Abstract
Reduction of plasma cholesterol by citrus flavonoids is associated with effects on specific liver functions related to lipid handling. In previous in vivo studies, polymethoxylated flavones (PMF) reduced plasma cholesterol levels at lower doses than required for flavanones. To delineate hepatic mechanisms that underlie this differential potency, we used HepG2 cells to quantitate effects on expression of the LDL receptor (LDLR) gene. A dose-response analysis showed that 200 μmol/L hesperetin, a flavanone present as a disaccharide in oranges, increased LDLR mRNA levels 3.6- to 4.7-fold of the untreated control. In contrast, nobiletin, a PMF found at the highest concentration in oranges and tangerines, achieved maximal stimulation of 1.5- to 1.6-fold of control at only 5 μmol/L. Transcriptional regulation of the LDLR gene by citrus flavonoids has been implicated but, to our knowledge, not directly demonstrated. Here, using transfection vector constructs containing the upstream region of the LDLR gene, we show differences in both potency and efficacy in the induction of transcription, with peak stimulation of 5.3- to 7.5-fold of control at 150-160 μmol/L hesperetin and 3- to 3.8-fold of control at 10-20 μmol/L nobiletin. Hesperetin sustains induction, whereas nobiletin is inhibitory at high doses, resulting in an inverted-U dose response. The sterol regulatory element (SRE) in the LDLR gene upstream region plays a crucial role, because mutation of this site strongly attenuated induction in response to hesperetin or nobiletin. Thus, citrus flavonoids are likely to act through the SRE-binding proteins, with PMF initially activating these mechanisms at considerably lower concentrations than flavanones.
Introduction
The nonnutritive constituents of citrus fruits reduce several risk factors for cardiovascular disease in humans and animals. Ingestion of citrus fruits or juices decreases levels of plasma cholesterol and triglycerides (TG)7, liver cholesterol and cholesteryl esters, and the circulating LDL:HDL ratio (1-8). The bioactive components in citrus include flavonoid compounds, which are present in thousands of different structural forms in a diversity of plants (9). The major citrus flavonoids include the flavanones, hesperetin and naringenin, and the polymethoxylated flavones (PMF), such as nobiletin and tangeretin (Fig. 1) (10). Oral administration of specific flavonoids decreases plasma cholesterol and/or TG levels in rabbits (11), rats (12,13), mice (14,15), and hamsters (16), with the PMF exhibiting greater potency than the flavanones in the hamster model. In humans, dietary supplementation with a flavanone lowers total and LDL cholesterol in the plasma (17) and recent clinical trials demonstrated that citrus PMF, in combination with palm tocotrienols, reduce plasma TG and total and LDL cholesterol (18). Because the liver plays a major role in cholesterol and lipid metabolism, studies have focused on the action of citrus flavonoids in this organ and have identified multiple effects on lipid-handling pathways in intact animals (11,13,15).
FIGURE 1.
Structures of citrus and soy flavonoids.
To analyze mechanisms of hepatic activity of flavonoids in greater detail than is possible in whole animals, the human HepG2 liver cell line has been extensively characterized. For example, using output of the protein apolipoprotein B (apoB) as an indicator of lipid secretion, it was shown that citrus flavonoids decrease the rate of apoB synthesis, secretion of newly synthesized apoB, and apoB mass in the medium (19-26). The reduction of apoB secretion from HepG2 cells occurs with lower concentrations of PMF compared with flavanones (22), analogous to the higher potency of the PMF in the hamster model (16). Flavonoid treatment also decreases the availability of lipids for export by inhibiting cholesteryl ester synthesis, microsomal TG transfer protein enzyme activity, and/or TG accumulation in the microsomal lumen (19-21,23-25).
In conjunction with establishing physiological parallels between intact liver and HepG2 cells, it is important to consider the type of flavonoids available to the liver in vivo. PMF, such as nobiletin and tangeretin, are present in the fruit as aglycones (10), allowing this form to be used both in vivo and in cultured liver cells (16,22,25). In contrast, the glycosylated flavanones, hesperidin and naringin, naturally occur in citrus and, consequently, are used in human and animal studies (11-17). However, deglycosylation takes place in the intestines before absorption into the circulation (27-30), warranting the administration of the aglycones, hesperetin and naringenin, to isolated liver cells (19-24,26).
In addition to inhibition of lipid export, effects of citrus flavanones on LDL receptor (LDLR) activity have been demonstrated through enhancement of binding, uptake, and degradation of LDL in HepG2 cells (20). Consistent with these stimulatory effects on the LDLR protein, hesperetin and naringenin act at the mRNA level to increase the concentration of LDLR mRNA (20). It is plausible that these effects on LDLR mRNA are due to regulation of gene transcription by sterol regulatory element (SRE)-binding proteins (SREBP), because the LDLR gene is a positive target of these transcription factors (31). In support of this mechanism, naringenin increases the mass of both nuclear and cytoplasmic SREBP-1 (24) and the soy isoflavones genistein and daidzein elevate mature SREBP-2 levels (32). We have found no studies that investigated the role of extended native DNA sequences in the regulation of LDLR gene transcription by citrus flavonoids in liver cells. Thus, because the physiological relevance of citrus effects on LDLR are established and the LDLR gene is one of the most well-characterized SREBP target genes, we have examined the effects of nobiletin and hesperetin on both LDLR mRNA levels and gene transcription in HepG2 cells and have investigated the role of the SRE. We compared PMF to the flavanone to determine whether there were differences in potency, efficacy, and overall patterns of expression.
Materials and Methods
HepG2 cell culture
HepG2 cells from the American Type Culture Collection were maintained at 37 °C, 5% CO2 in Eagle’s minimum essential medium with Earle’s balanced salt solution and 2 mmol/L glutamine (GIBCO), 7-10% (v:v) fetal bovine serum (Atlanta Biologicals or US Biotech), 1 mmol/L sodium pyruvate (GIBCO), 0.1 mmol/L MEM nonessential amino acids (GIBCO), and 17 mg/L gentamicin sulfate (Sigma). Frozen stocks in the above medium containing 5% (v:v) dimethyl sulfoxide (DMSO) were prepared as recommended by the American Type Culture Collection and, to ensure uniformity, cells from a single frozen stock tube were maintained in culture for no longer than 10 wk. Uncoated T-75 flasks (Corning) were used for propagation of the cells for preparation of total cellular RNA (∼25 × 106 cells per flask at confluency) and 24-well Primaria microtiter plates (Falcon) were used for transfections.
