Abstract
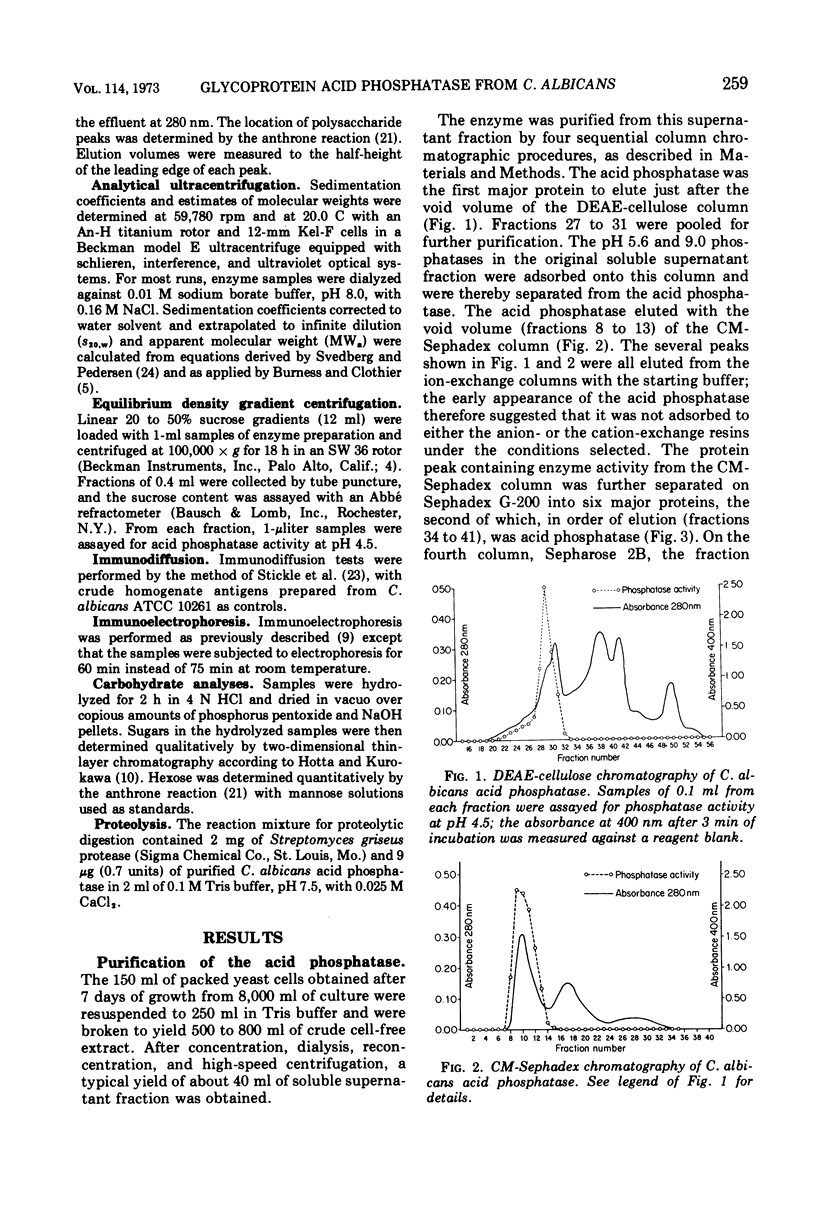
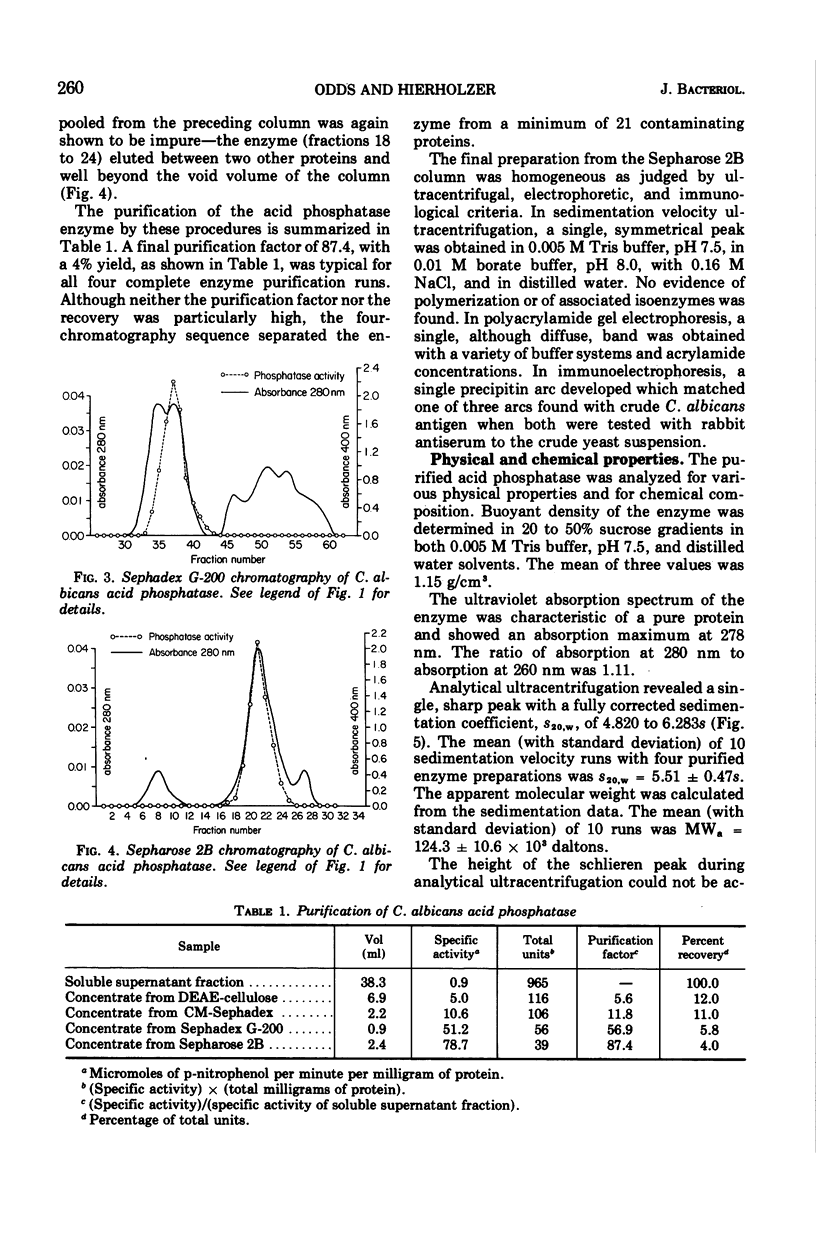
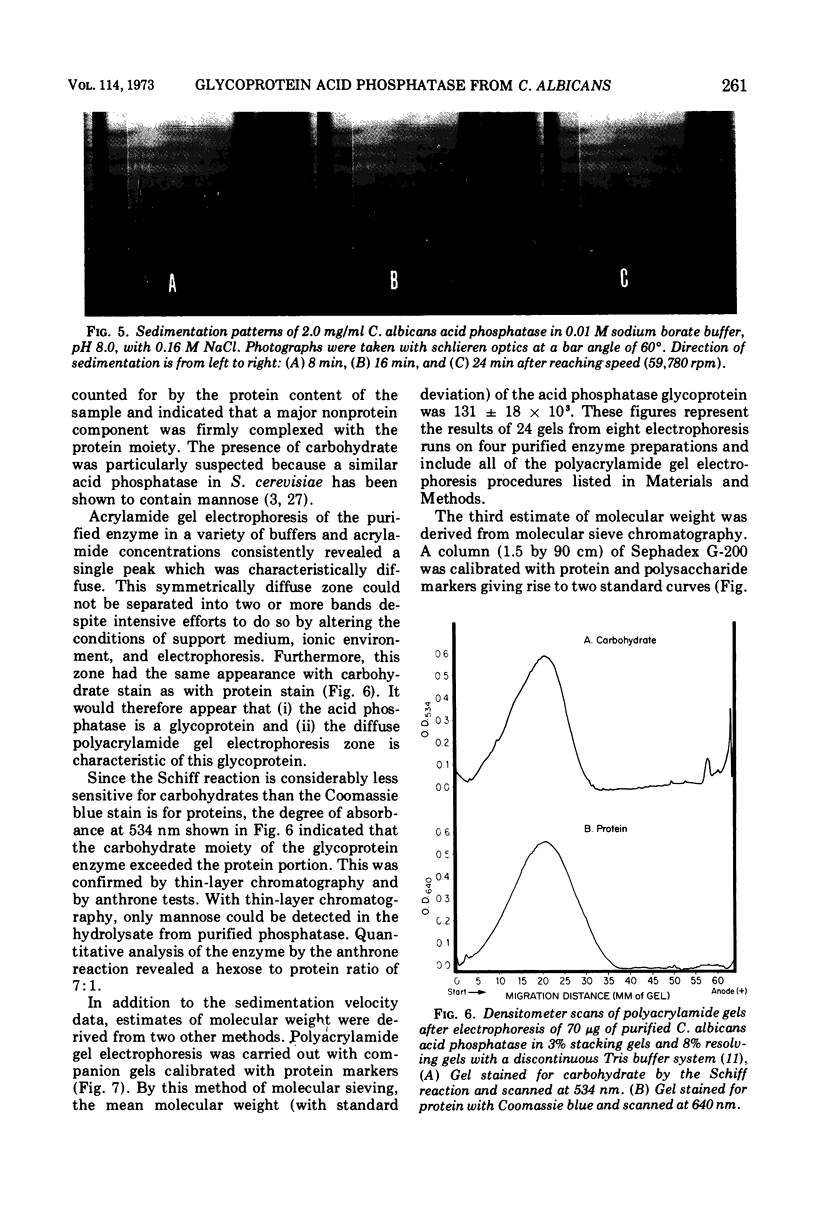
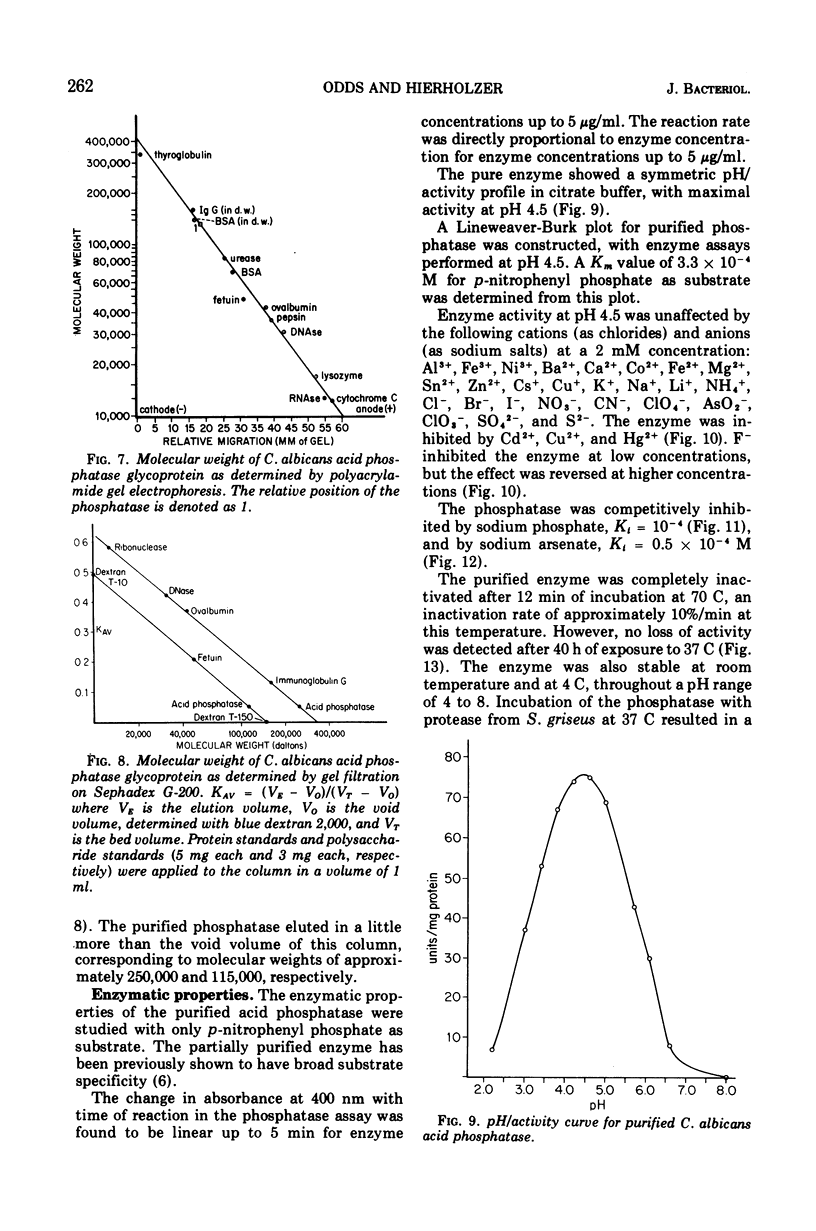
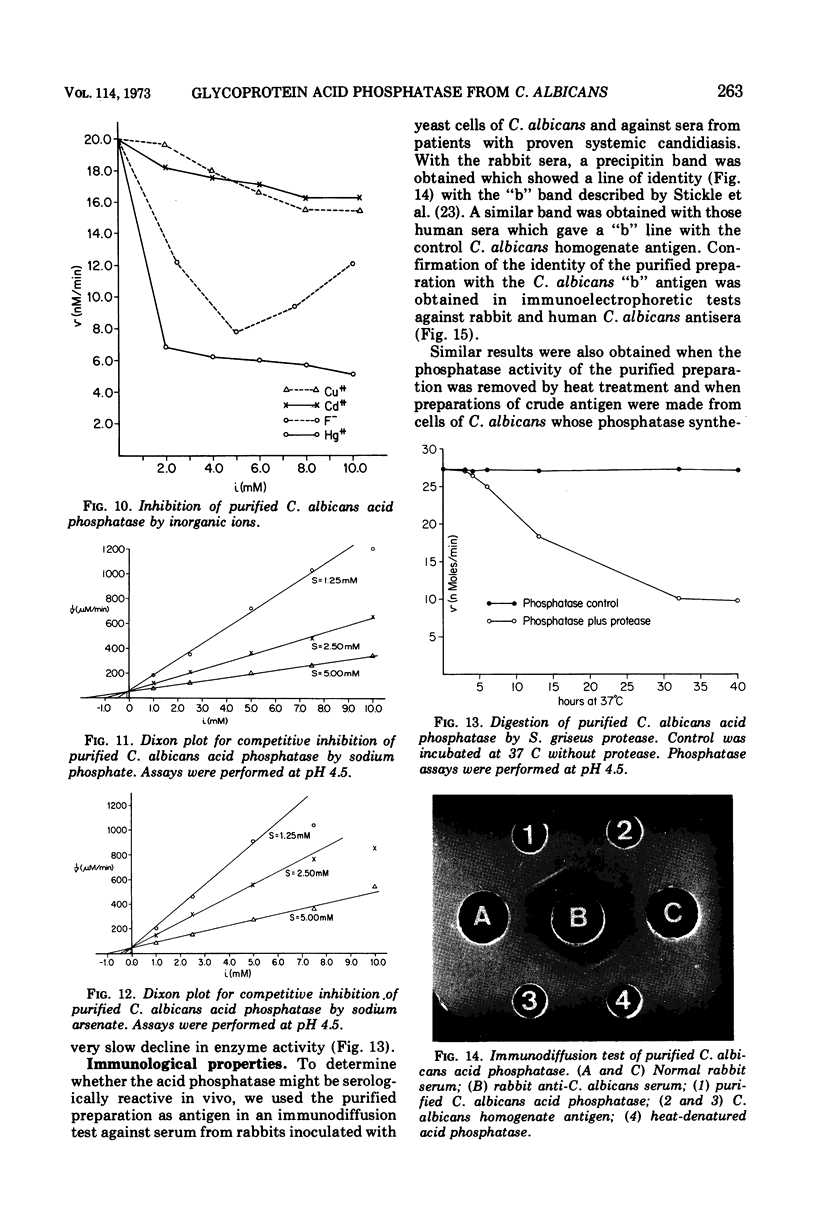
An acid phosphomonoesterase was purified 87-fold with a 4% recovery from disintegrated cells of Candida albicans by four stages of column chromatography. The purified enzyme was homogeneous by ultracentrifugal, electrophoretic, and immunological analyses. The fully corrected sedimentation coefficient, s20,w, was calculated to be 5.51s. Molecular weight estimated from ultracentrifugal data was 124.3 × 103, from gel chromatography was 115 × 103, and from acrylamide gel electrophoretic data was 131 × 103. Buoyant density in sucrose was 1.15 g/cm3. The enzyme was a mannoprotein with a hexose to protein ratio of 7: 1. The Michaelis constant of the enzyme was 3.3 × 10−4 M for p-nitrophenyl phosphate as substrate, and the pH optimum was 4.5. The enzyme was competitively inhibited by inorganic phosphate (Ki = 10−4 M) and by arsenate (Ki = 0.5 × 10−4 M). A wide range of inorganic cations and anions did not affect enzyme activity, but Hg2+, Cd2+, and Cu2+ were inhibitory. F− was