h (Fig. 4 F and J), in contrast with markedly slower kinetics of disappearance in WB-exposed mice, in which apoptotic neural progenitors decreased out at 48 h (0.26%) (Fig. 4 G and J). Consistently, cell repopulation and return to normal EGL thickness was slower and still incomplete in WB- vs. SH-irradiated mice at 48 h (Fig. 4 G and H). No significant apoptosis was induced in the EGL post-0.036Gy (Table S1), or in sham-irradiated mice (data not shown) at all times.
Notably, although shielded cerebella received a scatter dose that was estimated to be as much as two orders of magnitude lower relative to unshielded cerebellum (0.036 vs. 3 Gy), only 5.5-fold lower apoptosis was detected at 6 h postirradiation in cerebella of SH mice (Fig. 4 C, D, and J). This suggests a significant bystander component of damage to shielded brains.
Radiation Damage by Expected Scatter Dose in Exposed vs. Bystander EGL.
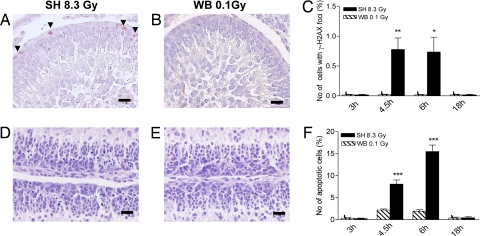
The obvious disparity, at tissue and whole-animal levels, between biological effects of the estimated scatter dose (i.e., 0.036 Gy) from 3 Gy to the whole body and the effects of the same dose to shielded cerebellum, prompted us to confirm that the genetic damage to cerebellum suffered by shielded mice did not result from insufficient shielding or dose scatter. To test this hypothesis, and to maximize downstream bystander responses, we compared the magnitude and kinetics of DNA DSBs and apoptosis in mice that had been SH-irradiated with 8.3 Gy (SH-8.3Gy), or WB exposed to the estimated 0.1 Gy scatter dose to cerebellum (WB-0.1Gy). Based on the delayed apoptotic wave in SH cerebellum (Fig. 4 D and J), analyses were carried out starting from 3h postirradiation. Lacking additional indirect effects, shielded EGL receiving 0.1 Gy through dose scatter should theoretically suffer biological damage similar to the EGL of mice receiving 0.1 Gy to the total body. γ-H2AX focus formation was very low or undetectable in cerebellum directly exposed to 0.1 Gy (Fig. 5 B and C). Instead, highly significant induction of γ-H2AX foci occurred in SH cerebellum at 4.5 (P = 0.0015) and 6 h (P = 0.0139) after exposure to 8.3 Gy of the remainder of the body (Fig. 5 A and C), with decrease to control level at 18 h. Apoptosis was also significantly up-regulated in cerebella of SH-8.3Gy mice (Fig. 5 D and F). A statistically significant response in SH relative to 0.1-Gy exposed EGL was already evident at 4.5 h (P = 0.0001), with a 3.2-fold bystander-related enhancement. Apoptosis was further increased at 6 h in the EGL of SH-8.3Gy mice, reaching 7-fold over the scatter-dose group (P = 0.0001), and declined at 18 h postirradiation (Fig. 5F). Thus, significant bystander genotoxic responses occur in shielded cerebellum in vivo after irradiation of the remainder of the body.
Fig. 5.
Radiation damage by expected scatter dose in exposed vs. bystander EGL. (A and B) γ-H2AX positivity in the outer EGL of SH-8.3 Gy mice at 6h postirradiation compared with undetectable staining after exposure to the scatter dose (0.1 Gy). (D and E) Increased apoptosis in EGL of SH-8.3Gy mice at 6 h postirradiation compared with very rare apoptosis after a 0.1 Gy dose. (C and F) Percentage of γ-H2AX-positive and apoptotic cells in cerebellum at 3, 4.5, 6, and 18 h post-8.3-Gy (SH) and 0.1 Gy (WB) irradiation. *, P = 0.0139; **, P = 0.0015; ***, P = 0.0001. (Scale bars, 20 μm.)
