Abstract
The tyrosine kinase receptor c-kit and its ligand stem cell factor (SCF) have not been explored in prostate cancer (PC) bone metastasis. Herein, we found that three human PC cell lines and bone marrow stromal cells express a membrane-bound SCF isoform and release a soluble SCF. Bone marrow stromal cells revealed strong expression of c-kit, whereas PC cells showed very low levels of the receptor or did not express it all. Using an experimental model of PC bone metastasis, we found that intraosseous bone tumors formed by otherwise c-kit-negative PC3 cells strongly expressed c-kit, as demonstrated using immunohistochemical and Western blot analyses. Subcutaneous PC3 tumors were, however, c-kit-negative. Both bone and subcutaneous PC3 tumors were positive for SCF. Immunohistochemical analysis of human specimens revealed that the expression frequency of c-kit in epithelial cells was of 5% in benign prostatic hyperplasia, 14% in primary PC, and 40% in PC bone metastases, suggesting an overall trend of increased c-kit expression in clinical PC progression. Stem cell factor expression frequency was more than 80% in all the cases. Our data suggest that the bone microenvironment up-regulates c-kit expression on PC cells, favoring their intraosseous expansion.
Introduction
Skeletal metastasis is the biggest cause of morbidity and mortality in prostate cancer (PC) patients [1]. Once the disease metastasizes, treatment available at present is palliative and not curative. Therefore, the identification of new molecular targets is urgently required for the development of innovative strategies aimed at eliminating or delaying bone metastases in PC patients.
Overexpression and/or structural alterations of many receptor tyrosine kinase (RTK) family members are frequently associated to several types of cancers [2], and the activation of RTKs ultimately lead to an ample spectrum of cellular processes known to be crucial to cancer growth and progression, such as proliferation, migration, angiogenesis, and survival. The proto-oncogene c-kit (CD117, or stem cell factor [SCF] receptor) is one of these RTKs and belongs to class III subfamily, which also includes the platelet-derived growth factor receptors and the macrophage colony-stimulating factor 1 receptor [3]. c-kit was first identified as the cellular homolog of the oncogene v-kit present in the genome of Hardy-Zuckerman 4-feline sarcoma virus [4] and was then found to be allelic with the murine white spotting (W) locus [5,6]. The ligand for c-kit is the SCF, also known as mast cell growth factor, kit ligand (KL), or steel factor [7,8]. Alternative splicing of the pre-mRNA involving the region corresponding to exon 6 gives rise to two SCF transcript forms initially expressed at the cell membrane [9]. The exon 6-containing SCF isoform (SCF248 or KL-1) is a 45-kDa glycoprotein that is readily cleaved and released from the cell membrane as a soluble SCF (sSCF) of 31 kDa [9,10] by different proteases [11–13]. Conversely, the SCF isoform lacking exon 6 (SCF220 or KL-2) is mostly present as a membrane-bound species of 32 kDa [10]. Both membrane-bound SCF (mSCF) and sSCF are bioactive [7], although they show different efficiency in activating c-kit tyrosine kinase [14]. Activation of c-kit occurs by binding to noncovalent SCF homodimers, which mediates dimerization of the receptor followed by autophosphorylation of its intracellular tyrosine kinase domains, leading to activation of different signaling transduction pathways [10,15].
From studies in mice with loss-of-function mutations in c-kit and SCF (W- and Sl-mutants), it was concluded that these factors constitute an important signal transduction system regulating normal cell growth and differentiation in hematopoiesis, gametogenesis, and melanogenesis [16,17]. Stem cell factor has been found to be expressed in normal glandular myoepithelium of the breast, bladder and cervical smooth muscle, and in bone marrow and prostate stromal cells, whereas the c-kit protein was found on tissue mast cells, basal cells of skin, hematopoietic stem cells, and glandular breast epithelial cells, among other cell types [18]. Some malignancies have been reported to express constitutive gain-of-function mutations in c-kit that induce ligand-independent activation of the receptor [19–22] or mutations that encode for truncated c-kit proteins with still not clear functions [23,24]. However, other tumor types coexpress c-kit and SCF [25–29], suggesting the existence of an autocrine loop.
