Abstract
Alveolar soft-part sarcoma (ASPS) is a rare neoplasm with chromosomal translocation that results in ASPL-TFE3 fusion. It is a slow-growing lesion associated with a high incidence of pulmonary and brain metastases indicating poor survival. We demonstrated that the ASPS metastases include also stromal myofibroblasts. These cells proliferate, express smooth-muscle genes, and synthesize extracellular matrix proteins, all of which are characteristics of activated myofibroblasts. The tumor cells also exhibited stromal components such as transforming growth factor beta (TGFβ)-dependent, hypoxia-regulated cytoglobin (stellate cell activation association protein, cytg/STAP) and prolyl 4-hydroxylase, a collagen cross-linking enzyme. The pulmonary ASPS myofibroblasts synthesize serum response factor (SRF), a repressor of Smad3-mediated TGFβ signaling essential for myofibroblast differentiation and Smad3. The phosphorylated active Smad3 was found mostly in the tumor cells. The brain tumor cells express cytg/STAP, but in contrast to the lung metastases, they also express SRF, Smad3, and phospho-Smad3. Halofuginone, an inhibitor of myofibroblasts' activation and Smad3 phosphorylation, inhibited tumor development in xenografts derived from renal carcinoma cells harboring a reciprocal ASPL-TFE3 fusion transcript. This inhibition was associated with the inhibition of TGFβ/SRF signaling, with the inhibition of myofibroblasts' activation, and with the complete loss in TFE3 synthesis by the tumor cells. These results suggest that the myofibroblasts may serve as a novel target for treatment of ASPS metastases.
Introduction
Alveolar soft-part sarcoma (ASPS) is a rare soft tissue neoplasm that can arise in any region of the body. In young adults, the neoplasm often develops in the upper or lower extremities; in young children and adolescents, the tumor commonly originates in the head and neck where the orbit or tongue are the most favorable sites [1,2]. The neoplasm is characterized cytogenetically by an unbalance recurrent chromosomal translocation resulting in a consistent der(17)t(X;17)(p11; q25). This translocation results in the fusion of the ASPL gene on chromosome 17 to the TFE3 gene on chromosome X [1,3]. Alveolar soft-part sarcoma is typically a slow-growing lesion, but frequently, it is associated with high incidence of pulmonary and brain metastases early in the course of the disease [4]. Treatment of ASPS patients includes surgical resection of the primary tumor and of themetastases when possible, the use of chemotherapy, and, in some cases, the use of radiotherapy [2,5]. The curative potential of surgery alone has remained unclear. Local recurrences have been reported to occur in up to 20% of patients with ASPS [6], althoughmost of the patients operated with wide surgical margins did not develop local recurrence [7]. Few, if any, of the patients showed a clinical response to chemotherapy, and chemotherapy or immunotherapy for metastatic tumor, including cisplatin, ifosfamide, doxorubicin, carboplatin, methotrexate, interferon, and interleukin 2, regimens was without any clinical response [7,8]. Tumor size, bone involvement, and especially metastases at presentation indicate a significantly poorer prognosis [7]. Therefore, alternative treatment approaches are needed especially for patients with a metastatic disease.
Most solid tumors consist of a mixture of neoplastic and nonneoplastic cells with extracellular matrix (ECM) components. This cellular microenvironment directly modulates tissue architecture, cell morphology, and cell fate [9], and the ECM-stromal cell interaction contributes to the neoplastic phenotype [10]. The conversion of fibroblasts to myofibroblasts, as mediated by transforming growth factor beta (TGFβ) and its inducible transcription factor the serum response factor (SRF), is the most prominent stromal reaction in a large number of epithelial lesions [11–13]. After binding of TGFβ to its receptor, signaling to the nucleus occurs predominantly by the phosphorylation of cytoplasmatic mediators of the Smad family [14]. The regulation of matrix proteins, in general, and of collagen type I gene expression, in particular, involved the Smad3 signaling pathway [15]. In addition to the major increase in ECM components, the fibroblasts that acquire an activated phenotype are characterized by expressing smooth muscle genes such as α smooth-muscle actin (αSMA) and transgelin [16]. Smad3 directly links TGFβ signaling to an SRF-associated regulatory network in controlling muscle-specific gene transcription [17]. The myofibroblasts are associated with the tumor cells at all stages of cancer progression [18], and in various malignancies, tumor-dependent differentiation of fibroblasts toward myofibroblasts further promotes neoplastic progression [19–21]. It is well established that collagen type I, the major ECM component produced by myofibroblasts, not only functions as a scaffold for the tissue but also regulates the expression of genes associated with cellular signaling and metabolism, gene transcription, and translation, thus affecting fundamental cellular processes that are essential for tumor progression. For example, collagen type I was found to be a signal for invasion, and its intratumoral expression level has been associated with increased tumor invasiveness [22]. Stellate cell activation association protein, also known as cytoglobin (cygb/STAP) is another TGFβ-regulated and collagen-related gene expressed by the myofibroblasts [23]. Although its specific role is yet to be elucidated, a potential role in the detoxification of reactive oxygen species in tumors and other pathologies has been suggested [24,25].