Significantly, short-term cellular responses were not specific of radiosensitive Ptch1+/− mice, because WT siblings showed identical bystander phenomena in neural precursors of P2 cerebellum (data not shown). To ascertain that effects were not dependent on genetic background hosting the Ptch1 mutation, the SH-8.3Gy/WB-0.1Gy experiment was replicated using the unrelated, carcinogen-resistant Car-R mouse strain (see SI Methods). Both DNA damage and apoptotic cell death in shielded EGL revealed similar magnitude of bystander-related enhancements in cerebellum of SH-8.3Gy relative to WB-0.1Gy Car-R mice (data not shown).
In all assays, we observed a non-random distribution of damaged cells in SH-exposed cerebellum, with γ-H2AX-positive and apoptotic nuclei located preferentially in the highly-proliferative outer compartment of the EGL (Fig. 4 A, C, and D and Fig. 5 A and D), suggesting proliferation-dependent bystander effects. These results are consistent with published studies showing spatial dependence of the bystander effects (21).
Inhibiting Cell–Cell Communication via Gap Junctions Reverses Bystander Effects.
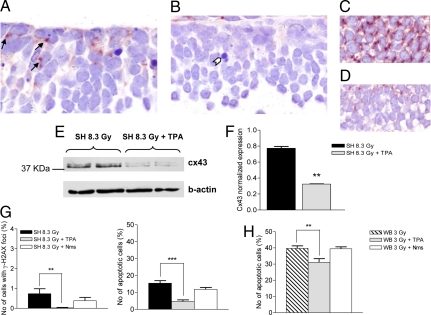
If GJICs are essential in mediating bystander responses, it should be possible to reduce transmission of damage from directly hit to unexposed tissues by using inhibitors of GJIC. Thus, we examined whether modulation of GJICs can be elicited by TPA in neural precursors of P2 cerebellum, and whether this may prevent bystander responses in shielded cerebellum. Expression of connexin43 protein (Cx43), the most abundant gap junction protein in the CNS (22), was evident in cerebellum post-8.3Gy SH irradiation (Fig. 6A). By contrast, Cx43 was almost undetectable in the EGL upon TPA treatment of SH-8.3Gy irradiated mice (Fig. 6B). For comparison, TPA influence on Cx43 expression is shown in the subventricular zone (SVZ) of neonatal cerebellum (Fig. 6 C and D). In the EGL, protein blotting confirmed a 2.6-fold decrease of Cx43 expression (P = 0.0025; Fig. 6 E and F). Remarkably, TPA treatment abrogated DNA-DSB responses in the EGL post-8.3Gy SH irradiation. Moreover, apoptotic damage was reduced by 3.3-fold (Fig. 6G). This suggests that physiologic Cx43 expression is associated with communication of damaging signals between adjacent cells in CNS. As a control of TPA effects on irradiated tissues, we tested its influence on cerebellum post-3Gy-WB irradiation and found a TPA-related 1.2-fold reduction (39.6 vs. 31.1%) in apoptosis (Fig. 6H and Table S2). Thus, ≈8% of directly-hit GCPs were “protected” by TPA, a value surprisingly similar to the 7.1% apoptosis in the SH-3Gy group (Fig. 6J). This may imply that TPA is responsible for inhibition of the bystander component of damage in cerebellum of WB-irradiated mice, and that this component may account for ≈20% of total apoptotic damage.
Fig. 6.
