Abstract
Sharon Peacock and colleagues discuss management of adult patients with sepsis in low- and middle-income settings, with a particular emphasis on tropical regions.
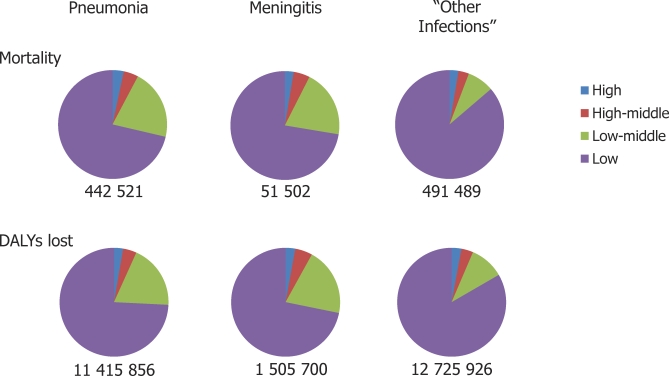
Sepsis is a progressive injurious process resulting from a systemic inflammatory response to infection [1]. In developed countries, sepsis is an important cause of mortality: in the United States alone, up to 750,000 people annually suffer from severe sepsis—mostly bacterial in aetiology—of whom 29% may die [2,3]. Unfortunately, data on bacterial sepsis in developing countries are notably lacking, particularly in adults. Estimates of the burden of lower respiratory tract infections, meningitis, and “other infections”, of which a significant proportion are associated with severe sepsis, show that the majority of deaths and disability-adjusted life years lost occur in low-income countries (Figure 1) [4]. Additionally, severe sepsis is likely to complicate a varying proportion of cases of malaria, HIV/AIDS, diabetes, maternal conditions, and cancer deaths globally.
Figure 1. Mortality and Disability-Adjusted Life Years (DALYs) Lost in Adults 15–60 Years Old in 2002 for Three Selected Indicator Diseases, by World Bank Income Level [4].
The standard of care varies significantly across lower- and middle-income developing countries, but published reports suggest that outcomes are poor even at major hospitals [5–10]. Melioidosis, a serious tropical infection caused by Burkholderia pseudomallei that often presents with sepsis, is endemic in a region containing both high- and low-income countries [11]. Outcomes vary significantly: the case fatality rate for melioidosis is higher in Thailand (40%–50%) than in Australia (10%–20%) [11–13], and the case-fatality rate for melioidosis with severe sepsis is approximately 50% in Singapore compared with 90% in a Thai clinical trial [14,15]. The burden of disease and case fatality of patients with melioidosis in less developed countries such as Cambodia and Myanmar are unknown. Although melioidosis is not common outside of southeast Asia, extrapolating this experience suggests that the outcomes from all-cause bacterial sepsis in underdeveloped regions are likely to be poor.
The recently updated “Surviving Sepsis Campaign” guidelines have been widely disseminated in the developed world as a model of optimal sepsis management [16]. Although there has been some controversy regarding the recommendations and the development of the guidelines [17–19], most interventions based on improving early management of septic patients are less controversial. Crucially, most of the studies on which these recommendations are based were undertaken in the developed world and may not be applicable to the majority of the world's population who live in poorer regions. The purpose of this paper is to highlight the paucity of epidemiological or management data on bacterial sepsis in the developing world, discuss current management approaches to sepsis in adults, and examine how clinical sepsis management guidelines could be best adapted to provide improved care at low cost in under-resourced regions. We use the term “developing country” to refer to lower- or middle-income countries as defined by the World Bank [20].
Summary Points.
The burden of sepsis is understudied but likely to be high in developing countries.
Most previous studies have been disease-specific rather than syndrome-focused.
The recently updated “Surviving Sepsis” guidelines have defined the standard of care for patients with severe sepsis in the developed world but do not incorporate the realities of health care in resource-constrained settings.
A focus on early management of severe sepsis, including fluid management, blood pressure control, timely administration of antibiotics, and source control, is likely to be the most cost-effective intervention for critically ill, septic patients in resource-constrained settings. The efficacy of particular strategies of care needs to be evaluated in clinical studies.
An integrated programme of management for adults, which includes training for health care workers on the prevention, recognition, and management of severe sepsis, is required.