In this study, we demonstrated the presence of activated myofibroblasts and demonstrated the existence of the TGFβ/SRF-dependent Smad3 pathway in human ASPS lung and brain metastases. In xenografts produced by cells harboring the reciprocal ASPL-TFE3 fusion transcript, inhibition of the Smad3 phosphorylation by halofuginone, an inhibitor of fibroblast-to-myofibroblast transition [26–28], resulted in the reduction in tumor growth that was accompanied by the inhibition of myofibroblast activation and reduction in TFE3 expression by the tumor cells.
Patient and Methods
Patients
Brain metastases were obtained from 18- and 20-year-old male ASPS patients with thigh tumors and lung metastases. After the removalof the primary tumors, one of the patients was clinically stable with no therapy for 4 years, when a single brain metastasis was identified by magnetic resonance imaging and removed surgically. Additional brain metastasis appears during the next 2 years, despite cranial irradiation. The second patient developed brain metastasis soon after diagnosis, and tissue was obtained from a resection. The pulmonary biopsy was taken from a lower lobe of a 16-year-old female who underwent a thoracotomy to lessen tumor burden. The primary tumor at the left scapula developed to multiple pulmonary nodules bilaterally.
Materials
Fetal calf serum, Dulbecco's modified Eagle's medium, and trypsin-EDTA solution (0.02–0.25%) were obtained from Biochemical Industries (Bet-HaEmek, Israel). Sirius red F3B was obtained from BDH Laboratory Supplies (Poole, England). Halofuginone bromhydrate was obtained from Collgard Biopharmaceuticals Ltd (Tel Aviv, Israel). Monoclonal antibodies to αSMA were from Dako A/S (Glostrup, Denmark), and antibodies to cygb/STAP were a gift from N. Kawada from the Department of Hepatology, Osaka City University, Japan. Smad3 antibodies were from Abcam (Cambridge, UK); TFE3, mMET, phospho-Smad3, and SRF antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Animals and Experimental Design
The human renal cell carcinoma cell line FU-UR-1 (a generous gift fromDr Masako Ishiguro, Department of Pathology, FukuokaUniversity School of Medicine, Fukuoka, Japan) with the reciprocal ASPL-TFE3 fusion transcript [29] was cultured in Dulbecco's modified Eagle's medium with 10% fetal calf serum. All animal experiments were carried out according to the guidelines of the Volcani Center Institutional Committee for Care and Use of Laboratory Animals. Nude (CD1 nu/nu) male mice (Harlen Laboratories, Israel) were housed in cages (four mice per cage) under conditions of constant photoperiod (12-hour light/12-hour dark) with free access to food and water. Xenografts were established by implanting the FU-UR-1 cells in Matrigel (2 x 106 cells/ml) subcutaneously using a 27-gauge needle. Halofuginone (5 µg per mouse) was administered intraperitoneally three times a week starting at the time that the tumor volume reached 40mm3. During the experiments, tumor volume was determined with a caliper according to the following formula: length x width x depth x 0.5236; and it is presented as the mean ± SE.
Preparation of Sections, In Situ Hybridization, and Immunohistochemistry
At the end of the experiments, tumors were collected and fixed overnight in 4% paraformaldehyde in PBS at 4°C. Serial 5-µm sections were prepared from the xenograft tumors and from the ASPS brain and lung metastases. Samples were stained with Sirius red for collagen. In situ hybridization for collagen α1(I) was performed as previously described [26]. Immunohistochemistry was performed with anti-Cygb/STAP (1:500), monoclonal anti-αSMA antibodies (1:200), Smad3 (1:200), TFE3 (1:100), mMET (1:50), phospho-Smad3 (1:50), and SRF (1:500) antibodies. Peroxidase activity was revealed by using 3,3′-diaminobenzidine as chromogen.
Results
Alveolar Soft-Part Sarcoma Pulmonary and Brain Metastases
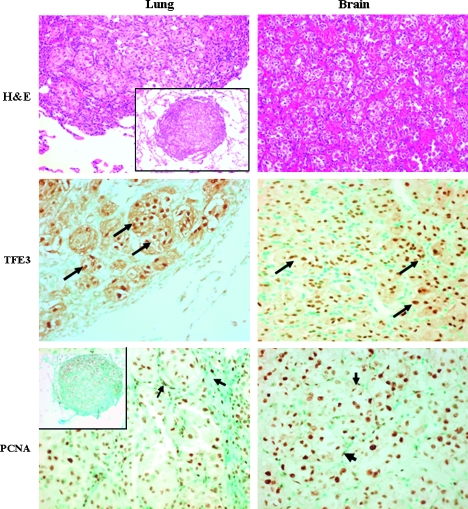
Tumor cells in both lung and brain metastases were large but uniform, with abundant granular-to-vacuolated cytoplasm and well-defined cell borders. The nuclei were round-to-polygonal and vesicular, often with prominent nucleoli. The nuclei were positive for TFE3, and in both the lung and the brain specimens, extensive cell division was observed (Figure 1). In both pulmonary and brain metastases, the stroma and the tumor cells were positive for proliferating cell nuclear antigen. No MET receptor tyrosine kinase expression was observed in the pulmonary and brain ASPS metastases (data not shown), although it was identified in few malignancies with TFE3 translocations and was significantly overexpressed in some primary ASPS tumors [30].