Inhibition of GJICs reverses bystander effects in cerebellum. (A) Immunohistochemical analysis showing widespread expression of Cx43 protein in the EGL at 6h post-8.3Gy SH irradiation. Arrows: Cx43 expression at cell–cell contacts. (B) Lack of Cx43 positivity in cerebellum of mice receiving combined TPA and SH-8.3Gy treatment. (C) Strong Cx43 expression in the postnatal SVZ, and (D) reduced expression in the SVZ of SH-8.3Gy mice injected with TPA are shown for comparison. (E and F) Western blot analysis showing decrease of Cx43 expression in TPA-treated relative to -untreated mice (P = 0.0025). (G) TPA (gray columns) dramatically reduced the levels of γ-H2AX-positive (P = 0.0075) and apoptotic cells (P < 0.0001) in shielded cerebellum; note numerous apoptotic figures in A, compared with sporadic apoptosis (white arrowhead) in B. No significant effect of nimesulide (Nms; white columns) administration on levels of short-term cellular responses. (H) TPA, but not Nms, significantly reduced apoptosis in cerebellum directly exposed to 3 Gy.
Effect of Cyclooxygenase-2 (COX-2) Inhibitor on Bystander Damage.
Non-irradiated bystander cells overexpress the COX-2 gene (23), a downstream target of mitogen-activated protein kinase (MAPK) pathways involved in radiation responses and linked to bystander processes (24). We therefore assessed responses in shielded cerebellum using a specific COX-2 inhibitor (25), nimesulide, combined with SH-8.3Gy. We show that nimesulide (1.5 μg/g body weight) caused a non-statistical reduction in DSBs, and had no effect on levels of apoptotic damage in shielded cerebellum. Similarly, no significant modulation of apoptosis by nimesulide was detected in cerebellum post-3Gy WB irradiation (Fig. 6H).
Discussion
It is clear that the array of non-targeted radiation effects, e.g., sister chromatid exchanges (3), DNA DSBs (10), micronucleus formation (9), mutations (8), chromosomal aberrations and instability (5, 7), reported to date mostly from in vitro systems, may have implications in cancer development. However, evidence that radiation-associated bystander responses are effectual in vivo has been limited (26), and there has been no proof that non-targeted radiation effects contribute to carcinogenesis in bystander tissues. Here, we show that genetic damage caused by bystander responses contributes to cancer risk in mouse CNS, with drastic acceleration of medulloblastoma in Ptch1+/− mice irradiated with shielded brains.
In the developing brain, radiosensitivity is highly dependent on developmental stage, being higher in neural precursors compared with postmitotic neurons (20). In proliferating GCPs of P2 cerebellum we have analyzed induction of γ-H2AX foci. Whereas massive DSB damage was present in directly-exposed EGL within 0.5 h postirradiation, γ-H2AX-positive cells were rare in shielded cerebellum of mice exposed to 3 Gy. The finding of a highly increased tumor response in shielded tissue, despite low levels of γ-H2AX induction, suggests that a persisting low level of DNA damage in CNS after low radiation doses (<0.04 Gy) may facilitate chromosomal events relevant for carcinogenesis. This hypothesis is consistent with findings that low levels of DNA damage (<10–20 DSBs) fail to initiate the G2/M checkpoint in human fibroblasts (27), allowing genetically altered cells to proliferate. In addition, slower kinetics of disappearance of DSBs in neural progenitors relative to neurons (20) may contribute to a persistent low level of unrepaired DSBs. As evidence of persisting damage, a steep increase in apoptosis was detected at 6 h in the EGL of shielded mice relative to internal controls (WB-0.036) or sham-irradiated mice. Apoptotic cell death might be considered protective, because it selectively eliminates damaged cells that may contribute to carcinogenic effects. However, cell death significantly stimulated compensatory hyperplasia in the EGL, which is potentially a tumor-promoting factor in the presence of residual DNA damage.
