Abstract
Late-life loss of independence in daily living is a central concern for the aging individual and for society. The implications of increased survival to advanced age may be different at the population level than at the individual level. Here we used a longitudinal multi-assessment survey of the entire Danish 1905 cohort from 1998 to 2005 to assess the loss of physical and cognitive independence in the age range of 92 to 100 years. Multiple functional outcomes were studied, including independence, which was defined as being able to perform basic activities of daily living without assistance from other persons and having a MiniMental State Examination (MMSE) score of 23 or higher. In the aggregate, the 1905 cohort had only a modest decline in the proportion of independent individuals at the 4 assessments between age 92 and 100 years: 39%, 36%, 32%, and 33%, with a difference between first and last assessment of 6% [95% confidence interval (CI), −1–14%]. For participants who survived until 2005, however, the prevalence of independence was reduced by more than a factor of 2, from 70% in 1998 to 33% in 2005 (difference, 37%; 95% CI, 28–46%). Similar results were obtained for the other functional outcomes. Analyses of missing data resulting from nonresponse and death suggest that the discrepancy between the population trajectory and the individual trajectory is caused by increased mortality among dependent individuals. For the individual, long life brings an increasing risk of loss of independence. For society, mortality reductions are not expected to result in exceptional levels of disability in cohorts of the very old.
Keywords: centenarians, nonagenarians, survival, independence
The oldest-old is the fastest growing segment of the population in the Western world, and the increase results mainly from a reduction in mortality rates among the oldest-old (1). There has been a longstanding debate within gerontology as to whether longer life is associated with a “compression of morbidity” (2), an “expansion of morbidity” (3, 4), or a combination of both, with an increased prevalence of chronic diseases counterbalanced by a decrease in the severity and consequences of the same diseases (5). The evidence supporting these different perspectives is mixed, perhaps because of differences in research settings (e.g., cohorts, countries, and ethnicities) and methodology (e.g., response rates and assessment instruments) (6–8). Nonetheless, there are accumulating data that the prevalence of chronic disability is decreasing among the elderly (6, 9). Although there is evidence that successive cohorts are living not only longer but also better (10–13), there still is considerable concern, both at the individual and societal level, that an extension of life into the highest ages in any birth cohort of elderly, now or in the future, will be accompanied by very high rates of loss of independence, with great personal and societal costs. Cross-sectional data indicate that dependency is considerably more prevalent in the oldest-old than in the younger elderly (14), but these studies are unable to disentangle age effects from cohort effects, and only a few longitudinal studies have a substantial sample size of the oldest-old (15, 16). Furthermore, many of these studies exclude institutionalized individuals, and this exclusion severely biases the estimation of the frequency of independence at the highest ages (12).
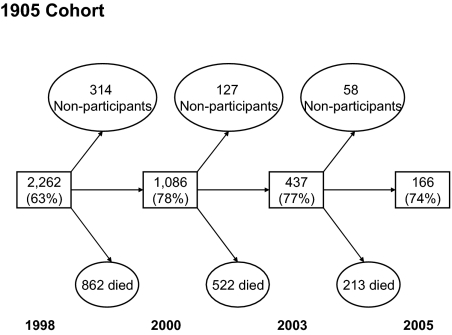
Here we used a longitudinal multi-assessment survey of the entire Danish 1905 cohort from 1998 to 2005 to assess the loss of physical and cognitive independence in the age range 92–100 years. The complete Danish 1905 cohort was contacted in 1998, when 3600 individuals in this cohort were still alive; 2262 (63%) participated in the survey. The participants were reassessed in 2000, 2003, and 2005 with participation rates between 74% and 78% (Fig. 1). Numerous outcomes were studied, including physical functioning [activity-of-daily-living (ADL) score, disability score, and grip strength}, cognitive functioning [MiniMental State Examination (MMSE) and cognitive composite score], and depression symptomatology. Further, this information can be used to determine whether a person is independent, defined as being able to perform basic activities of daily living without assistance from other persons and having an MMSE score of 23 or higher.
Fig. 1.
Flow-chart of the longitudinal study of the Danish 1905 cohort. The square boxes give the number of participants and participation rates.
The 1905-Cohort study is not able to address cohort differences (e.g., whether disability occurs later in more recent cohorts), but it tests whether living to an exceptional age will result in an exceptional level of disability and whether the health trajectory in the 10th decade of life differs between individuals and the population.
Results
In the 4 waves, a total of 4041 assessments were made, 20.1% of which were via proxy-participants. Nearly half the participants (48%) were living in a house or apartment at the time of the interview, a third were in a nursing home (33%), and the rest were living in special dwellings with professional care that was less intensive than provided in nursing homes. About 1% of participants did not have a valid assessment of the 4 ADLs because of missing data. Three-quarters of the proxy responses were the result of dementia, illness, and sensory impairments. The reasons for nonparticipation in the 3 follow-up assessments were unknown for 49.1% (no reason given); for 21.6% of the nonparticipants the reasons were disease or illness; and 17.5% died just before or during the survey period.
