Abstract
Background
Anti-epiligrin cicatricial pemphigoid (AECP) is a mucosal predominant subepidermal blistering disease associated with an increased relative risk of cancer. In contrast to prior reports showing that anti-L-332 autoantibodies are a reliable marker for patients with AECP, a recent report suggested that as many as 40% of patients with bullous pemphigoid (BP) have IgG reactive with this laminin isoform.
Objective
To determine if patients with BP possess circulating IgG anti-L-332 autoantibodies.
Methods
Sera from 100 adults with BP were analyzed by indirect IF testing of intact skin, immunoblot studies of human keratinocyte (HK) extracts, and a new L-332 ELISA. Sera showing reactivity suggestive of anti-L-332 autoantibodies in these assays were further analyzed in immunoblot studies of HK extracellular matrix (ECM) and immunoprecipitation studies of biosynthetically radiolabeled HK extracts.
Results
IgG from all BP patients bound intact epidermal basement membrane by indirect IF microscopy and immunoblotted BPAG1 and/or BPAG2 in HK extracts. None of these sera immunoblotted L-332 in HK extracts, though 13 did score above the cut-point of a new IgG4 L-332 ELISA (sensitivity=0.91, specificity=0.98, Youden index=0.89). Further analysis of sera from these 13 patients found: 1) all had IgG that bound the epidermal side of 1 M NaCl split skin by indirect IF microscopy; 2) none immunoblotted L-332 purified from HK ECM; and 3) none immunoprecipitated L-332 from biosynthetically radiolabeled HK extracts.
Limitations
The basis of false-positive ELISA determinations for anti-L-332 IgG among patients with BP is unknown.
Conclusion
Anti-L-332 autoantibodies remain a reliable marker for patients with AECP.
Keywords: Laminin-332, autoimmunity, immunobullous disease, immunopathology
Introduction
Anti-epiligrin cicatricial pemphigoid (AECP) is an autoimmune subepithelial blistering disease characterized by IgG anti-basement membrane (BM) autoantibodies directed against laminin-332 (L-332, previously termed laminin 5, epiligrin, kalinin, nicein, and BM600 [designations indicating that separate groups independently identified and characterized this protein almost simultaneously]) 1-6. The demonstration that this form of mucous membrane pemphigoid is associated with an increased relative risk (RR) for cancer has enhanced the need to identify patients with AECP 7, 8. It has also prompted the need to develop sensitive and specific screening assays that can, in contrast to traditional immunoprecipitation and immunoblot studies, be used widely and quickly to detect IgG anti-L-332 autoantibodies in patients with low grade mucosal diseases (e.g., desquamative gingivitis, periodontal disease, or chronic conjunctivitis) that may represent subclinical or early forms of AECP. Interestingly, a recently developed ELISA that can be used for such purposes found that as many as 40% of patients with bullous pemphigoid (BP) may have IgG reactive with this laminin isoform 9. This finding differs notably from prior studies suggesting that anti-L-332 autoantibodies are a reliable marker for patients with AECP. To explore this issue further, the sera of 100 adults with BP were rigorously analyzed using a series of immunoassays shown to display great sensitivity for detection of anti-L-332 autoantibodies as well as a new sensitive and specific ELISA capable of detecting IgG reactive with L-332 in the purified extracellular matrix (ECM) of cultured human keratinocytes (HKs).
Methods
Reagents
Affinity purified fluorescein isothiocyanate-conjugated goat F(ab′)2 anti-human IgG (Biosource International, Camarillo, CA), horse radish peroxidase-conjugated goat F(ab′)2 anti-mouse IgG (Biosource), alkaline phosphatase-conjugated goat F(ab′)2 anti-human IgG (Biosource), alkaline phosphatase-conjugated goat F(ab′)2 anti-rabbit IgG (Biosource), and ascites fluids containing mouse monoclonal anti-human IgG1 (clone HP6001), anti-human IgG2 (clone HP6014), anti-human IgG3 (clone HP6050), anti-human IgG4 (clone HP6025) (all from Sigma, St Louis, MO) were used in this study.
Indirect IF microscopy studies
Indirect IF microscopy of intact and 1M NaCl split skin was performed as described previously 10.
Immunoblot studies
L-332 was isolated from the ECM of cultured HKs and studied by immunoblotting with sera from patients and controls as described previously 11. Alkaline phosphatase-conjugated goat F(ab′)2 anti-human IgG (1:1000) was used as the second-step antibody in these studies. Immunoblots were developed for 3 min with AP-conjugate substrate kit (Bio-Rad Laboratories).
Immunoprecipitation studies
Subconfluent monolayers of HKs were biosynthetically radiolabeled with 35S-methionine (50 uCi/mL; Amersham Biosciences Corp., Arlington, IL) for 2 hours to yield cell extracts that were processed and studied by immunoprecipitation using sera from patients and controls as described previously 10.