Previous work (28, 29) has stressed the importance of DNA-DSB repair in bystander phenomena, and the possibility that unrepaired or misrepaired DSBs underlie bystander induction of chromosomal aberrations and mutations involving large-scale genetic changes. In the Ptch1 model, time-course studies suggest a steadily increase of Ptch1-LOH rate in early preneoplastic cerebellar lesions, supporting a critical role for biallelic Ptch1 loss in cerebellum tumorigenesis. Moreover, different Ptch1 LOH patterns were shown in radiation-induced and spontaneous medulloblastomas (14). Here, we report a clear and consistent pattern of chr-13 interstitial deletions in tumors from bystander cerebellum, resembling tumors from whole-body-exposed mice. Mechanistically, this suggests that bystander radiation damage inherently has the potential to induce alteration and loss of genetic material and, in the CNS, promote tumorigenesis by facilitating LOH. Hence, genetic damage is a critical component of in vivo oncogenic bystander responses. Although there are no known reports of bystander-related tumorigenesis, long-term epigenetic alterations have recently been described in mouse bystander skin and spleen after partial-body irradiation (11, 30). Thus, the nature of long-term bystander responses may be tissue specific.
We observed spatial effects in the distribution of damaged cells in bystander mouse cerebellum, with damage located preferentially in the outer compartment of the EGL. This could be explained by rapid GCP proliferation in that region. Interestingly, studies of bystander effects in primary urothelial explants after 3He2+ traversal of single cells revealed non-targeted damage at the proliferating periphery of explants, regardless of location of targeted cells (21). This suggested that only actively dividing cells were susceptible to bystander effects. Our in vivo results in mouse CNS confirm that the proliferative or differentiation state is important for expression of bystander damage.
We also observed a dose-response effect for bystander damage, because the magnitude of both endpoints analyzed increased about proportionally with the dose delivered to shielded animals. Previous in vitro studies involving different techniques and radiation types have shown that the bystander effect is an all-or-nothing process with no apparent dose-effect relationship (31, 32). However, production of transmissible cell-to-cell effects can be substantially different in an in vivo context, in which physiologic cellular connections within a tissue, or cross talk among tissues, allow gated diffusion of bystander signals. This type of intercellular communication permits coordinated responses to cellular injury, a critical feature for homeostasis during development and adult life of multicellular organisms.
One of the major challenges in the field is to understand the mechanisms of non-targeted effects. In vitro studies have shown that, in confluent cultures, physical contacts through GJICs between irradiated and non-irradiated cells are essential for the process (33, 34). However, in low-density cultures bystander effects may rely on soluble factors released in the culture medium by irradiated cells (35). In vivo, evidence that acute radiation exposures result in the release of clastogenic soluble factors into the blood stream comes from several observations (36). For instance, clastogenic activity in human blood has been found after therapeutic or accidental exposure to high radiation doses. The results described here strongly support the view that a Cx43-mediated gap-junction transfer of the bystander signal in vivo mediates short-term cellular responses that may subsequently trigger long-term carcinogenic effects in mouse CNS. In fact, by TPA-mediated inhibition of GJICs we have shown suppression of bystander responses for both endpoints analyzed. As TPA, however, may have effects other than inhibition of Cx43-mediated intercellular communication, further studies testing different GJIC inhibitors will be needed to assess the generality of the response. However, suppression of COX-2 activity in bystander cerebellum by the chemical inhibitor nimesulide did not influence bystander damage, suggesting that inflammation/oxidative stress is not central in the effects observed here. GJICs and their constituent connexin proteins are known to play a significant role in mediating radiation-induced bystander effects (33). Because no structure is more interconnected than the mammalian CNS, it is conceivable that Cx43 gap junctions are crucial in mediating bystander responses in mouse CNS in vivo. Although the nature of factor(s) transmitted through GJICs and connecting irradiated and bystander neural cells remains unknown, only molecules <1 kDa (i.e., ions, small signaling molecules, some endogenous metabolites) could be possible candidates (37).
The similarity of initial cellular responses in shielded cerebellum of different mouse strains demonstrates that bystander effects occurring in CNS by exposure of distant tissues are not specific of Ptch1-mutant mice, and represent a cross-strain phenomenon. Thus, our findings are potentially important to estimate bystander signals in other species, and to assess their relevance for human health.