Flavonoid treatment of HepG2 cells
Hesperetin (≥95% pure), a flavanone from oranges, and synthetic genistein (≥98% pure), a soy isoflavone, were obtained from Sigma. Nobiletin was purified from tangerine peel and recrystallized twice to yield a final purity of >99% (33). Concentrated 50 mmol/L flavonoid stock solutions were prepared in DMSO. The final concentration of DMSO introduced into the cultured cells, either with the flavonoids or as the vehicle in control cells, was ≤0.4% (v:v). To ensure that basal levels of SREBP were available to mediate flavonoid action, the cells were not preexposed to low sterol conditions, which would tend to mobilize SREBP stores, or to high sterol conditions, which would more strongly sequester SREBP in the endoplasmic reticulum (34).
Transfections
DNA vectors were introduced into the HepG2 cells by transfection with Lipofectamine Reagent (Invitrogen). To obviate well-to-well variation in transfection efficiency, we developed a batch-wise transfection procedure, which is described in more detail in the Online Supporting Material. In each experiment (n), there were 2 replicates for each flavonoid dose and 2-4 replicates for the untreated control.
Statistical methods
The t test was performed as described (35). For the experiments in Fig. 3C,D, Bartlett’s test indicated equal variance and Dunnett’s test was performed (35). For the experiments in Figs. 3B and 4, standard ANOVA followed by tests of normality and equal variance (36,37) of the residuals were conducted on natural log-transformed data. The overall F-test and Dunnett’s test were then carried out. For Fig. 5A, the data from nobiletin-treated cells were analyzed as described in the preceding sentence, because the residuals from the log-transformed data were normal and had equal variances. The untransformed data from hesperetin-treated cells were analyzed using a mixed linear model approach, which allowed for unequal variances for each dose in this data set, followed by the overall F-test and Dunnett’s test.
FIGURE 3.
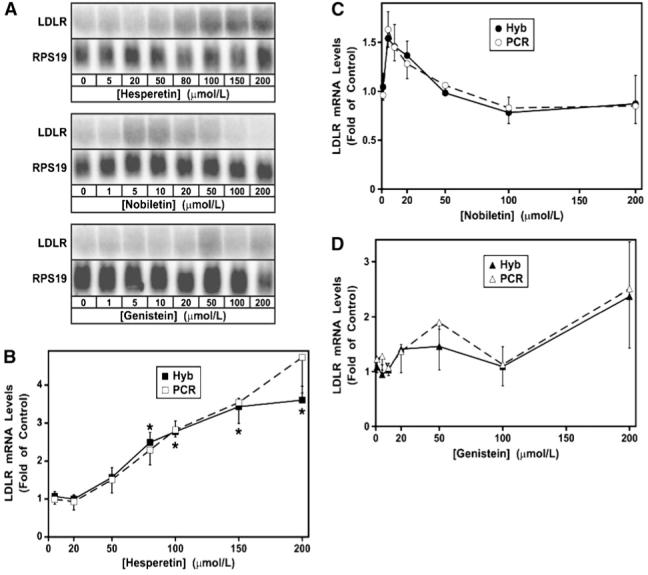
The impact of 24-h flavonoid treatment on LDLR mRNA levels shown by hybridization (A) and quantified by hybridization and qRT-PCR (B-D). Hybridizations were conducted using ribosomal protein S19 mRNA as an internal control. Both hybridization and qRT-PCR were used to corroborate the results from each method independently. Quantitation and normalization of LDLR mRNA levels from 4 separate experiments (except n = 3 for 2 nobiletin doses) were conducted for each technique. Error bars represent +SEM for the hybridizations and -SEM for the qRT-PCR data. *Hybridization data different from the untreated control, P ≤ 0.05 (Dunnett’s test).
FIGURE 4.
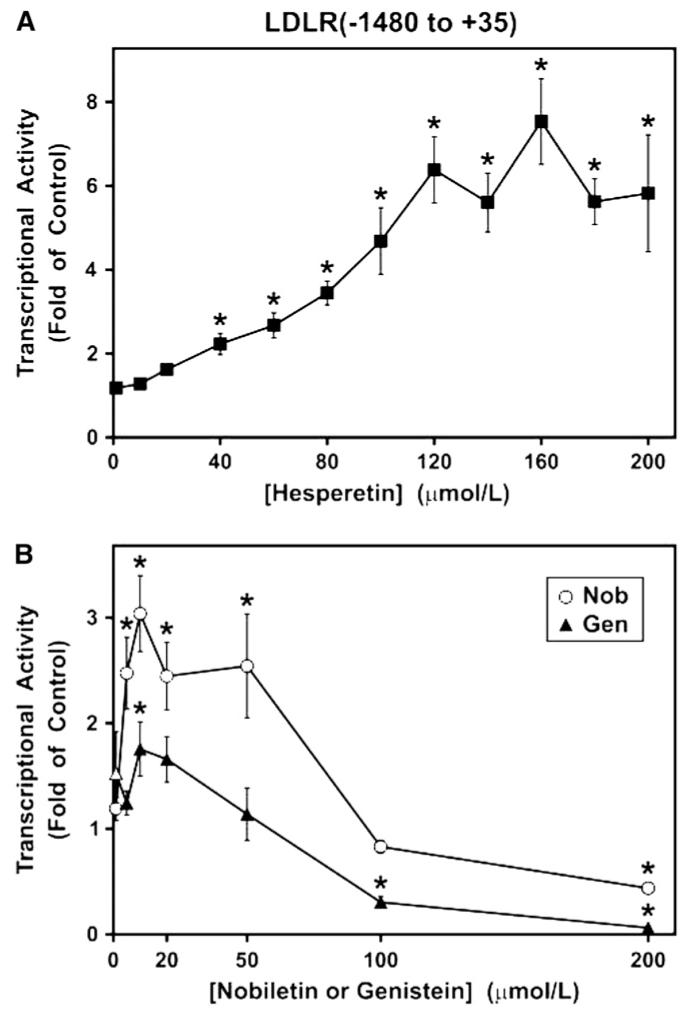
Dose effects of hesperetin (A) and nobiletin or genistein (B) on luciferase reporter vectors containing regulatory sequences of the human LDLR gene from -1480 to +35 relative to the transcription start site. Transfected HepG2 cells were treated with hesperetin (n = 5), nobiletin (n = 5), or genistein (n = 3) for 24 h over a range of concentrations. Luciferase values were normalized as described in the Online Supporting Material. Error bars are ± SEM. *Different from the untreated control, P ≤ 0.05 (Dunnett’s test).