Identification of Sepsis
Interventions performed soon after diagnosis of sepsis in developed regions have been shown to improve survival [21,22], and in developing countries, interventions to identify and treat pneumonia in children reduce mortality [23]. Thus, prompt identification of sepsis in developing countries is an essential component of any management strategy. Most studies of infection have focused on specific diseases, but sepsis itself is a clinically recognisable syndrome despite its heterogeneous causes. From a practical standpoint, sepsis is largely a clinical diagnosis [24], and implementation of strategies to promote recognition of sepsis as a clinical syndrome should be feasible even in the most resource-challenged areas where supportive radiographic imaging or laboratory measurements are not available. Education of health care providers about sepsis is critical to enhance the early identification of sick patients and may help facilitate transfer to available health care facilities. Simple algorithms tailored to local medical capacities that comprise the basic components of sepsis, such as diagnosed or suspected infection and the systemic manifestations of infection, may be useful.
Initial Treatment of Sepsis
Volume resuscitation is a well-established initial therapy of sepsis. Many studies have demonstrated that tissue perfusion in sepsis is partly impaired by hypovolaemia [25,26], and patients may have substantial fluid deficits requiring 6–10 l within the first 24 hours [21,27]. Guidelines suggest that hypotensive patients should receive an initial challenge of 20 ml/kg or boluses of 500–1000 ml of crystalloid with ongoing monitoring of volume status [16,28,29]. Observational evidence suggests that aggressive fluid resuscitation was associated with decreased early mortality from typhoid with ileal perforation in a rural African hospital [30].
Although albumin is at least as effective as crystalloids for volume resuscitation in sepsis, the latter is cheaper and more widely available [31]. Judicious enteral fluid loading with standard oral rehydration solution has been used in other forms of hypovolaemia, but is as yet untested as a strategy in sepsis [32,33]. In severely compromised patients, oral fluids may be associated with a risk of pulmonary aspiration and are less likely to be effective with severe intravascular hypovolaemia. Invasively measured endpoints of fluid resuscitation have been defined [28], but clinical endpoints such as blood pressure and heart rate measurements, skin colour and capillary refill, mental status, or urinary output are the most feasible measures for monitoring in underdeveloped regions. Central venous access, where available, may be helpful for monitoring of central venous pressure and administration of vasopressors. Simpler non-invasive devices such as tissue perfusion monitors may be more practical but are not yet used widely [33].
Because septic shock is often characterised by inappropriate peripheral vasodilation, initiation of vasopressor therapies is indicated for persistent hypotension following fluid loading [16,34]. Although norepinephrine and (suprarenal dose) dopamine are accepted first-line agents [16,28], both may be associated with extravasation-related tissue necrosis if infused into a peripheral vein when central venous access is not possible. Accurate titration of intravenous vasopressors is also problematic in the absence of infusion pumps. Finally, non-invasive monitoring of blood pressure may be less accurate than intra-arterial blood pressure monitoring [16]. We have identified very few studies of non-intravenous vasopressor agents, such as oral midodrine [35], yet conceivably such therapies may play a role in sepsis management when intravenous medications are not feasible.
Haemodynamic optimisation measures in the resuscitation of ill septic patients often require extensive nursing and medical resources. An invasive goal-directed haemodynamic optimisation therapy approach to early sepsis management, while effective, has proven challenging to implement even in large hospitals in the United States, and is not practical to consider in this form for health care facilities in most developing settings [21,36]. Nonetheless, in regions where basic sepsis therapies exist yet early management is suboptimal, enhanced care could be achieved with simple, clearly defined fluid resuscitation and blood pressure management protocols, incorporating clinical endpoints and non-invasive tools if available. In areas where paramedical staff are available, simply engaging an assistant to observe patient status may be a cost-effective substitute for electronic monitoring. In better staffed facilities, designation of a dedicated nurse for at least the initial resuscitation period may be beneficial. However, more studies are required to determine optimal fluid resuscitation strategies where invasive haemodynamic monitoring and fall-back therapies (such as mechanical ventilation and dialysis) may not be available.
Antibiotic Regimens
The timely and appropriate use of antibiotics in the early management period is associated with survival from sepsis and pneumonia [22,37]. A potential barrier to the formulation of an effective empiric antimicrobial regimen is that the spectrum of bacterial pathogens in the tropics is often diverse. In one study in Kenyan children, 16 individual pathogens, each of which accounted for less than 10% of cases, accounted for over a third of bacteraemias [38]. Similarly, in a study of adults in Nepal, no single pathogen accounted for more than 13% of patients where a pathogen was identified [39].
Many regions do not have access to diagnostic microbiology laboratories, so the causes of sepsis and their susceptibility profiles may not be known. The list of common bacterial pathogens may also show significant variation, even between neighbouring countries. An example of this is between the adjacent countries of Thailand and Laos. In northeast Thailand, common causes of community-acquired sepsis include Staphylococcus aureus, pneumococci and other streptococci, Escherichia coli and other Enterobacteriaceae, Pseudomonas spp., and B. pseudomallei [40], as well as leptospirosis, scrub typhus, and dengue [41]. In adjacent Vientiane, Laos, the commonest cause of community-acquired bacteraemia in one study was Salmonella enterica serovar typhi (50.9%), followed by S. aureus and E. coli [42]. Causes of hospital-acquired sepsis are also poorly characterised in resource-constrained settings where culture facilities are limited or absent, but lack of infection control infrastructure is likely to be associated with a significant burden of nosocomial sepsis. Because improved outcomes in sepsis management depend on early and appropriate antibiotic administration, it is critical that these issues be addressed in future epidemiological studies.