Patients
Serum samples were obtained from 32 patients who met the following criteria for the diagnosis of AECP: 1) the presence of subepidermal blistering and/or erosive lesions on mucosal surfaces; 2) continuous deposits of IgG (± C3) in epidermal BM; 3) circulating IgG anti-BM autoantibodies that bound the dermal side of 1M NaCl split skin; and 4) circulating IgG that immunoprecipitated L-332 from extracts of biosynthetically radiolabeled HKs. Details regarding some of these AECP patients have been published previously 7; five of these patients had an underlying solid cancer (lung [n=1], gastric [n=3], colon [n=1]). Control samples used to standardize the IgG4 L-332 ELISA used in this study included sera from healthy donors (n= 87) as well as patients with other immunobullous diseases (specifically, PV [n=31], PF [n=21], and BP [n=34]). Criteria for the diagnosis of pemphigus included the presence of intraepidermal acantholytic blisters as well as IgG that bound desmoglein (Dsg) 3 or Dsgs 3 and 1 (patients with PV) or Dsg 1 alone (patients with PF) (commercial ELISAs, Medical & Biological Laboratories, Nagoya, Japan). Criteria for the diagnosis of BP among patients whose sera was used to standardize the IgG4 anti-L332 ELISA included the presence of subepidermal blisters and circulating anti-BM IgG that bound the epidermal side of 1M NaCl split skin with a titer ≥ 10 as determined by indirect IF microscopy. To assess the incidence of IgG anti-L-332 autoantibodies in patients with BP, sera from an additional 100 patients with this disorder were studied by indirect IF microscopy (test substrate, intact and/or 1 M NaCl split human skin), immunoblot (antigen source, HK extracts), and ELISA (antigen source L-332 in HK ECM). These human studies were approved by the Authors' Institutional Review Board.
ELISA studies
Normal HKs were isolated from neonatal foreskins and grown in serum-free media (Invitrogen Life Technologies, Carlsbad, CA). Third passage HKs were plated in 96-well plates (Costar Corporation, Cambridge, MA) at a density 5×104 cells per well for 24 hours. Studies demonstrated that HKs were confluent at this time point and thus suitable for sequential extraction to yield plates coated with only L-332. Confluent HKs were sequentially extracted with 1% Triton X-100 in phosphate-buffered saline (PBS), 2 M urea in 1 M NaCl, and 8 M urea (10 minutes in each buffer, all buffers containing 1 mM phenylmethylsulfonyl fluoride and 2 mM N-ethylmaleimide) to yield L-332 coated plates 3. Preliminary ELISA studies defined optimal working conditions for the assays by testing various “checkerboard” dilutions of human sera (patient and normal), mouse monoclonal antibodies directed against IgG subclasses, and HRP-conjugated goat F(ab′)2 anti-mouse IgG. All serum samples were tested in duplicate; each plate contained the same positive (i.e., serum from an AECP patient) and negative (serum from a normal volunteer) standards. The optimized ELISA was run under the following conditions: experimental sera were diluted 1:50 in 0.05% Tween-20 in PBS and incubated with immobilized L-332 for 2 hours at 37° C; wells were washed five times with 0.05% Tween-20 in PBS; a 1:500 dilution of subclass-specific monoclonal antibody was added for another 2 hours at 37° C; wells were again washed five times with 0.05% Tween-20 in PBS; HRP-conjugated goat F(ab′)2 anti-mouse IgG (1:5000) was added to each well for 2 hours at 37° C; following a final series of washes, 100 ul of freshly dissolved substrate (0-phenylenediamine in 0.1% H2O2) was added to each well; ten minutes after the addition of substrate, the reaction was stopped with 100 ul of 4 M H2SO4 and OD490 was measured using a microplate reader (Bio-Rad, Hercules, CA). Preliminary studies demonstrated that anti-L-332 IgG in AECP patients' sera was predominantly of the IgG4 subclass. Accordingly, this particular assay was standardized for day-to-day, plate-to-plate, and measurement error as well as various statistical determinations listed below.
Statistical analysis
Proc Logistic was used to calculate sensitivity and specificity for ROC curves for detection of anti-L-332 IgG4 autoantibodies. Exact binominal confidence intervals for sensitivity and specificity were calculated using Proc Freq. Proc Means and Proc Varcomp with maximum likelihood estimation method were used to estimate the variability of ELISA measurements. All statistical analysis was carried out using SAS statistical software (SAS Institute, Cary, NC).
Results
Analysis of Sera from Patients with AECP
Summary data regarding the serologic reactivity of patients with AECP is shown in Table 1. Selected data regarding some of these patients has been published previously 7. All patients immunoprecipitated L-332 from extracts of biosynthetically radiolabeled HKs; 30 of 32 patients had circulating IgG that immunoblotted one or more subunits of L-332 isolated from HK ECM. More specifically, IgG from 26 of 30 patients immunoblotted the α subunit alone (n=23) or in combination with reactivity against the γ (n=2) or β (n=1) subunits of this laminin isoform; three patients had IgG that immunoblotted the β subunit; and one patient had IgG that immunoblotted the β and γ subunits of L-332. To profile the subclass distribution of circulating anti-L-332 autoantibodies under non limiting dilutions of IgG, a series of ELISA studies were performed. These studies, like a prior report concerning a smaller number of patients, found that virtually all patients with AECP possess notable levels of IgG4 that is reactive with L-332 12. Of three sera that showed little evidence of anti-L332 IgG4 by ELISA, all immunoprecipitated L-332 from biosynthetically radiolabeled extracts of HKs, two immunoblotted the unprocessed (200 kD) and processed (165 kD) α subunit of L-332 in HK ECM, two demonstrated low levels of anti-L-332 IgG2, and one demonstrated low levels of anti-L-332 IgG1. While preliminary ELISA studies found that some AECP sera contained IgG1, IgG2, or IgG3 reactive with L-332, the levels of these immunoglobulins were low, approaching or exceeding those of the IgG4 subclass only rarely. Table 1. There was no difference in anti-L-332 IgG level or subclass distribution among AECP patients with underlying solid cancers. Based on these comprehensive studies of 32 patients with AECP, the IgG4 subclass-specific L-332 ELISA was standardized for its performance with patient and control sera as well as its variability on a day-to-day and plate-to-plate basis.