FIGURE 5.
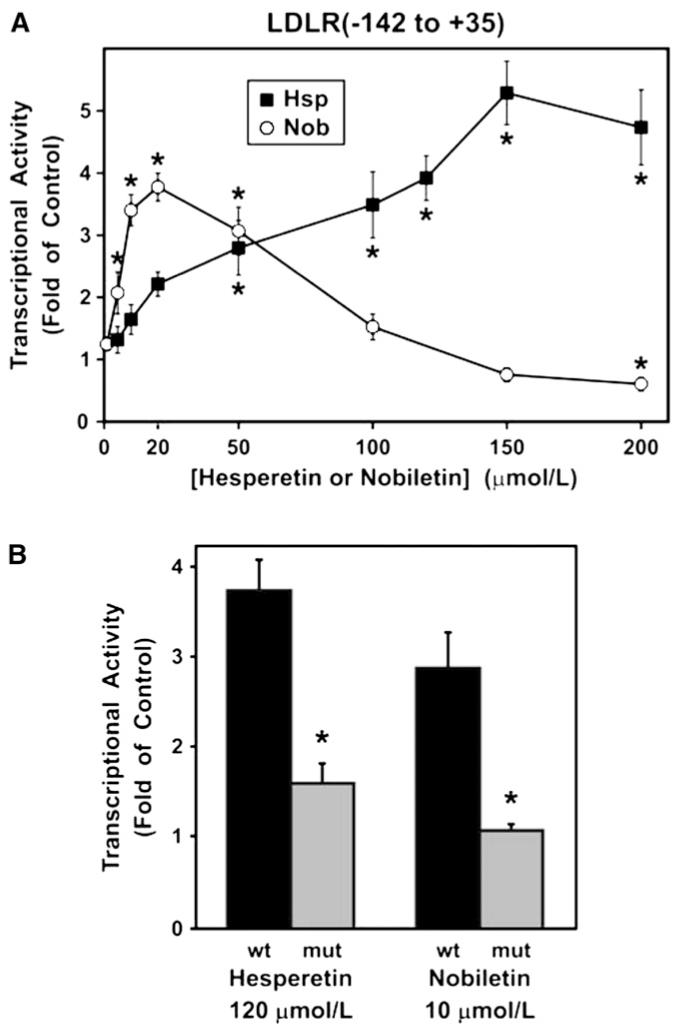
Dose effects of 24-h treatment with hesperetin or nobiletin on transcriptional activity of the LDLR promoter sequence from -142 to +35 (A) and reduction of flavonoid responsiveness by mutation of the SRE (B). Luciferase values were normalized and expressed as a fold of the corresponding untreated sample as described in the Online Supporting Material. Error bars are ±SEM. (A) Transfected HepG2 cells were treated with hesperetin (n = 4-6 at each dose) or nobiletin (n = 5-7 at each dose) over a range of concentrations. *Different from the untreated control, P ≤ 0.05 (Dunnett’s test). (B) The SREBP binding site was either the native sequence (wt) or mutated at a single nucleotide (mut) (n = 4). *Different between the mut and the wt constructs for each compound at P ≤ 0.0001 (ANOVA).
Because a more complex analysis was necessary for Fig. 5B, ANOVA with mixed effects and associated tests of contrasts were conducted. The mixed linear model was:
where Ln(yijkl) is the natural log-transformed measure of luciferase expression for treatment i (none, hesperetin, or nobiletin), type j (wild type or mutant), run k, and replicate l. The fixed effects were treatment and type and the random effects were run (γ) and error (ε). The random terms were assumed to be uncorrelated random variables having 0 means and variances σ2γ and σ2ε. This model was fit using SAS software (v9.1.3) and the GLIMMIX procedure. Estimation was carried out using restricted maximum likelihood and degrees of freedom were based on the Kenward-Roger method. Model diagnostics were thoroughly evaluated to ensure proper model fit and appropriateness of underlying theoretical assumptions (i.e. normality and unequal group variances).
Other experimental procedures
RNA was purified with the RNeasy system (Qiagen). RNA was size fractionated on a denaturing formaldehyde gel and transferred to GeneScreen (New England Nuclear/Perkin Elmer) for molecular hybridization. Quantitative RT-PCR (qRT-PCR) utilized SYBR Green chemistry and was run on the iQ5 RT-PCR Detection system (Bio-Rad). Luciferase assays were done with the Dual-Luciferase Reporter Assay system (Promega) (see Online Supporting Material).
Results
Identification of 3 size variants of LDLR mRNA induced by hesperetin in HepG2 cells
Three potential forms of human LDLR mRNA have been described (Fig. 2A): 1) the full-length molecule of 5175 bases, excluding the 3′ polyA tail (GenBank accession no. NM_000527.2) (38); 2) a shorter RNA that uses a polyadenylation signal at nucleotides 4579-4584 to terminate slightly downstream of position 4584 (GenBank accession no. NM_000527.2); and 3) a molecule ending at position 3521 (relative to the numbering in NM_000527.2), identified in Burkitt lymphoma (Genbank accession no. BC014514). (See Note Added in Proof for minor changes to RNA sizes.) Previous studies showed that 200 μmol/L hesperetin induced LDLR mRNA levels in HepG2 cells, but the particular size variants were not identified (20). To determine which LDLR mRNA species were regulated by flavonoid treatment in HepG2 cells, we used a set of complementary probes that differentiated between the 3 potential mRNAs (Supplemental Table 1; Fig. 2A). We show by hybridization to size-fractionated RNA from HepG2 cells that Probe 3 detected a broad band at ∼5000 bases, as well as a sharper band at ∼3500 bases (Fig. 2B, lane 6). To clarify the identity of the broad band, we hybridized the RNA with either probe 1 that recognized the largest species of 5175 bases exclusively, or probe 2 that hybridized to both the 5175 and the 4584 base mRNAs (Fig. 2A). In lane 2 of Fig. 2B, probe 1 hybridized to RNA corresponding to the upper part of the broad band in lane 6, consistent with the full-length LDLR mRNA. Probe 2 hybridization was more diffuse, consistent with the detection of both the 5175 and the truncated species of 4584 bases (Fig. 2B, lane 4). Thus, we conclude that all 3 forms of LDLR mRNA are produced in HepG2 cells and induced by 200 μmol/L hesperetin (compare lanes 2 to 1, 4 to 3, and 6 to 5). For subsequent investigations, we used probe 3, because it appeared to interact with the RNA more efficiently than the other 2 probes.