The longitudinal data in Table 1 show a clear association between intake score and status at first follow-up. Across all quantitative scores, the mean scores at intake were significantly better for individuals who participated in the first follow-up than for living nonparticipants, whose intake scores, in turn, were significantly better than those of participants who died by first follow-up.
Table 1.
The Danish 1905-Cohort mean intake score (SD) on physical and cognitive functioning and depression symptomatology, stratified by status at first follow-up
| First follow-up | Grip strength | Disability score | MMSE score | Cognitive composite | Depression symptomatology |
|---|---|---|---|---|---|
| Participants | 16.7 (6.8) n = 850 | 2.97 (0.77) n = 1053 | 23.4 (4.7) n = 846 | 0.30 (0.96) n = 854 | 7.3 (5.5) n = 810 |
| Nonparticipants | 15.6 (6.1) n = 204 | 3.15 (0.66) n = 269 | 20.1 (6.1) n = 336 | −0.22 (0.95) n = 323 | 8.7 (6.4) n = 342 |
| Dead | 14.8 (6.7) n = 453 | 3.42 (0.62) n = 906 | 19.4 (6.7) n = 616 | −0.31 (0.97) n = 607 | 9.8 (6.8) n = 587 |
| F test | F (2,1504) = 11.9; P < .001 | F (2,2225) = 101.9; P < .001 | F (2,1795) = 978; P < .001 | F (2,1781) = 80.0; P < .001 | F (2,1736) = 28.7; P < .001 |
Note: All means are significantly different.
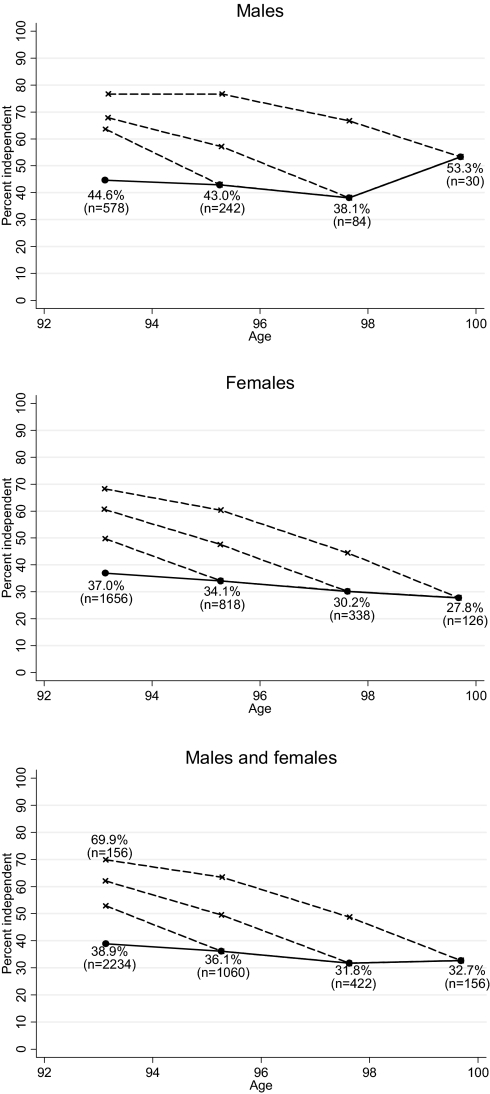
Fig. 2 illustrates the very different health trajectories for the overall population and for the individuals surviving to various ages. In the aggregate, the 1905 cohort had only a modest decline in the proportion of independent individuals at the 4 assessments between age 92 and 100 years: 39%, 36%, 32%, and 33%. The difference between first and last assessment was 6% [95% confidence interval (CI), −1–14%]. However, for participants who survived until 2005, the prevalence of independence was reduced by more than a factor of 2, declining from 70% in 1998 to 33% in 2005 (difference 37%, 95% CI 28–46%). As expected, a higher proportion of men than women remained independent. All the trajectories in Fig. 2 show similar patterns except the fourth assessment for men, which showed a nonsignificant increase in independence which probably results from the small sample. There was a clear difference in prevalence of independence related to housing: less than 10% of those living in nursing homes were independent according to the criteria used in this study (a few of the residents live in nursing homes because they want to continue living with a spouse who needs nursing home care).
Fig. 2.
Percentages of the individuals born in 1905 classified as independent at four assessments in the period 1998–2005. Dotted lines show the “history” of individuals completing at least 2, 3, and 4 waves, respectively. For example, the graphs show that among the 156 persons (30 men and 126 women) who participated in all four assessments, 33% were independent at the last assessment at age 99–100 years, but ≈70% were independent at the intake assessment at age 92–93 years. The graph thus shows that, although the level of independence in the overall 1905 cohort remained nearly stable from age 92–93 years through age 99–100 years, most of the individuals experienced a loss of abilities after age 92–93 years. The explanation for this apparent paradox is a very high mortality among the dependent participants.