Table 1. Summary Data Regarding Patients With AECP.
| Screening anti-L-332 ELISAs* | ||||||
|---|---|---|---|---|---|---|
| Patient | IP | IB | IgG1 | lgG2 | lgG3 | IgG4 |
| 1 | L-332 | α3 | 0.093 | 0.298 | 0.080 | 0.755 |
| 2** | L-332 | α3 | 0.195 | 0.208 | 0.097 | 0.510 |
| 3 | L-332 | α3 | 0.128 | 0.202 | 0.172 | 1.123 |
| 4** | L-332 | α3 | 0.078 | 0.109 | 0.073 | 0.307 |
| 5 | L-332 | α3 | 0.074 | 0.134 | 0.064 | 0.525 |
| 6 | L-332 | α3 | 0.088 | 0.268 | 0.078 | 0.688 |
| 7** | L-332 | α3 | 0.171 | 0.143 | 0.123 | 0.555 |
| 8 | L-332 | α3 | 0.076 | 0.217 | 0.063 | 0.083 |
| 9 | L-332 | α3, γ2 | 0.087 | 0.345 | 0.069 | 0.345 |
| 10 | L-332 | α3 | 0.170 | 0.334 | 0.087 | 0.640 |
| 11** | L-332 | α3 | 0.102 | 0.206 | 0.065 | 0.768 |
| 12 | L-332 | β3, γ2 | 0.099 | 0.174 | 0.078 | 0.597 |
| 13 | L-332 | α3 | 0.107 | 0.102 | 0.072 | 0.563 |
| 14 | L-332 | α3, γ2 | 0.089 | 0.156 | 0.074 | 0.381 |
| 15** | L-332 | α3, β3 | 0.207 | 0.177 | 0.079 | 0.584 |
| 16 | L-332 | neg | 0.235 | 0.151 | 0.077 | 0.186 |
| 17 | L-332 | β3 | 0.166 | 0.207 | 0.076 | 0.351 |
| 18 | L-332 | β3 | 0.115 | 0.299 | 0.081 | 0.631 |
| 19 | L-332 | α3 | 0.110 | 0.347 | 0.065 | 0.583 |
| 20 | L-332 | α3 | 0.125 | 0.110 | 0.071 | 0.746 |
| 21 | L-332 | α3 | 0.101 | 0.159 | 0.076 | 0.334 |
| 22 | L-332 | α3 | 0.095 | 0.202 | 0.077 | 0.476 |
| 23 | L-332 | α3 | 0.094 | 0.159 | 0.066 | 0.924 |
| 24 | L-332 | β3 | 0.089 | 0.183 | 0.065 | 0.415 |
| 25 | L-332 | α3 | 0.094 | 0.144 | 0.080 | 0.461 |
| 26 | L-332 | α3 | 0.094 | 0.172 | 0.080 | 0.387 |
| 27 | L-332 | α3 | 0.147 | 0.251 | 0.108 | 0.606 |
| 28 | L-332 | neg | 0.161 | 0.254 | 0.089 | 0.387 |
| 29 | L-332 | α3 | 0.158 | 0.222 | 0.137 | 0.758 |
| 30 | L-332 | α3 | 0.119 | 0.118 | 0.102 | 0.745 |
| 31 | L-332 | α3 | 0.099 | 0.190 | 0.094 | 0.786 |
| 32 | L-332 | α3 | 0.079 | 0.252 | 0.070 | 0.075 |
Reactivity of patient IgG subclasses versus L-332 as scored by OD490 in screening ELISAs. IP, results of immunoprecipitation studies of biosynthetically radiolabeled HK extracts; IB, results of immunoblot studies examining extracts of cultured HKs.
Denotes an AECP patient with cancer.
IgG4 anti-L-332 ELISA
Serum samples from 32 patients with AECP, 31 patients with pemphigus vulgaris (PV), 21 patients with pemphigus foliaceus (PF), 34 patients with BP (none of whom were included in the studies listed below), and 87 normal controls were tested for evidence of anti-L332 IgG4 by ELISA. Consistency and reproducibility of ELISA scores derived in these studies were validated using variance components analysis. The means and standard deviations of negative and positive standards were calculated for all plates on each day. The standard deviation estimates for day-to-day, plate-to-plate, and measurement error for negative and positive standards were all less than 0.03. Receiver Operating Characteristic (ROC) methodology was used to select a cut point score (i.e., a decision rule) for detecting L-332-specific IgG4 in sera from patients with AECP. When a cut point score of OD490 > 0.2 was used, three negative results were recorded in AECP sera (Patients 8, 16, and 32). All AECP sera that were negative in the IgG4 anti-L-332 ELISA demonstrated IgG directed against the dermal side of 1 M NaCl split skin at titers of 40 or less; as noted above, sera from all of these patients immunoprecipitated L-332 from extracts of biosynthetically radiolabeled HKs. Using the same cut point score, low levels of anti-L-332 IgG4 (OD490 ≤ 0.35) were identified in 3 of 86 control sera from patients with immunobullous diseases. Figure 1. All positive sera were from patients with BP. None of these sera immunoprecipitated L-332 from extracts of biosynthetically radiolabeled HKs or immunoblotted L-332 in HK ECM, indicating that such reactivity was not specific. ELISA studies found no evidence of L-332-specific IgG4 in sera from 87 normal controls. In sum, the diagnostic accuracy of the IgG4 L-332 ELISA was deemed to be high with a sensitivity of 0.91 (confidence interval 0.75 – 0.98) and a specificity of 0.98 (confidence interval 0.95 – 0.99). The corresponding Youden index of this ELISA was 0.89. The demonstration that the IgG4 anti-L-332 ELISA has great sensitivity and specificity justified its use in studies analyzing the reactivity of sera from patients with BP.