also inhibitory at low concentrations, but the effect was reversed at higher concentrations. Phosphatase activity was completely destroyed by exposure of the enzyme to 70 C for 12 min, but was destroyed only slowly by proteolytic hydrolysis. The purified glycoprotein enzyme gave a line of identity with the “b” antigen of crude C. albicans homogenates in immunodiffusion and immunoelectrophoresis tests with sera from rabbits inoculated with intact C. albicans cells and from humans with proven candidiasis. Preliminary evidence suggests that the mannan and not the protein portion of the enzyme molecule is responsible for this antigenicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers H., Büsing G., Schudt G. Uber die aktive Gruppe der sauren Hefephosphatase (3,5-Phosphatase) Enzymologia. 1966 Mar 31;30(3):149–178. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer P., Steyn-Parvé E. P. Isolation and purification of an acid phosphatase from baker's yeast (Saccharomyces cerevisiae). Biochim Biophys Acta. 1966 Nov 15;128(2):400–402. doi: 10.1016/0926-6593(66)90189-5. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Clothier F. W. Particle weight and other biophysical properties of encephalomyocarditis virus. J Gen Virol. 1970 Mar;6(3):381–393. doi: 10.1099/0022-1317-6-3-381. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Odds F. C., Barlow A. J. An examination of the production of hydrolytic enzymes and toxins by pathogenic strains of Candida albicans. J Gen Microbiol. 1971 Aug;67(3):255–263. doi: 10.1099/00221287-67-3-255. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Palmer E. L., Whitfield S. G., Kaye H. S., Dowdle W. R. Protein composition of coronavirus OC 43. Virology. 1972 May;48(2):516–527. doi: 10.1016/0042-6822(72)90062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K., Kurokawa M. Separation of fucose and acetylhexosamines by two-dimensional thin-layer chromatography. Anal Biochem. 1968 Dec;26(3):472–474. doi: 10.1016/0003-2697(68)90216-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MCLELLAN W. L., Jr, LAMPEN J. O. The acid phosphatase of yeast. Localization and secretion by protoplasts. Biochim Biophys Acta. 1963 Feb 12;67:324–326. doi: 10.1016/0006-3002(63)91832-8. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Summers D. F., Scharff M. D. SDS-acrylamide gel electrophoresis and its application to the proteins of poliovirus- and adenovirus-infected human cells. J Cell Physiol. 