Figure 1.
Pulmonary and brain ASPS metastases. Hematoxylin and eosin staining shows an organoid, pseudoalveolar large round to polyhedral eosinophilic cells displaying large nucleus with a uniform “small nest” pattern separated by fine fibrous septa (original magnification, x100; insert's original magnification, x40). The pulmonary and brain tumor cells express high levels of TFE3 (arrows; original magnification, x200). Both the metastatic cells and the stroma cells (arrows) stain positive for proliferating cell nuclear antigen (original magnification, x200) suggesting that they are at their proliferative state.
Stromal Components in ASPS Pulmonary Metastases
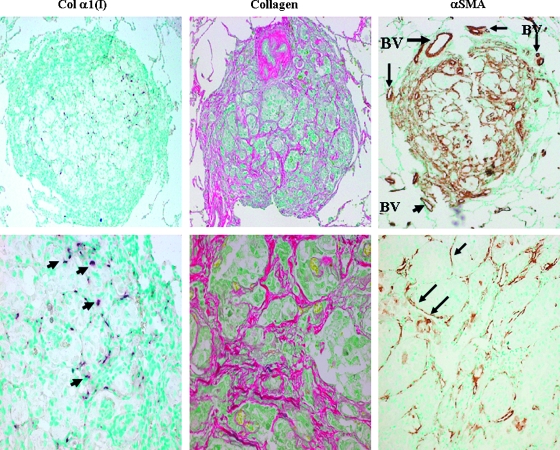
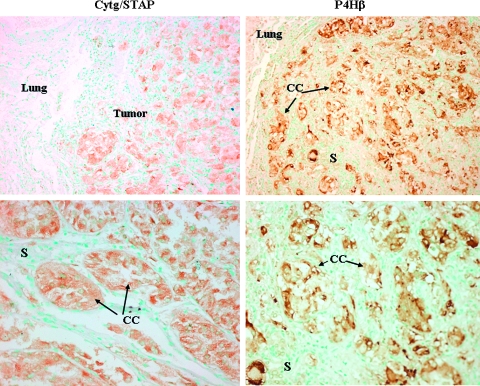
The cancer cells of the pulmonary metastasis were surrounded by myofibroblasts that express the collagen α1(I) gene that resulted in high levels of collagen synthesis (Figure 2). The collagen is organized in fibrils that encircle clusters of cancer cells, which resulted in fibrous septa. No expression of the collagen α1(I) gene was observed either in the surrounding lung tissue or in the primary tumor (data not shown). The myofibroblasts also exhibited high levels of αSMA, a hallmark of activated myofibroblasts as found in ASPS tumors from other locations [31]. High levels of αSMA were also observed in the blood vessels in the periphery of the metastasis. The cancer cells also display characteristic components characteristic of the stroma. They synthesized cytg/STAP that is usually expressed by myofibroblasts from various origins [27,28] and prolyl 4-hydroxylase (P4H), one of the major enzymes required for collagen cross-linking and maturation (Figure 3). Both cytg/STAP and P4H were exclusively synthesized by the tumor cells and not by the myofibroblasts within the tumor or by the surrounding lung tissue.
Figure 2.
Pulmonary ASPS metastases contain activated myofibroblasts. The myofibroblasts surrounding clusters of cancer cells express the collagen α1(I) gene (arrows) and synthesize large amounts of collagen (stained by Sirius red). These cells and the endothelial cells of the blood vessels (BV) at the periphery of the metastasis synthesize αSMA as determined by immunohistochemistry. Original magnifications: upper panel, x40; lower panel, x400.
Figure 3.
Pulmonary metastatic ASPS tumor cells express stromal components. The tumor cells, but not the stromal cells, synthesize cygb/STAP and the β subunit of prolyl 4-hydroxylase (P4Hβ) as demonstrated by immunohistochemistry (arrows). CC indicates cancer cells; S, stroma populated with myofibroblasts. Original magnifications: upper panel, x100; lower panel, x400.
Smad3 Signaling in ASPS Pulmonary Metastases
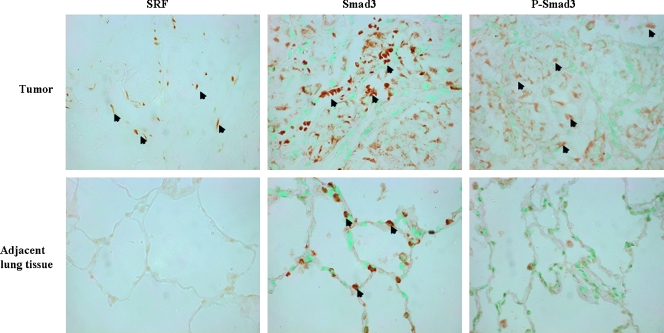
Serum response factor, which acts as a nuclear repressor of Smad3-mediated TGFβ signaling, is essential for myofibroblast differentiation [32]. Smad3, in turn, links TGFβ signaling to SRF-associated regulatory network [17]. The stromal cells within the pulmonary metastasis, but not the tumor cells or the adjacent lung tissue, express SRF (Figure 4). Transforming growth factor beta possesses dual tumor-suppressive and oncogenic effects. During tumorigenesis, malignantly transformed cells often lose the response to the tumor-suppressive effects of TGFβ, which, in turn, starts to act as an autocrine tumor-promoting factor by enhancing cancer invasion and metastasis. The stroma cells within the neoplastic tissue and the lung epithelial cells in the adjacent tissue stain positive for Smad3, whereas the phosphorylated active form of Smad3 was found mostly in the tumor cells, although some of the stromal cells exhibited phospho-Smad3 as well.