Although previous studies have investigated c-kit and SCF expression in PC cell lines [30,31], the results reported by the different groups are to some extent discrepant. Additionally, analyses of human benign and malignant prostatic tissue have found altered patterns of expression of c-kit and SCF [30], but no studies have been made in PC bone metastasis. In an attempt to enhance the knowledge in this area and clarify the disparate results obtained in other studies, here we investigated the expression of c-kit and SCF in vitro and in vivo. In addition, an immunohistochemical study in archival tissue human specimens that include PC bone metastases was performed.
Materials and Methods
Cell Culture
The human PC cell lines PC3 [32], LNCaP [33], and DU145 [34], derived from metastases to the bone, supraclavicular lymph node, and brain, respectively, were originally obtained from American Type Culture Collection (Manassas, VA). Bone marrow stromal (BMS) cells were obtained by scraping the bone marrow cavity of 22- to 24-week gestating human male fetal femurs derived from elective pregnancy terminations in compliance with state and federal regulations (Advanced Bioscience Resources, Alameda, CA). PC3, LNCaP, and BMS cells were cultured in RPMI-1640 (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA). DU145 cells were maintained in DMEM culture medium (Invitrogen) with 10% FBS. Bone marrow stromal cells displayed a fibroblastoid phenotype, as we have previously shown [35].
Real-time Polymerase Chain Reaction
Total RNA was extracted from cultured cells using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Two micrograms of total RNA were used to synthesize a firststrand complimentary DNA (cDNA) with an oligo(dT) primer and Super Script II reverse transcription (Invitrogen). The real-time polymerase chain reaction (PCR) analysis was performed with SYBER green PCR core reagents (Stratagene, La Jolla, CA) in a Stratagene Mx4000 cycler, and the data were analyzed using Mx4000 v3.01 software. Fold changes for each gene were normalized to glyceraldehyde phosphate dehydrogenase (GAPDH) and then expressed as relative to gene message expression in BMS cells (arbitrarily set as 1), according to the 2-ΔΔCT T method [36]. The following primers were used: human SCF, forward (5′-ACT GAC TCT GGA ATC TTT CTC AGG-3′) and reverse (5′-GAT GTT TTG CCA AGT CAT TGT TGG-3′); human c-Kit, forward (5′-ATG AGA GGC GCT CGC GGC GC-3′) and reverse (5′-AGC TTG GCA GGA TCT CTA AC-3′); and human GAPDH, forward (5′-AAG GTC ATC CCT GAG CTG AA-3′) and reverse (5′-TGA CAA AGT GGT CGT TGA GG-3′). For each sample, real-time PCR was performed in triplicate.
Cell Surface Biotinylation and Immunoblot Analysis
Specific labeling of cell surface proteins was performed using EZ-Link Sulfo-NHS-Biotin (Pierce, Rockford, IL) according to the manufacturer's instructions. Briefly, cells were washed twice with phosphate-buffered saline (PBS) containing MgCl2 and CaCl2 and then biotinylated with the Sulfo-NHS-biotin solution. After quenching and washing, cells were lysed in NP-40 lysis buffer (50 mM Tris pH 7.5, 150 nM NaCl, 1% NP-40) containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and centrifuged at 13,000 rpm. Protein concentrations were measured and normalized in the obtained supernatants. Biotinylated proteins were precipitated with NeutrAvidin Protein (Pierce), washed with buffer, separated on 4% to 12% Bis-Tris gel (Invitrogen), and transferred to a nitrocellulose membrane. A monoclonal antibody against the extracellular N-terminal domain of human c-kit (clone 28, 1:1000; BD Biosciences, Burlington, MA) and a rabbit antibody that cross-reacts with mouse and human SCF (1:250; Chemicon, Temecula, CA) were used as primary antibodies. The membranes were then incubated with 1:2500 antimouse (for c-kit) or 1:4000 goat antirabbit (for SCF) horseradish peroxidase conjugates (Cell Signaling Technology, Danvers, MA) at room temperature for 1 hour and then developed with SuperSignal West Femto Maximum and Pico Chemiluminescent substrates (Pierce), respectively. Whole HeLa and CCRF-HSB cell lysates (Santa Cruz Biotechnology, Santa Cruz, CA) were used as positive control for SCF and c-kit, respectively. Tissue homogenates derived from PC3 bone tumors, control human fetal bone xenografts, or subcutaneous PC3 tumors obtained as described below were used for Western blot analysis of c-kit and SCF. To confirm the presence of PC in the tissue extracts, the membranes were stripped and reprobed with a mixture of anticytokeratin monoclonal antibodies (1:500; Sigma Chemical Co., St Louis, MO) that recognize cytokeratins 1, 4, 5, 6, 8, 10, 13, 18, and 19. The cytokeratins detected by these antibodies range in molecular weight (MW) between 40 and 68 kDa. Glyceraldehyde phosphate dehydrogenase (Trevigen, Inc., Gaithersburg, MD) was used as a loading control.