Figure 1.
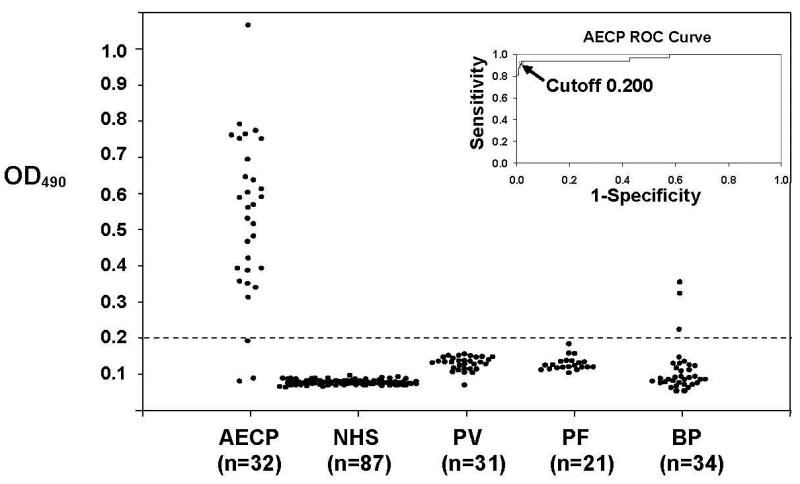
The IgG4 anti-L-332 ELISA detects autoantibodies in patients with AECP with great sensitivity and specificity. Scatter plot representation of ELISA results concerning the performance of sera from 32 patients with AECP and 173 controls. All sera were tested in duplicate; plotted dots represent the average of the OD490 reading obtained for each sample. The dashed line indicates the cut point score determined by ROC methodology. The insert depicts the ROC curve of the assay (sensitivity 0.91 [confidence interval 0.75 – 0.98], specificity 0.98 [confidence interval 0.95 – 0.99], Youden index 0.89).
Analysis of Sera from Patients with BP
Summary data regarding the serologic reactivity of 100 patients with BP is shown in Table 2. As shown, these patients, all seropositive for IgG versus intact human epidermal BM, had autoantibodies that bound bullous pemphigoid antigen 1 (BPAG1, n=17) 13, bullous pemphigoid antigen 2 (BPAG2, n=9) 14, or both (n=73) in immunoblot studies of HK extracts. While none of these sera immunoblotted L-332 in HK extracts, 13 scored above the cut-point of the IgG4 L-332 ELISA. Further analysis of these 13 samples found that all had IgG that bound the epidermal side of 1 M NaCl split skin by indirect IF microscopy in a manner characteristic of autoantibodies from BP patients; none had IgG reactive with the dermal side of 1 M NaCl split skin like that characteristic of autoantibodies from patients with AECP. In addition, none of these 13 patients had IgG that immunoblotted L-332 purified from HK ECM (the most sensitive known immunoblot substrate for detection of autoantibodies in patients with AECP), or IgG that immunoprecipitated L-332 from extracts of biosynthetically radiolabeled HKs (the most sensitive known technique for detection of autoantibodies in patients with AECP) 15. Figure 2.
Table 2. Summary Data Regarding Patients with BP*.
| Patient | IB | IgG4 |
|---|---|---|
| 1 | 230, 180 | 0.060 |
| 2 | 230, 180 | 0.073 |
| 3 | 230, 180 | 0.093 |
| 4 | 230, 180 | 0.146 |
| 5 | 230, 180 | 0.083 |
| 6 | 230, 180 | 0.087 |
| 7 | 230, 180 | 0.095 |
| 8 | 230, 180 | 0.124 |
| 9 | 180 | 0.091 |
| 10 | 230, 180 | 0.076 |
| 11 | 230, 180 | 0.096 |
| 12 | 230, 180 | 0.069 |
| 13 | 230, 180 | 0.111 |
| 14 | 180 | 1.137 |
| 15 | 230, 180 | 0.086 |
| 16 | 230, 180 | 0.081 |
| 17 | 230, 180 | 0.200 |
| 18 | 230 | 0.123 |
| 19 | 230, 180 | 0.089 |
| 20 | 230 | 0.063 |
| 21 | 230, 180 | 0.063 |
| 22 | 230, 180 | 0.172 |
| 23 | 230, 180 | 0.086 |
| 24 | 230 | 0.066 |
| 25 | 230, 180 | 0.098 |
| 26 | 230, 180 | 0.090 |
| 27 | 230, 180 | 0.123 |
| 28 | 230, 180 | 0.095 |
| 29 | 230 | 0.080 |
| 30 | 230, 180 | 0.095 |
| 31 | 230, 180 | 0.169 |
| 32 | 230, 180 | 0.075 |
| 33 | 230, 180 | 0.070 |
| 34 | 230, 180 | 0.187 |
| 35 | 230, 180 | 0.083 |
| 36 | 230 | 0.126 |
| 37 | 230, 180 | 0.344 |
| 38 | 230 | 0.064 |
| 39 | 180 | 0.109 |
| 40 | 230, 180 | 0.135 |
| 41 | 230, 180 | 0.096 |
| 42 | 230 | 0.077 |
| 43 | 230, 180 | 0.080 |
| 44 | 230, 180 | 0.091 |
| 45 | 180 | 0.371 |
| 46 | 180 | 0.123 |
| 47 | 230, 180 | 0.466 |
| 48 | 230, 180 | 0.111 |
| 49 | 230, 180 | 0.071 |
| 50 | 230 | 0.477 |
| 51 | 230, 180 | 0.068 |