1970 Dec;76(3):273–287. doi: 10.1002/jcp.1040760307. [DOI] [PubMed] [Google Scholar]
- SCHMIDT G., BARTSCH G., LAUMONT M. C., HERMAN T., LISS M. Acid phosphatase of bakers' yeast: an enzyme of the external cell surface. Biochemistry. 1963 Jan-Feb;2:126–131. doi: 10.1021/bi00901a022. [DOI] [PubMed] [Google Scholar]
- SUOMALAINEN H., LINKO M., OURA E. Changes in the phosphatase activity of Baker's yeast during the growth phase and location of the phosphatases in the yeast cell. Biochim Biophys Acta. 1960 Jan 29;37:482–490. doi: 10.1016/0006-3002(60)90505-9. [DOI] [PubMed] [Google Scholar]
- Schurr A., Yagil E. Regulation and characterization of acid and alkaline phosphatase in yeast. J Gen Microbiol. 1971 Mar;65(3):291–303. doi: 10.1099/00221287-65-3-291. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stickle D., Kaufman L., Blumer S. O., McLaughlin D. W. Comparison of a newly developed latex agglutination test and an immunodiffusion test in the diagnosis of systemic candidiasis. Appl Microbiol. 1972 Mar;23(3):490–499. doi: 10.1128/am.23.3.490-499.