The role of the clinical microbiology laboratory in developing countries has been discussed extensively elsewhere, and issues relating to problems with clinical misdiagnosis, poor use of existing resources, and quality assurance are well documented [43,44]. Possible solutions include the development of low-cost laboratories, or the intermittent use of mobile diagnostic “clinics” that can define the range of pathogens and their susceptibility patterns in a given area to inform a rational empiric prescribing policy. These data should be widely disseminated to clinicians within the region. Evidence for the impact on mortality of accurate diagnosis can be seen from mortality rates from melioidosis in northeast Thailand over time. The burden of melioidosis became apparent after diagnostic laboratories were introduced into this area in the early 1970s [45]. This led to the first clinical treatment trial that reported a reduction in mortality from 74% to 37% for patients with acute melioidosis treated with ceftazidime compared with the previous combination antibiotic therapy [46]. Similarly, recent clinical trials have been prompted by the recognition of emerging resistance in Salmonella typhi [47].
There are a number of concerns with respect to antibiotic therapy in developing regions. In many developing countries, antibiotics are not regulated and are freely available, particularly in urban areas. It is becoming widely appreciated that a significant proportion of patients in rural Asia will have taken antibiotics prior to presentation to hospital [48,49]. This has major implications both for antibiotic resistance and the sensitivity of diagnostic culture when the patient presents to hospital. The quality of antibiotics is a further consideration. Fake drugs are widespread in the tropics [50] and have been implicated in deaths from malaria. Furthermore, many antibiotics are stored at room temperature, but ambient temperatures may reach 40° C in the tropics.
Five Key Papers in the Field.
Dellinger et al., 2008 [16] These international guidelines detail the current standard of care for patients with severe sepsis in developed countries.
Cheng et al., 2007 [15] This trial demonstrated poor outcomes from septic shock due to suspected melioidosis despite hospital care in Thailand, with in-hospital mortality exceeding 80%. Granulocyte colony stimulating factor, as an adjuvant to antibiotics, was not associated with a mortality benefit.
Phu et al., 2002 [63] This trial in Vietnam demonstrated that haemodialysis was more effective than peritoneal dialysis in patients with infection-related acute renal failure predominantly due to malaria. Despite the increased cost, the mortality benefit was large, suggesting that haemodialysis was more cost-effective.
Rivers et al., 2001 [21] This single-centre randomised controlled trial demonstrated a 16% absolute decrease in in-hospital mortality associated with early goal-directed management compared with standard management.
Gove S, 1997 [73] This paper describes the development and evaluation of the integrated management of childhood illness guidelines and training course for health workers in developing countries.
Supportive and Adjunctive Therapeutic Agents
Prophylaxis for deep venous thrombosis and for peptic “stress” ulcers can be readily implemented in many developing countries. Histamine blockers such as ranitidine are inexpensive and can be administered via nasogastric tube to intubated patients. Although some have argued that the incidence of venous thromboembolism is lower in populations of non-European origin [51–53], more recent evidence suggests that this may not be the case in post-operative and medical patients [54,55]. In the absence of studies in critically unwell patients with prolonged immobilisation, the use of subcutaneous unfractionated heparin seems warranted.
Other interventions often used in the developed setting for patients with severe sepsis are renal replacement therapy and mechanical ventilation. Respiratory failure is a frequent complication of severe sepsis, and progression to acute lung injury is most commonly caused by sepsis [56,57]. In a trial of granulocyte colony stimulating factor in severe sepsis due to melioidosis in Thailand [15], 70% of patients required intubation and ventilation. Where available, mechanical ventilation of septic patients in developing countries is associated with extremely high mortality rates [6], and in some countries, patients with respiratory failure are ventilated by hand by relatives (A. C. Cheng, personal experience). Many ventilators in developing countries are simple devices that do not have minute ventilation alarms or allow for adjunct ventilator functions such as positive end-expiratory pressure. Sub-optimal ventilator care may lead to ventilator-associated pneumonia [58,59], and the lack of low-volume “lung-protective” strategies may be associated with poor outcomes from acute lung injury [60]. The reliance on non-invasive monitoring using peripheral oxygen saturation may reduce the ability to monitor the adequacy of ventilation. Although non-invasive positive-pressure ventilation is mainly used for respiratory failure in non-septic patients, it has been used successfully in developing countries [61,62], and strategies incorporating non-invasive positive-pressure ventilation should be evaluated further where invasive mechanical ventilation cannot be managed adequately.
