Abstract
Erythropoietin (Epo), a known hematopoietic growth factor, has been reported to promote tumor growth and angiogenesis in Epo receptor (EpoR)-positive tumors, but its effects on EpoR-negative tumors have not been clearly shown. Here, we show that Epo accelerates the growth of EpoR-negative tumors by promoting tumor angiogenesis. Mice were inoculated with Lewis lung carcinoma cells and treated with Epo. Erythropoietin accelerated tumor growth and increased intratumoral microvessel density, although it did not accelerate Lewis lung carcinoma cell tumor proliferation in vitro. To observe the direct effect of Epo on endothelial cells, we examined human dermal microvascular endothelial cells (HMVECs) that expressed EpoR. Erythropoietin induced the proliferation of HMVECs and protected them from H2O2-induced cell death. Erythropoietin activated the extracellular signal-regulated kinase signaling pathway and up-regulated the expression of the downstream antiapoptotic protein Bcl-xL in HMVECs. Moreover, in both the absence and presence of tumors, in vivo treatment of mice with Epo increased circulating endothelial progenitor cells. To investigate the role of Epo in a primary tumor model, we inoculated the chemical carcinogen methylcholanthrene (MCA) subcutaneously into mice at two doses, a high or a low dose, which induced fibrosarcoma, and treated them with Epo. Erythropoietin promoted tumor growth after MCA inoculation at both doses and decreased the overall survival of the mice inoculated with the high-dose MCA. However, Epo did not increase the incidence of fibrosarcoma at either dose. Lewis lung carcinoma cells and MCA-induced fibrosarcomas did not express EpoR. These results suggest that Epo accelerates the growth of tumors that lack EpoR expression by promoting tumor angiogenesis.
Introduction
Hematopoietic growth factors are often used in intensive cancer chemotherapy to help overcome myelosuppression, a reduction in blood cell numbers caused by anticancer treatment. However, recent studies in mice suggest that hematopoietic growth factors such as macrophage colony-stimulating factor (M-CSF) and granulocyte colony-stimulating factor (G-CSF) can cause tumor growth by promoting angiogenesis [1–3], which supplies blood to solid tumors [4,5]. Erythropoietin (Epo) is one of the hematopoietic growth factors often used in cancer treatment. It normally regulates the proliferation, survival, and differentiation of the erythroid lineage, but recent studies have shown that Epo can act on nonhematopoietic organs including solid tumors [6]. The effect of Epo on the survival rate of cancer patients seems variable: Epo decreases the survival of cancer patients with head and neck, metastatic breast, or non-small cell lung cancer [7–9], but by contrast, erythropoiesis-stimulating agents (ESAs), which include Epo, does not reduce the survival rate of patients with small cell lung cancer [10]. In 2007, the Food and Drug Administration convened a meeting to discuss the risks of administrating ESAs to cancer patients. However, no clear conclusion was reached, and the Food and Drug Administration simply advised caution in the use of ESAs [10]. A clearer understanding of Epo's effect on tumor growth is therefore urgently needed to help clinicians decide whether to prescribe Epo to their cancer patients.
One way in which Epo could trigger tumor growth is by acting directly on the tumor cells because many tumor cells express the Epo receptor (EpoR). However, the response of EpoR-expressing tumor cells to Epo varies. Some studies found that Epo treatment can increase tumor cell numbers in vitro [11–13], although others found no effect [6]. In vivo studies found that blocking Epo function can inhibit the progression of certain tumors [13,14], although other studies found that Epo treatment had no effect on tumor growth [15]. Whereas the effect of Epo on EpoR-positive tumors is still controversial, even less is known about the effects of Epo on EpoR-negative tumors.
Several studies suggest that Epo could act indirectly on tumor growth. For instance, Epo has been reported to act on tumor angiogenesis [6,16] and some studies have shown a direct effect of Epo on some endothelial cells (ECs) [17,18]. Moreover, Epo can increase the number of circulating endothelial progenitor cells (EPCs) in tumor-free humans and mice [19,20]. However, although EPCs have recently been found to contribute to tumor vessel formation [21,22], the effect of Epo on circulating EPCs has not been reported in cancer models. In addition, because most previous studies used EpoR-positive tumors, they could not distinguish between a direct effect of Epo on tumors and an indirect effect through angiogenesis.
In the present study, we examined the effect of Epo on two types of EpoR-negative tumors: implanted Lewis lung carcinoma cells (LLCs) and primary fibrosarcoma induced by a chemical carcinogen methylcholanthrene (MCA) [23]. We found that Epo could trigger growth of these tumors by stimulating angiogenesis and examined which pathway responded to Epo in the ECs.