Table 2 shows a similar pattern of results for the more quantitative disability score, grip strength, cognitive composite score, and depression symptomatology score. The table gives a breakdown of the mean scores for each quantitative scale overall and as a function of the number of assessments completed. Also given is the regression coefficient (95% CI) associated with regressing each of the scale scores on the number of years since study inception. The regression coefficient for the total means provides a summary measure of aging at the population level; the regression coefficients conditional on the number of waves of participation are longitudinal and consequently estimate the rate of aging at the individual level. A consistent pattern of results emerges for grip strength and the three scores: individuals who participated only in the intake assessment have the poorest physical and cognitive function and the highest level of depression symptomatology at intake; participants' scores at intake improved with every additional follow-up the participants completed. The rate-of-change also is positively associated with number of follow-up participations. Consequently, participants who remained in the study for the longest time had the best average functioning at intake, the slowest rate of decline in cognitive and physical abilities, and the smallest increase in depression symptomatology score.
Table 2.
Longitudinal assessment of physical and cognitive functioning and depression symptomatology in the Danish 1905-cohort
| Study sample | 1998 | 2000 | 2003 | 2005 | n | Decline: regression slope unit/year(95% confidence interval) |
|---|---|---|---|---|---|---|
| Mean disability score (SD) | ||||||
| Intake only | 3.4 (0.6) | 1,175 | — | |||
| Intake plus 1 follow-up | 3.1 (0.7) | 3.4 (0.6) | 633 | 0.15 (0.12–0.19) | ||
| Intake plus 2 follow-ups | 2.9 (0.8) | 3.2 (0.7) | 3.6 (0.5) | 265 | 0.14 (0.11–0.16) | |
| Intake plus 3 follow-ups | 2.6 (0.8) | 2.8 (0.7) | 3.1 (0.7) | 3.4 (0.6) | 155 | 0.12 (0.10–0.14) |
| Total means | 3.2 (0.7) | 3.3 (0.7) | 3.4 (0.6) | 3.4 (0.6) | 2,228 | 0.04 (0.03–0.05) |
| Participants, no. | 2,228* | 1053 | 420 | 155 | ||
| Nonparticipants, no. | 269 | 114 | 46 | |||
| Dead, no. | 906 | 519 | 219 | |||
| Weighted means† | 3.2 (0.7) | 3.3 (0.7) | 3.5 (0.6) | 3.5 (0.6) | 0.05 (0.04–0.06) | |
| Mean grip strength (SD) | ||||||
| Intake only | 14.9 (6.4) | 832 | — | |||
| Intake plus 1 follow-up | 16.7 (7.0) | 14.7 (6.3) | 443 | −0.94 (−1.35,−0.54) | ||
| Intake plus 2 follow-ups | 18.2 (6.7) | 17.2 (6.7) | 14.6 (6.3) | 150 | −0.73 (−1.06,− 0.39) | |
| Intake plus 3 follow-ups | 18.3 (6.6) | 17.8 (5.8) | 16.8 (5.3) | 14.5 (6.1) | 82 | −0.53 (−0.80,−0.27) |
| Total means | 16.0 (16.7) | 15.7 (16.5) | 15.4 (6.1) | 14.5 (6.1) | 1,507 | −0.17 (−0.31,−0.02) |
| Participants, no. | 1507 | 675 | 232 | 82 | ||
| Nonparticipants, no. | 342 | 290 | 163 | |||
| Dead, no. | 490 | 985 | 1262 | |||
| Weighted means† | 16.0 (6.7) | 15.0 (6.2) | 14.3 (5.7) | 12.6 (5.8) | −0.45 (−0.35,−0.55) | |
| Mean cognitive composite score (SD) | ||||||
| Intake only | −0.28 (0.96) | 930 | — | |||
| Intake plus 1 follow-up | 0.17 (0.97) | −0.22 (1.12) | 565 | −0.18 (−0.23, −0.12) | ||
| Intake plus 2 follow-ups | 0.40 (0.93) | 0.34 (1.13) | −0.15 (0.98) | 197 | −0.11 (−0.15, −0.07) | |
| Intake plus 3 follow-ups | 0.87 (0.80) | 0.77 (1.04) | 0.45 (0.96) | 0.11 (1.10) | 92 | −0.11 (−0.15, −0.07) |
| Total means | 0.00 (1.00) | 0.02 (1.17) | 0.04 (1.01) | 0.11 (1.10) | 1,784 | 0.01 (−0.01, 0.03) |
| Participants, no. | 1784 | 854 | 289 | 92 | ||
| Nonparticipants, no. | — | 323 | 302 | 192 | ||
| Dead, no. | — | 607 | 263 | 5 | ||
| Weighted means† | 0.00 (1.00) | −0.08 (1.17) | −0.15 (1.00) | −0.51 (1.19) | −0.05 (−0.07, −0.04) | |
| Mean depression symptomatology score (SD) | ||||||
| Intake only | 9.4 (6.7) | 929 | ||||
| Intake plus 1 follow-up | 7.8 (5.8) | 8.7 (6.1) | 518 | 0.38 (0.05, 0.71) | ||
| Intake plus 2 follow-ups | 6.7 (5.1) | 7.1 (5.2) | 8.9 (6.4) | 189 | 0.45 (0.20, 0.70) | |
| Intake plus 3 follow-ups | 5.8 (4.6) | 6.1 (5.1) | 6.0 (5.1) | 7.1 (5.6) | 103 | 0.16 (−0.04, 0.36) |
| Total means | 8.4 (6.3) | 8.0 (5.9) | 7.9 (6.1) | 7.1 (5.6) | 1,739 | −0.17 (−0.30, −0.05) |
| Participants, no. | 1739 | 810 | 292 | 103 | ||
| Nonparticipants, no. | 342 | 294 | 174 | |||
| Dead, no. | 587 | 224 | 15 | |||
| Weighted means† | 8.4 (6.3) | 8.3 (6.0) | 8.5 (6.3) | 8.1 (6.0) | −0.04 (−0.13, 0.05) | |
*Disability scores were assessed for all participants including proxy-participants but still the number of participants is slightly different from the total number of participants because values for a few participants were missing.