Stem Cell Factor ELISA
PC3, LNCaP, DU145, and BMS cells were plated in their respective complete culture medium in 24-well plates at a concentration of 1 x 105 cells per well. Media were replaced 24 hours later by FBS-free corresponding culture media supplemented with 0.1% bovine serum albumin (Sigma Chemical Co.; 500 µl per well). Conditioned media (CM) were collected 48 hours later, centrifuged to remove cell debris, and used undiluted to quantitate sSCF using Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Concentrations were calculated using a standard curve generated with recombinant human SCF included in the kit and were expressed as picograms per 105 cells per milliliter. The ELISA assay was performed in triplicate.
Mice
Five-week-old male homozygous C.B-17 scid/scid (severe combined immunodeficient; SCID) mice were purchased from Taconic Farms (Germantown, NY) and were maintained under aseptic conditions. All procedures involving mice were performed according to the National Institutes of Health standards established in the Guidelines for the Care and Use of Experimental Animals and were approved by the Animal Investigation Committee at Wayne State University School of Medicine.
Establishment of PC3 Human Bone Tumors
One fourth human male fetal femurs (Advanced Bioscience Resources) were implanted under the skin of SCID mice as described previously for the SCID-human model of PC bone metastasis [37]. Four weeks later, 1 x 105 PC3 were injected through the mouse skin directly into the marrow side of the previously implanted bone. Bone implants injected with FBS-free culture medium served as controls. Mice were euthanized 4 weeks later, and the xenografts were harvested, fixed in 4% paraformaldehyde, decalcified with 10% ethyl-enediaminetetraacetic acid (pH 6.5) in PBS, and processed for immunohistochemistry. Some specimens were used instead to obtain tissue extracts for Western blot analysis of c-kit and SCF, as described previously. Briefly, harvested tissues were immediately homogenized in a mortar (CoorsTek, Golden, CO) with 500 µl of tissue lysis buffer (150 mM NaCl, 20 mM Tris, pH 7.5, 1% NP-40, and 5 mM EDTA), the homogenate obtained was centrifuged at 10,000 rpm at 4°C for 10 minutes, and the supernatant was collected and stored at -80°C for ulterior analysis. Subcutaneous PC3 tumors obtained in SCID mice and harvested 14 days after tumor inoculation were used as nonosseous control tumors.
Human Tissue Samples
Clinical samples were obtained from patients between 54 and 78 years of age who underwent radical prostatectomy for primary PC (n = 21) or transurethral resection for benign prostatic hyperplasia (BPH, n = 22). Forty-three percent of PC specimens had Gleason scores 5 to 6, whereas the rest had a Gleason score of 7 or higher. Tissues were fixed in 10% buffered formalin and paraffin-embedded after surgery. All procedures were performed by the Department of Urology, Johannes Gutenberg-University, Mainz, Germany. Bone metastasis tissue samples (n = 20) from rapid autopsies of PC patients were obtained as previously described [38], in the Department of Urology, University of Washington, Seattle, WA.