Materials and Methods
Cell Culture
Lewis lung carcinoma cells, H9c2 cells, and KLN 205 cells were purchased from American Type Culture Collection (Manassas, VA). Lewis lung carcinoma cells were cultured in a high-glucose Dulbecco's modified Eagle's medium containing 10% fetal calf serum (FCS) and 100 µg/ml kanamycin. H9c2 cells were cultured in a high-glucose Dulbecco's modified Eagle's medium containing 10% FCS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. H9c2 cells were differentiated to H9c2 myotubes as previously shown [24]. KLN 205 cells were cultured in minimum essential medium containing 10% FCS, 1% nonessential amino acids, and 100 µg/ml kanamycin. Normal adult human dermal microvascular endothelial cells (HMVECs), originally derived from foreskins, and human umbilical vein endothelial cells (HUVECs) were purchased from Kurabo (Osaka, Japan) and were cultured in HuMedia-MvG medium (Kurabo).
Lewis Lung Carcinoma Cell Tumor Model
Lewis lung carcinoma cells (3 x 105 cells per mouse) were injected subcutaneously into the hind flank of male C57BL/6 mice (6–9 weeks old) on day 0. Tumor size was quantified daily as width2 x length x 0.52 [1]. For tumor growth rate models, human recombinant Epo (epoetin beta, 200 IU/kg; Chugai Seiyaku, Tokyo, Japan) was injected into mice subcutaneously once a week from day 1. The mice were killed on day 25 (total four Epo injections per mouse). For the culture assay of EPCs, Epo was injected subcutaneously for 3 days daily from day 18, and the mice were killed on day 21. Controls were subcutaneously injected with phosphate-buffered saline (PBS).
Histology
When the diameter of the tumors reached approximately 1.2 cm, tumors were fixed in 10% formalin, embedded in paraffin and sectioned [1]. Control mice were killed on day 21, and the Epo-treated mice were killed on day 19 (total three Epo injections). The sections were stained with hematoxylin and eosin (H&E). The intratumoral microvessel density was determined as previously described by immunohistochemical staining with polyclonal anti-human factor VIII-related antigen antibody (DakoCytomation, Carpinteria, CA) [1,25,26].
Cell Proliferation and Cell Death Assays
The assays were performed as previously shown [27]. In short, LLCs (5 x 103 cells) were incubated with 0, 0.2, 1, or 5 IU/ml Epo for 48 hours. Human dermal microvascular endothelial cells (3 x 103 cells) were incubated with 0, 0.008, 0.04, 0.2, or 1 IU/ml Epo for 36 or 48 hours. Then, the cell numbers were determined by water-soluble tetrazolium (WST) assay using a Cell Counting Kit (Dojindo, Tokyo, Japan). For cell death assays, HMVECs were incubated with 0, 0.04, 0.2, or 1 IU/ml Epo for 16 hours. Then, the cells were stimulated with H2O2 for 8 hours. The WST assay determined the cell viability.
Flow Cytometry
Fluorescein isothiocyanate-labeled anti-CD34 and purified rat anti-CD144 (VE-cadherin) antibodies were purchased from BD Pharmingen (San Diego, CA), PE-labeled anti-EpoR was purchased from DakoCytomation, and control rat IgG2a was purchased from eBioscience (San Diego, CA). Flow cytometry for EPCs was performed as previously shown [1]. For EpoR detection, the cells were first incubated with unlabeled anti-CD16/32 mAb (eBioscience) and then with the anti-EpoR antibody. Flow cytometry was performed with a FACScan (BD Bioscience, San Jose, CA), and data were analyzed with CellQuest software (BD Bioscience) [1].
Western Blot Analysis
Western blot analysis was performed as previously shown [28]. Human dermal microvascular endothelial cells (1 x 106 cells) were stimulated with Epo (1 IU/ml) for indicated periods. Methylcholanthrene-induced fibrosarcoma cells (3 x 106 cells) or LLCs (2 x 106 cells) were plated onto a culture dish, cultured overnight, and lysed. H9c2 cells were differentiated to H9c2 myotubes, stimulated with M-CSF (100 ng/ml) for 10 minutes, and lysed as previously shown [24]. HeLa cell lysates were purchased from Cell Signaling Technology (Beverly, MA). The cell lysates were subjected to gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membranes were blotted with antibodies to phospho-extracellular signal-regulated kinase (ERK), phospho-Akt, phospho-signal transducer and activator of transcription 5 (Stat5), Bcl-xL (Cell Signaling Technology), or EpoR (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes blotted with antibodies to detect phosphorylation were then reblotted with antibodies to total ERK, Akt, and Stat5 (Cell Signaling Technology).