†Weighted means are based on inverse probability weighting.
Correcting the disability score for data missing because of nonparticipation (but not because of death) using inverse probability weights (17) gave results very similar to the unadjusted score, indicating that in this case the discrepancy between the individual trajectories and the population trajectory is caused mainly by death among the most disabled and not primarily by nonparticipation of individuals still alive at the assessment.
Recontacting individuals in 2005 who were nonparticipants in earlier waves of the survey yielded an extra 90 persons at the last assessment. Including these additional persons in the analyses resulted in a statistically nonsignificant decline in the prevalence of independence in 2005, from 32.7% to 27.5%. This is in agreement with the missing-data analyses that indicate that nonparticipants have somewhat poorer health than participants.
When we re-did the analysis of independence including the fifth ADL item (getting outside), we found the same pattern of results, albeit with a slightly lower overall level of independence reflecting the need to meet the additional standard. The classification of proxy-participants as “dependent” was supported by survival analyses that showed that the mortality in the proxy-participant group corresponded with the mortality in the most physically disabled group of responders. In another set of analyses in which we classified the proxy-participants afflicted with dementia as “dependent” and the rest of the nonparticipants as “independent,” we obtained the same overall pattern of results, albeit with higher overall levels of independence.
Discussion
Nonagenarians have a high risk of losing independence, but the prevalence of independence still declines only very modestly from age 92 to 100 years, suggesting little societal care cost is associated with the extension of lifespan at the highest ages. The reason for this discrepancy is the high rate of mortality among the most disabled at any given time.
Our analyses comprised a general measure of independence and more specific assessments of physical and cognitive functioning and depression symptomatology, but all analyses showed the same pattern, with little decline in functionality and mood on a population level but marked decline on an individual level (i.e., conditional on survival to a given time point in the future, the nonagenarians experienced a marked decline). Our finding that 30% to 40% of the cohort were independent from age 92 to age 100 years also may be valid beyond age 100 years, because a recent study of 32 supercentenarians (age 110–119 years) in the United States showed that about 40% required minimal assistance or were independent (18). Analysis of the cognitive composite on a population level revealed a stable level, but correcting for missing data revealed a slight decline (5% of an SD per year); this decline, however, was less than half the size of the decline observed at the individual level. Similarly, the uncorrected data for depression symptomatology showed slight improvements in mood with age, and the corrected data showed stable means at the population level, even though analysis of individual-level data showed significant increases in depression.
The strength of the present study is that it is population based, with complete mortality follow-up and with extensive possibilities to test for differences between participants and nonparticipants. This is essential in studies of independence among the oldest-old, because any result will be very sensitive to the characteristics of nonparticipants. Many studies on disabilities and functioning exclude institutionalized individuals (12). Our finding that a third of the participants lived in nursing homes and that less than 10% of these participants are independent suggests that there is a bias in most studies of the oldest-old.
In the present study, it was reassuring that the various approaches for assessing the impact of nonparticipants pointed in the same direction: 1) at the intake assessment, hospitalization patterns before 1998 were the same for participants and nonparticipants, but in the 6-month period after the initiation of the survey nonparticipants had higher mortality, suggesting that terminal illness is one of the main reasons for nonparticipation (19–21); 2) both the recorded reasons for nonparticipation at intake and the reassessment of individuals who previously had dropped out of the study suggested that nonparticipants had somewhat poorer health than participants, and 3) the statistical correction for missing data indicated that there was a group of terminally ill patients among the nonparticipants. However, the main finding is that decline on the population level, even when missing-data correction is used, was minimal compared with the decline observed in individuals participating in multiple assessments. This large difference and the convergence of the various approaches suggest that the overall finding of a marked population versus individual difference is very robust. This was also confirmed by the various tests of the robustness of the classification of both the independence measure and the proxy-participant definition.