FIGURE 2.
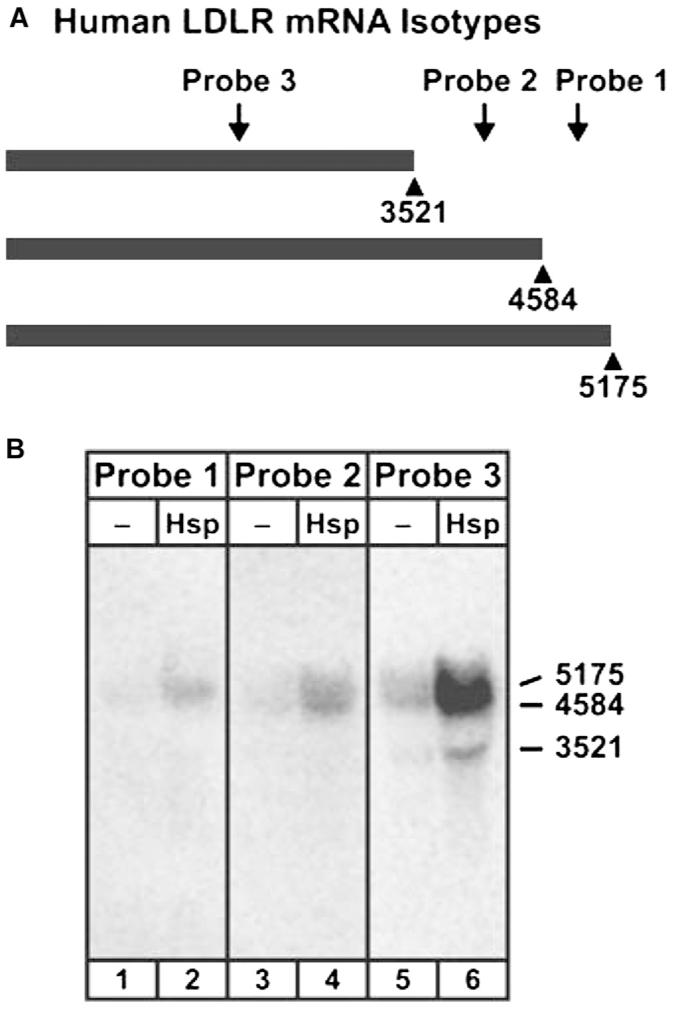
Schematic representation (A) and experimental detection (B) of 3 LDLR mRNA isotypes induced by hesperetin in HepG2 cells. Hybridization analyses were performed on size-fractionated total RNA from untreated and hesperetin-treated cells. The sizes of the 3 mRNA isotypes are denoted on the right.
Dose-dependent regulation of LDLR mRNA levels by flavonoids of 3 structural classes
Citrus PMF are physiologically effective at lower doses than flavanones in reducing plasma cholesterol in animals (16) and, at the cellular level, in decreasing apoB secretion from HepG2 cells (22). Also, intermediate concentrations of genistein, a soy isoflavone, have been shown to inhibit apoB secretion and to increase LDLR mRNA amounts in HepG2 cells (39). Therefore, we compared the potency and efficacy of a PMF (nobiletin) to those of a flavanone (hesperetin) and an isoflavone (genistein) at the molecular level of LDLR gene expression. These representative compounds were chosen based upon higher activity compared with other members of their respective class (20,39, our unpublished data). For these experiments, HepG2 cells were treated with a concentration range of 5-200 μmol/L hesperetin, 1-200 μmol/L nobiletin, or 1-200 μmol/L genistein and total RNA was purified 24 h later. No systematic differences were found in the total amount of RNA recovered from untreated vs. flavonoid-treated cells across the dose ranges tested (see Online Supporting Material), suggesting that the compounds did not cause considerable cell death. The RNA was analyzed for changes in LDLR mRNA levels both by hybridization of probe 3 to size-fractionated RNA to ensure the integrity of the LDLR mRNA and by qRT-PCR for more rapid quantitation of relative RNA amounts. Like the hybridization probe, the qRT-PCR primers detected all 3 isoforms of the LDLR mRNA (see Supplemental Table 1).
Representative hybridization data are shown from 1 experiment (Fig. 3A). For each sample, the LDLR mRNA was normalized to ribosomal protein S19 mRNA. Four independent experiments were performed with all 3 flavonoid treatments and the data from both hybridization and qRT-PCR are shown (Fig. 3B,C,D). The citrus flavanone, hesperetin, induced LDLR mRNA by 3.6- to 4.7-fold of control, with maximal stimulation occurring at 150-200 μmol/L (Fig. 3B). Compared with the untreated control, all hesperetin doses ≥40 μmol/L elevated LDLR mRNA levels (P ≤ 0.05). The full dose-range response is consistent with previous results showing induction of 6.6-fold of control at 200 μmol/L hesperetin (20). Genistein effects were quite variable over the dose range (Fig. 3D) and not significant at any dose by Dunnett’s test (P ≤ 0.05); however, other investigators have found that genistein induced LDLR mRNA in HepG2 cells by 3.1- and 5.7-fold of control at 50 and 100 μmol/L, respectively (39). In contrast to hesperetin, nobiletin maximally induced LDLR mRNA by a relatively modest 1.5- to 1.6-fold of control, but this stimulation occurred at 5-10 μmol/L, while higher concentrations were ineffective (Fig. 3C). When we compared the 10 μmol/L nobiletin dose to the untreated control by t-test, the difference was significant at P ≤ 0.05 but it was not when we used the correct and more stringent Dunnett’s test for multiple comparisons (35). Nonetheless, because of the consistency of the pattern at several doses and the reproducibility of the results between experiments by 2 independent methods of quantitation, further investigation of the underlying molecular mechanisms was warranted.
