Abstract
Deficits in learning and memory are among the most robust correlates of schizophrenia. It has been hypothesized that these deficits are in part due to reduced conscious recollection and increased reliance on familiarity assessment as a basis for retrieval. The Remember-Know (R-K) paradigm was administered to 35 patients with chronic schizophrenia and 35 healthy controls. In addition to making “remember” and “know” judgments, the participants were asked to make forced choice recognition judgments with regard to details about the learning episode. Analyses comparing response types showed a significant reduction in “remember” responses and a significant increase in “know” responses in schizophrenia patients relative to controls. Both patients and controls recalled more details of the learning episode for “remember” compared to “know” responses, although, in particular for “remember” responses, patients recalled fewer details compared with controls. Notably, patients recognized fewer inter-item but not intra-item stimulus features compared with controls. These findings suggest deficits in organizing and integrating relational information during the learning episode and/or using relational information for retrieval. A Dual-Process Signal Detection interpretation of these findings suggests that recollection in chronic schizophrenia is significantly reduced, while familiarity is not. Additionally, a unidimensional Signal Detection Theory interpretation suggests that chronic schizophrenia patients show a reduction in memory strength, and an altered criterion on the memory strength distribution for detecting new compared with old stimuli but not for detecting stimuli that are remembered versus familiar. Taken together, these findings are consistent with a deficit in recollection and increased reliance on familiarity in making recognition memory judgments in chronic schizophrenia.
Keywords: schizophrenia, psychosis, chronic, memory, episodic, recognition, recollection, familiarity, context, remember, know
1. Introduction
Patients with schizophrenia show robust deficits in declarative memory (Aleman et al. 1999; Cirillo and Seidman 2003; Heinrichs and Zakzanis 1998; Kuperberg and Heckers 2000; Pelletier et al. 2005), but the precise nature of these deficits and their neural underpinnings remain unresolved (Cirillo and Seidman 2003). A prominent model posits that the declarative memory deficits in schizophrenia are in part due to reduced conscious recollection and increased reliance on familiarity assessment as a basis for retrieval (Achim and Lepage 2005; Danion et al. 1999; Huron et al. 1995). In this framework, recollection is thought to involve retrieval of the target item as well as some of the specific details of the encoding context, such as sensory-perceptual features, and spatial and temporal information, etc., while familiarity represents a dissociable process involving retrieval of the target item but without retrieval of such contextual information. Functional neuroimaging and lesion studies provide convergent evidence that these two processes are in part dissociable at the neural level (Skinner and Fernandes 2007).
Several studies using the Remember-Know (R-K) paradigm have provided evidence consistent with the view that patients with schizophrenia exhibit greater reduction in the use of conscious recollection than in the use of familiarity assessment as a basis for retrieval compared with controls (Danion et al. 2005; Danion et al. 2003; Danion et al. 1999; Grillon et al. 2005; Huron and Danion 2002; Huron et al. 1995; Huron et al. 2003; Neumann et al. 2006; Ribeiro et al. 2005; Sonntag et al. 2003; Souchay et al. 2006; Tendolkar et al. 2002; Thoma et al. 2006). In the R-K paradigm, when recognizing a memorandum as previously encountered, participants are asked to make a “remember” response when they retrieve contextual information that was part of the learning episode and a “know” response when they do not. Guided by the Dual-Process Signal Detection model (Yonelinas 2002; Yonelinas et al. 1998), which assumes that “remember” and “know” responses represent independent processes, two studies reported reductions in recollection that were about twice as large as those for familiarity in patients with schizophrenia compared with healthy volunteers (Danion et al. 2003; Thoma et al. 2006). However, neither study explicitly tested whether this difference was statistically significant.
While it has been proposed that a single process involving memory strength may underlie both “remember” and “know” judgments (Donaldson 1996; Dunn 2004; Hirshman and Henzler 1998), it has recently been argued that a unidimensional (cf. single process) model is not incompatible with the phenomenological distinction between recollection and familiarity, but in fact that information obtained from both recollection and familiarity may aggregate on a unidimensional measure of memory strength (Wixted and Stretch 2004). In this model, the sources of the distinction of “remember” and “know” judgments are hypothetical thresholds on a memory strength continuum (see Figure 1), in which one threshold represents an ‘old-new’ criterion reflecting the lower bound memory strength at which an item is judged to be familiar, and another threshold represents a ‘remember-know’ criterion reflecting the lower bound memory strength at which an item is judged to be remembered. To our knowledge, while the validity of the different models is hotly debated (Parks and Yonelinas 2007; Wixted 2007a; Wixted 2007b), the unidimensional model has not previously been applied in relation to schizophrenia.
Figure 1.
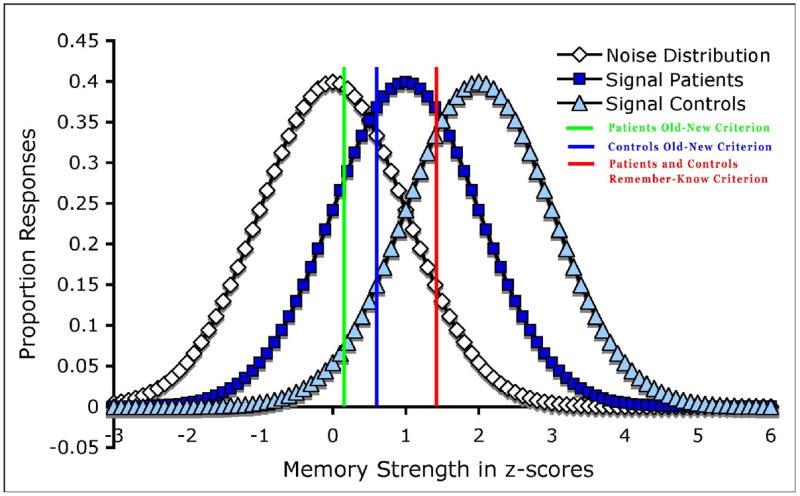
Signal Detection Theory or Unidimensional Model of Remember and Know Judgments. The first threshold is an ‘old-new’ criterion reflecting the lower bound memory strength at which an item is judged to be familiar, and the second threshold is a ‘remember-know’ criterion reflecting the lower bound memory strength at which an item is judged to be remembered.