The 1905-Cohort Study is not able to address cohort differences, e.g., whether disability occurs later in more recent cohorts. Disability prevalence rates have declined substantially since the 1980s in many countries, including Denmark (22), but the decline is best documented in the United States (23–25). Schoeni, et al.'s (2008) (24) comprehensive review into the reasons for this trend in the United States suggests that the substantial reductions in late-life disabilities are likely due to advances in medical care as well as from changes in socioeconomic factors. Those analyses showed that education was by far the most important of the socioeconomic factors considered. The causal pathway for this association remains to be determined, however. Perhaps surprisingly, during the last 20 years a change in smoking behavior (measured as “ever smoked”) did not contribute to the decline in disability rate among persons older than age 70 years, because these cohorts are still cohorts of high smoking prevalence. Considering the decline in disability rates, the stable population level of disability for the 1905 cohort could result partly from progress in medical treatment and living conditions for that cohort during the observation period from 1998 to 2005, although the changes within a 7- or 8-year period are likely to be modest. Currently there is no indication that the decline in disability rates is stopping, and with continuous improvement in medical treatment and with better-educated and lower-smoking cohorts entering the oldest-old population, further lowering of the disability level can be expected. Increases in the prevalence of obesity may counteract this development to some degree, however (24).
Considerable concern has emerged during the last decades about effects of the so-called “fourth age” for humans, because a substantial number of individuals in each birth cohort can be expected to survive into their 90s (26). It has been postulated that life extension would provide only increased chances of being frail and existing in a vegetative state, with huge personal and societal costs. Our study does not support this grim prediction. On the contrary, our findings suggest that the characteristics of a cohort do not change much in an age range from 92 to 100 years in central domains such as physical and cognitive functions and depression symptomatology. These cohort characteristics are stable because, even though individuals in this age range have an increased risk of disability for each additional year of life, the frailest and most disabled members of the cohort are those who are most likely to die at any given age. Our study suggests that the care costs per individual do not rise in the 10th decade of life, even though the current design did not directly assess the trajectory of the expenses for medical treatment. However, a study by Lubitz, et al. (2003) (27) showed that the expected cumulative health expenditures for individuals who were in good health at age 70 years were not greater than expected cumulative expenditures for less healthy persons, despite the greater longevity of healthier elderly persons. That study concluded that health-promotion efforts aimed at persons younger than 65 years of age may improve the health and longevity of the elderly without increasing health expenditures, and our study supports that conclusion for individuals having exceptional longevity.
So, at a first glance, our finding of only a slight decrease in the rate of independence with age at the population level but a much steeper decline at the individual level might seem to be good news for society as a whole but bad news for the individual. Nonetheless, our finding also suggests that individuals who survive into the highest ages have a health profile that is similar in many aspects to that of individuals who are 7 or 8 years younger. This suggests that most individuals can expect to experience physical decline before they die, but the postponement of this individual decline makes it possible for us to live into the fourth age.
Materials and Methods
Study Population.
The intake study took place from August to October 1998 and included all Danes born in 1905 and living in Denmark, a total of 3600 persons. There were no exclusion criteria: all individuals born in Denmark and still living in Denmark were approached irrespective of residence, health, and cognitive status (19–21). The cohort was identified through the Danish Civil Register System, which, since 1968, has kept a record of all persons living in Denmark. Each person has a unique 10-digit identification number, which is the key to individual information in all official registries covering the Danish population. The system ensures complete identification and follow-up of the participants, provided they had not emigrated (a negligible problem among the oldest-old).
A total of 2262 (62.8%) persons participated in the intake survey: 1814 (80.2%) in person and 448 (19.8%) via a proxy-participant. Participants and nonparticipants were compared using the extensive registration of the Danish population that made it possible to evaluate thoroughly differences between participants and nonparticipants. No differences were found in housing and marital status, but men and persons living in rural areas were more likely to participate than women and urban dwellers. An analysis of hospitalization patterns from 1973 to1998 indicated that participants were not healthier than nonparticipants. Nevertheless, in a 6-month period after the start of the survey, nonparticipants had higher mortality, suggesting that terminal illness was one of the reasons for nonparticipation (19–21).
Surviving participants were followed up subsequently in 2000, 2003, and 2005 with participation rates among survivors between 74% and 78% (Fig. 1). Each of the 4 waves was conducted within a period of approximately 3 months. We used a very conservative method for estimating the response rates, including as potential participants individuals who died just before or during the 3-month survey period. This group is considerable among nonagenarians and constituted 18% of the nonparticipants.
Survey Instrument.
The survey instrument was practically identical in the 4 waves of interviews (apart from the omission in the follow-up surveys of questions about fixed traits such as number of children and education). The assessment included an interview, physical and cognitive tests, and the collection of biological material (19–21, 28).