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
Methylcholanthrene-induced fibrosarcoma cells (3 x 106 cells), LLCs (2 x 106 cells), or KLN 205 cells (3 x 106 cells) were plated onto a culture dish and cultured overnight, and total RNA was isolated using RNAzol B reagent (Tel-Test, Friendswood, TX). Conventional reverse transcription-polymerase chain reaction (RT-PCR) was performed as previously shown [1] using primers as previously described [29].
Culture Assay of Circulating EPCs
Mononuclear cells were isolated, cultured, characterized, and counted as previously described [30] with some modifications [1]. Fluorescent microscopy identified cultured circulating EPCs as double-positive cells for acetylated low-density lipoprotein-Dil complex (Dil-acLDL; Molecular Probes, Eugene, OR) and fluorescein isothiocyanate-labeled lectin from Ulex europaus (Sigma-Aldrich, St. Louis, MO). Two independent investigators evaluated the number of double-positive cells by counting three randomly selected high-power fields.
Methylcholanthrene-Induced Fibrosarcoma
Six-week-old male C57BL/6 mice were injected subcutaneously into the hind flank with 25 (low dose) or 100 µg (high dose) of 3-MCA (Sigma-Aldrich) in 0.1 ml of maize oil. Development of the fibrosarcoma was assessed periodically for 100 to 150 days. Tumors more than 2 mm in diameter were recognized as positive. Tumor size was quantified as described in the Lewis Lung Carcinoma Cell Tumor Model section. For the MCA-induced fibrosarcoma cell isolation, when the diameter of 100-µg MCA-induced fibrosarcoma reached 1 cm, the mice were killed and the tumors were removed aseptically. Tumors were cut into small pieces and treated with collagenase (Sigma type IV) at 37°C for 1 hour. Clumps were removed, and single cells were cultured in RPMI 1640 medium supplemented with 10% FCS and 2 mM l-glutamine.
Data Analysis
Data are shown as mean ± SD. Statistical analysis was performed using analysis of variance with Fisher's least significant difference test. Statistical analysis of the overall survival of MCA-induced fibrosarcoma-bearing mice was performed using Mann-Whitney U test. P values < .05 were considered as significant.
Results
Erythropoietin Accelerates Tumor Growth In Vivo But Not In Vitro
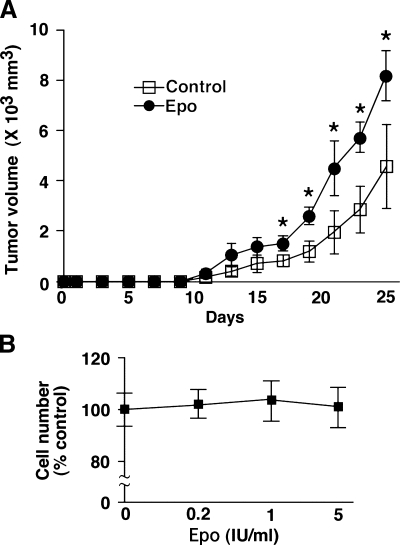
To test the effect of Epo in vivo, we inoculated LLCs into mice subcutaneously on day 0 and injected Epo or PBS into the mice once a week starting at day 1. Erythropoietin significantly accelerated tumor growth (Figure 1A). To test whether this growth might reflect a direct effect of Epo on LLCs, we examined the response of LLCs to Epo in vitro. We found that Epo did not increase LLC proliferation in vitro (Figure 1B). Because the FCS might contain some growth factors that made high-growing background, we examined the response of LLCs to Epo under low-FCS culture medium condition that contained 5% FCS. We found that Epo did not increase LLC proliferation under this condition (data not shown). The effect of Epo on LLC tumor growth therefore seems to be indirect.
Figure 1.
Epo accelerates tumor growth in vivo but not in vitro. (A) Mice were inoculated with LLCs on day 0 and treated with Epo or PBS from day 1 once a week. Erythropoietin significantly accelerated tumor growth (n = 8 per group, *P < .03). (B) Lewis lung carcinoma cells (5 x 103 cells) were cultured with the indicated amounts of Epo for 48 hours. Water-soluble tetrazolium assay determined the cell number. Shown is representative of three independent experiments.