| 52 | 230, 180 | 0.091 |
| 53 | 230, 180 | 0.066 |
| 54 | 230 | 0.080 |
| 55 | 230, 180 | 0.077 |
| 56 | 230, 180 | 0.096 |
| 57 | 230, 180 | 0.135 |
| 58 | 230, 180 | 0.076 |
| 59 | 230, 180 | 0.063 |
| 60 | 230, 180 | 0.096 |
| 61 | 230, 180 | 0.106 |
| 62 | 230 | 0.071 |
| 63 | 230, 180 | 0.097 |
| 64 | 230, 180 | 0.066 |
| 65 | 230, 180 | 0.096 |
| 66 | 230 | 0.851 |
| 67 | 230, 180 | 0.099 |
| 68 | 230, 180 | 0.129 |
| 69 | 180 | 0.092 |
| 70 | 230, 180 | 0.075 |
| 71 | 230, 180 | 0.070 |
| 72 | 230, 180 | 0.076 |
| 73 | 230 | 0.092 |
| 74 | 230 | 0.097 |
| 75 | 230, 180 | 0.093 |
| 76 | 230, 180 | 0.089 |
| 77 | 230 | 0.370 |
| 78 | 180 | 0.907 |
| 79 | 180 | 0.087 |
| 80 | 230, 180 | 0.071 |
| 81 | 230, 180 | 0.936 |
| 82 | 180 | 0.090 |
| 83 | 230, 180 | 0.141 |
| 84 | 230, 180 | 0.111 |
| 85 | 230, 180 | 0.084 |
| 86 | 230, 180 | 0.124 |
| 87 | 230, 180 | 0.116 |
| 88 | 230 | 0.156 |
| 89 | 230, 180 | 0.127 |
| 90 | 230, 180 | 0.083 |
| 91 | 230, 180 | 0.248 |
| 92 | 230, 180 | 0.093 |
| 93 | 230, 180 | 0.089 |
| 94 | 230 | 0.074 |
| 95 | 230, 180 | 0.106 |
| 96 | 230 | 0.808 |
| 97 | 230, 180 | 0.206 |
| 98 | 230, 180 | 0.911 |
| 99 | 230, 180 | 0.116 |
| 100 | 230, 180 | 0.083 |
IB, results of immunoblot studies examining extracts of cultured HKs. IgG4, denotes results of IgG4 anti-L-332 ELISA (OD490).
Figure 2.
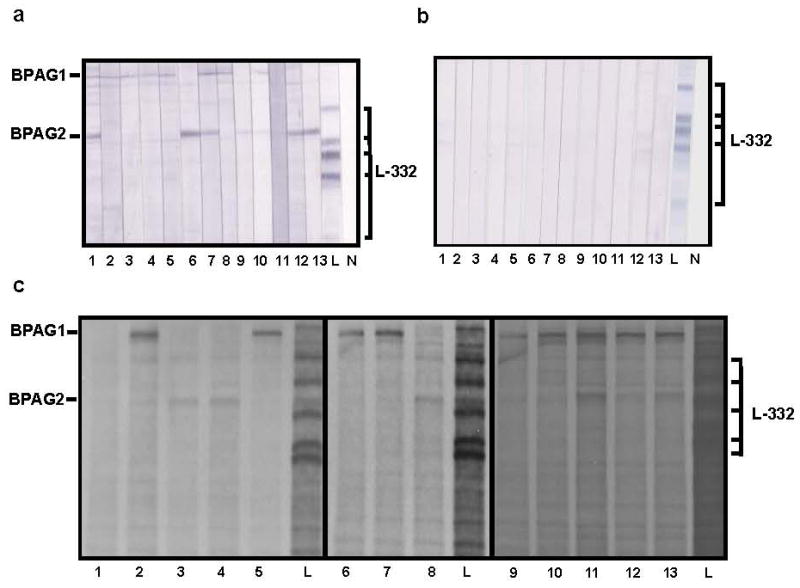
Sera from patients with BP do not contain IgG autoantibodies directed against L-332. a. Sera from 13 BP patients scoring above the cut point in the IgG4 anti-L-332 ELISA (lanes 1-13) immunoblotted either BPAG1 or BPAG2 in HK extracts. None of these samples displayed reactivity against L-332. Lane L represents a positive control where L-332 was immunoblotted with a well characterized rabbit antiserum and developed with alkaline phosphatase-conjugated goat anti-rabbit IgG (Biosource); lane N represents a negative control where L-332 was immunoblotted with normal human serum and developed with the same second-step antibody used in immunoblot studies of sera from patients with BP. Ticks in the left margin correspond to BPAG1 and BPAG2; brackets in the right margin correspond to various L-332 subunits (specifically, the unprocessed and processed α subunit, the unprocessed and processed γ subunit, and the β subunit). b. Sera from 13 BP patients scoring above the cut point in the IgG4 anti-L-332 ELISA (lanes 1-13) showed no evidence of reactivity to L-332 in HK ECM (the most sensitive antigen source for detection of autoantibodies in patients with AECP) 15. Lanes L and N as well as the brackets in the right margin are the same as noted in the description of panel a. c. Sera from all BP patients scoring above the cut point in the IgG4 anti-L-332 ELISA (lanes 1-13) immunoprecipitated either BPAG1 and/or BPAG2 from extracts of biosynthetically radiolabeled HKs. None of these samples immunoprecipitated L-332 from this antigen source (the most sensitive known technique for detection of autoantibodies in patients with AECP) 15. Lane L represents a positive control where L-332 was immunoprecipitated with a well characterized rabbit antiserum. Annotations in the panel margins are the same as those depicted in panel a.