In this study we combined the standard R-K procedure with assessment of retrieval of multiple (one intra-item and one inter-item) stimulus features at encoding in order to validate subjects’ subjective “remember” and “know” responses and to examine patient-control differences in memory performance according to the Dual-Process Signal Detection (DPSD) model and the unidimensional Signal Detection Theory (SDT) model. Furthermore, there is some evidence from clinical (Mayes et al. 2007), electrophysiological (Jager et al. 2006), and neuroimaging (Staresina and Davachi 2006) studies that suggests that “intra-item” and “inter-item” learning have separate neural correlates, with “intra-item” learning relying on the perirhinal cortex and familiarity processes, and “inter-item” learning involving the hippocampus and relational/recollection processes.
Based on the findings reviewed above, we hypothesized that: 1) schizophrenia patients will make fewer “remember” responses and more “know” responses compared with healthy controls; 2) both patients and controls will recognize more stimulus features on “remember” compared with “know” responses; 3) patients will recognize an overall lower number of stimulus features than controls and that this effect will be stronger for the inter-item compared with the intra-item stimulus features; and 4) patients will show a larger reduction in the Dual-Process Signal Detection index of recollection than that of familiarity. Groups were also compared on signal detection metrics based on the SDT (unidimensional) model to determine whether the predicted reduction in the number of “remember” responses and predicted increase in the number of “know” responses in the patients reflects an overall downward shift in memory strength and either a reduced old-new criterion, an increased remember-know criterion, or both.
2. Methods
2.1 Participants
Thirty-five outpatients with diagnosis of schizophrenia (n=30) or schizoaffective disorder-depressive type (n=5), determined by a Structured Clinical Interview for DSM-IV [SCID] (First et al. 1997) along with confirmation of their diagnosis by consensus of the treating team of licensed clinical psychologists and psychiatrists, and 35 demographically (age and gender) matched controls participated in the study. All patients were prior participants of the Aftercare Research Program, an outpatient schizophrenia research clinic at UCLA in which patients, who are referred from a number of local public and private hospitals, receive treatment and participate in ongoing research projects of the UCLA Center for Neurocognition and Emotion in Schizophrenia. All patients had their first psychotic episode more than five years prior to this study, were between 18 and 60 years of age, understood spoken English sufficiently to comprehend testing procedures, had estimated IQ greater than 70, and exhibited no physical, cognitive, or language impairment that could adversely affect task performance. Patients with histories of traumatic brain injury, and clinically significant neurological disorder were excluded from the study. Patients were also excluded if there was evidence of alcohol and or substance abuse in the past six months, if psychotic episodes were drug-induced, or if substance use was considered a dominant factor in the course of illness. At the time of testing patients were clinically stabilized on a variety of first-generation (n=4) and second-generation antipsychotic (n=30) medications (missing medication data: n=1). In addition, eight patients received secondary anti-cholinergic medications [Artane (n=2), range 2–6mg; Cogentin (n=6): range 1–4mg, daily], one a benzodiazepine, one a barbiturate, and seven a serotonin reuptake inhibitor.
The 35 healthy participants were recruited through local newspaper and poster advertisements. Exclusion criteria for healthy controls included a diagnosis of any DSM-IV Axis I psychotic disorder, major mood disorder, post-traumatic stress disorder, current or past alcohol or substance dependence or current abuse, and/or avoidant, paranoid, schizotypal, or schizoid personality disorder, as assessed using the SCID (First et al. 1997). Controls were also excluded if they met criteria for a prodromal state, as assessed by the Structured Interview for Prodromal Syndromes [SIPS (Miller et al. 1999)]. Potential controls who had histories of neurological disorder, traumatic brain injury, or a first-degree relative with a psychotic disorder, or who were currently pregnant, also were excluded.
The patient and control groups did not differ significantly in terms of mean age [F(1,69)=.047, P=0.49], mean subject education [F(1,65)=0.89, P=0.35], gender (χ21=0.24, P=0.80), race (χ21=1.54, P=0.74), or handedness distribution (χ21=2.61, P=0.24), though patient’s mean level of parental education was higher than that of controls [F(1,65)= 4.81, P=0.03] (Table 1). The study was approved by the University of California Los Angeles Institutional Review Board, and all participants provided written informed consent after study procedures were fully explained.
Table 1.
Demographic Characteristics of the Participants
| Patients (N=33) | Controls (N=30) | |
|---|---|---|
| Age (years), mean ± SD | 33.3 ± 7.4 | 31.3 ± 5.3 |
| Sex, No. | ||
| Male | 21 | 23 |
| Female | 14 | 12 |
| Duration of Illness (years), mean ± SD | 9.3 ± 4.4 | |
| SANS Sum of Global Ratings ± SD | 8.7 ± 5.3 | |
| SAPS Sum of Global Ratings ± SD | 3.5 ± 3.2 | |
| Education, mean ± SD | ||
| Subject | 14.2 ± 1.7 | 13.8 ± 1.4 |
| Parent | 15.5 ± 3.3 | 13.8 ± 3.0* |
| Race, No. | ||
| Asian American | 4 | 2 |
| African American | 5 | 6 |
| Mixed Race | 3 | 1 |
| Caucasian | 23 | 22 |
P<0.05; estimates for mean education and race distribution based on available data of 31 of the 35 controls.