Erythropoietin Increases Tumor Microvessel Density In Vivo
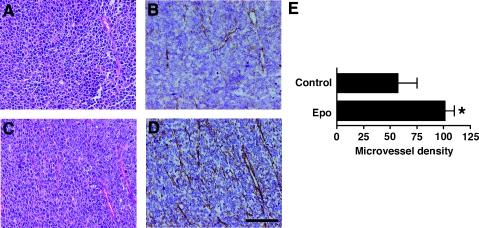
To analyze the mechanism of tumor growth acceleration by Epo, we stained the tumors with H&E and found that the number of tumor blood vessels was higher in Epo-treated mice than in PBS-treated mice (Figure 2, A and C). To quantify tumor angiogenesis, we stained tumors with an antibody against the factor VIII-related antigen, a blood vessel marker (Figure 2, B and D) [1,26]. Erythropoietin significantly increased microvessel density in tumors (Figure 2E).
Figure 2.
Epo increases tumor microvessel density in vivo. (A–D) Mice were inoculated with LLCs on day 0 and killed when the diameter of the tumors reached 1.2 cm. Control mice (A and B) were killed on day 21, and Epo-treated mice (C and D) were killed on day 19 (total three Epo injections). Sections were stained with H&E (A and C) and anti-factor VIII-related antigen antibody (B and D); scale bar, 100 µm. Images show one representative mouse of eight in each group. (E) Epo significantly increased microvessel density in tumors (n = 8 per group, *P < .01).
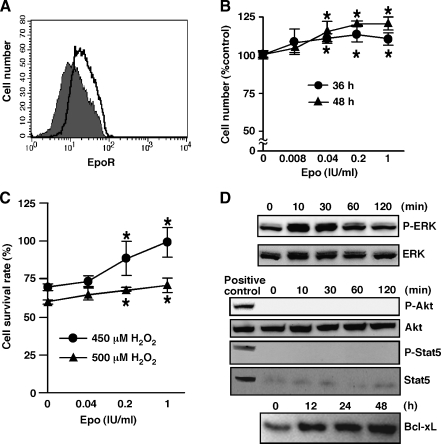
Erythropoietin Promotes Proliferation and Survival of HMVEC In Vitro, Activates ERK Signaling, and Increases Bcl-xL Expression
Because Epo promoted tumor angiogenesis, we examined effect of Epo on ECs more precisely in vitro. We examined EpoR expression on two types of ECs using FACS analysis. We found EpoR expression on HMVECs (Figure 3A) but not on HUVECs (data not shown). Because HMVECs expressed EpoR, we next examined HMVEC proliferation after various periods of exposure to Epo. Erythropoietin significantly promoted proliferation of HMVECs at 36 and 48 hours of exposure (Figure 3B) but not at 24 hours (data not shown). Another hematopoietic growth factor, M-CSF, supports the survival of cardiomyocytes and skeletal muscle cells in the presence of the toxic dose of H2O2 [24]. Therefore, we examined the effect of Epo on the survival of HMVECs exposed to H2O2. Erythropoietin significantly protected HMVECs from H2O2-induced cell death (Figure 3C). In erythroid cells, Epo activates cell signaling pathways such as the Akt pathway, the Janus-associated kinase 2 (Jak2)-Stat5 pathway, and the ERK pathway [31,32]. To elucidate the molecular mechanisms of Epo-induced proliferation and survival, the activation status of the ERK, Akt, and Stat5 signaling pathways was investigated in HMVECs after treatment with Epo. Erythropoietin activated ERK, as indicated by ERK phosphorylation, although it did not affect the total ERK protein levels in cell lysates (Figure 3D). In contrast, Epo did not activate Akt or Stat5 (Figure 3D). Cell lysates derived from H9c2 myotubes stimulated with M-CSF and HeLa cells stimulated with IFN-α were used as positive controls for the activated form of Akt and Stat5 respectively (Figure 3D). Extracellular signal-regulated kinase activation by Epo up-regulates the antiapoptotic protein Bcl-xL in a leukemia cell line and in erythroid progenitor cells [33]. Therefore, we examined Bcl-xL expression in Epo-stimulated HMVECs. Low-level Bcl-xL expression was detected in HMVECs without Epo stimulation (Figure 3D). Erythropoietin up-regulated Bcl-xL expression after 12 and 24 hours of exposure (Figure 3D) before Epo has any effect on cell proliferation. At later time points, Bcl-xL levels kept increasing, but we cannot interpret this increase because of Epo's effect on cell proliferation. These results suggest that Epo induces the proliferation and survival of HMVECs by activating ERK and their survival by up-regulating Bcl-xL.
Figure 3.