Discussion
AECP is a mucosal predominant subepithelial blistering disease that is clinically indistinguishable from other forms of cicatricial pemphigoid (CP) 1, 7. A longitudinal study of 35 patients with AECP followed for an average duration of 38 months (range 2-145 months) found that 10 members of this cohort (29%) developed a solid cancer 8. Studies comparing the incidence of cancer in this cohort with age- and gender-matched controls in the National Cancer Institute's Surveillance Epidemiology and End Results database identified a RR for solid cancer in patients with AECP of approximately 7. The RR for solid cancer among this cohort of 35 patients with AECP approximates that for adults with dermatomyositis 16, 17. Subsequent to this report, other patients with AECP and cancer have been described; such cases have included patients with non Hodgkins lymphoma, ovarian cancer, and cutaneous T cell lymphoma 18-20.
The diagnosis of AECP has been confined to patients who demonstrate mucosal predominant blistering lesions, in situ deposits of immunoreactants in epidermal BM, and circulating IgG autoantibodies that bind L-332. Traditionally, the seropositivity of these patients has been established in specialized immunopathologic studies assessing the ability of patient IgG to either immunoprecipitate L-332 from extracts or medium of biosynthetically radiolabeled HKs, or immunoblot L-332 in extracts or ECM of cultured HKs 1, 7. What is lacking at this time is a practical, sensitive, and specific assay that can quickly screen large numbers of samples for evidence of anti-L-332 autoantibodies. Cultured HKs produce significant amounts of L-332 and incorporate all subunits of this heterotrimer into the ECM beneath and around colonies of proliferating cells 3. It has been shown previously that L-332 can be recovered from the ECM of cultured HKs free of any contaminating proteins 3. Moreover, when such L-332 was used to develop polyclonal antibodies in rabbits, the resulting antisera were specific to this laminin isoform (i.e., the lack of such antisera's reactivity to other keratinocyte proteins confirming that pure L-332 was isolated from HK ECM) 10. Building on these findings, L-332 in HK ECM was isolated and used for antigen capture of specific IgG by ELISA. While initial studies found that polyclonal second-step (i.e., developing) antibodies directed against human IgG displayed high background reactivity in this ELISA (data not shown), IgG subclass-specific monoclonal antibodies were found to perform quite well. A previous analysis of the distribution of IgG subclasses of anti-L-332 autoantibodies in 12 patients with AECP revealed that IgG4 was the predominant species 12. This report confirms that observation and describes the development and standardization of an ELISA for detection of anti-L-332 IgG4. The diagnostic accuracy of this ELISA was high (sensitivity 0.91, specificity 0.98, Youden index 0.89). Because rapidly growing HKs incorporate large quantities of L-332 into their ECM in vitro, it is simple to prepare L-332 coated ELISA plates of high quality and reproducibility. Moreover, such plates can be stored for extended periods at −70° C, thawed as needed, and used for the routine detection of autoantibodies against this autoantigen. This approach avoids the need to isolate, process, and purify soluble L-322 or be concerned about how much of such protein may adhere to a given microtiter well on the same or different plates. The sensitive and specific IgG4 anti-L-332 ELISA described in this report represents a useful tool to evaluate the serologic reactivity of patients suspected to have AECP. This ELISA, like indirect IF microscopy studies of 1 M NaCl split skin, can be used to identify patients who merit rigorous and specialized testing (e.g., immunoprecipitation and/or immunoblot) for anti-L-332 IgG autoantibodies.
Prior studies have shown that circulating anti-L-332 IgG autoantibodies are largely specific to patients with AECP 1, 7, 8, 21-24. While bullous disease patients with autoantibodies against multiple autoantigens (including L-332) in epidermal BM have been described, such patients are relatively rare 25-30. In contrast, a recent study found that 29 of 72 patients with BP (40%) had circulating IgG reactive with L-332 by ELISA 9. The antigen source used in this ELISA consisted of L-332 affinity purified from the conditioned medium of SCC-25 cells; analysis of this material demonstrated that it contained the processed α, intact (yet partially degraded) β, and the processed and unprocessed γ subunits of L-332 as well as fibronectin. This ELISA used a conjugated anti-human IgG as its second step antibody - a polyclonal reagent that, as noted above, in our experience typically yields high background reactivity against L-332. Indeed, the performance characteristics of this particular ELISA (sensitivity 40.3%, specificity 88.2%, Youden index 0.285), coupled with these investigators demonstration that: 1) only 16 of their 72 BP patients (22%) had IgG that dot blotted L-332; 2) only 38% of BP patients scoring positive by ELISA also scored positive by dot blot; and 3) BP serum samples showing no reactivity to 1 M NaCl split skin by indirect IF microscopy sometimes scored positive by ELISA support the view that specialized immunochemical tests (sometimes used in combination) remain of great importance in the evaluation of patients with immunobullous diseases.