The cost of equipment and trained staff largely prohibits the use of renal replacement therapies in low-income settings, although they are used to a variable extent in rural regions of middle-income countries. In Vietnam, haemofiltration was more cost-effective than peritoneal dialysis in infection-related renal failure despite its significant cost [63]. This study primarily included patients with malaria, but such a study would be of significant interest in patients with sepsis in resource-constrained settings where haemofiltration is possible. In a study of severe sepsis in melioidosis, acute renal failure and acidosis were prominent on admission, suggesting that aggressive fluid resuscitation and/or renal replacement therapy would be potentially beneficial in this group [15]. There has been increasing awareness of the importance of acidosis and fluid and electrolyte imbalances in severe malaria, and despite differences in pathophysiology, such research may be relevant to severe bacterial sepsis [64,65].
There have been few trials of adjuvant therapies in developing countries, and results of studies may not be generalisable to this setting. For example, granulocyte colony stimulating factor appeared to be associated with significant benefit in treating melioidosis in Australia [13], but was not associated with a significant mortality benefit in Thailand in a clinical trial [15]. Conversely, high-dose steroids are not thought to be useful in severe sepsis generally, but may possibly have a specific application in severe typhoid [66–68]. Interventions that are of marginal benefit in developed countries, such as physiological dose steroid replacement [19,69] or intensive insulin therapy, are unlikely to be effective in developing countries in the absence of other standard intensive care interventions. The risk of adverse events, such as hypoglycaemia, associated with intensive insulin regimens are likely be greater in resource-poor settings, and the setting of less strict blood glucose targets may be warranted [16,18,70]. However, the safety and efficacy of such strategies require study. The effectiveness of activated protein C (drotrecogin alfa) has also been subject to intensive debate, but the cost of this therapy is prohibitive for most health care systems in developing countries [71,72].
Training
Implicit in this discussion is the need for appropriately trained health care providers at the local level. Ideally, sepsis identification and management training should be integrated into general adult health care. Such a strategy is analogous to the World Health Organization's Integrated Management of Childhood Illness training course, which included severe infection, chronic diseases, and preventative measures for all levels of health workers including doctors, nurses, medical assistants, and literate paramedical workers at both a primary, and more recently, hospital level [73,74]. This course was integrated into a comprehensive strategy that also included measures to improve drug supply, health care infrastructure, and family behaviour in relation to sick children.
Cost-Effectiveness
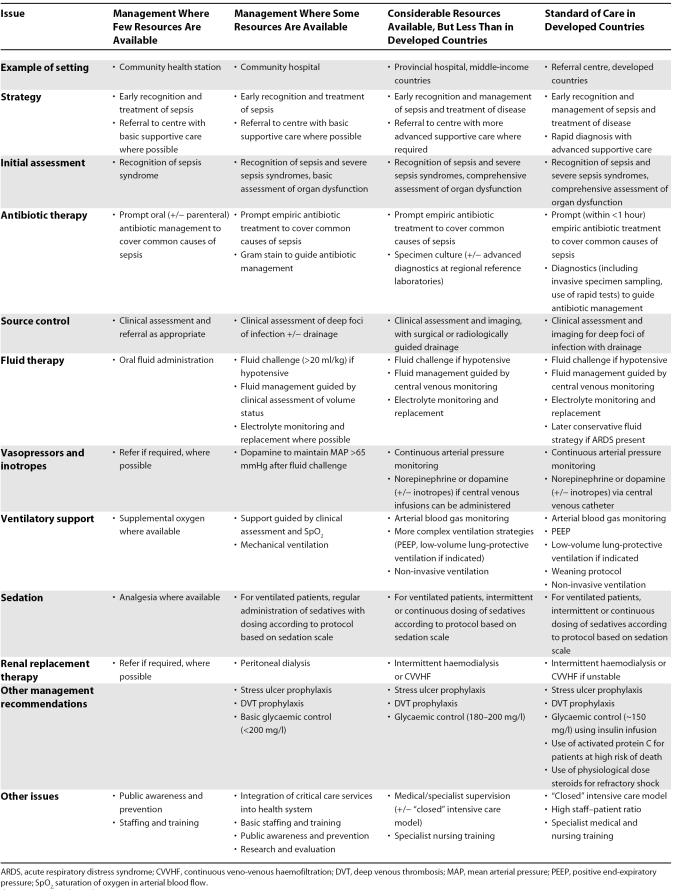
The development of critical care services has significant resource implications for developing countries. We propose a stepwise approach based on income level, from extremely limited services in Africa and parts of southeast Asia, to more extended services in lower-middle-income countries such as Thailand and some South American countries (Table 1). Some evidence suggests that critical care services may be cost-effective even in poor countries, but such a decision needs to be made on a case-by-case basis [75]. We feel that with the current paucity of evidence regarding the effectiveness of potential interventions for severe sepsis, such decisions cannot currently be made. We further note that factors other than cost-effectiveness must be considered in priority setting in health care resource allocation, including equity, ethical, and political considerations [76]. However, where such services already exist, the challenge is to integrate these into the broader health care system to ensure access and to provide a cost-effective and sustainable staffing model [77]. Further research is required to define the most effective interventions for sepsis in developing countries, as well as evaluation and quality control programmes for existing services.