Finally, whereas short-term bystander responses were reproducible in different WT strains, longer-term carcinogenic effects in cerebellum were specific of the Ptch1+/− genotype. The PTCH mutation underlies a recessive human syndrome characterized by cancer susceptibility and high radiosensitivity (13, 18). However, frequencies of PTCH mutant alleles within human populations are low, and the contribution that such extreme sensitivity makes to population risk is very small. Greater contribution to radiation risks is probably due to genes that are less penetrant but more frequent, causing individual variability in biological responses to radiation, and increased risk in susceptible individuals. If ongoing and future studies confirm the tumorigenic role of bystander effects, this will have significant impact on future assessment of cancer risks associated with occupational, diagnostic and environmental exposures to radiation.
Materials and Methods
Immunohistochemistry, Statistics, and Mice.
See SI Methods.
Animal Irradiation.
Ptch1+/− mice and WT siblings were irradiated with X rays at P2. Full methods can be found in SI Methods.
For tumor induction, Ptch1+/− mice (n = 37) were whole-body (WB) irradiated with 3 Gy. Control mice (n = 51) were left untreated. For shielded irradiation (SH), Ptch1+/− mice (n = 46) were exposed to 3 Gy with heads protected by individual custom-built lead cylinders that allowed exposing only the lower part of the body. A group of shielded mice (n = 34) was sham-irradiated at P2. To analyze short-term cellular responses, additional groups (3 per time point) were killed at 0.5, 3, 6, 18, and 48 h postirradiation.
Protection of shielded parts was verified by dosimetry and Monte Carlo simulation (SI Methods and Fig. S2). The dose due to primary photons beneath the shields was experimentally found to be <0.26%. In addition, shielded tissues receive scattered radiation due to x-ray deflection through the irradiated tissues. A conservative value of the total dose (attenuated + scattered) delivered to the shielded tissues of 1.2% of the total dose was estimated. Thus, another cohort of 25 mice was WB- exposed to 0.036 Gy (i.e., the estimated scattered and attenuated dose to shielded cerebellum from irradiation of the body with 3 Gy) at P2 and used as internal control.
For characterization of DNA-damage responses in the WB and SH settings, Ptch1+/−, Ptch1+/+, and Car-R mice for comparative purposes (n = 3 for each genotype/strain), were exposed at P2 to 8.3 Gy of X rays with shielded heads. Corresponding groups were WB-exposed to 0.1 Gy (the approximate scatter dose to SH cerebellum resulting from exposure of the body to 8.3 Gy) and used as internal controls. Mice were killed at 3, 4.5, 6, and 18 h after irradiation for detection of γ-H2AX foci and apoptosis. Mouse experimental procedures were carried out according to the Italian legislation on animal experimentation, and reviewed by the internal Institutional Animal Care and Use Committee.
Histological Analysis and Tumor Quantification.
The 3 Gy WB- and SH-exposed cohorts were observed daily for their lifetime. When moribund, mice were killed and autopsied. Brains were processed for histology by standard techniques. Macroscopic cerebellar tumors were partly frozen at −80°C.
LOH at the Ptch1 Locus.
DNA was extracted from tumors and normal tissue using Wizard SV Genomic DNA Purification System (Promega). LOH analysis was performed as described in SI Methods.
Detection and Counting of γ-H2AX.
To study kinetics of γ-H2AX formation in GCPs from WB- and SH-irradiated mice, we counted γ-H2AX-positive nuclei in the entire EGL from midsagittal sections of P2 cerebellum (n = 3 per time point) at 3, 4.5 and 6 h after exposure to 3 or 8.3 Gy. Full methods can be found in SI Methods.
Apoptosis.
Brains (n = 3 per time point) of Ptch1+/−, Ptch1+/+, or Car-R pups irradiated with 3 or 8.3 Gy at P2 in the WB or SH settings were collected and fixed at 0.5, 3, 6, 18, 48 and 72 h postirradiation. Quantification of apoptosis was performed as described in SI Methods.
Modulation of GJICs by TPA.
Mice (n = 6 per group) were injected i.p. with TPA dissolved in corn oil (200 ng/g body weight, 2-h intervals), or vehicle, at 0.5 h before, and up to 4.5 h post-8.3Gy SH irradiation (38). Mice showed no signs of acute toxicity. Sacrifice was at 6 h postirradiation, and brains were fixed. For immunoblot analysis, cerebellum was dissected and snap frozen.