2.2 Remember-Know Paradigm
The R-K paradigm was administered on a personal computer using E-Prime (Psychology Software Tools, www.pstnet.com). Stimuli were presented as word and picture pairs in order to provide opportunities for use of relational information at encoding (Figure 2). The paradigm comprised three phases: 1) encoding, 2) item recognition, and 3) stimulus feature recognition. Before the task began, participants were instructed to remember as much of the information presented on each trial as possible and were given examples of the types of questions that would be asked in the item and stimulus feature recognition phases. The explanation of “remember” and “know” responses was based on those by (Gardiner and Java 1990), and those with regard to the stimulus feature recognition resembled those by (Dudukovic and Knowlton 2006). As a pre-test measure, participants were given six example sentences describing memories and asked whether each was reflective of a “remember” or a “know” memory. Participants who got fewer than four out of six questions correct were eliminated from the analyses (one patient). A full set of instructions can be requested from the corresponding author. The target pictures were drawn from the Snodgrass and Vanderwart “Like” objects (Rossion and Pourtois 2004) colored in blue, green, orange, or pink with Adobe Photoshop (Adobe Systems Incorporated). The line drawings were a subset of the 244 freely available drawings from the International Picture Naming project (Bates et al. 2003; Szekely et al. 2003). Subjects responded using the one, two, and three keys on the keyboard. E-prime data (responses and reaction times) were scored using Excel Visual Basic macros.
Figure 2. Task Structure for Encoding, Item Recognition, and Stimulus Feature Recognition Phases.
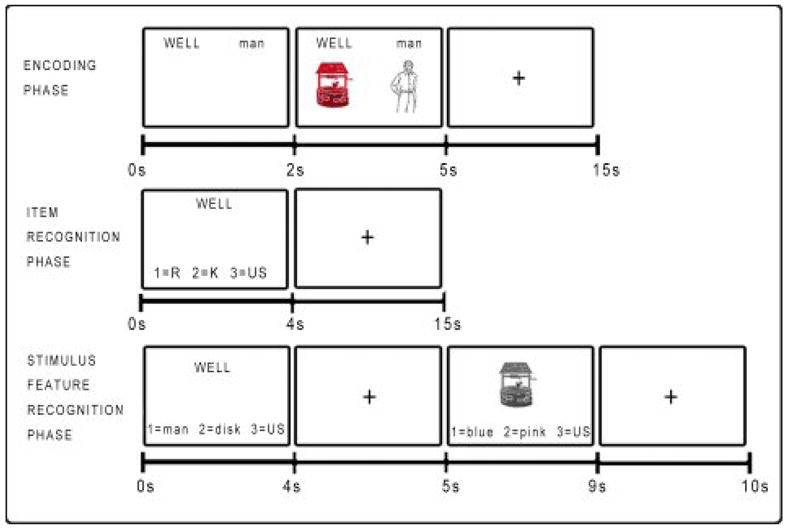
In the experiment “R” is listed as “remember”, “K” is listed as “know”, and “US” is listed as unstudied on the computer screen.
During the encoding phase, participants were shown sixty stimulus pairs, and explicitly asked to memorize as much about the items on the screen as possible: the words, pictures, colors of the pictures, and their location on the screen; they were also encouraged to develop other associations. As a check on task engagement, participants were asked to judge whether the colored target picture appeared on the left or right.
During the item recognition phase, immediately following encoding, subjects were presented with the sixty previously presented targets and twenty new foil words. Participants were instructed to press one of three keys according to whether they remembered the word and could recall specific additional information presented with the word [“remember”], know that the word was previously presented, but do not recall additional information [“know”], or think the word was not previously presented [“unstudied”].
Immediately following the item recognition phase, subjects were given the feature recognition phase, in which they were first presented with a target word and asked to make a forced-choice recognition judgment between two possible paired words. A grayscale target picture was then presented and subjects were asked to make a forced-choice recognition judgment between two possible picture colors. Both judgments also offered the ‘unstudied’ option to indicate that the word or picture was not present in the encoding phase. The feature recognition phase was self-paced.
2.3 Measures
The primary performance measures were calculated using formulae provided by the Dual-Process Signal-Detection (DPSD) and Signal Detection Theory (SDT) models of remembering and knowing. With regard to the DPSD interpretation of the R-K paradigm, estimates of recollection and familiarity were calculated, taking into account possible differences in response bias or false-alarm rates [see Appendix A of (Yonelinas et al. 1998)]. To avoid issues with extreme proportions (i.e., hit or false-alarm rates of 0 or 1) we used the loglinear approach of adding 0.5 to the hits and false alarms and dividing by the number of signal + 1 and the number of noise + 1 trials [see (Hautus 1995) in (Stanislaw and Todorov 1999)].
With regard to the SDT interpretation of the R-K paradigm, estimates of the criterion separating old from new responses (k), the criterion separating R from K responses (r), and sensitivity (d′), were based on the equal variance normal distribution model [see equation 2 in (Dunn 2004)]. These estimates are in units of z-scores and were derived for each subject by minimizing the sum of squared differences between the observed and predicted data using a Visual Basic macro in Excel (Microsoft, Inc.) calling the Solver function. In addition, to examine possible group differences in signal and noise distributions between patients and controls, zROC slopes were computed (Rotello et al. 2004), which represent an estimate of signal versus noise standard deviation ratio (Wixted and Stretch 2004).
To examine the validity of the distinction between “remember” and “know” responses, the mean number of recognized stimulus features per true positive response was calculated for “remember” and “know “responses separately.