Table 1. Possible Interventions for the Management of Sepsis in Resource-Constrained Settings.
Prevention
Few vaccines are available against most of the common causes of severe sepsis in the tropics, and many vaccines with known substantial efficacy against common diseases such as typhoid and pneumococcal disease are not generally available to developing countries because of cost. Other preventative measures may be useful for specific diseases. In a case-control study in northeast Thailand, the use of protective clothing reduced the incidence of leptospirosis [78], and protective footwear may also help prevent melioidosis, scrub typhus, snake bite, and physical injury. Although anecdotal reports suggested that farmers found protective footwear uncomfortable, particularly during the ploughing and planting seasons (V. Wuthiekanun, personal communication), it is possible that this obstacle could be overcome through a combination of education and the development of comfortable and practical clothing. Predictive modelling has proven to be a useful tool in malaria control [79], and similar techniques have been developed for a variety of other diseases such as cholera [80] and arboviruses [81]. Such tools might allow for targeting of public health interventions that may reduce exposure or disease transmission in specific populations.
Conclusion
The burden of sepsis is greatest in developing countries, and there is a need to translate modern management strategies for adults with severe sepsis to this context. The majority of studies of infectious diseases to date have been pathogen-specific, but efforts are required to define the epidemiology of all-cause sepsis in developing countries and to define the most cost-effective interventions that are sustainable in these countries. Principles of management may be adapted from current guidelines, particularly low-cost interventions targeted at early sepsis. Critical care services need to be considered in the context of competing priorities for resource allocation, but where they currently exist, standardised protocols need to be developed and evaluated to make the best use of available resources.
Access to diagnostic facilities is fundamental to the care of the individual and to the development of logical and effective empiric prescribing regimens. Low-income countries rarely have access to diagnostic laboratories, and this is a major impediment to the improvement of care. Primary prevention is theoretically possible for a range of serious tropical infections, and studies are required to define acceptable measures and to validate their effectiveness.
Acknowledgments
We thank staff at the Sappasithiprasong Hospital and at the Mahidol-Oxford Tropical Medicine Research Unit for their support and advice.
Footnotes
Allen C. Cheng is with the Department of Medicine, University of Melbourne, and the Menzies School of Health Research, Charles Darwin University, Darwin, Northern Territory, Australia. T. Eoin West is with the Department of Medicine, Harborview Medical Center, University of Washington, Seattle, Washington, United States of America. Direk Limmathurotsakul and Sharon J. Peacock are with the Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Sharon J. Peacock is also with the Centre for Clinical Vaccinology and Tropical Medicine, Nuffield Department of Clinical Medicine, University of Oxford, Churchill Hospital, Oxford, United Kingdom.
Funding: ACC is supported by a Health Professional Research Fellowship from the Australian National Health and Medical Research Council. TEW is funded by National Institutes of Health grant U54AI057141. SJP is supported by the Wellcome Trust. The funding bodies played no role in the decision to submit the article or in its preparation.
Competing Interests: The authors have declared that no competing interests exist.
References
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [No authors listed] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Revised global burden of disease 2002 estimates. 2008. Available: http://www.who.int/healthinfo/bodgbd2002revised/en/index.html. Accessed 18 July 2008.