Immunohistochemistry
Five-micrometer tissue sections were deparaffinized, pretreated in Ag Citrus Plus Retrieval Solution (BioGenex, San Ramon, CA) in a microwave to achieve antigen retrieval, and incubated for 60 minutes at room temperature with rabbit antibodies generated against human SCF (K089; Immuno-Biological Laboratories Co, Japan) or the cytoplasmic C-terminal domain of human c-kit (DakoCytomation, Denmark), both diluted 1:100 in PBS. After incubation, the sections were washed with PBS and processed using the Vectastain Elite ABC (peroxidase) kit for rabbit IgG (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. The specimens were then incubated with diaminobenzidine (Sigma Chemical Co.)/H2O2 solution, and cell nuclei were lightly counterstained with Mayer's hematoxylin. Negative controls were obtained by replacing the primary antibodies with nonimmune rabbit immunoglobulin.
Cocultures and Immunomagnetic Cell Separation
Confluent monolayers of BMS cells obtained in six-well plates were overlaid with PC3 cells (2.5 x 105 cells/well) and cocultured for 24 hours. The culture medium was then replaced by serum-free medium, and cocultured cells were harvested 24 and 48 hours later using 2 mM EDTA. Cell suspensions were incubated with CD326 (antihuman EpCAM) MACS microBeads (Miltenyi Biotec Inc., Auburn, CA) and then applied on magnetic columns (MACS Cell Isolation kit; Miltenyi Biotec) following the manufacturer's instructions. Bone marrow stromal and PC3 cells cultured alone were used as controls. Cell lysates were obtained and analyzed for c-kit using Western blot as described previously.
Statistical Analysis
Fisher's exact test was used to assess differences in c-kit and SCF immunostaining between clinical tumor samples. Statistical analysis was performed using the GraphPad InStat version 3.0 (GraphPad Software, San Diego, CA). Differences were considered statistically significant when P < .05.
Results
SCF and c-kit mRNA Expression in Bone Marrow Stromal and PC Cells
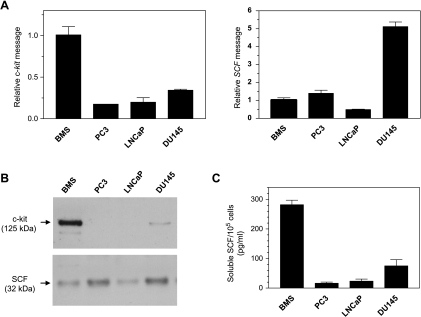
C-kit and SCF transcript levels of BMS and PC cells were assessed semiquantitatively by real-time PCR. As can be seen in Figure 1A, the c-kit mRNA level in BMS cells was three to five times higher than in PC cells, among which PC3 cells displayed the lowest level. As for SCF gene expression, DU145 cells showed the highest level. The mean relative SCF message was around five times lower in BMS and PC3 cells and 10 times lower in LNCaP cells than in DU145 cells (Figure 1A).
Figure 1.
C-kit and SCF mRNA expression in BMS and PC cells, as determined using real-time PCR. Relative quantitation of the SCF and c-kit genes was performed and normalized by GAPDH gene expression using the 2-ΔΔCT method. Values obtained for PC cells were then expressed as fold increase/decrease relative to that obtained for BMS cells, which was arbitrarily set as 1 (A). Cell surface expression of c-kit and SCF was analyzed in avidin-precipitated fractions of biotinylated samples separated on 4% to 12% Bis-Tris minigels followed by Western blot analysis. Samples were normalized for protein concentrations before precipitation with avidin beads. (B). Soluble SCF was quantitated in CM using ELISA, and the results were expressed as mean ± SE for triplicate determinations (C).