Epo promotes the proliferation and survival of HMVECs in vitro, activates ERK signaling, and increases Bcl-xL expression. (A) Human dermal microvascular endothelial cells expressed EpoR. The shaded histogram indicates staining with EpoR, and the blank histogram indicates staining with control IgG. Shown is representative of two independent experiments. (B) Human dermal microvascular endothelial cells were cultured with indicated amount of Epo for indicated periods, and WST assay determined the cell number. Erythropoietin (0.04, 0.2, and 1 IU/ml) significantly increased the number of HMVECs (*P < .05). (C) Human dermal microvascular endothelial cells were cultured with indicated amount of Epo for 16 hours and then stimulated with H2O2 for 8 hours. Erythropoietin (0.2 and 1 IU/ml) significantly protected HMVECs from H2O2-induced cell death (*P < .02). (D) Human dermal microvascular endothelial cells were stimulated with Epo (1 IU/ml) for the indicated periods, and then cell lysates were blotted with antibodies specific for the activated form of ERK (phospho-ERK), Akt (phospho-Akt), or Stat5 (phospho-Stat5). The membranes were reblotted with antibodies to total ERK, Akt, or Stat5, respectively. Cell lysates derived from M-CSF-stimulated H9c2 myotubes and IFN-α-stimulated HeLa cells were used as positive controls for the activated form of Akt and Stat5, respectively (D). Bcl-xL expression was confirmed by the specific antibody. Shown is representative of two independent experiments.
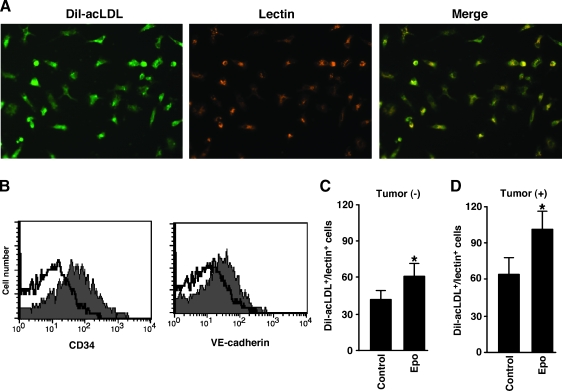
Erythropoietin Increases the Number of Circulating EPCs in Tumor-Bearing Mice
We hypothesized that Epo enhanced tumor angiogenesis by increasing the numbers of circulating EPCs in addition to directly stimulating ECs. To investigate the effects of Epo on the number of circulating EPCs, mice were injected with Epo for 3 days and circulating EPCs, characterized by Dil-acLDL uptake, lectin binding, and CD34 and VE-cadherin expression (Figure 4, A and B) [34–36], were counted. Erythropoietin significantly increased the number of circulating EPCs (Figure 4C). We next evaluated the effect of Epo on circulating EPCs in mice bearing LLCs. Injection of Epo once a week starting 1 day after LLC inoculation significantly increased the tumor volume after day 17 (Figure 1A). Previous study showed that a growing LLC tumor itself increased serum vascular endothelial growth factor (VEGF) level and the numbers of EPCs [1], which suggested that when the volume of LLC tumors increased, it could 2increase the number of circulating EPCs. To isolate circulating EPCs from mice bearing a similar volume of LLC tumors, we injected Epo from day 18 for three consecutive days and isolated EPCs on day 21. This treatment significantly increased the number of circulating EPCs (Figure 4D) without significantly affecting tumor size (data not shown).
Figure 4.
Epo increases the number of circulating EPCs in tumor-bearing mice. (A) Mononuclear cells were isolated from peripheral blood and cultured. Fluorescence microscopy determined Dil-acLDL uptake (left panel) and lectin binding (middle panel) of adherent cells. Double-positive cells (merge) were considered as cultured circulating EPCs (right panel). (B) Expression of CD34 and VE-cadherin on cultured circulating EPCs. The shaded histograms indicate staining with Abs to CD34 or VE-cadherin, and the blank histograms indicate background staining with control IgG. (C and D) Mice were injected with Epo for 3 days daily and were then killed (C). Mice were inoculated with LLCs on day 0, injected with Epo for 3 days daily from day 18, and killed on day 21 (D). Mononuclear cells (4 x 106 cells per mouse) were isolated from peripheral blood and cultured. Adherent Dil-acLDL and lectin double-positive cells were counted. Erythropoietin significantly increased the number of double-positive cells (n = 8 per group, *P < .01).