2.4 Statistical Analysis
Statistical analyses were conducted using Proc Mixed (SAS version 8.2, SAS Institute, Inc, Cary, NC). Group differences in the estimates of recollection and familiarity and the old-new and remember-know criteria were analyzed using repeated measures analysis of covariance models, with process (recollection, familiarity) and criterion (old-new, remember-know) entering the models as within-subject repeated measure variables, respectively. Group differences in the number of stimul us features recognized per true positive response were analyzed with a doubly repeated measures analysis of covariance model, with response type (“remember”, “know”) and feature (pair-word, target-color) entering the model as within-subject repeated measures variables. Group (patient, control) and sex (male, female) entered the models as between-subject variables. The statistical models included the interactions of all the between- and within-subject class variables. Age was included as a between-subject covariate. Whenever one of the terms contributed significantly to the prediction of the dependent variable, two-tailed contrast analyses were used to compare hypothesized mean differences within the term collapsing over the other terms in the model. This approach maintains the hypothesis-wise Type I error rate at 0.05 because a predictor’s contribution to particular dependent measures is evaluated only if its effect is found to vary at the multivariate level.
Additionally, univariate mixed model analyses were used to test for group differences in percent true and false positives, the number of omissions, and recognition accuracy based on data from the Stimulus Recognition Phase, and the number of “remember” and “know” responses with zero, one, or two recognized features based on the “remember” and “know” judgments from the Stimulus Recognition Phase and the feature recognition judgments from the Stimulus Feature Recognition Phase. To correct for skew and kurtosis in the distribution of false positives a square-root transform was applied to the data. Omissions were highly zero inflated and therefore analyzed using non-parametric statistics (Fisher’s Exact test).
3. Results
3.1 Univariate Analyses
Table 2 gives raw means and standard deviations for all of the response parameters in patients and controls. Compared with controls, schizophrenia patients had a lower percent true positives (t65=−2.15, P=0.04), a lower overall recognition accuracy (t65=−3.60, P=0.0006), a lower number of “remember” responses (t66=−3.52, P=0.0008), and a lower number of responses on which two stimulus features were recognized (t65=−4.56, P<0.0001), as well as a higher number of false positives (t65=2.15, P=0.04), a higher number of “know” responses (t66=2.12, P=0.04), and a higher number of responses on which no stimulus features were recognized (t65=2.51, P=0.007). Patients and controls did not differ in the number of omissions (χ21=0.00, P=0.67).
Table 2.
Raw Means (and Standard Deviations) of the Patient and Control Groups on the R-K Task Performance Indices.
| Patients (n=35) | Controls (n=35) | |
|---|---|---|
| Percent True Positives | 0.73 (0.14) | 0.79 (0.08) |
| Number of False Positives | ||
| Remember | 2.11 (2.25) | 1.74 (2.31) |
| Know | 5.60 (3.61) | 3.74 (2.31) |
| Number of Omissions** | 2.06 (4.23) | 1.40 (2.10) |
| Recognition Accuracy*** | 68.68 (10.40) | 76.68 (7.36) |
| Number of True Positives | ||
| Remember | 20.60 (11.67) | 30.00 (11.43) |
| Know | 22.46 (10.77) | 17.11 (11.30) |
| Number of Recognized Features**** | ||
| per Remember Response | 0.99 (0.35) | 1.31 (0.29) |
| per Know Response | 0.83 (0.28) | 1.06 (0.39) |
| Number of Recognized Pair Words | ||
| per Remember Response | 0.51 (0.23) | 0.72 (0.17) |
| per Know Response | 0.45 (0.19) | 0.55 (0.29) |
| Number of Recognized Target Colors | ||
| per Remember Response | 0.46 (0.20) | 0.59 (0.17) |
| per Know Response | 0.38 (0.15) | 0.38 (0.23) |
| Number of Remember Responses***** | ||
| with 0 Recognized Features | 5.49 (4.26) | 5.06 (4.93) |
| with 1 Recognized Feature | 8.86 (5.13) | 11.77 (5.84) |
| with 2 Recognized Features | 6.23 (5.60) | 13.17 (7.19) |
| Number of Know Responses | ||
| with 0 Recognized Features | 8.37 (8.03) | 4.26 (3.18) |
| with 1 Recognized Feature | 9.40 (5.40) | 7.17 (5.24) |
| with 2 Recognized Features | 4.69 (3.80) | 5.69 (4.90) |
| Estimate of Recollection | 0.50 (0.59) | 1.22 (1.29) |
| Estimate of Familiarity | 0.68 (0.64) | 0.81 (0.65) |
| Estimate of Remember-Know Criterion | 0.37 (0.74) | 0.70 (0.65) |
| Estimate of Old-New Criterion | 1.59 (0.92) | 1.58 (0.68) |
| Estimate of Sensitivity (D-Prime) | 1.07 (0.63) | 1.61 (0.69) |
| zROC slope****** | 0.60 (0.24) | 0.52 (0.38) |
Number of Omissions [targets – true positives – false negatives];
Recognition Accuracy or the probability of correctly identifying targets and foils [(true positives + true negatives)/(true positives + false positives + true negatives + false negatives + omissions) * 100],
Number of Stimulus Features Recognized per Remember and Know Response (e.g., [(number of “remember” responses on which zero stimulus features were recognized * zero + number of “remember” responses on which one stimulus feature was recognized * one + number of “remember” responses on which two stimulus features were recognized * two)/total number of “remember” responses],
Number of Remember Responses made during the Item Recognition Phase with zero, one, or two recognized features during the Stimulus Feature Recognition Phase,
zROC slope = [φ−1(proportion of true positive responses) - φ−1(proportion of remember responses)]/[φ−1(proportion of false positive responses) - φ−1(proportion of remember false positive responses)]; given that φ−1(0)=negative infinity, no zROC is available for subjects who’s recognition false positives or recognition remember false positives are zero, (therefore control n=21 and patients n=25 for this measure).