The present article focuses on basic activities of daily living, grip strength, cognitive functioning, and depression-symptomatology—all outcomes that we have studied extensively using the same reliable and validated instrument in this and other cohorts of elderly persons (29–36). A short description of the outcomes is given later in this article. More detailed descriptions have been published previously (37–42).
We defined “independence” as a combination of physical and cognitive functioning.
Physical Functioning.
Four questions were used to categorize persons as physically independent/dependent: 1) “Are you able to get up from a bed?” 2) “Are you able to get up from a chair?” 3) “Are you able to walk around in the house?” 4) “Are you able to go to the toilet?” There were 4 possible answers to all 4 questions: (1) yes; (2) yes, with aids; (3) yes, with the assistance of another person; (4) no. Subjects were classified as physically independent if they were able to perform all 4 items with or without aids; otherwise they were classified as physically dependent. We also performed the analyses including a fifth ADL (“Are you able to get outdoors, e.g., in the garden?”) to test whether the results were sensitive to the classification of physically dependent/independent.
Cognitive Function.
Cognitive function was measured using the MMSE (scale 0–30). Subjects were classified as cognitively dependent if they had a MMSE score less than 23 and as independent if their score was greater or equal to 23. Scores of 23 or less on the MMSE are widely considered to be indicative of cognitive impairment (37).
Independence.
Participants were classified as independent if they were both physically and cognitively independent according to the criteria described in the previous sections. Individuals who had another person answering for them in the interview (proxy-interviews) were regarded as dependent. The most common reasons for proxy participation were dementia (55%), sensory impairment (12%), and illness (10%). To test the robustness of the assumption that those who participated by proxy were all dependent, we re-did the analyses, counting as dependent only those who did not participate because of dementia.
Grip Strength.
Grip strength discriminates functioning in all adult age groups (32, 38), predicts incident disability (39), and is highly correlated with muscular power in other muscular groups (39). It can be measured easily and reliably, and it also correlates with ADL function and survival among the oldest-old (20, 21).
Disability Score.
To supplement our overall assessment of disability, an 11-item self-report measure of physical disability was administered at each wave of the study. The 11 items ranged broadly, from relatively simple physical tasks such as walking around the house and walking up and down 1 flight of stairs to more demanding activities such as running 100 m and carrying 5 kilos. Each item was answered on a scale ranging from 1 to 4 points (1 = can do the activity without fatigue, 2 = can do activity with fatigue or minor difficulties, 3 = can do the activity with aid or major difficulties, 4 = cannot do the activity). This scale has been shown to provide a sensitive quantitative measure of physical ability in our other studies of elderly Danes (30, 31, 40) and is highly internally consistent (α = 0.93 for both men and women) and stable (2-year retest correlations > 0.65).
Cognitive Composite.
To supplement the MMSE assessment of cognitive functioning, a battery of 5 cognitive tests was administered. The component cognitive measures were selected to represent tasks that are sensitive to normative age changes but that could be assessed reliably and briefly by lay interviewers. The specific tasks included in the cognitive battery are: 1) a fluency task, which involved the number of animals an individual could name in a 1-min interval, 2) forward and backward digit span, and 3) immediate and delayed recall of a 12-item list. Because the individual component tasks are significantly positively correlated (mean correlation, 0.39; range, 0.20–0.57), an overall cognitive composite was formed by summing the standard scores for each of the 5 component tasks. The cognitive composite is temporally stable (2-year stability correlation ≈0.60) and has been shown in other research involving elderly Danish populations to provide a sensitive measure of cognitive functioning (33, 34). To facilitate the interpretation of mean changes, cognitive composite scores were linearly transformed so that at intake they had a mean of 0 and a standard deviation of 1.0
Depression Symptomatology.
Depression symptomatology was assessed using an adaptation of the depression section of the Cambridge Mental Disorders of the Elderly Examination (41). A complete description of the development and contents of the specific depression scale administered in the Danish 1905 project is given by McGue and Christensen (35, 42). Briefly, the depression scale consists of 11 items designed to assess both dominant mood (e.g., “Do you feel sad, depressed, or miserable?”) and the associated somatic complications of depression (e.g., “Do you find it difficult to concentrate?”). Respondents were asked to report how they were feeling currently or recently, and most of the items had a 3-point response format (i.e., 0 = no or never, 1 = yes, sometimes, and 2 = yes, most of the time). The measure used in the present study is highly internally consistent (α > 0.85) and stable (r > 0.60 over a 2-year interval) (35).
Missing Data.
We empirically assessed the influence of nonparticipants by obtaining the reason for nonparticipation in each of the 3 follow-up waves. Also, at the last assessment in 2005 we contacted survivors who had opted out in previous waves to determine whether including their data changed the results for the final assessment. Ninety of the 233 surviving dropouts completed the final assessment.
