Abstract
Telomerase is thought to play an essential role in tumorigenesis and progression. Its activity is directly correlated with the expression of its catalytic subunit, human telomerase reverse transcriptase (hTERT). A correlation of transcript expression with a poor prognosis has been detected in different human malignancies. However, data on hTERT in pancreatic ductal adenocarcinoma (PDAC) are purely descriptive so far. Therefore, we evaluated the impact of hTERT expression on patients' prognosis. Human telomerase reverse transcriptase mRNA isolates from 56 human microdissected PDAC tissues were analyzed by quantitative reverse transcription-polymerase chain reaction and multivariate Cox regression hazard test. Elevated hTERT transcript levels were measured in 23 of 56 PDAC tissues, 33 patients showed no detectable transcripts. Unexpectedly, a low expression of hTERT mRNA levels was associated with a worse prognosis for overall survival (relative risk = 5.33; P = .013) when compared to high levels, whereas undetectable expression showed an intermediate risk of tumor-related death. These data challenge previous findings outlining hTERT's negative impact on overall survival. The risk pattern obtained in PDAC suggests a more complex regulation of hTERT.
Introduction
Current molecular oncology strongly suggests that aberrant reactivation of telomerase is one of the key features of the malignant phenotype of a somatic cell [1–4]. Telomerase catalyzes the synthesis and extension of telomeric DNA, thus leading to inactivation of apoptosis and senescence [3,5]. To detect and measure telomerase activity, the mRNA expression of its catalytic subunit human telomerase reverse transcriptase (hTERT) has been proposed as a surrogate marker, given the fact that mRNA levels correlate directly with telomerase activity [6,7]. Data on the clinical relevance of hTERT expression in malignant neoplasias are yet irresolute, although hTERT expression levels suggest a correlation with a poor prognosis in non-small cell lung cancer, Wilm tumor, B-chronic lymphocytic leukemia, acute myelogenous leukemia, colorectal cancer [8–10], and in soft-tissue sarcoma [11,12].
Pancreatic ductal adenocarcinoma (PDAC) is a dismal disease because of its aggressive biologic phenotype, characterized by an early local invasion and high metastatic potential, late clinical presentation, very poor overall prognosis with a short median survival time of only a few months after diagnosis (ranging from a few weeks to years), and high resistance to radiation and chemotherapy [13].
Most recently, telomerase and hTERT activity have been reported in tumors and pancreatic juice of patients with PDAC [14–18], and their possible role in early diagnosis has been proposed. So far, there are no studies on the impact of hTERT expression on the prognosis of patients with PDAC. Therefore, we investigated precisely this in a cohort of 56 PDAC patients from our institution. We microdissected fresh-frozen tumor tissues to highly enrich neoplastic cells, isolated mRNA, and applied a quantitative polymerase chain reaction analysis for hTERT message. The impact of the gene expression on prognosis was determined by a multivariate Cox regression hazard model.
Materials and Methods
Patients
The study comprised a cohort of 56 patients (20 females and 36 males; age range, 34–80 years; mean age, 61.7 years), who were monitored for a mean observation time of 15.8 months (range, 1–61 months) and whose median survival rate was 14 months (range, 1–49 months). All patients included in the study underwent primary surgery in the years 2001–2005 at our hospital (Department of Surgery 1, University of Ulm, Ulm, Germany). Surgical pancreatic resection specimens were immediately placed on ice and subsequently snap-frozen and stored at -80°C. All patients gave written informed consent, and approval of the ethics committee was obtained.
Microdissection
RNA was isolated using the Innuprep RNA mini-kit (AJ Innuscreen GmbH, Berlin, Germany). The integrity of the isolated RNA was confirmed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The cryostat tissues were cut into 8- to 10-µm sections. Microdissection of selected areas containing approximately 50 to 300 neoplastic ductal epithelial cells per area (>4000 cells per tissue per patient) was carried out by means of laser microdissection and pressure catapulting (LCM) technique (PALM Microlaser Technologies, Bernried, Germany) on cresyl violet-stained sections.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Measurement of hTERT transcript expression levels was performed by quantitative real-time reverse transcription-polymerase chain reaction as described previously [11,12]. The cutoff levels for low expression of hTERT were set at 0.00 ag/fg hypoxanthine-phospho-ribosyl-transferase (HPRT), whereas the cutoff for high expression was set at the mean value of hTERT expression (Table W1). Accordingly, three categories of expression levels were set up: 1, “no expression” (meaning no detectable mRNA levels); 2, “low expression” (>0.00 and ≤61 ag hTERT mRNA/fg HPRT mRNA); 3, “high expression” (>61 ag hTERT/fg HPRT mRNA). Additionally, the “low-expression” and “high-expression” groups were merged into a joint group comprising patients with detectable hTERT levels (>0.00 ag hTERT/fg HPRT mRNA).