Figure 4.
Smad3 signaling in pulmonary ASPS metastasis. The myofibroblasts in the pulmonary metastasis, but not in the surrounding lung tissue, exhibited SRF (arrows), whereas Smad3 is present both in the tumor stromal cells and lung epithelial cells. Phospho-Smad3 was demonstrated mostly in the tumor and some of the stroma cells but not by the lung epithelial cells. Original magnification, x400.
Alveolar Soft-Part Sarcoma Brain Metastases
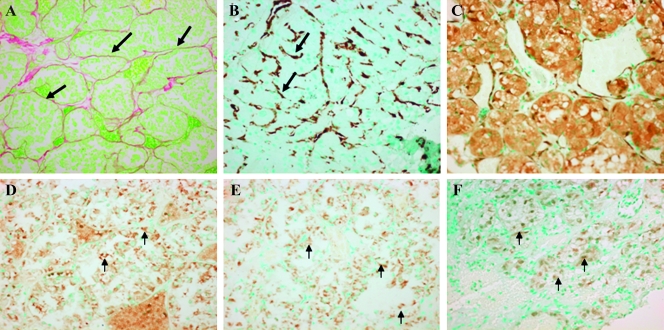
The ASPS brain myofibroblasts, as detected by their ability to exhibit αSMA, synthesize much less collagen than the pulmonary ASPS cells, resulting in much thinner septa surrounding the tumor cells (Figure 5). Similar to the pulmonary tumor cells, the metastatic brain cancer cells express cytg/STAP, but in contrast to the lung metastases, they also express SRF, Smad, and the phosphorylated active form of Smad3. The same results were obtained from both ASPS patients' brain tumor specimens.
Figure 5.
Stroma components and Smad signaling in the ASPS brain metastases. The tumor cells in the brain metastases are surrounded by fibrous septa (A; original magnification, x100) and populated by myofibroblasts displaying a large amount of αSMA (B; original magnification, x100). The tumor cells exhibited high levels of cytg/STAP (C; original magnification, x100), SRF (D; original magnification, x400) Smad3 (E; original magnification, x400), and phospho-Smad3 (F; original magnification, x100; insert's original magnification, x400).
Inhibition of Tumor Growth Derived from Cells with Reciprocal ASPL-TFE3 Fusion Transcript
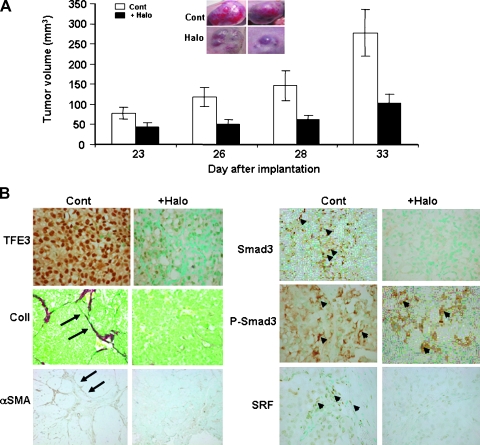
Xenografts were established in the nude mice through subcutaneous implantation of FU-UR-1 renal carcinoma cell line that contain the reciprocal ASPL-TFE3 fusion transcript [29]. In the untreated mice, the tumors reached the volume of 277 mm3 at 33 days after implantation (Figure 6A). Halofuginone, an inhibitor of myofibroblast activation and of Smad3 phosphorylation [28,33,34], inhibited tumor growth and development and, at 33 days after cell implantation, had only grown to 38% of the volume of the control mice (Figure 6A). The inhibition in tumor volume by halofuginone was associated with the near complete reduction in TFE3 synthesis by the tumor cells and with the reduction in activated myofibroblasts as demonstrated by abrogation of collagen and αSMA synthesis (Figure 6B). Halofuginone also inhibited SRF synthesis, and in agreement with other studies [27,33,34], halofuginone inhibited the phosphorylation of Smad3 without affecting Smad3 levels.
Figure 6.
Inhibition of tumor growth in an ASPL-TFE3 fusion transcript xenografts by halofuginone. (A) Halofuginone treatment inhibited tumor growth and development in xenografts derived from the FU-UR-1 cell with reciprocal ASPL-TFE3 fusion transcript. Insert: Tumors of control and halofuginone-treated mice at 33 days after FU-UR-1 cell implantation. (B) Inhibition of TFE3, myofibroblasts' activation, and Smad3 signaling in FU-UR-1 xenograft by halofuginone. At day 33 after FU-UR-1 cell implantation, tumors from control and halofuginone-treated mice were taken for histopathology analysis. Almost all of the tumor cells of the untreated mice exhibit high levels of TFE3, which was abrogated by halofuginone treatment (original magnification, x400). Halofuginone treatment inhibited the collagen and αSMA synthesis observed in the control mice (arrows; original magnification, x200) suggesting inhibition of myofibroblasts' activation. Halofuginone also inhibited the SRF and phosphorylation of Smad3 without affecting Smad3 levels (arrows; original magnification, x400).