Our studies of BP sera - both those used to standardize our IgG4 ELISA (i.e., studies involving 34 BP patients) as well as those used to characterize the autoantibodies present in 100 additional patients with BP - found that roughly 10% of BP patients displayed evidence of reactivity to L-332 by IgG4 ELISA. Subsequent immunoprecipitation and immunoblot studies of sera from these BP patients found that they contained IgG reactive with BPAG1 and/or BPAG2, but not L-332. False-positive determinations in the IgG4 ELISA were not observed using sera from patients with PV, PF, other immunobullous diseases (data not shown), or normal volunteers. While the basis for the elevated frequency of false positive determinations among BP patients is unknown, quantitation of total circulating IgG4 by radial immunodiffusion in our BP patients with false-positive IgG4 L-332 ELISA results found that many had elevated serum levels of this IgG subclass (data not shown). Screening sera for evidence of IgG4 reactive with L-332 by ELISA represents a sensitive and practical method to identify patients who may have AECP.
Acknowledgments
Funding Source: This work was supported in part by NIH Grant RO1 AR048982 to KBY.
Abbreviations
- AECP
anti-epiligrin cicatricial pemphigoid
- L-332
laminin 332
- BP
bullous pemphigoid
- IF
immunofluorescence
- HK
human keratinocyte
- BM
basement membrane
- RR
relative risk
- ECM
extracellular matrix
- PV
pemphigus vulgaris
- PF
pemphigus foliaceus
- ROC
receiver operating characteristic
- BPAG1
bullous pemphigoid antigen 1
- BPAG2
bullous pemphigoid antigen 2
- CP
cicatricial pemphigoid
- PBS
phosphate buffered saline
Footnotes
Disclosures: There are no potential conflicts of interest or competing interests to disclose.
Conflict of Interest The authors state no conflicts of interest.
References
- 1.Domloge-Hultsch N, Anhalt GJ, Gammon WR, Lazarova Z, Briggaman R, Welch M, et al. Anti-epiligrin cicatricial pemphigoid. A subepithelial bullous disorder. Arch Dermatol. 1994;130:1521–9. [PubMed] [Google Scholar]
- 2.Domloge-Hultsch N, Gammon WR, Briggaman RA, Gil SG, Carter WG, Yancey KB. Epiligrin, the major human keratinocyte integrin ligand, is a target in both an acquired autoimmune and an inherited subepidermal blistering skin disease. J Clin Invest. 1992;90:1628–33. doi: 10.1172/JCI116033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- 4.Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–76. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verrando P, Blanchet-Bardon C, Pisani A, Thomas L, Cambazard F, Eady RA, et al. Monoclonal antibody GB3 defines a widespread defect of several basement membranes and a keratinocyte dysfunction in patients with lethal junctional epidermolysis bullosa. Lab Invest. 1991;64:85–92. [PubMed] [Google Scholar]
- 6.Verrando P, Hsi BL, Yeh CJ, Pisani A, Serieys N, Ortonne JP. Monoclonal antibody GB3, a new probe for the study of human basement membranes and hemidesmosomes. Exp Cell Res. 1987;170:116–28. doi: 10.1016/0014-4827(87)90121-2. [DOI] [PubMed] [Google Scholar]
- 7.Egan CA, Lazarova Z, Darling TN, Yee C, Yancey KB. Anti-epiligrin cicatricial pemphigoid: clinical findings, immunopathogenesis, and significant associations. Medicine (Baltimore) 2003;82:177–86. doi: 10.1097/01.md.0000076003.64510.00. [DOI] [PubMed] [Google Scholar]
- 8.Egan CA, Lazarova Z, Darling TN, Yee C, Cote T, Yancey KB. Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet. 2001;357:1850–1. doi: 10.1016/S0140-6736(00)04971-0. [DOI] [PubMed] [Google Scholar]
- 9.Bekou V, Thoma-Uszynski S, Wendler O, Uter W, Schwietzke S, Hunziker T, et al. Detection of laminin 5-specific auto-antibodies in mucous membrane and bullous pemphigoid sera by ELISA. J Invest Dermatol. 2005;124:732–40. doi: 10.1111/j.0022-202X.2005.23646.x. [DOI] [PubMed] [Google Scholar]
- 10.Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB. Passive transfer of anti-laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest. 1996;98:1509–18. doi: 10.1172/JCI118942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirtschig G, Marinkovich MP, Burgeson RE, Yancey KB. Anti-basement membrane autoantibodies in patients with anti-epiligrin cicatricial pemphigoid bind the alpha subunit of laminin 5. J Invest Dermatol. 1995;105:543–8. doi: 10.1111/1523-1747.ep12323431. [DOI] [PubMed] [Google Scholar]
- 12.Hsu R, Lazarova Z, Yee C, Yancey KB. Noncomplement fixing, IgG4 autoantibodies predominate in patients with anti-epiligrin cicatricial pemphigoid. J Invest Dermatol. 1997;109:557–61. doi: 10.1111/1523-1747.ep12337073. [DOI] [PubMed] [Google Scholar]
- 13.Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv Immunol. 1993;53:291–325. doi: 10.1016/s0065-2776(08)60503-9. [DOI] [PubMed] [Google Scholar]