3.2 Dual-Process Signal Detection Model Estimates of Recollection and Familiarity
There were significant main effects of group [F(1,65)=11.34, P=0.001] and sex [F(1,65)=4.58, P=0.03], and significant group × process (recollection, familiarity) interaction [F(1,66)=4.52, P=0.04] on the Dual-Process Signal Detection estimates of recollection and familiarity. Overall (collapsing across process indices), patients had poorer memory performance compared with controls (LSM±SE=0.62±0.09, 1.07±0.10, respectively, t65=−3.37, P=0.001), and males had poorer performance than females [LSM±SE=0.70±0.08, 0.99±0.10, respectively, t65=−2.14, P=0.04). As shown in Figure 3, patients had significantly lower recollection compared with controls (t66=−3.80, P=0.0003), but the two groups did not differ in terms of familiarity (t66=−0.55, P=0.58).
Figure 3.
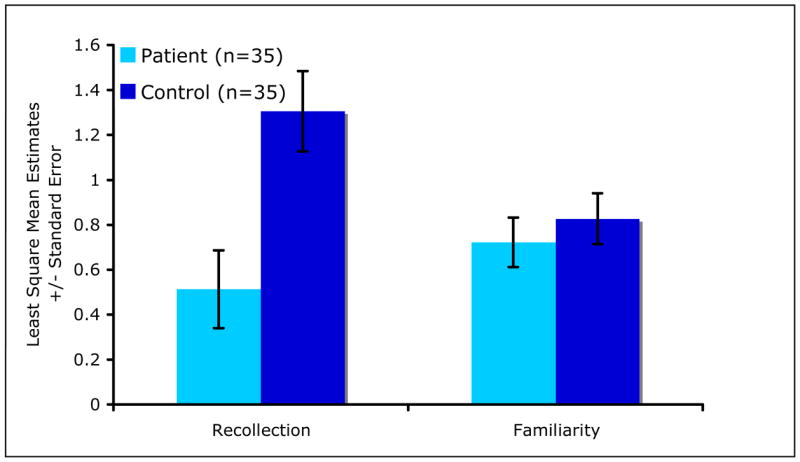
Estimates of Recollection and Familiarity Based on the Dual-Process Signal Detection Model.
3.3 Signal Detection Theory Estimates of Old-New and Remember-Know Criteria
There were significant main effects of criterion (old-new, remember-know) [F(1,66)=170.87, P<0.0001] and a significant group × criterion interaction [F(1,66)=5.14, P=0.03] on the performance estimates derived from the SDT (unidimensional) model. The overall estimate of the remember-know criterion was higher than that of the old-new criterion (LSM±SE=1.61±0.09, 0.58±0.09, respectively, t66=13,07, P<0.0001). As shown in Figure 1 and Figure 4, patients had significantly lower estimates on the old-new criterion compared with controls (t66=−1.91, P=0.06), while the groups did not differ in terms of the remember-know criterion (t66=0.02, P=0.99). Furthermore, patients [LSM±SE=1.12±0.11] had significantly lower sensitivity (d-prime) compared with controls [LSM±SE=1.67±0.11] (t65=−3.49, P=0.0009), though zROC slopes were similar (t41=1.18, P=0.25).
Figure 4.
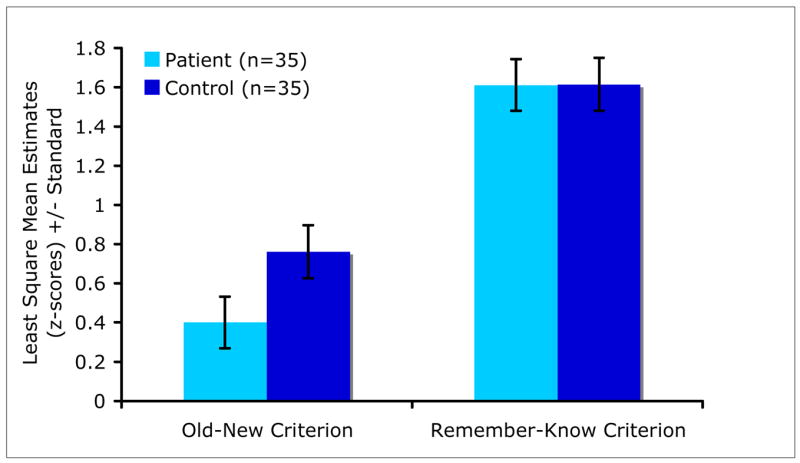
Estimates of the Old-New and Remember-Know Criteria Based on the Equal-Variance Normal Distribution Model by Group.
3.4 Number of Recognized Stimulus Features per True Positive Response
Doubly repeated measures analysis of covariance showed significant main effects of group [F(1,65)=7.00, P=0.01], response (“remember”, “know”) [F(1,66)=48.86, P<0.0001], and feature (word, color) [F(1,66)=35.62, P<0.0001], along with significant group × response [F(1,66)=11.49, P=0.001] and group × feature [F(1,66)=5.54, P=0.02] interactions on the number of recognized stimulus features per true positive response.
Comparisons of least square means showed that patients [LSM±SE=0.46±0.03] recognized fewer features per true positive response than controls [LSM±SE=0.57±0.03] (t65=−2.64, P=0.01), and that the number of recognized features per true positive was larger for “remember” [LSM±SE=0.58±0.02] than for “know” [LSM±SE=0.45±0.02] responses (t66=6.99, P=<0.0001), and larger for the “word” [LSM±SE=0.57±0.02] than the “color” [LSM±SE=0.46±0.02] feature (t66=5.97, P=<0.0001).