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONINO G. J., STEYN-PARVE E. P. Localization of some phosphatases in yeast. Biochim Biophys Acta. 1963 Mar 12;67:453–469. doi: 10.1016/0006-3002(63)91851-1. [DOI] [PubMed] [Google Scholar]
- Taschdjian C. L., Kozinn P. J., Cuesta M. B., Toni E. F. Serodiagnosis of Candidal infections. Am J Clin Pathol. 1972 Feb;57(2):195–205. doi: 10.1093/ajcp/57.2.195. [DOI] [PubMed] [Google Scholar]
- Van Rijn H. J., Boer P., Steyn-Parvé E. P. Biosynthesis of acid phosphatase of baker's yeast. Factors influencing its production by protoplasts and characterization of the secreted enzyme. Biochim Biophys Acta. 1972 May 12;268(2):431–441. doi: 10.1016/0005-2744(72)90339-7. [DOI] [PubMed] [Google Scholar]
- WARD D. N., ARNOTT M. S. GEL FILTRATION OF PROTEINS, WITH PARTICULAR REFERENCE TO THE GLYCOPROTEIN, LUTEINIZING HORMONE. Anal Biochem. 1965 Aug;12:296–302. doi: 10.1016/0003-2697(65)90094-1. [DOI] [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. EVIDENCE FOR AN EXOCELLULAR SITE FOR THE ACID PHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1964 Dec;88:1743–1754. doi: 10.1128/jb.88.6.1743-1754.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]