Flavonoid effects on LDLR gene transcription
Because LDLR mRNA levels can be affected through changes in mRNA stabilization (40,41) and/or gene transcription, we used gene transfection assays to determine whether LDLR mRNA regulation was primarily due to effects on gene transcription. The greater sensitivity of this approach allows for a more thorough statistical evaluation to corroborate the dose response patterns observed at the mRNA level. The LDLR(-1480 to +35) luciferase construct, containing the regulatory region of the human LDLR gene spanning from 1480 nucleotides upstream to 35 nucleotides downstream of the transcription start site (position +1), was introduced into HepG2 cells, which were then treated with a dose range of the flavonoids for 24 h. In response to hesperetin, LDLR gene transcription increased steadily until essentially reaching a plateau at ∼120 μmol/L, with maximal induction of 7.5-fold of control (Fig. 4A). In contrast, nobiletin was effective at much lower doses, achieving maximal stimulation of 3.0-fold of control at 10 μmol/L (Fig. 4B). However, the higher doses of 100 and 200 μmol/L nobiletin were distinctly inhibitory in marked contrast to the sustained induction by hesperetin. This negative regulation is due to specific action on the LDLR gene, because normalization to the Renilla luciferase corrected for any general toxicity at higher flavonoid concentrations. The overall pattern of stimulation of LDLR gene transcription by genistein was similar to that of nobiletin, but maximal induction was only 1.8-fold of control at 10 μmol/L (Fig. 4B) and negative effects at high doses were more pronounced. Our studies with genistein were undertaken initially to determine whether this well-known soy isoflavone is more effective than the citrus flavonoids in regulating LDLR gene expression. The variable responses at the mRNA level (Fig. 3D), together with the lower induction of gene transcription (Fig. 4B), show that the soy compound is not superior to the citrus flavonoids. Therefore, we continued to investigate the molecular mechanisms of hesperetin and nobiletin without further analysis of genistein.
To delimit the location of DNA regulatory elements required for flavonoid action, the transcription assay was next conducted with a construct containing only 142 bp of the upstream regulatory sequence, designated LDLR(-142 to +35). This segment contains an SRE sequence at -65 to -56 upstream of the transcription start site (31,42). Transcription from the LDLR(-142 to +35) construct rose gradually with increasing doses of hesperetin until leveling off above 150 μmol/L, achieving a maximal response of 5.3-fold of control (Fig. 5A). Thus, the -142 to +35 segment upstream of the LDLR gene is sufficient to mediate responsiveness to hesperetin. With nobiletin treatment, transcription from this DNA reached maximal induction of 3.8-fold of control at 20 μmol/L, with repression at high doses (Fig. 5A), indicating that this DNA is sufficient not only for positive induction at low concentrations but also for inhibitory effects at high concentrations of nobiletin. The close agreement between the results in the mRNA and gene transcription assays indicates that transcriptional regulation is the major mechanism by which the citrus flavonoids control LDLR mRNA amounts.
Role of the SRE in flavonoid induction of LDLR gene transcription
The role of the SRE (ATCACCCCAC) at -65 to -56 in mediating flavonoid regulation of the LDLR gene was examined by mutating position -59 (ATCACCgCAC), because this single point mutation disrupts modulation of LDLR gene transcription by SREBP (31,42). Transcription of the wild-type LDLR(-142 to +35) construct was stimulated 3.7-fold of control by 120 μmol/L hesperetin, whereas the responsiveness of the SRE mutant was attenuated to 1.6-fold (Fig. 5B). Likewise, the response to 10 μmol/L nobiletin markedly declined from 2.9-fold of control for the wild type to 1.1-fold for the mutant. In addition, basal expression in the mutant was reduced to 23% of the wild-type level, but expression was still >27-fold above the assay background.
Discussion
Role of the SRE in citrus flavonoid regulation of LDLR gene transcription and potential mechanisms of SREBP activation
The SRE at positions -65 to -56 (31,42) of the human LDLR gene regulatory region mediates induction of LDLR gene transcription by a number of stimuli, including low sterol concentrations, insulin, estradiol, and interleukin-6 (31,42-44). Furthermore, involvement of SREBP in flavonoid action in HepG2 cells has been suggested (24,32). Hence, we demonstrated here for the first time, to our knowledge, that stimulation of LDLR gene transcription by citrus flavonoids is dependent on the binding site for SREBP.
The most straightforward mechanisms to account for stimulation of gene transcription through an SRE are to increase SREBP total amount, nuclear availability, or inherent activity. A previous study showed that total SREBP-1 rises in response to naringenin (24). However, both the SREBP-1a isoform and SREBP-2 can induce LDLR mRNA in transgenic mice (45-48) and LDLR gene transcription in HepG2 cells (47). We have preliminary data from mRNA microarray experiments that show that SREBP-2 mRNA levels increased within 2 h of hesperetin treatment, attaining ∼100% increase after 6 h (data not shown). SREBP-1 mRNA, on the other hand, was increased only slightly after 6 h of treatment. The increase in SREBP-2 mRNA is likely due, at least in part, to stimulation of gene transcription.
Because SREBP-2 regulates transcription of its own gene (45,47), we propose that the likely initial step in activation of this gene is either an increase in nuclear localization of SREBP-2 or stimulation of intrinsic SREBP-2 activity. Either mechanism could be controlled by signal transduction pathways, which is in agreement with the general concept that flavonoids act predominantly as cellular signaling molecules (49). In support of a role for kinase cascades in citrus flavonoid action, enhancement of LDLR mRNA levels by naringenin was blocked when phosphatidylinositol 3-kinase (PI3K) activity was inhibited (24). We have preliminary evidence suggesting that hesperetin and nobiletin induction of LDLR mRNA levels are also dependent on PI3K activity, because both responses were reduced in the presence of the PI3K inhibitor, wortmannin (data not shown). There is precedent for involvement of kinases through PI3K effects on the transport of SREBP-2 out of the endoplasmic reticulum in response to low sterol conditions (50) and enhancement of inherent transcriptional activity of both SREBP-1a and -2 through direct phosphorylation by mitogen-activated protein kinase in response to insulin (51,52). Therefore, our future studies will analyze the role of signal transduction pathways in citrus flavonoid enhancement of SREBP-2 activity and translocation to the nucleus and the resulting stimulation of transcription of the SREBP-2 and LDLR genes.
Potential mechanisms underlying the inverted-U dose response to nobiletin
We are not aware of any other investigations that have analyzed a biphasic dose response to flavonoids at the molecular level, but positive and negative effects across a dose range have been reported for hyperoside, a flavonoid found in St. John’s wort (53), and the general phenomenon of an inverted-U response is well known for a variety of other stimuli (54-56). One possible explanation for this duality is that different metabolites of nobiletin are responsible for different effects, because conversion of PMF to a variety of forms in the liver has been found in hamsters (16). However, genistein gave a similar pattern of repression at high doses in the transfection assay (although results at the mRNA level were erratic and more difficult to interpret). Thus, the phenomenon does not appear to be unique to PMF, suggesting that a more general mechanism is likely.