Decomposition of the group × response interaction effect showed that, while for both patients (t66=2.59, P=0.01) and controls (t66=7.23, P<0.0001) the number of recognized features per “remember” response was larger than that per “know” response, patients recognized a similar number of features per “know” response [LSM±SE=0.43±0.03 and LSM±SE=0.47±0.03] (t66=−0.95, P=0.34), but fewer features per “remember” response [LSM±SE=0.50±0.03, LSM±SE=0.66±0.03] (t66=−3.83, P=0.0003) compared with controls (Figure 5).
Figure 5.
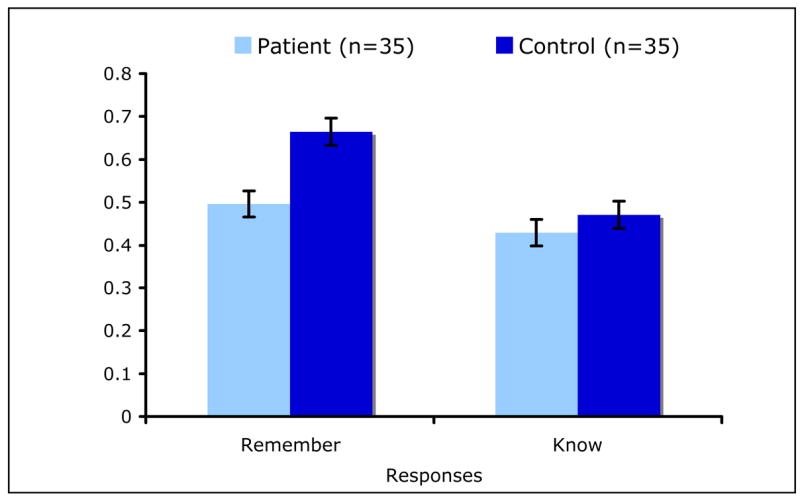
The Number of Stimulus Features Recognized per Remember and Know Response by Group
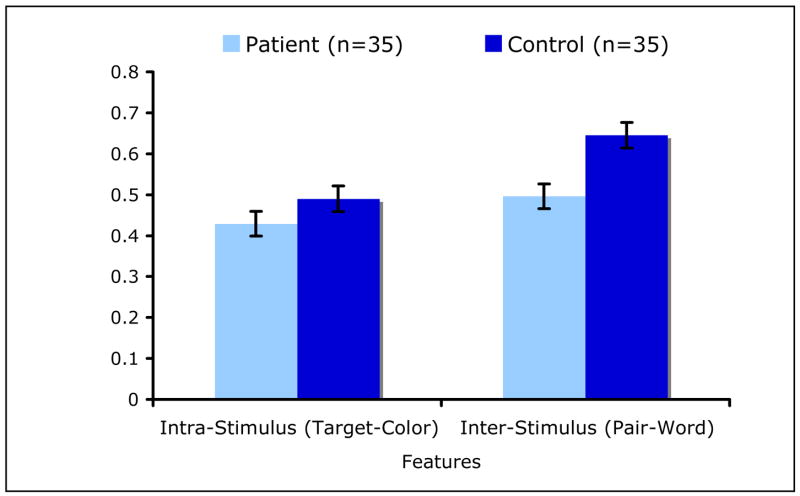
Decomposition of the group × feature interaction effect showed that controls recognized more inter-stimulus features (pair-words) than patients [LSM±SE=0.65±0.03, LSM±SE=0.50±0.03] (t66=3.39, P=0.001), while recognition of intra-stimulus features (target-colors) did not differ between the groups [LSM±SE=0.49±0.03, LSM±SE=0.43±0.03] (t66=1.39, P=0.17); controls also recognized more pair-words than target-colors (t66=5.79, P<0.0001) [Figure 6].
Figure 6.
The Number of Intra-Stimulus (Target-Color) and Inter-Stimulus (Pair-Word) Features Recognized per Remember and Know Response by Group
4. Discussion
This study used a translational behavioral science paradigm – the Remember-Know task – to probe the cognitive architecture of declarative memory disruption in patients with schizophrenia. Consistent with two prior studies (Danion et al. 2003; Thoma et al. 2006), patients gave significantly fewer Remember responses and significantly more Know responses than controls, resulting in significantly lower estimates of the Dual-Process Signal Detection measures of recollection but not of familiarity. These results are consistent with the hypothesis that declarative memory deficits in schizophrenia are associated with a relatively greater disruption of processes associated with conscious recollection than of those associated with familiarity assessment. Further exploration of the response pattern within the unidimensional Signal Detection Theory framework revealed that schizophrenia patients have an overall reduction in sensitivity (d′), and a significantly lowered old-new criterion, but no difference in the remember-know criterion, and no difference in relative spread of the signal compared to the noise distributions (as assessed by zROC curves) compared to controls. Thus, patients and controls require a similar minimum level of memory strength to make “remember” judgments, but, since patients’ overall memory strength distribution is lower, they make fewer “remember” responses than controls. In addition, because the patients’ distribution of memory strength is shifted downward and because their criterion for old-new discrimination is lower on the memory strength continuum compared with controls, they rely more on familiarity assessments during recognition judgments, and are more inclusive in these judgments, such that non-target items that seem familiar are judged to have been part of the study set, resulting in a higher number of false positive responses. Our results with regard to the retrieval of stimulus feature information corroborate those of others in suggesting that patients with schizophrenia have a deficit in binding information into a coherent episodic memory (Danion et al. 1999; Waters et al. 2004). More specifically, in terms of a deficit in associative learning and consistent with a larger deficit in recollection than familiarity (Jager et al. 2006; Mayes et al. 2007; Staresina and Davachi 2006), this study, to our knowledge, is the first to show a deficit in inter-item, but not intra-item associative learning in patients with schizophrenia compared with controls.