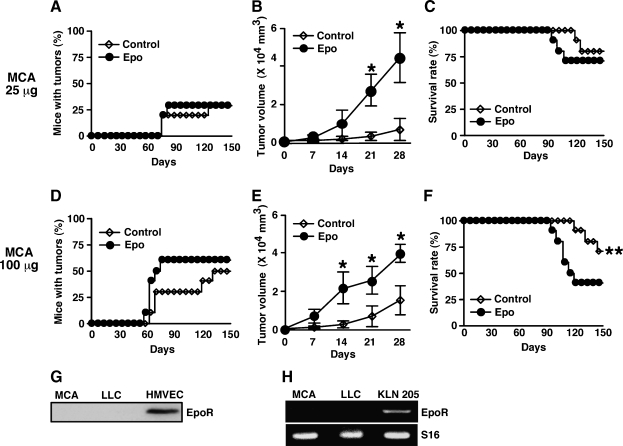
Erythropoietin Promotes Tumor Growth But Does Not Increase the Incidence of MCA-Induced Fibrosarcoma and Impairs Overall Survival of Mice Inoculated with High-Dose MCA
We next investigated whether Epo impaired overall survival and increased the incidence of primary tumor development after treatment with the chemical carcinogen MCA. We injected 25 (low dose) or 100 µg (high dose) of MCA into mice and injected Epo once a week for 100 to 150 days starting 1 day after MCA injection. Erythropoietin did not significantly increase tumor incidence at either dose of MCA (Figure 5, A and D). However, it significantly promoted tumor growth after the onset of the tumors at both doses (Figure 5, B and E). Erythropoietin significantly impaired the overall survival of mice inoculated with the high dose of MCA but not the lower dose (Figure 5, C and F). Western blot analysis showed EpoR in HMVECs but not in MCA-induced fibrosarcoma cells or in LLCs (Figure 5G), which was consistent with the result in Figures 1B and 2F. Conventional RT-PCR showed mRNA of EpoR in squamous carcinoma cell line KLN 205 cells, which Epo significantly increased proliferation rate in vitro (data not shown), but not in MCA-induced fibrosarcoma cells or in LLCs (Figure 5H).
Figure 5.
Epo promotes tumor growth but does not increase the incidence of MCA-induced fibrosarcoma and impairs overall survival of mice inoculated with high-dose MCA. Mice were subcutaneously inoculated with 25 (low dose) or 100 µg (high dose) of MCA into the hind flank on day 0, treated with Epo or PBS from day 1 once a week, and observed for fibrosarcoma development during the course of 100 to 150 days (n = 10 mice per group). (A and D) Percentages of mice with tumors are shown. (B and E) Tumor volumes after the onset of tumor development are shown (n = 3–6 per group). Erythropoietin significantly accelerated tumor growth (*P < .03). (C and F) Overall survival of mice is shown. Erythropoietin significantly decreased overall survival of 100-µg MCA inoculated mice but not of 25-µg MCA-inoculated mice (**P < .04). (G) Cell lysates were isolated from MCA-induced fibrosarcoma, LLCs, or HMVECs and blotted with specific antibody for EpoR. Methylcholanthrene-induced fibrosarcoma or LLC did not express EpoR. (H) Total RNA was isolated from MCA-induced fibrosarcoma, LLCs, or KLN 205 cells, and conventional RT-PCR was performed for EpoR and S16. KLN 205 cells expressed mRNA of EpoR but MCA-induced fibrosarcoma or LLCs did not.
Discussion
In this study, we found that Epo accelerated the growth of EpoR-negative tumors in vivo by promoting angiogenesis, both in a primary tumor model (MCA-induced tumor) and a conventional tumor model (LLC inoculation). Erythropoietin directly increased EC proliferation and decreased EC death by activating ERK and up-regulating the downstream antiapoptotic protein Bcl-xL. In addition, Epo increased the number of circulating EPCs in tumor-bearing mice. In the MCA-induced primary tumor model, Epo impaired the overall survival of the 100-µg MCA-inoculated mice but not of the 25-µg MCA-inoculated mice. However, Epo did not increase MCA-induced tumor incidence.
We have previously reported that other hematopoietic growth factors other than Epo could accelerate tumor growth by promoting angiogenesis. Macrophage colony-stimulating factor up-regulated serum concentration of VEGF, a potent angiogenic factor [1], and G-CSF increased the number of a type of endothelial cell precursors known as Gr1+CD11b+ cells [2] and promoted tumor angiogenesis and growth in mice. Unlike these hematopoietic growth factors, Epo did not increase VEGF or Gr1+CD11b+ cell numbers in mice (data not shown). However, we showed that Epo could act on ECs directly.
Erythropoietin significantly impaired the overall survival of mice inoculated with the higher dose but not the lower dose of MCA. This difference might be due to the insufficient number of tumor-positive mice in 25-µg MCA low-dose groups. The high dose ofMCA induced 5 to 6 tumor-positive mice among 10 mice, whereas the 25-µg dose induced only 3 tumor-positive mice among 10 mice. Therefore, to analyze the overall survival, the number of tumor-positive mice in low-dose MCA-inoculated groups might be insufficient.