Suppression of COX-2 Activity by Nimesulide.
Mice (n = 3 per group) were treated with i.p. injections of nimesulide dissolved in water (1.5 mg/kg), or vehicle 1 h before 8.3-Gy SH irradiation and killed at 6 h postirradiation.
Western Blot.
Protein concentrations were determined by Bradford assay (Bio-Rad). Proteins (15 μg) from 3 cerebella were pooled for each lane and separated on 12% SDS/PAGE. Antibodies included rabbit polyclonal antibody against Cx43 (Cell Signaling Technology Inc.), 1:1000, detecting endogenous levels of total Cx43, and monoclonal antibody against β-actin (Sigma–Aldrich), 1:4000.
Supplementary Material
Acknowledgments.
We thank Orsio Allegrucci and Maurizio Quini for technical assistance. This work was supported by European Union Contract FI6R-CT-2003-508842 RISC-RAD.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804186105/DCSupplemental.
References
- 1.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 2.Sowa M, Arthurs BJ, Estes BJ, Morgan WF. Effects of ionizing radiation on cellular structures, induced instability and carcinogenesis. EXS. 2006;96:293–301. doi: 10.1007/3-7643-7378-4_12. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 4.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 5.Huang L, Kim PM, Nickoloff JA, Morgan WF. Targeted and nontargeted effects of low-dose ionizing radiation on delayed genomic instability in human cells. Cancer Res. 2007;67:1099–1104. doi: 10.1158/0008-5472.CAN-06-3697. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov OV, et al. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc Natl Acad Sci USA. 2005;102:14203–14208. doi: 10.1073/pnas.0505020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedelnikova OA, et al. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 2007;67:4295–4302. doi: 10.1158/0008-5472.CAN-06-4442. [DOI] [PubMed] [Google Scholar]
- 8.Wu LJ, et al. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc Natl Acad Sci USA. 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prise KM, Belyakov OV, Folkard M, Michael BD. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int J Radiat Biol. 1998;74:793–798. doi: 10.1080/095530098141087. [DOI] [PubMed] [Google Scholar]
- 10.Sokolov MV, Dickey JS, Bonner WM, Sedelnikova OA. gamma-H2AX in bystander cells: not just a radiation-triggered event, a cellular response to stress mediated by intercellular communication. Cell Cycle. 2007;6:2210–2212. doi: 10.4161/cc.6.18.4682. [DOI] [PubMed] [Google Scholar]
- 11.Koturbash I, et al. In vivo bystander effect: cranial X-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen. Int J Radiat Oncol Biol Phys. 2008;70:554–562. doi: 10.1016/j.ijrobp.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Rowitch DH, et al. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn H, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 14.Pazzaglia S, et al. Two-hit model for progression of medulloblastoma preneoplasia in Patched heterozygous mice. Oncogene. 2006;25:5575–5580. doi: 10.1038/sj.onc.1209544. [DOI] [PubMed] [Google Scholar]
- 15.Mancuso M, et al. Basal cell carcinoma and its development: insights from radiation-induced tumors in Ptch1-deficient mice. Cancer Res. 2004;64:934–941. doi: 10.1158/0008-5472.can-03-2460. [DOI] [PubMed] [Google Scholar]
- 16.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husøy T, Cruciani V, Sanner T, Mikalsen SO. Phosphorylation of connexin43 and inhibition of gap junctional communication in 12-O-tetradecanoylphorbol-13-acetate-exposed R6 fibroblasts: Minor role of protein kinase C beta I and mu. Carcinogenesis. 2001;22:221–231. doi: 10.1093/carcin/22.2.221. [DOI] [PubMed] [Google Scholar]
- 18.Hahn H, et al. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4:619–622. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- 19.Oliver TG, et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132:2425–2439. doi: 10.1242/dev.01793. [DOI] [PubMed] [Google Scholar]