In this study, subjects’ judgments did not include guess responses in addition to “remember” and “know” responses, and did not use a two-step procedure in which subjects are first asked to make an old-new judgment followed by a remember-know judgment. Eldridge and colleagues (2002) have suggested that without the use of these procedures, remember-know judgments may be reflective of trace-strength.
Given that patients produced a lower number of true positives than controls, we cannot rule out that differences in motivation or effort may account for some of the findings. While we cannot fully exclude the possibility that antipsychotic medications influenced memory performance in the patients, several reviews have concluded that memory dysfunction in schizophrenia is not significantly influenced by such agents (Aleman et al. 1999; Goldberg and Weinberger 1996). Patients who are unmedicated (Saykin et al. 1994) or on a drug reduction (Seidman et al. 1993) show memory impairment, and none of the reports employing the R-K procedure in patients with schizophrenia showed significant associations with either typical or atypical antipsychotic, nor with anticholinergic agents (Danion et al. 1999; Huron and Danion 2002; Huron et al. 1995). Furthermore, while the study may be underpowered to show the effects, when including anti-cholinergic medication (yes, no) as a covariate in the analyses it did not contribute significantly to predictions, nor did it significantly alter the reported findings. With regard to associations of the memory deficits with clinical symptoms, consistent with an earlier report of lower recollection in patients with predominantly negative symptoms (Thoma et al. 2006), recollection showed a trend (r33=−0.29, P=0.11), and feature recognition showed a significant negative association (r33=−0.44, P=0.01) with the SANS Sum of Global Ratings.
In conclusion, as hypothesized, based on the Dual-Process Signal Detection model, successful recognition of a target in patients with schizophrenia was less likely to be based on an explicit memory of the moment of learning (recollection) and more likely based on an impression of familiarity. However, these results are also accounted for by a unidimensional model, in which recollection and familiarity are additive, suggesting an overall reduction in memory strength, along with an altered criterion on the memory strength distribution for detecting new compared with old stimuli but not for detecting stimuli that are remembered versus familiar, in patients with schizophrenia.
Furthermore, while both patients and controls recognized more features on remember compared with know responses, validating patient’s ability to make “remember” and “know” judgments, patients recognized fewer stimulus details of the learning episode compared with controls, suggesting a deficit in organizing and integrating information during the learning episode and using such information for retrieval. Taken together, these results encourage the use of encoding and retrieval strategies to try to boost schizophrenia patients’ memory performance, and the use of functional magnetic imaging to dissociate the physiological abnormalities underlying the declarative memory deficits in schizophrenia on the R-K paradigm.
Finally, in our opinion, the current task design unfortunately does not allow for the adequate dissociation of the two memory models described and an in depth consideration of possible experiments that would allow such dissociations is beyond the scope of this discussion. Nevertheless, future experiments should attempt to make clearly dissociable predictions based on these memory models (for reviews see Parks and Yonelinas 2007; Wixted 2007a; Wixted 2007b) in order to aid in the interpretation of the nature of the memory deficits observed in schizophrenia.
Supplementary Material
Acknowledgments
The authors like to thank Malin McKinley from the Staglin Family Music Festival Center for the Assessment and Prevention of Prodromal States (CAPPS) and Samantha Swain and Michael DeGroot from the Aftercare Center at the University of California Los Angeles, U.S.A., for data management, Dr. Tara Niendam from CAPPS, UCLA for aid with coding of medications, professor John C. Dunn from the School of Psychology, University of Adelaide, Australia, for help with the estimation of the old-new and remember-know criteria, professor Andrew P. Yonelinas from the Center for Mind and Brain, University of California Davis, U.S.A., for checking the formulas used for estimation of recollection and familiarity using the dual-process signal-detection model, and the participants for taking part in the study. This research was funded by NIMH Grant P50 MH066286 to K.H.N., NIH Grants MH65079, MH066286, GM072978 and RR021992 to T.D.C., and a gift to the UCLA Foundation by Garen and Shari Staglin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Bates E, D’Amico S, Jacobsen T, Szekely A, Andonova E, Devescovi A, Herron D, Lu CC, Pechmann T, Pleh C, Wicha N, Federmeier K, Gerdjikova I, Gutierrez G, Hung D, Hsu J, Iyer G, Kohnert K, Mehotcheva T, Orozco-Figueroa A, Tzeng A, Tzeng O. Timed picture naming in seven languages. Psychon Bull Rev. 2003;10(2):344–380. doi: 10.3758/bf03196494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13(2):43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Danion JM, Cuervo C, Piolino P, Huron C, Riutort M, Peretti CS, Eustache F. Conscious recollection in autobiographical memory: an investigation in schizophrenia. Conscious Cogn. 2005;14(3):535–547. doi: 10.1016/j.concog.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Danion JM, Kazes M, Huron C, Karchouni N. Do patients with schizophrenia consciously recollect emotional events better than neutral events? Am J Psychiatry. 2003;160(10):1879–1881. doi: 10.1176/appi.ajp.160.10.1879. [DOI] [PubMed] [Google Scholar]