SCF and c-kit Protein Expression and SCF Shedding In Vitro
To determine the protein expression and cell localization of c-kit and SCF in the different cell lines, we performed cell surface biotinylation followed by Western blot. In agreement with the results obtained by real-time PCR, the highest expression of c-kit protein with a MW of 125 kDa was found in BMS cells. DU145 cells revealed a less intense band with the same MW than BMS cells, whereas no expression was evident for LNCaP and PC3 cells (Figure 1B). This apparent MW of 125 kDa matched with the single band shown by CCRF-HSB cell lysates used as a positive control (data not shown).
The expression of SCF protein and its localization on the cell surface of the various cell types were verified by immunoblot analysis of the biotinylated samples pulled down by the avidin beads (Figure 1B). Stem cell factor was detected as a band of ∼32 kDa, corresponding to the reported MW of the mSCF isoform lacking exon 6 (SCF220 or KL-2). Protein and mRNA SCF expression patterns followed the same trend.
Stem cell factor shedding from the cell surface was verified by analysis of CM using an enzyme-linked immunoassay. The level of sSCF released from BMS cells was between 3.8 and 17 times higher than levels found in CM obtained from the same number of PC cells (Figure 1C).
SCF and c-kit in Experimental PC3 Human Bone Tumors and Clinical Prostatic Tissue Samples
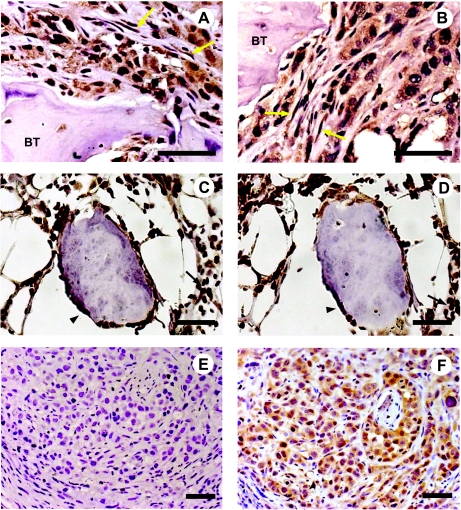
To study c-kit and SCF in PC growing intraosseously, we selected the PC3 cell line, based on the fact that it was originally established from skeletal metastasis [32]. Immunohistochemical analysis of PC3 bone tumors generated in the SCID-human model demonstrated strong positivity for c-kit and SCF. These epitopes were expressed not only by PC3 cells growing within the bone but also by bone stromal cells with fibroblast morphology adjacent to tumor cells (Figure 2, A and B, respectively). Hematopoietic and osteoblast cells present in control bone xenografts devoid of tumor cells also immunostained positive for c-kit and SCF (Figure 2, C and D, respectively). Conversely, subcutaneous PC3 tumors, used as a microenvironmental control, revealed expression of SCF but not of c-kit (Figure 2, E and F, respectively).
Figure 2.
Immunohistochemical staining of c-kit (A) and SCF (B) in bone tumors obtained by injection of PC3 cells into human bone xenografts in SCID mice. The immunostaining for c-kit (C) and SCF (D) in control human fetal bone xenografts show reactivity in osteoblasts and hematopoietic cells. Subcutaneous PC3 tumors used as controls were also immunostained for c-kit (E) and SCF (F). BT indicates bone trabeculae; yellow arrow, stromal cells; black arrowhead, osteoblasts; black arrow, hematopoietic cells. Scale bars, 50 µm.