To our knowledge, our study is the first to examine the effect of Epo on HMVECs. Our observations suggest that different ECs respond to Epo in different manners. For instance, a previous study using EA.hy926 endothelial cells, which are derived from the fusion of HUVEC with A549 lung carcinoma cells, showed that Epo activated Jak2 [17]. Instead of Jak2, we examined its downstream effector Stat5 and found that it was not activated by Epo in HMVECs, suggesting that Epo does not activate Jak2 in these cells. Very recently, another study showed that Epo activated Akt and ERK in HUVECs [18]. By contrast, we could only find evidence for ERK activation after Epo treatment of HMVECs. Interestingly, we could not detect EpoR on HUVECs we looked at by FACS analysis (data not shown), whereas we could detect it on HMVECs. Consistent with this finding, Epo treatment accelerated the proliferation of HMVECs but not of HUVECs (data not shown). Difference in culture conditions or in the source of HUVECs might explain why we failed to detect any response from HUVECs to Epo. Further experiments are required for pursuing the effect of Epo on ECs using other types of ECs.
Furthermore, our study shows that Epo increases the number of 2circulating EPCs in tumor-bearing mice. The role of circulating EPCs in tumor angiogenesis has been controversial. Using bone marrow transplantation, some studies reported large contributions of circulating EPCs to tumor angiogenesis, but other studies reported that these contributions were negligible [22]. Moreover, another study showed that bone marrow-derived cells mainly contributed to the formation of periendothelial vascular mural cells but not to the formation of endothelial cells [37]. On the basis of our observations, we suggest that both a direct effect of Epo on ECs and the mobilization of circulating EPCs by Epo contribute to Epo-induced tumor angiogenesis. However, we could not determine the extent of the contribution of circulating EPCs to Epo-induced tumor angiogenesis. Further study is required for identifying this issue.
The effect of Epo on EpoR-negative tumors seems very dose-dependent. Whereas administering 200 IU/kg once a week increased the rate of tumor growth, administering 2000 IU/kg once a week had no detectable effect on tumor growth in our mice models (data not shown). A large dose of Epo can cause thromboembolic disease [10]. It is therefore possible that the 2000-IU/kg injection, which is 20 times the recommended dose, causes thromboembolism in tumor blood vessels, impairing blood supply and preventing acceleration of the tumor growth. The 200-IU/kg once a week injection is two times the recommended dose for humans. Therefore, we suggest proceeding with caution while administrating Epo to cancer patients.
We performed immunohistochemical analysis of LLC tumors and MCA-induced fibrosarcomas but could not obtain clear staining. As a previous study reported limited use of anti-EpoR antibodies for immunohistochemical analysis [38], this analysis will be performed when an antibody suitable for mice EpoR immunohistochemical analysis is available.
In summary, Epo directly protected ECs, stimulated EC proliferation, and increased the number of circulating EPCs. Erythropoietin promoted tumor growth and angiogenesis in EpoR-negative tumors and impaired overall survival of the primary tumor-bearing mice. The effect of Epo on tumor progression might include other mechanisms. However, our results suggest that clinicians should be careful while using Epo, even with patients carrying EpoR-negative tumors.
Acknowledgments
The authors thank Peter Baluk, Hiroya Hashizume, and Francoise Chanut for helpful comments and correcting the manuscript.
Abbreviations
- Dil-acLDL
acetylated low-density lipoprotein-Dil complex
- EC
endothelial cell
- EPC
endothelial progenitor cell
- Epo
erythropoietin
- EpoR
Epo receptor
- ERK
extracellular signal-regulated kinase
- ESA
erythropoiesis-stimulating agent
- FCS
fetal calf serum
- G-CSF
granulocyte colony-stimulating factor
- H&E
hematoxylin and eosin
- HMVEC
human dermal microvascular endothelial cell
- HUVEC
human umbilical vein endothelial cell
- Jak
Janus-associated kinase
- LLC
Lewis lung carcinoma cell
- M-CSF
macrophage colony-stimulating factor
- MCA
methylcholanthrene
- PBS
phosphate-buffered saline
- Stat
signal transducer and activator of transcription
- VEGF
vascular endothelial growth factor
- WST
water-soluble tetrazolium
Footnotes
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of the Japanese government to Satoru Ebihara (no. 15590795) and to Masanori Asada (no. 17790526) by the Research Grant for Longevity Science (16C-1) from the Ministry of Health, Labor, and Welfare to Satoru Ebihara.