Statistical Analysis
Multivariate analysis according to Cox proportional hazards regression model (with adjustment for tumor staging, type of tumor resection, and patients' age) was performed for the analysis of hTERT mRNA expression. For statistical analyses, SPSS 15.0 software was used. P < .05 was considered significant.
Results
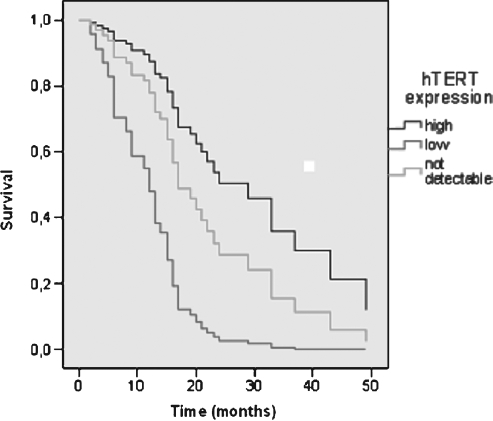
Of our cohort, 33 had undetectable, 17 had low, and 6 had high hTERT mRNA levels. The clinical data of the resulting three sub-groups are listed in Table 1. The data on the impact of the gene expression on prognosis—as determined by a multivariate Cox regression hazard model—are plotted in Figure 1.
Table 1.
Clinical and Histopathologic Data.
| Total (n = 56) | Low hTERT (n = 17) | Not Detectable hTERT (n = 33) | High hTERT (n = 6) | Patients at Follow-up | ||
| Alive* (n = 6) | Dead† (n = 50) | |||||
| Men/Women | 36:20 | 11:6 | 20:13 | 5:1 | 4:2 | 32:18 |
| Tumor stage | ||||||
| I | 2 | 1 | 1 | 0 | 1 | 1 |
| II | 12 | 2 | 8 | 2 | 1 | 11 |
| III | 31 | 7 | 21 | 3 | 4 | 27 |
| IV | 11 | 7 | 3 | 1 | 0 | 11 |
| Tumor resection | ||||||
| Radical (R0) | 38 | 9 | 25 | 4 | 5 | 33 |
| Not radical (R1) | 9 | 2 | 6 | 1 | 1 | 8 |
| Not radical (R2) | 9 | 6 | 2 | 1 | 0 | 9 |
| Patients at follow-up | ||||||
| Alive* | 6 | 0 | 5 | 1 | 6 | |
| Dead† | 50 | 17 | 28 | 5 | 50 | |
Data are number of patients.
After an average observation time of 15.8 months (range, 1–61 months).
Patients died after an average of 14 months (range, 1–49 months), 2 patients died of non-tumor-related reasons.
Figure 1.
Prognostic relevance of hTERT mRNA expression, analyzed with multivariate Cox proportional hazard regression model for overall survival for 56 PDAC patients (UICC stages I–IV, R0–2 resection), adjusted for tumor stage, type of tumor resection, and patients' age. Cutoffs for expression of hTERT were the mean value (61 ag) and undetectable mRNA levels (0.00 ag): >61 ag (high); >0.00 to ≤61 ag (low), and 0.00 ag mRNA (not detectable) standardized to HPRT transcript levels.