Discussion
Alveolar soft-part sarcoma is a rare and uncommon tumor with a characteristic histopathology, and despite overall indolent clinical behavior, patients eventually develop distant metastases [31]. The ASPS lung and brain metastases consists of at least two cell types: the myofibroblasts and the ASPS tumor cells that express TFE3 (Figure 1) with a probable cross talk between the two. The ASPS metastatic myofibroblasts, like in other tumors [28,35], acquire muscle-specific phenotype as demonstrated by high levels of αSMA, synthesize high levels of ECM constituents especially collagen type I (Figure 2), and demonstrate proliferation capabilities (Figure 1), all of which are characteristic of activated myofibroblasts. The myofibroblasts within the pulmonary ASPS tumor exhibited SRF (Figure 4), which is a transcription factor that controls many mitogen-responsive and muscle-specific genes such as αSMA and MyoD through the regulatory elements-CArG boxes in their promoter [36,37]. In the brain, however, SRF is expressed by the tumor cells and not by the stromal cells, although the stroma cells synthesized high levels of αSMA (Figure 5), which may indicate a cross talk between the two cell types. The tumor cells are most likely involved in the collagen metabolism as well, which suggests again an intimate relationship between the two cell types. They express P4H, an enzyme required for collagen cross-linking (Figure 3), as was found in other tumors [38,39]. The brain and lung metastatic tumor cells also expressed cygb/STAP (Figures 3 and 5) that is involved in collagen metabolism as well [23]. It is interesting to note that the synthesis of these two proteins is under the control of hypoxia [24,40]. Cygb/STAP is probably involved in cellular oxygen homeostasis and supply and plays a role as an oxygen reservoir that is used under hypoxic conditions. In contrast to many tissues and tumors in which cygb/STAP is synthesized by the stroma cells [27,28,41], in ASPS lung and brain metastases, only the cancer cells exhibited high levels of cygb/STAP. Whether this localization of cytg/STAP is unique to ASPS is still to be determined.
The origin of ASPS is controversial, and based on reports that ASPS cells express muscle-specific genes such as αSMA, MyoD1, and myogenin, it has been suggested that ASPS cells represent an unusual form of myogenic tumor [1,42,43]. Conversely, other studies could not detect muscle-specific genes in ASPS tumors, which do not support a myogenic origin [44–46]. Our findings suggest that the muscle-specific genes observed in ASPS may, in certain cases, be associated with the stroma fibroblast-to-myofibroblast transition and SRF function controlling muscle-specific gene transcription [17] rather than with an indication of its origin. These myofibroblasts that are unique mesenchymal cells with properties inherent to both muscle and nonmuscle cells express skeletal muscle structural and regulatory proteins. These include sarcomeric myosin heavy chain, αSMA, and MyoD, and despite the presence of such myogenic regulatory proteins, these cells do not terminally differentiate into skeletal muscle [47].
Recently, overexpression of the MET receptor tyrosine kinase gene was found in some ASPS primary tumors. It was demonstrated that ASPL-TFE3 mediated transcriptional up-regulation of the MET receptor tyrosine kinase, which results in its autophosphorylation and activation of downstream signaling in the presence of hepatocyte growth factor. Thus, MET signaling was suggested as a target for pharmacological intervention for ASPS patients [30]. The ASPS pulmonary and brain metastases were negative for MET, suggesting that different treatment strategies may be required for ASPS primary tumors and metastases.
The myofibroblasts together with ECM components provide the microenvironment that is pivotal for tumor cell growth, tumor invasion, and metastasis [18]. Thus, inhibition of fibroblast activation may become a viable approach for ASPS tumor treatment. Halofuginone, which at present is being evaluated in clinical trials [48], inhibits the fibroblast-to-myofibroblast transition of the tumor microenvironment [28,49] by inhibiting Smad3 phosphorylation downstream of the TGFβ/SRF signaling [33,34]. Previously, we reported that targeting the fibroblast-to-myofibroblast transition with halofuginone may synergize with low doses of chemotherapy in achieving a significant antitumoral effect, avoiding the need of a high dose of chemotherapy and its toxicity without impairing treatment efficacy [28]. In xenografts that contain the reciprocal ASPL-TFE3 fusion transcript, halofuginone inhibited myofibroblasts' activation as demonstrated by the abrogation of αSMA and collagen synthesis probably due to the TGFβ/SRF-Smad3 signaling inhibition (Figure 6). This resulted in a major decrease in tumor growth and development (Figure 6A). The role of TGFβ in tumor development is controversial and may be dependent on the origin of the tumor. Serum response factor, which is part of the TGFβ signaling pathway, is implicated in cancer progression, specifically at the epithelial-mesenchymal transition [50,51]. Overexpression of SRF accelerates migration and invasion of tumor cells with subsequent acquisition of mesenchymal phenotype and activation of immediate early genes.We demonstrated that in ASPS metastasis, Smad3 downstream of TGFβ and SRF were found in the tumor cells and in the myofibroblasts. Thus, functional antagonism of SRF or inhibition of Smad3 phosphorylation may provide a therapeutic approach by controlling tumor cell invasion and metastasis on one hand and fibroblast-to-myofibroblast transition on the other. Moreover, halofuginone treatment caused an almost complete inhibition in TFE3 synthesis by the tumor cells (Figure 6B). TFE3 has been found to bind Smad3 and Smad4 and to synergistically trans-activate the transcription of Smad7 in response to TGFβ [52]. Thus, TFE3-Smad3 response elements may represent a common target for TGFβ-induced gene expression, a feature that may be shared with various TFE3 fusions including ASPS.