References
- 1.Okazaki T, Ebihara S, Takahashi H, Asada M, Kanda A, Sasaki H. Macrophage colony-stimulating factor induces vascular endothelial growth factor production in skeletal muscle and promotes tumor angiogenesis. J Immunol. 2005;174:7531–7538. doi: 10.4049/jimmunol.174.12.7531. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M. Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int Immunol. 2006;18:1–9. doi: 10.1093/intimm/dxh334. [DOI] [PubMed] [Google Scholar]
- 3.Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. GCSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297:1058–1061. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 6.Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–339. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- 7.Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 8.Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 9.Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, Szechtman B, Roa W, Mulroy L, Rudinskas L, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25:1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 10.Khuri FR. Weighing the hazards of erythropoiesis stimulation in patients with cancer. N Engl J Med. 2007;356:2445–2448. doi: 10.1056/NEJMp078101. [DOI] [PubMed] [Google Scholar]
- 11.Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- 12.Westenfelder C, Baranowski RL. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000;58:647–657. doi: 10.1046/j.1523-1755.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, Onozaki M, Hashimoto M, Musha T, Ogawa K, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis. 2003;24:1021–1029. doi: 10.1093/carcin/bgg060. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda Y, Musha T, Tanaka H, Fujita Y, Fujita H, Utsumi H, Matsuo T, Masuda S, Nagao M, Sasaki R, et al. Inhibition of erythropoietin signalling destroys xenografts of ovarian and uterine cancers in nude mice. Br J Cancer. 2001;84:836–843. doi: 10.1054/bjoc.2000.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardee ME, Kirkpatrick JP, Shan S, Snyder SA, Vujaskovic Z, Rabbani ZN, Dewhirst MW, Blackwell KL. Human recombinant erythropoietin (rEpo) has no effect on tumour growth or angiogenesis. Br J Cancer. 2005;93:1350–1355. doi: 10.1038/sj.bjc.6602846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda Y, Fujita Y, Masuda S, Musha T, Ueda K, Tanaka H, Fujita H, Matsuo T, Nagao M, Sasaki R, et al. Erythropoietin is involved in growth and angiogenesis in malignant tumours of female reproductive organs. Carcinogenesis. 2002;23:1797–1805. doi: 10.1093/carcin/23.11.1797. [DOI] [PubMed] [Google Scholar]
- 17.Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell'Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- 18.Zhande R, Karsan A. Erythropoietin promotes survival of primary human endothelial cells through PI3K-dependent, NF-kappaB-independent upregulation of Bcl-xL. Am J Physiol Heart Circ Physiol. 2007;292:H2467–H2474. doi: 10.1152/ajpheart.00649.2006. [DOI] [PubMed] [Google Scholar]
- 19.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 20.Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 21.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 22.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195:161–169. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okazaki T, Ebihara S, Asada M, Yamanda S, Saijo Y, Shiraishi Y, Ebihara T, Niu K, Mei H, Arai H, et al. Macrophage colony-stimulating factor improves cardiac function after ischemic injury by inducing vascular endothelial growth factor production and survival of cardiomyocytes. Am J Pathol. 2007;171:1093–1103. doi: 10.2353/ajpath.2007.061191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 26.Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506–511. [PubMed] [Google Scholar]
- 27.Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, Hashimoto H, Mak TW, Yagita H, Okumura K, et al. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem. 2001;276:36530–36534. doi: 10.1074/jbc.M104837200. [DOI] [PubMed] [Google Scholar]
- 28.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogilvie M, Yu X, Nicolas-Metral V, Pulido SM, Liu C, Ruegg UT, Noguchi CT. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- 30.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribatti D, Vacca A, Roccaro AM, Crivellato E, Presta M. Erythropoietin as an angiogenic factor. Eur J Clin Invest. 2003;33:891–896. doi: 10.1046/j.1365-2362.2003.01245.x. [DOI] [PubMed] [Google Scholar]
- 32.Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11(Suppl 1):S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- 33.Mori M, Uchida M, Watanabe T, Kirito K, Hatake K, Ozawa K, Komatsu N. Activation of extracellular signal-regulated kinases ERK1 and ERK2 induces Bcl-xL up-regulation via inhibition of caspase activities in erythropoietin signaling. J Cell Physiol. 2003;195:290–297. doi: 10.1002/jcp.10245. [DOI] [PubMed] [Google Scholar]
- 34.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 36.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 37.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott S, Busse L, Bass MB, Lu H, Sarosi I, Sinclair AM, Spahr C, Um M, Van G, Begley CG. Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood. 2006;107:1892–1895. doi: 10.1182/blood-2005-10-4066. [DOI] [PubMed] [Google Scholar]