- Siddiqui S. Not “surviving sepsis” in the developing countries. J Indian Med Assoc. 2007;105:221. [PubMed] [Google Scholar]
- Tanriover MD, Guven GS, Sen D, Unal S, Uzun O. Epidemiology and outcome of sepsis in a tertiary-care hospital in a developing country. Epidemiol Infect. 2006;134:315–322. doi: 10.1017/S0950268805004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frikha N, Mebazaa M, Mnif L, El Euch N, Abassi M, et al. [Septic shock in a Tunisian intensive care unit: Mortality and predictive factors. 100 cases] Tunis Med. 2005;83:320–325. [PubMed] [Google Scholar]
- Smith C, Arregui LM, Promnitz DA, Feldman C. Septic shock in the Intensive Care Unit, Hillbrow Hospital, Johannesburg. S Afr Med J. 1991;80:181–184. [PubMed] [Google Scholar]
- Degoricija V, Sharma M, Legac A, Gradiser M, Sefer S, et al. Survival analysis of 314 episodes of sepsis in medical intensive care unit in university hospital: Impact of intensive care unit performance and antimicrobial therapy. Croat Med J. 2006;47:385–397. [PMC free article] [PubMed] [Google Scholar]
- Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, et al. Brazilian Sepsis Epidemiological Study (BASES study) Crit Care. 2004;8:R251–R260. doi: 10.1186/cc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- Currie BJ, Fisher DA, Howard DM, Burrow JN, Lo D, et al. Endemic melioidosis in tropical northern Australia: A 10-year prospective study and review of the literature. Clin Infect Dis. 2000;31:981–986. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Stephens DP, Anstey NM, Currie BJ. Adjunctive granulocyte colony-stimulating factor for treatment of septic shock due to melioidosis. Clin Infect Dis. 2004;38:32–37. doi: 10.1086/380456. [DOI] [PubMed] [Google Scholar]
- Chan KP, Low JG, Raghuram J, Fook-Chong SM, Kurup A. Clinical characteristics and outcome of severe melioidosis requiring intensive care. Chest. 2005;128:3674–3678. doi: 10.1378/chest.128.5.3674. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Limmathurotsakul D, Chierakul W, Getchalarat N, Wuthiekanun V, et al. A randomized controlled trial of granulocyte colony-stimulating factor for the treatment of severe sepsis due to melioidosis in Thailand. Clin Infect Dis. 2007;45:308–314. doi: 10.1086/519261. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- Eichacker PQ, Natanson C, Danner RL. Surviving sepsis—Practice guidelines, marketing campaigns, and Eli Lilly. N Engl J Med. 2006;355:1640–1642. doi: 10.1056/NEJMp068197. [DOI] [PubMed] [Google Scholar]
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- World Bank. Country classification. 2008. Available: go.worldbank.org/K2CKM78CC0. Accessed 18 July 2008.
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: A meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- Carroll GC, Snyder JV. Hyperdynamic severe intravascular sepsis depends on fluid administration in cynomolgus monkey. Am J Physiol. 1982;243:R131–R141. doi: 10.1152/ajpregu.1982.243.1.R131. [DOI] [PubMed] [Google Scholar]
- Winslow EJ, Loeb HS, Rahimtoola SH, Kamath S, Gunnar RM. Hemodynamic studies and results of therapy in 50 patients with bacteremic shock. Am J Med. 1973;54:421–432. doi: 10.1016/0002-9343(73)90038-7. [DOI] [PubMed] [Google Scholar]
- Rackow EC, Falk JL, Fein IA, Siegel JS, Packman MI, et al. Fluid resuscitation in circulatory shock: A comparison of the cardiorespiratory effects of albumin, hetastarch, and saline solutions in patients with hypovolemic and septic shock. Crit Care Med. 1983;11:839–850. [PubMed] [Google Scholar]
- Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32:1928–1948. doi: 10.1097/01.ccm.0000139761.05492.d6. [DOI] [PubMed] [Google Scholar]
- Hurtado FJ, Nin N. The role of bundles in sepsis care. Crit Care Clin. 2006;22:521–529. doi: 10.1016/j.ccc.2006.03.005. x. [DOI] [PubMed] [Google Scholar]
- Mock C, Visser L, Denno D, Maier R. Aggressive fluid resuscitation and broad spectrum antibiotics decrease mortality from typhoid ileal perforation. Trop Doct. 1995;25:115–117. doi: 10.1177/004947559502500309. [DOI] [PubMed] [Google Scholar]
- Finfer S, Bellomo R, Boyce N, French J, Myburgh J, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- Michell MW, Oliveira HM, Kinsky MP, Vaid SU, Herndon DN, et al. Enteral resuscitation of burn shock using World Health Organization oral rehydration solution: A potential solution for mass casualty care. J Burn Care Res. 2006;27:819–825. doi: 10.1097/01.BCR.0000245422.33787.18. [DOI] [PubMed] [Google Scholar]