In conclusion, our data demonstrated for the first time the existence of the TGFβ/SRF-dependent Smad3 pathway and the presence of activated myofibroblasts in pulmonary and brain ASPS metastases that may serve as a novel target for treatment of metastatic lesions in ASPS.
Acknowledgments
Special thanks to Sara Vargas, Larisa Debelenko, and the Children's Hospital Boston Pathology Department for providing pulmonary tissue specimens.
Abbreviations
- ASPS
alveolar soft-part sarcoma
- ECM
extracellular matrix
- TGFβ
transforming growth factor beta
- SRF
serum response factor
- αSMA
α smooth-muscle actin
- cygb/STAP
cytoglobin, stellate cell activation association protein
- P4H
prolyl 4-hydroxylase
Footnotes
This paper is a contribution from the ARO, the Volcani Center, Bet Dagan, Israel. The work was supported in part by the Cure Alveolar Soft Part Sarcoma International (iCureASPS) foundation and the Crosby Family Foundation, Inc.
References
- 1.Folpe AL, Deyrup AT. Alveolar soft-part sarcoma: a review and update. J Clin Pathol. 2006;59:1127–1132. doi: 10.1136/jcp.2005.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kayton ML, Meyers P, Wexler LH, Gerald WL, LaQuaglia MP. Clinical presentation, treatment, and outcome of alveolar soft part sarcoma in children, adolescents, and young adults. J Pediatr Surg. 2006;41:187–193. doi: 10.1016/j.jpedsurg.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Ladanyi M, Lui MY, Antonescu CR, Krause-Boehm A, Meindl A, Argani P, Healey JH, Ueda T, Yoshikawa H, Meloni-Ehrig A, et al. The der (17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene. 2001;20:48–57. doi: 10.1038/sj.onc.1204074. [DOI] [PubMed] [Google Scholar]
- 4.Azizi AA, Haberler C, Gupper A, Prayer D, Breitschopf H, Acker T, Slavc I. Vascular-endothelial-growth-factor (VEGF) expression and possible response to angiogenesis inhibitor bevacizumab in metastatic alveolar soft part sarcoma. Lancet Oncol. 2006;7:521–523. doi: 10.1016/S1470-2045(06)70729-X. [DOI] [PubMed] [Google Scholar]
- 5.Benetos IS, Mavrogenis AF, Soultanis KCh, Zoubos AB, Papagelopoulos PJ, Soucacos PN. Alveolar soft part sarcoma of the forearm: a case report. J Surg Orthop Adv. 2006;15:209–213. [PubMed] [Google Scholar]
- 6.Liberman PH, Brennan F, Kimmel M, Erlandson RA, Garin-Chesa P, Flehinger BY. Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer. 1989;63:1–13. doi: 10.1002/1097-0142(19890101)63:1<1::aid-cncr2820630102>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Ogose A, Yazawa Y, Ueda T, Hotta T, Kawashima H, Hatano H, Morita T. Alveolar soft part sarcoma in Japan: multi-institutional study of 57 patients from the Japanese Musculoskeletal Oncology Group. Oncology. 2003;65:7–13. doi: 10.1159/000071199. [DOI] [PubMed] [Google Scholar]
- 8.Portera CA, Jr, Ho V, Patel SR, Hunt KK, Feig BW, Respondek PM, Yasko AW, Benjamin RS, Pollock RE, Pisters PW. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer. 2001;91:585–591. doi: 10.1002/1097-0142(20010201)91:3<585::aid-cncr1038>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82:539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 10.Shekhar MPV, Pauley R, Heppner G. Host microenvironment in breast cancer development: extracellular matrix-stromal cell contribution to neoplastic phenotype of epithelial cells in the breast. Breast Cancer Res. 2003;5:130–135. doi: 10.1186/bcr580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ. Tumor-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90:822–832. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer. 2004;45:S163–S175. doi: 10.1016/j.lungcan.2004.07.977. [DOI] [PubMed] [Google Scholar]
- 13.Chai J, Norng M, Tarnawski AS, Chow J. A critical role of serum response factor in myofibroblast differentiation during experimental oesophageal ulcer healing in rat. Gut. 2007;56:621–630. doi: 10.1136/gut.2006.106674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 15.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 16.Untergasser G, Gander R, Lilg C, Lepperdinger G, Plas E, Berger P. Profiling molecular targets of TGF-beta1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech Ageing Dev. 2005;126:59–69. doi: 10.1016/j.mad.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Qiu P, Feng XH, Li L. Interaction of Smad3 and SRF-associated complex mediates TGF-beta1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol. 2003;35:1407–1420. doi: 10.1016/j.yjmcc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 19.De Wever O, Mareel M. Role of myofibroblasts at the invasion front. Biol Chem. 2002;383:55–67. doi: 10.1515/BC.2002.006. [DOI] [PubMed] [Google Scholar]