Surprisingly, the analysis of patients of all stages and resection types showed that patients with low expression levels of hTERT message in their PDAC did worse than those with high hTERT mRNA [relative risk (RR) = 3.39; P = .046; Figure 1]. Unexpectedly again, patients with undetectable hTERT took an intermediate course (RR = 1.60; P = .392) when compared with patients presenting high hTERT levels. Aiming at the analysis of patients treated with a curative intent, we excluded patients with advanced disease (UICC stage IVb—concomitance of metastases and/or local R2-resection). Within this group of patients, the presence of metastases and/or non-resectable tumor mass might be a more decisive factor of survival than the expression of hTERT. Interestingly, on such analysis, an even more explicit, significant association of a worse prognosis in patients with low expression of hTERT was observed (RR = 5.33; P = .013) when compared to patients with a high expression profile. Undetectable expression was again associated with a slightly worse outcome compared with patients with high hTERT levels (RR = 1.83; P = .32). Furthermore, in comparison with the patients showing undetectable expression of hTERT, the risk of tumor-related death in those with the low expression profile showed a highly significant increase to 3.8-fold (P = .002). Additionally, we analyzed the impact of the overall expression of hTERT (merged “low-expression” and “high-expression” groups; n = 23) on survival. The comparison of this group with patients presenting undetectable hTERT mRNA levels showed a tendency—however, not significant—for an overall increased risk of tumor-related death for patients expressing hTERT mRNA (RR = 1.358; P = .340).
Accordingly, analysis of the three hTERT expression profile groups with regard to the 18-month, and the average survival rate was performed. Of 33 patients with undetectable hTERT expression, 14 were still alive 18 months after diagnosis and surgery or had died within this time span of non-tumor-related causes. Interestingly, only 1 of 17 patients with low hTERT expression survived this period, whereas 3 of 6 patients with high hTERT expression were either still alive or had died due to non-tumor-related causes. The average survival rate in each group was 9.5 months in the low expression profile group, 18.2 months for patients with undetectable hTERT expression, and 20.8 months for patients with high hTERT expression.
Discussion
The data from this study challenge the present view that telomerase activity, strictly and in general, correlates with the biologic behavior of a malignant tumor when it comes to complex parameters such as survival after surgery. The expression of low levels of hTERT mRNA in PDAC does have a negative impact on patients' prognosis and a tendency for an association of hTERT expression in general with an unfavorable outcome can also be observed in our study. These data are at first consistent with observations made on other tumor entities. Among others, hTERT overexpression is (solely) associated with a significant poor outcome in soft-tissue sarcoma [11,12], non-small cell lung cancer [10] and also in combination with cytokeratins 19/20 and carcinoembryonic antigen in colorectal cancer [9]. However, the somewhat better prognosis of patients accompanying a high hTERT expression profile has not been reported for other malignant tumors up-to-date. This intriguing, more favorable outcome might indicate a complex and precise regulation of hTERT, affirming evidence on the complex biology of telomerase regulation.
Current evidence suggests that hTERT expression and its mRNA levels are mainly controlled at the level of transcription by such means as hypoxia (through hypoxia response element sites), mitogens, hormones (e.g., estrogen), chromatin remodeling, and cell signaling pathways [1,19]. Thus, hTERT's interrelation with different oncogenes, including c-Myc, Bcl-2, Her-2, Ras [20–22], p53 [23], and survivin [11,12,24], and the transcription factors Sp1 and Sp3 [19] has been suggested to play an important role in cancer progression and telomerase control.
However, additional ways of the regulation of hTERT expression have been proposed and provide possible explanations for the observations in our study. Thus, regulation of transcript processing, changes in mRNA half-life [19], specific activation of hTERT promoter by enhancer binding protein-2β [25], or hTERT gene regulation in the form of alternative splicing (e.g., with the active wild type and inactive b variant, as shown under hypoxic conditions) [26–28] might account for low hTERT activity despite high mRNA levels and effectively result in a better prognosis for PDAC patients. Thus, some alternately spliced deletion variants of hTERT were shown to be functionally inactive or even impair the function of telomerase (e.g., the hTERTα- splice variant), causing telomere shortening and cell death [29,30]. Tumors with a high hTERT expression profile might have either less or inactive variants of its mRNA, be subject to faster degradation or ineffective translation processes, resulting in a less functional protein expression.
In summary, measurement of hTERT transcription levels identifies groups of PDAC patients with different prognoses, with a patient group showing a 5.33-fold increased risk of tumor-related death. This might have an important potential as a predictor of survival. The oncobiologic role of telomerase activity in PDAC, intriguingly, remains elusive so far.
Supplementary Material
Acknowledgments
The authors thank T. Köhler, A. Rost (AJ Roboscreen GmbH, Leipzig, Germany), and T. Hillebrand (AJ Innuscreen GmbH, Berlin, Germany) for assistance with RNA analysis; S. Stöhr (Institute of Medical Biometrics, University of Ulm, Ulm, Germany) for help with statistical analysis; U. Bhanot and C. Hasel (Institute of Pathology, The University Hospital of Ulm, Ulm, Germany) for assistance with histopathologic examination; and N. Süssner for tissue preparation for microdissection.