- 14.Van den Bergh F, Giudice GJ. BP180 (type XVII collagen) and its role in cutaneous biology and disease. Adv Dermatol. 2003;19:37–71. [PubMed] [Google Scholar]
- 15.Lazarova Z, Sitaru C, Zillikens D, Yancey KB. Comparative analysis of methods for detection of anti-laminin 5 autoantibodies in patients with anti-epiligrin cicatricial pemphigoid. J Am Acad Dermatol. 2004;51:886–92. doi: 10.1016/j.jaad.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Airio A, Pukkala E, Isomaki H. Elevated cancer incidence in patients with dermatomyositis: a population based study. The Journal of rheumatology. 1995;22:1300–3. [PubMed] [Google Scholar]
- 17.Sigurgeirsson B, Lindelof B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. The New England journal of medicine. 1992;326:363–7. doi: 10.1056/NEJM199202063260602. [DOI] [PubMed] [Google Scholar]
- 18.Sadler E, Lazarova Z, Sarasombath P, Yancey KB. A widening perspective regarding the relationship between anti-epiligrin cicatricial pemphigoid and cancer. J Dermatol Sci. 2007;47:1–7. doi: 10.1016/j.jdermsci.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain AJ, Cooper SM, Allen J, Dean D, Baxter KF, Goodfield MJ, et al. Paraneoplastic immunobullous disease with an epidermolysis bullosa acquisita phenotype: two cases demonstrating remission with treatment of gynaecological malignancy. Australas J Dermatol. 2004;45:136–9. doi: 10.1111/j.1440-0960.2004.00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Shannon JF, Mackenzie-Wood A, Wood G, Goldstein D. Cicatricial pemphigoid in non-Hodgkin's lymphoma. Intern Med J. 2003;33:396–7. doi: 10.1046/j.1445-5994.2003.t01-1-00430.x. [DOI] [PubMed] [Google Scholar]
- 21.Oyama N, Setterfield JF, Powell AM, Sakuma-Oyama Y, Albert S, Bhogal BS, et al. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: the pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol. 2006;154:90–8. doi: 10.1111/j.1365-2133.2005.06998.x. [DOI] [PubMed] [Google Scholar]
- 22.Leverkus M, Schmidt E, Lazarova Z, Brocker EB, Yancey KB, Zillikens D. Antiepiligrin cicatricial pemphigoid: an underdiagnosed entity within the spectrum of scarring autoimmune subepidermal bullous diseases? Arch Dermatol. 1999;135:1091–8. doi: 10.1001/archderm.135.9.1091. [DOI] [PubMed] [Google Scholar]
- 23.Lazarova Z, Hsu R, Yee C, Yancey KB. Anti-epiligrin cicatricial pemphigoid represents an autoimmune response to subunits present in laminin 5 (alpha3beta3gamma2) Br J Dermatol. 1998;139:791–7. doi: 10.1046/j.1365-2133.1998.02502.x. [DOI] [PubMed] [Google Scholar]
- 24.Chan LS, Yancey KB, Hammerberg C, Soong HK, Regezi JA, Johnson K, et al. Immune-mediated subepithelial blistering diseases of mucous membranes. Pure ocular cicatricial pemphigoid is a unique clinical and immunopathological entity distinct from bullous pemphigoid and other subsets identified by antigenic specificity of autoantibodies. Arch Dermatol. 1993;129:448–55. doi: 10.1001/archderm.129.4.448. [DOI] [PubMed] [Google Scholar]
- 25.Izumi R, Fujimoto M, Yazawa N, Nakashima H, Asashima N, Watanabe R, et al. Bullous pemphigoid positive for anti-BP180 and anti-laminin 5 antibodies in a patient with graft-vs-host disease. J Am Acad Dermatol. 2007;56:S94–7. doi: 10.1016/j.jaad.2006.10.986. [DOI] [PubMed] [Google Scholar]
- 26.Mulyowa GK, Jaeger G, Sitaru C, Brocker EB, Zillikens D, Schmidt E. Scarring autoimmune bullous disease in a Ugandan patient with autoantibodies to BP180, BP230, and laminin 5. J Am Acad Dermatol. 2006;54:S43–6. doi: 10.1016/j.jaad.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 27.Baican A, Hirako Y, Lazarova Z, Yancey KB, Zillikens D, Sitaru C. IgG autoantibodies to type VII collagen and an exclusive IgG3 reactivity to the laminin alpha3 chain in a patient with an autoimmune subepidermal blistering disease. J Am Acad Dermatol. 2005;53:517–22. doi: 10.1016/j.jaad.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Shimanovich I, Petersen EE, Weyers W, Sitaru C, Zillikens D. Subepidermal blistering disease with autoantibodies to both the p200 autoantigen and the alpha3 chain of laminin 5. J Am Acad Dermatol. 2005;52:S90–2. doi: 10.1016/j.jaad.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Umemoto N, Demitsu T, Toda S, Ohsawa M, Noguchi T, Kakurai M, et al. A case of nonscarring subepidermal blistering disease associated with autoantibodies reactive with both type VII collagen and laminin 5. Dermatology. 2003;207:61–4. doi: 10.1159/000070945. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara Y, Amagai M, Ohata Y, Ishii K, Hasegawa Y, Hsu R, et al. A case of cicatricial pemphigoid with simultaneous IgG autoantibodies against the 180 kd bullous pemphigoid antigen and laminin 5. J Am Acad Dermatol. 1998;38:624–7. doi: 10.1016/s0190-9622(98)70129-x. [DOI] [PubMed] [Google Scholar]