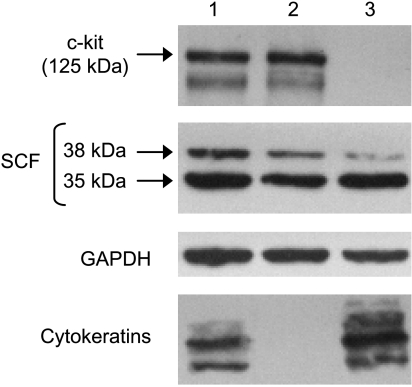
Western blot analysis of tissue homogenates obtained from PC3 bone tumors, and control bone xenografts confirmed the immunohistochemical findings. A 125-kDa protein was detected by the antibody against c-kit, whereas the anti-SCF antibody detected 35- and 38-kDa proteins (Figure 3, lanes 1 and 2), which could represent products with different glycosylation, also found in whole cell lysates of BMS and PC3 cells grown in vitro (data not shown). The presence of proteins from tumor cells was confirmed in the lysates using cytokeratin immunoblot analysis. Despite the high number of PC3 cells in the bone tumors (Figure 2, A and B), the intensity of the immunoreactive bands obtained for c-kit and SCF was similar to those obtained for the control bone xenografts lysates with an equivalent total protein content, as evidenced by GAPDH loading (Figure 3, lanes 1 and 2). This suggests that the tumor cells contributed to c-kit and SCF production in the bone tumors. In the case of c-kit, the results were unexpected because we predicted that the absence of c-kit protein in PC3 cells, as revealed in vitro, would have diluted the levels of c-kit derived from bone tissue in the homogenates of PC3 bone tumors. In addition, Western blot analysis of tissue homogenates obtained from subcutaneous PC3 tumors revealed SCF expression but nondetectable c-kit (Figure 3, lane 3), displaying an expression pattern similar to that observed in vitro. Taken together, these results suggest an induction of c-kit in PC3 cells by the bone microenvironment. To investigate this phenomenon, we cocultured PC3 and BMS cells for 24 hours and then separated them by immunomagnetic enrichment using microbeads bound to an antibody against EpCAM, a cell adhesion molecule constitutively expressed by epithelial cells [39]. Cell lysates derived from both cell populations were analyzed by immunoblot analysis using antibodies against c-kit. No c-kit expression was detected in either PC3 cells cultured alone or together with BMS following immunomagnetic separation, whereas the level of c-kit expression on BMS cells was found to be the same in both fractions (data not shown). We should acknowledge, however, that the conditions found in vivo when PC3 interact with the bone microenvironment may not be replicated in the coculture performed and/or that cells other than BMS could induce c-kit expression in the PC cells.
Figure 3.
Western blot analysis for c-kit and SCF in tissue homogenates obtained from PC3 tumor growing within a human bone xenograft (lane 1), control human bone xenograft injected with culture medium (lane 2), and PC3 subcutaneous tumor (lane 3). Glyceraldehyde phosphate dehydrogenase was used as loading control. Human cytokeratin was used as an epithelial marker to detect PC3 cells in the lysates.
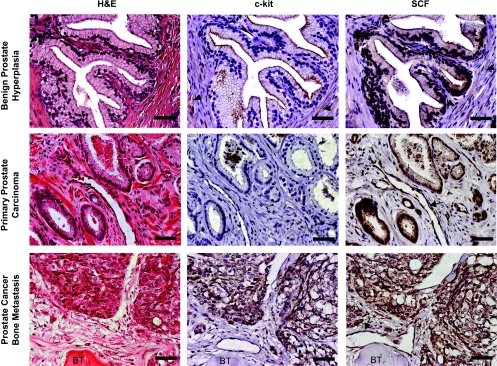
To determine whether the c-kit and SCF expression patterns displayed by the experimental bone metastasis model were similar to those present in human bone metastasis, we performed an immunohistochemical analysis of autopsy specimens from PC patients. We also analyzed archival samples of BPH and primary prostate adenocarcinoma, aimed to evaluate the different specimens under the same methodological procedures and to compare our results with others obtained in previous studies. We found c-kit expression in epithelial cells in only 5% and 14% of patients with BPH and primary PC, respectively, whereas c-kit was positive in 40% of patients with bone metastasis (Table 1 and Figure 4). C-kit expression was not detected in primary prostate PCs with Gleason score lower than 7, and only 25% of the tumors with higher Gleason score showed c-kit positivity in the epithelial compartment (P > .05). In contrast, positive SCF expression was observed in the glandular epithelium of 95% of BPH and primary PC samples studied and in 85% of patients with bone metastasis. Positive immunostaining for SCF was also noticed in blood vessels found in primary carcinomas and bone metastasis.
Table 1.