- Nager AL, Wang VJ. Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration. Pediatrics. 2002;109:566–572. doi: 10.1542/peds.109.4.566. [DOI] [PubMed] [Google Scholar]
- Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Weippl G. [Infectious toxic hypotension—Effect and dosage of midodrine (author's transl)] Padiatr Padol. 1979;14:211–216. [PubMed] [Google Scholar]
- Carlbom DJ, Rubenfeld GD. Barriers to implementing protocol-based sepsis resuscitation in the emergency department—Results of a national survey. Crit Care Med. 2007;35:2525–2532. doi: 10.1097/01.ccm.0000298122.49245.d7. [DOI] [PubMed] [Google Scholar]
- Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529–535. doi: 10.1016/j.amjmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, et al. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–675. [PubMed] [Google Scholar]
- Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, et al. Melioidosis: A major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, et al. Causes of acute, undifferentiated, febrile illness in rural Thailand: Results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–370. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- Phetsouvanh R, Phongmany S, Soukaloun D, Rasachak B, Soukhaseum V, et al. Causes of community-acquired bacteremia and patterns of antimicrobial resistance in Vientiane, Laos. Am J Trop Med Hyg. 2006;75:978–985. [PMC free article] [PubMed] [Google Scholar]
- Archibald LK, Reller LB. Clinical microbiology in developing countries. Emerg Infect Dis. 2001;7:302–305. doi: 10.3201/eid0702.010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: A barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- Punyagupta S. Melioidosis. Review of 686 cases and presentation of a new clinical classification. In: Punyagupta S, Sirisanthana T, Stapatayavong B, . Melioidosis. Bangkok: Bangkok Medical Publisher; 1989. pp. 217–229. editors. [Google Scholar]
- White NJ, Dance DA, Chaowagul W, Wattanagoon Y, Wuthiekanun V, et al. Halving of mortality of severe melioidosis by ceftazidime. Lancet. 1989;2:697–701. doi: 10.1016/s0140-6736(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Parry CM, Ho VA, Phuong le T, Bay PV, Lanh MN, et al. Randomized controlled comparison of ofloxacin, azithromycin, and an ofloxacin-azithromycin combination for treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever. Antimicrob Agents Chemother. 2007;51:819–825. doi: 10.1128/AAC.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamolratanakul P, Dhanamun B, Thaithong S. Human behavior in relation to selection of malaria treatment. Southeast Asian J Trop Med Public Health. 1992;23:189–194. [PubMed] [Google Scholar]
- Thamlikitkul V. Antibiotic dispensing by drug store personnel in Bangkok, Thailand. J Antimicrob Chemother. 1988;21:125–131. doi: 10.1093/jac/21.1.125. [DOI] [PubMed] [Google Scholar]
- Wertheimer AI, Chaney NM, Santella T. Counterfeit pharmaceuticals: Current status and future projections. J Am Pharm Assoc. 2003;43:710–717. 717–718. doi: 10.1331/154434503322642642. quiz. [DOI] [PubMed] [Google Scholar]
- De Stefano V, Chiusolo P, Paciaroni K, Leone G. Epidemiology of factor V Leiden: Clinical implications. Semin Thromb Hemost. 1998;24:367–379. doi: 10.1055/s-2007-996025. [DOI] [PubMed] [Google Scholar]
- Nandi P, Wong KP, Wei WI, Ngan H, Ong GB. Incidence of postoperative deep vein thrombosis in Hong Kong Chinese. Br J Surg. 1980;67:251–253. doi: 10.1002/bjs.1800670407. [DOI] [PubMed] [Google Scholar]
- Lee HM, Suk KS, Moon SH, Kim DJ, Wang JM, et al. Deep vein thrombosis after major spinal surgery: Incidence in an East Asian population. Spine. 2000;25:1827–1830. doi: 10.1097/00007632-200007150-00014. [DOI] [PubMed] [Google Scholar]
- Chotanaphuti T, Ongnamthip P, Silpipat S, Foojareonyos T, Roschan S, et al. The prevalence of thrombophilia and venous thromboembolism in total knee arthroplasty. J Med Assoc Thai. 2007;90:1342–1347. [PubMed] [Google Scholar]
- Mannucci PM. Thrombosis and bleeding disorders outside Western countries. J Thromb Haemost. 2007;5(Suppl 1):68–72. doi: 10.1111/j.1538-7836.2007.02463.x. [DOI] [PubMed] [Google Scholar]
- Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144:124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- Leong JR, Huang DT. Ventilator-associated pneumonia. Surg Clin North Am. 2006;86:1409–1429. doi: 10.1016/j.suc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Jaimes F, De La Rosa G, Gomez E, Munera P, Ramirez J, et al. Incidence and risk factors for ventilator-associated pneumonia in a developing country: Where is the difference. Respir Med. 2007;101:762–767. doi: 10.1016/j.rmed.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [No authors listed] [DOI] [PubMed] [Google Scholar]