Because the LDLR gene construct containing only 142 bp of upstream DNA is capable of mediating negative regulation by high doses of nobiletin (Fig. 5), a feasible scenario is that the SRE mediates both aspects of the biphasic response, with activation of SREBP at low doses and inhibition at high doses (e.g. by affecting different signaling cascades). However, we are not aware of any precedents in the literature for such effects on either SREBP-1 or -2. It is certainly possible that other transcription factors besides SREBP are affected by high doses of nobiletin. Little is known, however, about negative regulation of the LDLR gene. One case of repression has been reported, but the DNA sequence necessary for this effect was ∼1500 nucleotides upstream (i.e. not within the promoter-proximal region of our small construct) and a specific transcription factor was not identified (57). Another case of repression occurred through inhibition of the level of a positive transcription factor, CCAAT/enhancer binding proteinβ, which binds to a site very close to the start site of transcription (58,59), so it is possible that high doses of PMF could affect this factor.
Parallels between flavonoid effects in HepG2 cells and animal models
In our LDLR gene transcription assays, hesperetin was more efficacious, based on its higher maximal stimulation of LDLR gene transcription, than nobiletin (5.3- to 7.5-fold vs. 3.0- to 3.8-fold). Nobiletin satisfies the classical definition of being more potent, i.e. achieving maximal induction at lower doses than for hesperetin (10-20 μmol/L vs. 150-160 μmol/L). There is good agreement between this difference in potency at the molecular level and previously reported differences between citrus flavanones and PMF at the physiological level. In the in vivo studies using dietary supplementation in hypercholesterolemic hamsters, citrus PMF were more potent than citrus flavanones at reducing serum cholesterol and TG (16). The total concentration of PMF in the livers of these animals reached ∼16-67 μmol/L, which is in good agreement with the low-to mid-micromolar concentrations of tangeretin found to be effective in inhibiting apoB secretion, as well as other processes related to cholesterol and TG handling in HepG2 cells (25). Here, we found the PMF nobiletin to have peak activity at even lower doses.
The relatively high concentrations of flavanones in oranges and grapefruit have focused attention on the potential healthful effects of these citrus flavonoids. Although rather high dietary doses are sometimes used to maximize the effects in animal studies, flavanone concentrations in the plasma have been measured at 0.2-6 μmol/L in humans after 1-time ingestion of 0.5-1.0 L of citrus juice (28,29) and higher amounts of the flavonoids may accumulate in the liver compared with the plasma levels (60). These concentrations of individual flavanones are low compared with the levels we show are needed for activity in isolated liver cells, but it is certainly possible that the combinations of flavonoids and metabolites that are present in the liver after ingestion of natural juice could act synergistically, which is consistent with the observation that daily consumption of 1 or 2 citrus fruits or 200 mL of juice reduces plasma cholesterol levels in humans (4-6). However, a potential disadvantage of fruit or juice consumption is maintaining consistent and high daily ingestion. In addition, the calories associated with high dietary intake could increase cardiovascular disease risk through weight gain. PMF are present in citrus fruit and juice in lesser amounts than flavanones, but, because the PMF are active at much lower doses, attainment of effective in vivo concentrations of these compounds by dietary supplementation is more plausible. In fact, supplements containing citrus PMF have shown promising cardioprotective effects in humans (18), although our data at the molecular level suggest that beneficial effects could be lost at very high doses. It will be important to reveal the underlying mechanisms of both the positive and negative effects of flavonoids in the liver to understand the potential advantages and disadvantages of the different classes of compounds and to develop the citrus flavonoids to their full potential as therapeutic agents for combating heart disease, diabetes, and other chronic diseases.
Note Added in Proof. The latest update of GenBank accession no. NM_000527 (version 3 dated April 20, 2008) yields LDLR mRNA sizes of 5262, 4656, and 3597 bases, due to addition of 75 bases at the 5′ end, 13 bases at the 3′ end, and a few internal insertions and deletions. However, the sizes in Fig. 2 from NM_000527.2 are consistent with the numbering of the transcriptional regulatory region of the LDLR gene (42).
Supplementary Material
Footnotes
Supported by grants to L.J.H. from the NIH (R01-DK56822) and the University of Missouri Research Board (RB-05-025), Research Council (URC-07-001), and Alumni Association, and by a fellowship to K.M.Z. from the University of Missouri Life Sciences Undergraduate Research Opportunity Program.
Author disclosures: B. Morin, L. A. Nichols, K. M. Zalasky, J. W. Davis, J. A. Manthey, and L. J. Holland, no conflicts of interest.
Supplemental Table 1 and details of the protocols for RNA isolation, RNA gel electrophoresis and molecular hybridization, quantitative RT-PCR, transfection vector assembly, DNA purification, and transfections and luciferase assays are available with the online posting of this paper at jn.nutrition.org.
- apoB
- apolipoprotein B
- DMSO
- dimethyl sulfoxide
- LDLR
- LDL receptor
- PI3K
- phosphatidylinositol 3-kinase
- PMF
- polymethoxylated flavone
- qRT-PCR
- quantitative RT-PCR
- SRE
- sterol regulatory element
- SREBP
- sterol regulatory element-binding protein
- TG
- triglyceride.