For the continuous measurements we also applied statistical missing-data approaches. Nonparticipation at follow-up could be caused either by death or by nonresponse by living persons. Dufouil et al. (2004) (17) have argued that adjustment for dropout in longitudinal aging research should be based only on nonresponse among the living, because adjusting for nonparticipation caused by death would make statistical inferences applicable to a fictitious “immortal population.” Consequently, adjustment for nonparticipation among living members of the Danish 1905 cohort was made using the inverse probability weighting procedure described by Dufouil, et al. (2004) (17). This procedure adjusts for nonresponse by weighting each individual's response at a given assessment by the inverse of the probability of participation for individuals having the same score as that individual at the previous assessment.
Acknowledgments.
The Danish 1905-Cohort Survey was supported by grants from the Danish National Research Foundation and National Institutes of Health/National Institute on Aging Grant P01 AG08761. The Danish Aging Research Center is supported by a grant from the VELUX Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Vaupel JW, et al. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- 2.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 3.Kramer M. The rising pandemic of mental disorders and associated chronic diseases and disabilities. Acta Psychiatr Scand. 1980;62:282–297. [Google Scholar]
- 4.Olshansky SJ, Rudberg MA, Carnes BA, Cassel CK, Brody JA. Trading off longer life for worsening health: The expansion of morbidity hypothesis. J Aging Health. 1991;3:194–216. [Google Scholar]
- 5.Manton KG. Changing concepts of morbidity and mortality in the elderly population. Milbank Mem Fund Q. 1982;60(2):183–244. [PubMed] [Google Scholar]
- 6.Robine JM, Michel JP. Looking forward to a general theory on population aging. J Gerontol A Biol Sci Med Sci. 2004;59(6):M590–M597. doi: 10.1093/gerona/59.6.m590. [DOI] [PubMed] [Google Scholar]
- 7.Guralnik JM. Population aging across time and cultures: Can we move from theory to evidence? J Gerontol A Biol Sci Med Sci. 2004;59:M606–M608. doi: 10.1093/gerona/59.6.m606. [DOI] [PubMed] [Google Scholar]
- 8.Melzer D, Lan TY, Tom BD, Deeg DJ, Guralnik JM. Variation in thresholds for reporting mobility disability between national population subgroups and studies. J Gerontol A Biol Sci Med Sci. 2004;59(12):1295–1303. doi: 10.1093/gerona/59.12.1295. [DOI] [PubMed] [Google Scholar]
- 9.Manton KG, Gu X, Lamb VL. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proc Natl Acad Sci USA. 2006;103(48):18374–18379. doi: 10.1073/pnas.0608483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manton KG, Corder L, Stallard E. Chronic disability trends in elderly United States populations: 1982–1994. Proc Natl Acad Sci USA. 1997;94(6):2593–2598. doi: 10.1073/pnas.94.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manton KG, Gu X. Changes in the prevalence of chronic disability in the United States black and nonblack population above age 65 from 1982 to 1999. Proc Natl Acad Sci USA. 2001;98(11):6354–6359. doi: 10.1073/pnas.111152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the United States: A systematic review. JAMA. 2002;288(24):3137–3242. doi: 10.1001/jama.288.24.3137. [DOI] [PubMed] [Google Scholar]
- 13.Áijänseppä S, et al. Physical functioning in elderly Europeans: 10 Year changes in the north and south: The HALE project. J Epidem Comm Health. 2005;59:413–419. doi: 10.1136/jech.2004.026302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen-Ranberg K, et al. Declining physical abilities with age: A cross-sectional study of older twins and centenarians in Denmark. Age Ageing. 1999;28:373–377. doi: 10.1093/ageing/28.4.373. [DOI] [PubMed] [Google Scholar]
- 15.Zeng Y, Vaupel J. Association of late childbearing with healthy longevity among the oldest-old in China. Popul Stud (Camb) 2004;58(1):37–53. doi: 10.1080/0032472032000175437. [DOI] [PubMed] [Google Scholar]
- 16.Melzer D, et al. Profile of disability in elderly people: Estimates from a longitudinal population study. BMJ. 1999;318:1108–1111. doi: 10.1136/bmj.318.7191.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dufouil C, Brayne C, Clayton D. Analysis of longitudinal studies with death and drop-out: A case study. Stat Med. 2004;23(14):2215–2226. doi: 10.1002/sim.1821. [DOI] [PubMed] [Google Scholar]
- 18.Schoenhofen EA, et al. Characteristics of 32 supercentenarians. J Am Geriatr Soc. 2006;54(8):1237–1240. doi: 10.1111/j.1532-5415.2006.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nybo H, et al. The Danish 1905-cohort. A genetic-epidemiological nationwide survey. J Aging Health. 2001;13(1):32–46. doi: 10.1177/089826430101300102. [DOI] [PubMed] [Google Scholar]