- 20.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 21.Zidar N, Gale N, Kambic V, Fischinger J. Proliferation of myofibroblasts in the stroma of epithelial hyperplastic lesions and squamous carcinoma of the larynx. Oncology. 2002;62:381–385. doi: 10.1159/000065071. [DOI] [PubMed] [Google Scholar]
- 22.van Hoorde L, van Aken E, Mareel M. Collagen type I: a substrate and a signal for invasion. Prog Mol Subcell Biol. 2000;25:105–134. doi: 10.1007/978-3-642-59766-4_7. [DOI] [PubMed] [Google Scholar]
- 23.Nakatani K, Okuyama H, Shimahara Y, Saeki S, Kim DH, Nakajima Y, Seki S, Kawada N, Yoshizato K. Cytoglobin/STAP, its unique localization in splanchnic fibroblast-like cells and function in organ fibrogenesis. Lab Invest. 2004;84:91–101. doi: 10.1038/labinvest.3700013. [DOI] [PubMed] [Google Scholar]
- 24.Fordel E, Thijs L, Martinet W, Schrijvers D, Moens L, Dewilde S. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene. 2007;398:114–122. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Xu R, Harrison PM, Chen M, Li L, Tsui TY, Fung PC, Cheung PT, Wang G, Li H, Diao Y, et al. Cytoglobin overexpression protects against damage-induced fibrosis. Mol Ther. 2006;13:1093–1100. doi: 10.1016/j.ymthe.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Bruck R, Genina O, Aeed H, Alexiev R, Nagler A, Pines M. Halofuginone to prevent and treat thioacetamide-induced liver fibrosis in rats. Hepatology. 2001;3:379–386. doi: 10.1053/jhep.2001.21408. [DOI] [PubMed] [Google Scholar]
- 27.Gnainsky Y, Kushnirsky Z, Bilu G, Hagai Y, Genina O, Volpin H, Bruck R, Spira G, Nagler A, Kawada N, et al. Gene expression during chemically induced liver fibrosis: effect of halofuginone on TGF-beta signaling. Cell Tissue Res. 2007;328:153–166. doi: 10.1007/s00441-006-0330-1. [DOI] [PubMed] [Google Scholar]
- 28.Sheffer Y, Leon O, Pinthus JH, Nagler A, Mor Y, Genin O, Iluz M, Kawada N, Yoshizato K, Pines M. Inhibition of fibroblast to myofibroblast transition by halofuginone contributes to the chemotherapy-mediated antitumoral effect. Mol Cancer Ther. 2007;6:570–577. doi: 10.1158/1535-7163.MCT-06-0468. [DOI] [PubMed] [Google Scholar]
- 29.Ishiguro M, Iwasaki H, Ohjimi Y, Kaneko Y. Establishment and characterization of a renal cell carcinoma cell line (FU-UR-1) with reciprocal ASPL-TFE3 fusion transcript. Oncology Rep. 2004;11:1169–1175. [PubMed] [Google Scholar]
- 30.Tsuda M, Davis IJ, Argani P, Shukla N, McGill GG, Nagai M, Saito T, Laé M, Fisher DE, Ladanyi M. TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res. 2007;67:919–929. doi: 10.1158/0008-5472.CAN-06-2855. [DOI] [PubMed] [Google Scholar]
- 31.Zarrin-Khameh N, Kaye KS. Alveolar soft part sarcoma. Arch Pathol Lab Med. 2007;131:488–491. doi: 10.5858/2007-131-488-ASPS. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Zhe X, Phan SH, Ullenbruch M, Schuger L. Involvement of serum response factor isoforms in myofibroblast differentiation during bleomycininduced lung injury. Am J Respir Cell Mol Biol. 2003;29:583–590. doi: 10.1165/rcmb.2002-0315OC. [DOI] [PubMed] [Google Scholar]
- 33.McGaha TL, Phelps RG, Spiera H, Bona C. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforminggrowth- factor-beta-mediated Smad3 activation in fibroblasts. J Invest Dermatol. 2002;118:461–470. doi: 10.1046/j.0022-202x.2001.01690.x. [DOI] [PubMed] [Google Scholar]
- 34.Yee KO, Connolly CM, Pines M, Lawler J. Halofuginone inhibits tumor growth in the polyoma middle T antigen mouse via a thrombospondin-1 independent mechanism. Cancer Biol Ther. 2006;5:218–224. doi: 10.4161/cbt.5.2.2419. [DOI] [PubMed] [Google Scholar]
- 35.Surowiak P, Murawa D, Materna V, Maciejczyk A, Pudelko M, Ciesla S, Breborowicz J, Murawa P, Zabel M, Dietel M, et al. Occurrence of stromal myofibroblasts in the invasive ductal breast cancer tissue is an unfavourable prognostic factor. Anticancer Res. 2007;27:2917–2924. [PubMed] [Google Scholar]