Abbreviations
- HPRT
hypoxanthine-phospho-ribosyl-transferase
- hTERT
human telomerase reverse transcriptase
- PDAC
pancreatic ductal adenocarcinoma
- RR
relative risk
Footnotes
H.T.'s work was supported by the Deutsche Krebshilfe Miltred Scheel Stiftung (project: 107590).
This article refers to supplementary material, which is designated by Table W1 and is available online at www.neoplasia.com.
References
- 1.Cairney CJ, Keith WN. Telomerase redefined: integrated regulation of hTR and hTERT for telomere maintenance and telomerase activity. Biochimie. 2008;90:13–23. doi: 10.1016/j.biochi.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Tauchi T, Shin-ya K, Sashida G, Sumi M, Okabe S, Ohyashiki JH, Ohyashiki K. Telomerase inhibition with a novel G-quadruplex-interactive agent, telomestatin: in vitro and in vivo studies in acute leukemia. Oncogene. 2006;25:5719–5725. doi: 10.1038/sj.onc.1209577. [DOI] [PubMed] [Google Scholar]
- 3.Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–931. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 4.Verdun RE, Karlseder J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell. 2006;127:709–720. doi: 10.1016/j.cell.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JE, Gettings EJ, Schwalm J, Pei J, Testa JR, Litwin S, von Mehren M, Broccoli D. Whole-genome profiling in liposarcomas reveals genetic alterations common to specific telomere maintenance mechanisms. Cancer Res. 2007;67:9221–9228. doi: 10.1158/0008-5472.CAN-07-1133. [DOI] [PubMed] [Google Scholar]
- 6.Ducrest AL, Amacker M, Mathieu YD, Cuthbert AP, Trott DA, Newbold RF, Nabholz M, Lingner J. Regulation of human telomerase activity: repression by normal chromosome 3 abolishes nuclear telomerase reverse transcriptase transcripts but does not affect c-Myc activity. Cancer Res. 2001;61:7594–7602. [PubMed] [Google Scholar]
- 7.Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Tahara E, Ide T, Ishikawa F. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet. 1998;18:65–68. doi: 10.1038/ng0198-65. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick KL, Mokbel K. The significance of human telomerase reverse transcriptase (hTERT) in cancer. Eur J Surg Oncol. 2001;27:754–760. doi: 10.1053/ejso.2001.1151. [DOI] [PubMed] [Google Scholar]
- 9.Uen YH, Lin SR, Wu DC, Su YC, Wu JY, Cheng TL, Chi CW, Wang JY. Prognostic significance of multiple molecular markers for patients with stage II colorectal cancer undergoing curative resection. Ann Surg. 2007;246:1040–1046. doi: 10.1097/SLA.0b013e318142d918. [DOI] [PubMed] [Google Scholar]
- 10.Zhu CQ, Cutz JC, Liu N, Lau D, Shepherd FA, Squire JA, Tsao MS. Amplification of telomerase (hTERT) gene is a poor prognostic marker in non-small-cell lung cancer. Br J Cancer. 2006;94:1452–1459. doi: 10.1038/sj.bjc.6603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taubert H, Würl P, Greither T, Kappler M, Bache M, Bartel F, Kehlen A, Lautenschläger C, Harris LC, Kaushal D, et al. Stem cell-associated genes are extremely poor prognostic factors for soft-tissue sarcoma patients. Oncogene. 2007;26:7170–7174. doi: 10.1038/sj.onc.1210530. [DOI] [PubMed] [Google Scholar]
- 12.Würl P, Kappler M, Meye A, Bartel F, Köhler T, Lautenschläger C, Bache M, Schmidt H, Taubert H. Co-expression of survivin and TERT and risk of tumour-related death in patients with soft-tissue sarcoma. Lancet. 2002;359:943–945. doi: 10.1016/S0140-6736(02)07990-4. [DOI] [PubMed] [Google Scholar]
- 13.Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI, et al. Pancreatic cancer. Curr Probl Cancer. 2002;26:176–275. doi: 10.1067/mcn.2002.129579. [DOI] [PubMed] [Google Scholar]
- 14.Ohuchida K, Mizumoto K, Ogura Y, Ishikawa N, Nagai E, Yamaguchi K, Tanaka M. Quantitative assessment of telomerase activity and human telomerase reverse transcriptase messenger RNA levels in pancreatic juice samples for the diagnosis of pancreatic cancer. Clin Cancer Res. 2005;11:2285–2292. doi: 10.1158/1078-0432.CCR-04-1581. [DOI] [PubMed] [Google Scholar]