- Danion JM, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiatry. 1999;56(7):639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- Donaldson W. The role of decision processes in remembering and knowing. Mem Cognit. 1996;24(4):523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- Dudukovic NM, Knowlton BJ. Remember-Know judgments and retrieval of contextual details. Acta Psychol (Amst) 2006;122(2):160–173. doi: 10.1016/j.actpsy.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Dunn JC. Remember-Know: A Matter of Confidence. Psychological Review. 2004;111(2):524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- Gardiner JM, Java RI. Recollective experience in word and nonword recognition. Mem Cognit. 1990;18(1):23–30. doi: 10.3758/bf03202642. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Effects of neuroleptic medications on the cognition of patients with schizophrenia: a review of recent studies. Journal of Clinical Psychiatry. 1996;57(Suppl 93):62–65. [PubMed] [Google Scholar]
- Grillon ML, Johnson MK, Danion JM, Rizzo L, Verdet C, Huron C. Assessing a minimal executive operation in schizophrenia. Psychiatry Res. 2005;137(1–2):37–48. doi: 10.1016/j.psychres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Hautus M. Corrections for extreme proportions and their biasing effects on estimated valuds of d′. Behav Res Methods Instrum Comput. 1995;27:46–51. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hirshman E, Henzler A. The role of decision processes in conscious recollection. Psychological Science. 1998;9(1):61–65. [Google Scholar]
- Huron C, Danion JM. Impairment of constructive memory in schizophrenia. Int Clin Psychopharmacol. 2002;17(3):127–133. doi: 10.1097/00004850-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Huron C, Danion JM, Giacomoni F, Grange D, Robert P, Rizzo L. Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am J Psychiatry. 1995;152(12):1737–1742. doi: 10.1176/ajp.152.12.1737. [DOI] [PubMed] [Google Scholar]
- Huron C, Danion JM, Rizzo L, Killofer V, Damiens A. Subjective qualities of memories associated with the picture superiority effect in schizophrenia. J Abnorm Psychol. 2003;112(1):152–158. [PubMed] [Google Scholar]
- Jager T, Mecklinger A, Kipp KH. Intra- and inter-item associations doubly dissociate the electrophysiological correlates of familiarity and recollection. Neuron. 2006;52(3):535–545. doi: 10.1016/j.neuron.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kuperberg G, Heckers S. Schizophrenia and cognitive function. Curr Opin Neurobiol. 2000;10(2):205–210. doi: 10.1016/s0959-4388(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11(3):126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Neumann A, Blairy S, Lecompte D, Philippot P. Specificity deficit in the recollection of emotional memories in schizophrenia. Conscious Cogn. 2006;16(2):469–484. doi: 10.1016/j.concog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Parks CM, Yonelinas AP. Moving beyond pure signal-detection models: comment on Wixted (2007) Psychol Rev. 2007;114(1):188–202. doi: 10.1037/0033-295X.114.1.188. discussion 203-189. [DOI] [PubMed] [Google Scholar]
- Pelletier M, Achim AM, Montoya A, Lal S, Lepage M. Cognitive and clinical moderators of recognition memory in schizophrenia: a meta-analysis. Schizophr Res. 2005;74(2–3):233–252. doi: 10.1016/j.schres.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Ribeiro MA, Zuardi AW, Hetem LA. Method for evaluating subjective states of awareness that accompany recognition: adaptation for use in Portuguese-speaking patients with schizophrenia. Rev Bras Psiquiatr. 2005;27(4):278–284. doi: 10.1590/s1516-44462005000400005. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33(2):217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Reeder JA. Sum-difference theory of remembering and knowing: a two-dimensional signal-detection model. Psychol Rev. 2004;111(3):588–616. doi: 10.1037/0033-295X.111.3.588. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Pepple JR, Faraone SV, Kremen WS, Green AI, Brown WA, Tsuang MT. Neuropsychological performance in chronic schizophrenia in response to neuroleptic dose reduction. Biol Psychiatry. 1993;33(8–9):575–584. doi: 10.1016/0006-3223(93)90095-u. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia. 2007;45(10):2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Sonntag P, Gokalsing E, Olivier C, Robert P, Burglen F, Kauffmann-Muller F, Huron C, Salame P, Danion JM. Impaired strategic regulation of contents of conscious awareness in schizophrenia. Conscious Cogn. 2003;12(2):190–200. doi: 10.1016/s1053-8100(03)00016-3. [DOI] [PubMed] [Google Scholar]
- Souchay C, Bacon E, Danion JM. Metamemory in Schizophrenia: an exploration of the feeling-of-knowing state. J Clin Exp Neuropsychol. 2006;28(5):828–840. doi: 10.1080/13803390591000846. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26(36):9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely A, D’Amico S, Devescovi A, Federmeier K, Herron D, Iyer G, Jacobsen T, Bates E. Timed picture naming: extended norms and validation against previous studies. Behav Res Methods Instrum Comput. 2003;35(4):621–633. doi: 10.3758/bf03195542. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Ruhrmann S, Brockhaus A, Pukrop R, Klosterkotter J. Remembering or knowing: electrophysiological evidence for an episodic memory deficit in schizophrenia. Psychol Med. 2002;32(7):1261–1271. doi: 10.1017/s0033291702006335. [DOI] [PubMed] [Google Scholar]
- Thoma P, Zoppelt D, Wiebel B, Daum I. Recollection and familiarity in negative schizophrenia. Neuropsychologia. 2006;44(3):430–435. doi: 10.1016/j.neuropsychologia.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Waters FA, Maybery MT, Badcock JC, Michie PT. Context memory and binding in schizophrenia. Schizophr Res. 2004;68(2–3):119–125. doi: 10.1016/S0920-9964(03)00221-4. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev. 2007a;114(1):152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Spotlighting the Probative Findings: Reply to Parks and Yonelinas (2007) Psychol Rev. 2007b;114(1):203–209. [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev. 2004;11(4):616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The Nature of Recollection and Familiarity: A Review of 30 Years of Research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12(3):323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.