- 20.Nowak E, et al. Radiation-induced H2AX phosphorylation and neural precursor apoptosis in the developing brain of mice. Radiat Res. 2006;165:155–164. doi: 10.1667/rr3496.1. [DOI] [PubMed] [Google Scholar]
- 21.Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. A proliferation-dependent bystander effect in primary porcine and human urothelial explants in response to targeted irradiation. Br J Cancer. 2003;88:767–774. doi: 10.1038/sj.bjc.6600804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li WE, Nagy JI. Activation of fibres in rat sciatic nerve alters phosphorylation state of connexin-43 at astrocytic gap junctions in spinal cord: evidence for junction regulation by neuronal-glial interactions. Neuroscience. 2000;97:113–123. doi: 10.1016/s0306-4522(00)00032-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H, et al. Mechanism of radiation-induced bystander effect: Role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci USA. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat Res. 2006;165:400–409. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- 25.Nam KT, et al. The selective cyclooxygenase-2 inhibitor nimesulide prevents Helicobacter pylori-associated gastric cancer development in a mouse model. Clin Cancer Res. 2004;10:8105–8113. doi: 10.1158/1078-0432.CCR-04-0896. [DOI] [PubMed] [Google Scholar]
- 26.Kovalchuk O, Baulch JE. Epigenetic changes and nontargeted radiation effects–is there a link? Environ Mol Mutagen. 2008;49:16–25. doi: 10.1002/em.20361. [DOI] [PubMed] [Google Scholar]
- 27.Löbrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer. 2007;7:861–869. doi: 10.1038/nrc2248. [DOI] [PubMed] [Google Scholar]
- 28.Nagasawa H, et al. Role of homologous recombination in the alpha-particle-induced bystander effect for sister chromatid exchanges and chromosomal aberrations. Radiat Res. 2005;164:141–147. doi: 10.1667/rr3420. [DOI] [PubMed] [Google Scholar]
- 29.Little JB, Nagasawa H, Li GC, Chen DJ. Involvement of the nonhomologous end joining DNA repair pathway in the bystander effect for chromosomal aberrations. Radiat Res. 2003;159:262–267. doi: 10.1667/0033-7587(2003)159[0262:iotnej]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Koturbash I, et al. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25:4267–4275. doi: 10.1038/sj.onc.1209467. [DOI] [PubMed] [Google Scholar]
- 31.Schettino G, Folkard M, Michael BD, Prise KM. Low-dose binary behavior of bystander cell killing after microbeam irradiation of a single cell with focused c(k) x rays. Radiat Res. 2005;163:332–336. doi: 10.1667/rr3319. [DOI] [PubMed] [Google Scholar]
- 32.Mothersill C, Seymour C. Radiation-induced bystander effects–implications for cancer. Nat Rev Cancer. 2004;4:158–164. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- 33.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu B, et al. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis. 2006;27:245–251. doi: 10.1093/carcin/bgi224. [DOI] [PubMed] [Google Scholar]
- 35.Ryan LA, Smith RW, Seymour CB, Mothersill CE. Dilution of irradiated cell conditioned medium and the bystander effect. Radiat Res. 2008;169:188–196. doi: 10.1667/RR1141.1. [DOI] [PubMed] [Google Scholar]
- 36.Lehnert BE, Goodwin EH, Deshpande A. Extracellular factor(s) after exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997;57:2164–2171. [PubMed] [Google Scholar]
- 37.Qu Y, Dahl G. Function of the voltage gate of gap junction channels: selective exclusion of molecules. Proc Natl Acad Sci USA. 2002;99:697–702. doi: 10.1073/pnas.022324499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng X, et al. Inhibitory effect of 12-O-tetradecanoylphorbol-13-acetate alone or in combination with all-trans-retinoic acid on the growth of LNCaP prostate tumors in immunodeficient mice. Cancer Res. 2004;64:1811–1820. doi: 10.1158/0008-5472.can-03-2848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.