- 36.L'honore A, Lamb NJ, Vandromme M, Turowski P, Carnac G, Fernandez A. MyoD distal regulatory region contains an SRF binding CArG element required for MyoD expression in skeletal myoblasts and during muscle regeneration. Mol Biol Cell. 2003;14:2151–2162. doi: 10.1091/mbc.E02-07-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Matsui H, Kubochi K, Okazaki I, Yoshino K, Ishibiki K, Kitajima M. Collagen biosynthesis in gastric cancer: immunohistochemical analysis of prolyl 4-hydroxylase. J Surg Oncol. 1999;70:239–246. doi: 10.1002/(sici)1096-9098(199904)70:4<239::aid-jso8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Nissi R, Böhling T, Autio-Harmainen H. Immunofluorescence localization of prolyl 4-hydroxylase isoenzymes and type I and II collagens in bone tumours: type I enzyme predominates in osteosarcomas and chondrosarcomas, whereas type II enzyme predominates in their benign counterparts. Acta Histochem. 2004;106:111–121. doi: 10.1016/j.acthis.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Fähling M, Perlewitz A, Doller A, Thiele BJ. Regulation of collagen prolyl 4-hydroxylase and matrix metalloproteinases in fibrosarcoma cells by hypoxia. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:119–126. doi: 10.1016/j.cca.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Tateaki Y, Ogawa T, Kawada N, Kohashi T, Arihiro K, Tateno C, Obara M, Yoshizato K. Typing of hepatic nonparenchymal cells using fibulin-2 and cytoglobin/STAP as liver fibrogenesis-related markers. Histochem Cell Biol. 2004;122:41–49. doi: 10.1007/s00418-004-0666-0. [DOI] [PubMed] [Google Scholar]
- 42.Rosai J, Dias P, Parham DM, Shapiro DN, Houghton P. MyoD1 protein expression in alveolar soft part sarcoma as confirmatory evidence of its skeletal muscle nature. Am J Surg Pathol. 1991;15:974–981. doi: 10.1097/00000478-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Tallini G, Parham DM, Dias P, Cordon-Cardo C, Houghton PJ, Rosai J. Myogenic regulatory protein expression in adult soft tissue sarcomas. A sensitive and specific marker of skeletal muscle differentiation. Am J Pathol. 1994;144:693–701. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang NP, Bacchi CE, Jiang JJ, McNutt MA, Gown AM. Does alveolar soft-part sarcoma exhibit skeletal muscle differentiation? An immunocytochemical and biochemical study of myogenic regulatory protein expression. Mod Pathol. 1996;9:496–506. [PubMed] [Google Scholar]
- 45.Gomez JA, Amin MB, Ro JY, Linden MD, Lee MW, Zarbo RJ. Immunohistochemical profile of myogenin and MyoD1 does not support skeletal muscle lineage in alveolar soft part sarcoma. Arch Pathol Lab Med. 1999;123:503–507. doi: 10.5858/1999-123-0503-IPOMAM. [DOI] [PubMed] [Google Scholar]
- 46.Cessna MH, Zhou H, Perkins SL, Tripp SR, Layfield L, Daines C, Coffin CM. Are myogenin and MyoD1 expression specific for rhabdomyosarcoma? A study of 150 cases, with emphasis on spindle cell mimics. Am J Surg Pathol. 2001;25:1150–1157. doi: 10.1097/00000478-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Walker GA, Guerrero IA, Leinwand LA. Myofibroblasts: molecular crossdressers. Curr Top Dev Biol. 2001;51:91–107. doi: 10.1016/s0070-2153(01)51003-0. [DOI] [PubMed] [Google Scholar]
- 48.de Jonge MJ, Dumez H, Verweij J, Yarkoni S, Snyder D, Lacombe D, Marréaud S, Yamaguchi T, Punt CJ, van Oosterom A. Phase I and pharmacokinetic study of halofuginone, an oral quinazolinone derivative in patients with advanced solid tumours. Eur J Cancer. 2006;42:1768–1774. doi: 10.1016/j.ejca.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Pines M. Targeting TGFβ signaling to inhibit fibroblast activation as a therapy for fibrosis and cancer: effect of halofuginone. Exp Opin Drug Dis. 2008;3:11–20. doi: 10.1517/17460441.3.1.11. [DOI] [PubMed] [Google Scholar]
- 50.Psichari E, Balmain A, Plows D, Zoumpourlis V, Pintzas A. High activity of serum response factor in the mesenchymal transition of epithelial tumor cells is regulated by RhoA signaling. J Biol Chem. 2002;277:29490–29495. doi: 10.1074/jbc.M112368200. [DOI] [PubMed] [Google Scholar]
- 51.Park MY, Kim KR, Park HS, Park BH, Choi HN, Jang KY, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression of the serum response factor in hepatocellular carcinoma: implications for epithelial-mesenchymal transition. Int J Oncol. 2007;31:1309–1315. [PubMed] [Google Scholar]
- 52.Hua X, Miller ZA, Benchabane H, Wrana JL, Lodish HF. Synergism between transcription factors TFE3 and Smad3 in transforming growth factor-beta-induced transcription of the Smad7 gene. J Biol Chem. 2000;275:33205–33208. doi: 10.1074/jbc.C000568200. [DOI] [PubMed] [Google Scholar]