- 20.Nybo H, et al. Functional status and self-rated health in 2,262 nonagenarians: The Danish 1905 Cohort Survey. J Am Geriatr Soc. 2001;49(5):601–609. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- 21.Nybo H, et al. Predictors of mortality in 2,249 nonagenarians—the Danish 1905-Cohort Survey. J Am Geriatr Soc. 2003;51(10):1365–1373. doi: 10.1046/j.1532-5415.2003.51453.x. [DOI] [PubMed] [Google Scholar]
- 22.Jeune B, Brønnum-Hansen H. Trends in health expectancy at age 65 for various health indicators, 1987–2005, Denmark. Eur J Ageing. 2008 doi: 10.1007/s10433-008-0100-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crimmins EM. Trends in the health of the elderly. Annu Rev Public Health. 2004;25:79–98. doi: 10.1146/annurev.publhealth.25.102802.124401. [DOI] [PubMed] [Google Scholar]
- 24.Schoeni RF, Freedman VA, Martin LG . Why is late-life disability declining? Milbank Q. 2008;86(1):47–89. doi: 10.1111/j.1468-0009.2007.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai L, Lubitz J. Was there compression of disability for older Americans from 1992 to 2003? Demography. 2007;44(3):479–495. doi: 10.1353/dem.2007.0022. [DOI] [PubMed] [Google Scholar]
- 26.Baltes PB, Smith J. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology. 2003;49(2):123–135. doi: 10.1159/000067946. [DOI] [PubMed] [Google Scholar]
- 27.Lubitz J, Cai L, Kramarow E, Lentzner H. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349:1048–1055. doi: 10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 28.Bathum L, et al. No evidence for an association between extreme longevity and Microsomal Transfer Protein polymorphisms in a longitudinal study of 1651 nonagenarians. Eur J Hum Genet. 2005;13(10):1154–1158. doi: 10.1038/sj.ejhg.5201468. [DOI] [PubMed] [Google Scholar]
- 29.Christensen K, Holm NV, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on general health in the elderly. J Aging Health. 1999;11:49–64. doi: 10.1177/089826439901100103. [DOI] [PubMed] [Google Scholar]
- 30.Christensen K, Gaist D, Vaupel JW, McGue M. The genetic contribution to rate-of-change in functional abilities among Danish twins aged 75 years and older. Am J Epidemiol. 2002;155(2):132–139. doi: 10.1093/aje/155.2.132. [DOI] [PubMed] [Google Scholar]
- 31.Christensen K, Frederiksen H, Vaupel JW, McGue M. Age trajectories of genetic variance in physical functioning: A longitudinal study of Danish twins aged 70 years and older. Behav Genet. 2003;33(2):125–135. doi: 10.1023/a:1022501817781. [DOI] [PubMed] [Google Scholar]
- 32.Frederiksen H, et al. Age trajectories of grip strength: Cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006;16(7):554–562. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 33.McGue M, Christensen K. The heritability of cognitive functioning in very old adults: Evidence from Danish twins aged 75 and older. Psychol Aging. 2001;16:272–280. doi: 10.1037//0882-7974.16.2.272. [DOI] [PubMed] [Google Scholar]
- 34.McGue M, Christensen K. The heritability of level and rate-of-change in cognitive functioning in Danish twins aged 70 years and older. Exp Aging Res. 2002;28(4):435–451. doi: 10.1080/03610730290080416. [DOI] [PubMed] [Google Scholar]
- 35.McGue M, Christensen K. The heritability of depression symptoms in elderly Danish twins: Occasion-specific versus general effects. Behav Genet. 2003;33(2):83–93. doi: 10.1023/a:1022545600034. [DOI] [PubMed] [Google Scholar]
- 36.Johnson W, McGue M, Gaist D, Vaupel JW, Christensen K. Frequency and heritability of depression symptomatology in the second half of life: Evidence from Danish twins over 45. Psychol Med. 2002;32(7):1175–1185. doi: 10.1017/s0033291702006207. [DOI] [PubMed] [Google Scholar]
- 37.Cullen B, et al. Screening for dementia in an Irish community sample using MMSE: A comparison of norm-adjusted versus fixed cut-points. Int J Geriatr Psychiatry. 2005;20(4):371–376. doi: 10.1002/gps.1291. [DOI] [PubMed] [Google Scholar]
- 38.Mathiowetz V, et al. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69–74. [PubMed] [Google Scholar]
- 39.Rantanen T, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281(6):558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 40.Christensen K, et al. Genetic and environmental influences on functional abilities among Danish twins aged 75 years and older. J Gerontol Med Sci. 2000;55A(8):M446–M452. doi: 10.1093/gerona/55.8.m446. [DOI] [PubMed] [Google Scholar]
- 41.Roth M, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 42.McGue M, Christensen K. Genetic and environmental contributions to depression symptomatology: Evidence from Danish twins 75 years of age and older. J Abnorm Psychol. 1997;106:439–448. doi: 10.1037//0021-843x.106.3.439. [DOI] [PubMed] [Google Scholar]