- 15.Ohuchida K, Mizumoto K, Yamada D, Yamaguchi H, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. Quantitative analysis of human telomerase reverse transcriptase in pancreatic cancer. Clin Cancer Res. 2006;12:2066–2069. doi: 10.1158/1078-0432.CCR-05-1821. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto Y, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Fukuda E, Sueda T, Hiyama E. Detection of human telomerase reverse transcriptase (hTERT) expression in tissue and pancreatic juice from pancreatic cancer. Surgery. 2008;143:113–125. doi: 10.1016/j.surg.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 17.Kawahara R, Odo M, Kinoshita H, Shirouzu K, Aoyagi S. Analysis of hTERT mRNA expression in biliary tract and pancreatic cancer. J Hepatobiliary Pancreat Surg. 2007;14:189–193. doi: 10.1007/s00534-006-1132-2. [DOI] [PubMed] [Google Scholar]
- 18.Sawabu N, Watanabe H, Yamaguchi Y, Ohtsubo K, Motoo Y. Serum tumour markers and molecular biological diagnosis in pancreatic cancer. Pancreas. 2004;28:263–267. doi: 10.1097/00006676-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Ducrest AL, Szutorisz H, Lingner J, Nabholz M. Regulation of the human telomerase reverse transcriptase gene. Oncogene. 2002;21:541–552. doi: 10.1038/sj.onc.1205081. [DOI] [PubMed] [Google Scholar]
- 20.Goueli BS, Janknecht R. Upregulation of the catalytic telomerase subunit by the transcription factor ER81 and oncogenic HER2/Neu, Ras, or Raf. Mol Cell Biol. 2004;24:25–35. doi: 10.1128/MCB.24.1.25-35.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandal M, Kumar R. Bcl-2 modulates telomerase activity. J Biol Chem. 1997;272:14183–14187. doi: 10.1074/jbc.272.22.14183. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes Dev. 1998;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beliveau A, Yaswen P. Soothing the watchman: telomerase reduces the p53-dependent cellular stress response. Cell Cycle. 2007;6:1284–1287. doi: 10.4161/cc.6.11.4298. [DOI] [PubMed] [Google Scholar]
- 24.Endoh T, Tsuji N, Asanuma K, Yagihashi A, Watanabe N. Survivin enhances telomerase activity via up-regulation of specificity protein 1- and c-Myc-mediated human telomerase reverse transcriptase gene transcription. Exp Cell Res. 2005;305:300–311. doi: 10.1016/j.yexcr.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Deng WG, Jayachandran G, Wu G, Xu K, Roth JA, Ji L. Tumor-specific activation of human telomerase reverse transcriptase promoter activity by activating enhancer-binding protein-2beta in human lung cancer cells. J Biol Chem. 2007;282:26460–26470. doi: 10.1074/jbc.M610579200. [DOI] [PubMed] [Google Scholar]
- 26.Anderson CJ, Hoare SF, Ashcroft M, Bilsland AE, Keith WN. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene. 2006;25:61–69. doi: 10.1038/sj.onc.1209011. [DOI] [PubMed] [Google Scholar]
- 27.Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–4171. [PubMed] [Google Scholar]
- 28.Hisatomi H, Ohyashiki K, Ohyashiki JH, Nagao K, Kanamaru T, Hirata H, Hibi N, Tsukada Y. Expression profile of a γ-deletion variant of the human telomerase reverse transcriptase gene. Neoplasia. 2003;5:193–197. doi: 10.1016/S1476-5586(03)80051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colgin LM, Wilkinson C, Englezou A, Kilian A, Robinson MO, Reddel RR. The hTERT α splice variant is a dominant negative inhibitor of telomerase activity. Neoplasia. 2000;2:426–432. doi: 10.1038/sj.neo.7900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi X, White DM, Aisner DL, Baur JA, Wright WE, Shay JW. An alternate splicing variant of the human telomerase catalytic subunit inhibits telomerase activity. Neoplasia. 2000;2:433–440. doi: 10.1038/sj.neo.7900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.