Literature Cited
- 1.Kurowska EM, Borradaile NM, Spence JD, Carroll KK. Hypocholesterolemic effects of dietary citrus juices in rabbits. Nutr Res. 2000;20:121–9. [Google Scholar]
- 2.Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piché LA, Serratore P. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr. 2000;72:1095–100. doi: 10.1093/ajcn/72.5.1095. [DOI] [PubMed] [Google Scholar]
- 3.Gorinstein S, Leontowicz H, Leontowicz M, Drzewiecki J, Jastrzebski Z, Tapia MS, Katrich E, Trakhtenberg S. Red Star Ruby (Sunrise) and blond qualities of Jaffa grapefruits and their influence on plasma lipid levels and plasma antioxidant activity in rats fed with cholesterol-containing and cholesterol-free diets. Life Sci. 2005;77:2384–97. doi: 10.1016/j.lfs.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Gorinstein S, Caspi A, Libman I, Katrich E, Lerner HT, Trakhtenberg S. Preventive effects of diets supplemented with Sweetie fruits in hypercholesterolemic patients suffering from coronary artery disease. Prev Med. 2004;38:841–7. doi: 10.1016/j.ypmed.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Gorinstein S, Caspi A, Libman I, Katrich E, Lerner HT, Trakhtenberg S. Fresh Israeli Jaffa Sweetie juice consumption improves lipid metabolism and increases antioxidant capacity in hypercholesterolemic patients suffering from coronary artery disease: studies in vitro and in humans and positive changes in albumin and fibrinogen fractions. J Agric Food Chem. 2004;52:5215–22. doi: 10.1021/jf040139j. [DOI] [PubMed] [Google Scholar]
- 6.Gorinstein S, Caspi A, Libman I, Lerner HT, Huang D, Leontowicz H, Leontowicz M, Tashma Z, Katrich E, et al. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: studies in vitro and in humans. J Agric Food Chem. 2006;54:1887–92. doi: 10.1021/jf058171g. [DOI] [PubMed] [Google Scholar]
- 7.Gorinstein S, Leontowicz H, Leontowicz M, Krzeminski R, Gralak M, Martin-Belloso O, Delgado-Licon E, Haruenkit R, Katrich E, et al. Fresh Israeli Jaffa blond (Shamouti) orange and Israeli Jaffa red Star Ruby (Sunrise) grapefruit juices affect plasma lipid metabolism and antioxidant capacity in rats fed added cholesterol. J Agric Food Chem. 2004;52:4853–9. doi: 10.1021/jf040006y. [DOI] [PubMed] [Google Scholar]
- 8.Gorinstein S, Leontowicz H, Leontowicz M, Krzeminski R, Gralak M, Delgado-Licon E, Ayala ALM, Katrich E, Trakhtenberg S. Changes in plasma lipid and antioxidant activity in rats as a result of naringin and red grapefruit supplementation. J Agric Food Chem. 2005;53:3223–8. doi: 10.1021/jf058014h. [DOI] [PubMed] [Google Scholar]
- 9.Harborne JB. The flavonoids: advances in research since 1986. Chapman & Hall; New York: 1994. [Google Scholar]
- 10.Horowitz RM, Gentili B. Flavonoid constituents of citrus. In: Nagy S, Shaw PE, Veldhuis MK, editors. Citrus science and technology. Vol. 1. Avi Publishing; Westport (CT): 1977. pp. 397–426. [Google Scholar]
- 11.Jeon SM, Park YB, Choi MS. Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits. Clin Nutr. 2004;23:1025–34. doi: 10.1016/j.clnu.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Monforte MT, Trovato A, Kirjavainen S, Forestieri AM, Galati EM, Lo Curto RB. Biological effects of hesperidin, a citrus flavonoid. (note II): hypolipidemic activity on experimental hypercholesterolemia in rat. Il Farmaco. 1995;50:595–9. [PubMed] [Google Scholar]
- 13.Bok SH, Lee SH, Park YB, Bae KH, Son KH, Jeong TS, Choi MS. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA:cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr. 1999;129:1182–5. doi: 10.1093/jn/129.6.1182. [DOI] [PubMed] [Google Scholar]
- 14.Chiba H, Uehara M, Wu J, Wang X, Masuyama R, Suzuki K, Kanazawa K, Ishimi Y. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr. 2003;133:1892–7. doi: 10.1093/jn/133.6.1892. [DOI] [PubMed] [Google Scholar]
- 15.Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol. 2006;38:1134–45. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Kurowska EM, Manthey JA. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J Agric Food Chem. 2004;52:2879–86. doi: 10.1021/jf035354z. [DOI] [PubMed] [Google Scholar]
- 17.Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, Kim HK, Jeong TS, Choi MS. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr. 2003;22:561–8. doi: 10.1016/s0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 18.Roza JM, Xian-Liu Z, Guthrie N. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Altern Ther Health Med. 2007;13:44–8. [PubMed] [Google Scholar]
- 19.Borradaile NM, Carroll KK, Kurowska EM. Regulation of HepG2 cell apolipoprotein B metabolism by the citrus flavanones hesperetin and naringenin. Lipids. 1999;34:591–8. doi: 10.1007/s11745-999-0403-7. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox LJ, Borradaile NM, de Dreu LE, Huff MW. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J Lipid Res. 2001;42:725–34. [PubMed] [Google Scholar]
- 21.Borradaile NM, de Dreu LE, Barrett PHR, Huff MW. Inhibition of hepatocyte apoB secretion by naringenin: enhanced rapid intracellular degradation independent of reduced microsomal cholesteryl esters. J Lipid Res. 2002;43:1544–54. doi: 10.1194/jlr.m200115-jlr200. [DOI] [PubMed] [Google Scholar]
- 22.Kurowska EM, Manthey JA. Regulation of lipoprotein metabolism in HepG2 cells by citrus flavonoids. In: Buslig B, Manthey J, editors. Flavonoids in cell function. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 173–9. [DOI] [PubMed] [Google Scholar]
- 23.Borradaile NM, de Dreu LE, Barrett PHR, Behrsin CD, Huff MW. Hepatocyte apoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry. 2003;42:1283–91. doi: 10.1021/bi026731o. [DOI] [PubMed] [Google Scholar]
- 24.Borradaile NM, de Dreu LE, Huff MW. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes. 2003;52:2554–61. doi: 10.2337/diabetes.52.10.2554. [DOI] [PubMed] [Google Scholar]
- 25.Kurowska EM, Manthey JA, Casaschi A, Theriault AG. Modulation of HepG2 cell net apolipoprotein B secretion by the citrus polymethoxyflavone, tangeretin. Lipids. 2004;39:143–51. doi: 10.1007/s11745-004-1212-8. [DOI] [PubMed] [Google Scholar]
- 26.Allister EM, Borradaile NM, Edwards JY, Huff MW. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 2005;54:1676–83. doi: 10.2337/diabetes.54.6.1676. [DOI] [PubMed] [Google Scholar]
- 27.Choudhury R, Chowrimootoo G, Srai K, Debnam E, Rice-Evans CA. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem Biophys Res Commun. 1999;265:410–5. doi: 10.1006/bbrc.1999.1695. [DOI] [PubMed] [Google Scholar]