- Hussain SF, Haqqee R, Iqbal J. Non-invasive ventilation in the management of acute respiratory failure in Pakistan. Trop Doct. 2004;34:238–239. doi: 10.1177/004947550403400421. [DOI] [PubMed] [Google Scholar]
- George IA, John G, John P, Peter JV, Christopher S. An evaluation of the role of noninvasive positive pressure ventilation in the management of acute respiratory failure in a developing country. Indian J Med Sci. 2007;61:495–504. [PubMed] [Google Scholar]
- Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895–902. doi: 10.1056/NEJMoa020074. [DOI] [PubMed] [Google Scholar]
- Maitland K, Akech S, Gwer S, Idro R, Fegan G, et al. Phase III trials required to resolve clinical equipoise over optimal fluid management in children with severe malaria. PLoS Clin Trials. 2007;2:e2. doi: 10.1371/journal.pctr.0020002. doi: 10.1371/journal.pctr.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idro R, Aketch S, Gwer S, Newton CR, Maitland K. Research priorities in the management of severe Plasmodium falciparum malaria in children. Ann Trop Med Parasitol. 2006;100:95–108. doi: 10.1179/136485906X91459. [DOI] [PubMed] [Google Scholar]
- Punjabi NH, Hoffman SL, Edman DC, Sukri N, Laughlin LW, et al. Treatment of severe typhoid fever in children with high dose dexamethasone. Pediatr Infect Dis J. 1988;7:598–600. doi: 10.1097/00006454-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Rogerson SJ, Spooner VJ, Smith TA, Richens J. Hydrocortisone in chloramphenicol-treated severe typhoid fever in Papua New Guinea. Trans R Soc Trop Med Hyg. 1991;85:113–116. doi: 10.1016/0035-9203(91)90180-7. [DOI] [PubMed] [Google Scholar]
- Hoffman SL, Punjabi NH, Kumala S, Moechtar MA, Pulungsih SP, et al. Reduction of mortality in chloramphenicol-treated severe typhoid fever by high-dose dexamethasone. N Engl J Med. 1984;310:82–88. doi: 10.1056/NEJM198401123100203. [DOI] [PubMed] [Google Scholar]
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- Preiser J. Intensive glycemic control in med-surg patients (European Glucontrol trial) 2007. Society of Critical Care Medicine 36th Critical Care Congress; 17–21 February 2007; Orlando, Florida, United States.
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332–1341. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- Gove S. Integrated management of childhood illness by outpatient health workers: Technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull World Health Organ. 1997;75(Suppl 1):7–24. [PMC free article] [PubMed] [Google Scholar]
- Duke T, Kelly J, Weber M, English M, Campbell H. Hospital care for children in developing countries: Clinical guidelines and the need for evidence. J Trop Peds. 2006;52:1–2. doi: 10.1093/tropej/fmk006. [DOI] [PubMed] [Google Scholar]
- Topley JM, Nkrumah FK. Paediatric intensive care in Harare. Ann Trop Paediatr. 1987;7:282–286. doi: 10.1080/02724936.1987.11748526. [DOI] [PubMed] [Google Scholar]
- Musgrove P. Public spending on health care: How are different criteria related. Health Policy. 1999;47:207–223. doi: 10.1016/s0168-8510(99)00024-x. [DOI] [PubMed] [Google Scholar]
- Bhagwanjee S. Critical care in Africa. Crit Care Clin. 2006;22:433–438. doi: 10.1016/j.ccc.2006.03.008. viii. [DOI] [PubMed] [Google Scholar]
- Phraisuwan P, Whitney EA, Tharmaphornpilas P, Guharat S, Thongkamsamut S, et al. Leptospirosis: Skin wounds and control strategies, Thailand, 1999. Emerg Infect Dis. 2002;8:1455–1459. doi: 10.3201/eid0812.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Gouws E, Omumbo J, Rapuoda B, Craig MH, et al. Models to predict the intensity of Plasmodium falciparum transmission: Applications to the burden of disease in Kenya. Trans R Soc Trop Med Hyg. 1998;92:601–606. doi: 10.1016/s0035-9203(98)90781-7. [DOI] [PubMed] [Google Scholar]
- Matsuda F, Ishimura S, Wagatsuma Y, Higashi T, Hayashi T, et al. Prediction of epidemic cholera due to Vibrio cholerae O1 in children younger than 10 years using climate data in Bangladesh. Epidemiol Infect. 2008;136:73–79. doi: 10.1017/S0950268807008175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Wagatsuma Y, Emch M, Breiman RF. Use of a geographic information system for defining spatial risk for dengue transmission in Bangladesh: Role for Aedes albopictus in an urban outbreak. Am J Trop Med Hyg. 2003;69:634–640. [PubMed] [Google Scholar]