- 28.Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131:235–41. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 29.Manach C, Morand C, Gil-Izquierdo A, Bouteloup-Demange C, Rémésy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr. 2003;57:235–42. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 30.Spencer JPE, Rice-Evans CA, Srai SKS. Metabolism in the small intestine and gastrointestinal tract. In: Rice-Evans CA, Packer L, editors. Flavonoids in health and disease. Marcel Dekker; New York: 2003. pp. 363–89. [Google Scholar]
- 31.Briggs MR, Yokoyama C, Wang X, Brown MS, Goldstein JL. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. J Biol Chem. 1993;268:14490–6. [PubMed] [Google Scholar]
- 32.Mullen E, Brown RM, Osborne TF, Shay NF. Soy isoflavones affect sterol regulatory element binding proteins (SREBPs) and SREBP-regulated genes in HepG2 cells. J Nutr. 2004;134:2942–7. doi: 10.1093/jn/134.11.2942. [DOI] [PubMed] [Google Scholar]
- 33.Swift LJ. Flavones of the neutral fraction of the benzene extractables of an orange peel juice. J Agric Food Chem. 1965;13:431–3. [Google Scholar]
- 34.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Glantz SA. Primer of biostatistics. 4th ed. McGraw-Hill; New York: 1997. [Google Scholar]
- 36.Levene H. In: Contributions to probability and statistics: essays in honor of Harold Hotelling. Olkin I, Ghurye SG, Hoeffding W, Madow WG, Mann HB, editors. Stanford University Press; Stanford (CA): 1960. pp. 278–92. [Google Scholar]
- 37.D’Agostino RB, Belanger A, D’Agostino RB., Jr. A suggestion for using powerful and informative tests of normality. Am Stat. 1990;44:316–21. [Google Scholar]
- 38.Yamamoto T, Davis CG, Brown MS, Schneider WJ, Casey ML, Goldstein JL, Russell DW. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984;39:27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- 39.Borradaile NM, de Dreu LE, Wilcox LJ, Edwards JY, Huff MW. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem J. 2002;366:531–9. doi: 10.1042/BJ20020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson GM, Roberts EA, Deeley RG. Modulation of LDL receptor mRNA stability by phorbol esters in human liver cell culture models. J Lipid Res. 1997;38:437–46. [PubMed] [Google Scholar]
- 41.Abidi P, Chen W, Kraemer FB, Li H, Liu J. The medicinal plant goldenseal is a natural LDL-lowering agent with multiple bioactive components and new action mechanisms. J Lipid Res. 2006;47:2134–47. doi: 10.1194/jlr.M600195-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Smith JR, Osborne TF, Goldstein JL, Brown MS. Identification of nucleotides responsible for enhancer activity of sterol regulatory element in low density lipoprotein receptor gene. J Biol Chem. 1990;265:2306–10. [PubMed] [Google Scholar]
- 43.Streicher R, Kotzka J, Müller-Wieland D, Siemeister G, Munck M, Avci H, Krone W. SREBP-1 mediates activation of the low density lipoprotein receptor promoter by insulin and insulin-like growth factor-I. J Biol Chem. 1996;271:7128–33. doi: 10.1074/jbc.271.12.7128. [DOI] [PubMed] [Google Scholar]
- 44.Gierens H, Nauck M, Roth M, Schinker R, Schürmann C, Scharnagl H, Neuhaus G, Wieland H, März W. Interleukin-6 stimulates LDL receptor gene expression via activation of sterol-responsive and Sp1 binding elements. Arterioscler Thromb Vasc Biol. 2000;20:1777–83. doi: 10.1161/01.atv.20.7.1777. [DOI] [PubMed] [Google Scholar]
- 45.Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101:2331–9. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, et al. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res. 2002;43:1220–35. [PubMed] [Google Scholar]
- 48.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. 2003;100:12027–32. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams RJ, Spencer JPE, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–49. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Du X, Kristiana I, Wong J, Brown AJ. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link between a key cell proliferative pathway and membrane synthesis. Mol Biol Cell. 2006;17:2735–45. doi: 10.1091/mbc.E05-11-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotzka J, Müller-Wieland D, Roth G, Kremer L, Munck M, Schürmann S, Knebel B, Krone W. Sterol regulatory element binding proteins (SREBP)-1a and SREBP-2 are linked to the MAP-kinase cascade. J Lipid Res. 2000;41:99–108. [PubMed] [Google Scholar]
- 52.Kotzka J, Lehr S, Roth G, Avci H, Knebel B, Müller-Wieland D. Insulin-activated Erk-mitogen-activated protein kinases phosphorylate sterol regulatory element-binding protein-2 at serine residues 432 and 455 in vivo. J Biol Chem. 2004;279:22404–11. doi: 10.1074/jbc.M401198200. [DOI] [PubMed] [Google Scholar]
- 53.Butterweck V, Jürgenliemk G, Nahrstedt A, Winterhoff H. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 2000;66:3–6. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- 54.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–84. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 55.Ahn N-S, Hu H, Park J-S, Park J-S, Kim J-S, An S, Kong G, Aruoma OI, Lee Y-S, Kang K-S. Molecular mechanisms of the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced inverted U-shaped dose responsiveness in anchorage independent growth and cell proliferation of human breast epithelial cells with stem cell characteristics. Mutat Res. 2005;579:189–99. doi: 10.1016/j.mrfmmm.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 56.Calabrese EJ, Baldwin LA. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001;62:330–8. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- 57.Oh J, Choi YS, Kim J-W, Park J-Y, Kim S-W, Park K-K, Pak YK. Inhibition of low density lipoprotein receptor expression by long-term exposure to phorbol ester via p38 mitogen-activated protein kinase pathway. J Cell Biochem. 2005;96:786–94. doi: 10.1002/jcb.20551. [DOI] [PubMed] [Google Scholar]
- 58.deBruijn DRH, Allander SV, van Dijk AHA, Willemse MP, Thijssen J, van Groningen JJM, Meltzer PS, van Kessel AG. The synovial sarcoma-associated SS18-SSX2 fusion protein induces epigenetic gene (de)regulation. Cancer Res. 2006;66:9474–82. doi: 10.1158/0008-5472.CAN-05-3726. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Ahlborn TE, Briggs MR, Kraemer FB. Identification of a novel sterol-independent regulatory element in the human low density lipoprotein receptor promoter. J Biol Chem. 2000;275:5214–21. doi: 10.1074/jbc.275.7.5214. [DOI] [PubMed] [Google Scholar]
- 60.Murakami A, Koshimizu K, Ohigashi H, Kuwahara S, Kuki W, Takahashi Y, Hosotani K, Kawahara S, Matsuoka Y. Characteristic rat tissue accumulation of nobiletin, a chemopreventive polymethoxy-flavonoid, in comparison with luteolin. Biofactors. 2002;16:73–82. doi: 10.1002/biof.5520160303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.