Expression of c-Kit and SCF in Benign and Malignant Prostate Tumors and Bone Metastasis.
| Tissue | Expressing c-kit (%) | Expressing SCF (%) | Total |
| BPH | 1 (5) | 21 (95) | 22 |
| Primary PC | 3 (14) | 20 (95) | 21 |
| Bone Metastasis | 8 (40)* | 17 (85) | 20 |
P = .0077 between BPH and bone metastasis (Fisher's exact test).
Figure 4.
Immunohistochemical staining of c-kit and SCF in representative serial paraffin sections of human specimens. Note that the strongest immunostaining for c-kit and SCF is restricted to the epithelial cells. A diffuse cytoplasmic immunoreactivity for c-kit and SCF was consistently displayed by fibromuscular stromal cells (arrowhead) in BPH samples. Blood vessels (arrow) occasionally immunostained positive for SCF in primary prostate carcinomas and bone metastasis. BT indicates bone trabeculae. Scale bars, 50 µm.
Discussion
Prostate cancer is the second leading cause of cancer-related death and the most common cancer in males. Approximately 65% to 75% of PCs metastasize to the bone marrow where SCF expressed by stromal cells interacts with c-kit expressed on the surface of progenitor cells [11]. In addition, different tumors have been shown to express SCF and/or c-kit [25–29]. Therefore, in tumors that metastasize to bone, such is the case of PC, autocrine, juxtacrine, and/or paracrine mechanisms could act in concert favoring intraosseous tumor expansion directly or indirectly.
This study examines for the first time the status of c-kit and SCF in PC bone metastasis, both in an experimental model and in patient's specimens. The expression of c-kit has been previously investigated in PC cell lines [30,31], although with conflicting results. In accordance with Savarese et al. [31], we identified c-kit mRNA transcripts in LNCaP, PC3, and DU145 cells, which showed lower levels than in BMS cells. However, as opposed to their findings using immunocytochemical techniques in those PC cells [31], our Western blot analysis of surface biotinylated samples only found c-kit protein expressed on the cell membrane of DU145 cells (we also analyzed BMS cells, which revealed higher c-kit protein levels than DU-145 cells). The discrepancy between their results and ours at the protein level could be due not only to the different techniques used but also to the region of c-kit recognized by the antibodies used. We used an antibody generated against the extracellular domain of c-kit, which allows determining the expression of the receptor present on the cell surface susceptible of binding to SCF. The use of antibodies that recognize other domains of the receptor could account for the detection of other c-kit proteins only present in the cytoplasm, which could even be catalytically inactive. That is the case of a truncated c-kit protein, which contains part of the interkinase domain, the phosphotransferase domain, and the C-terminal region of the receptor, but lacks the ATP binding site, and that is found to be expressed in the cytoplasm of LNCaP cells but not in PC3 cells [24].
Our data regarding SCF release from the different PC cells to the extracellular milieu are mostly in agreement with those previously reported for the same cells [31], with significantly lower levels of sSCF than BMS cells. In addition, we found that only the mSCF isoform lacking exon 6 (SCF220 or KL-2) is expressed on the surface of the different cells in relative levels that match those observed for relative SCF message. Our results are in agreement with previous studies in which it has been demonstrated that the half-life of KL-2 at the cell surface is 10-fold or higher than that of KL-1 [9,40]. This suggests that most of the sSCF found in the CM of the cells derive from the larger splice form KL-1. Because both mSCF and sSCF are biologically active [7,41], it is probable that the membrane-bound KL-2 binds in a juxtacrine manner to c-kit receptors expressed by neighboring cells, whereas the soluble form of KL-1 acts in autocrine and/or paracrine manner/s. In addition, it is likely that binding of mSCF and sSCF to c-kit might affect different cell mechanisms, given their capacity to induce a more or less persistent activation of the receptor and probable differences in downstream cell signaling [41]. Moreover, the interaction between PC cells and different resident cells in the bone microenvironment would result in a complex network of interactions between SCF and c-kit of different sources that, ultimately, could disrupt the local homeostasis and affect the expansion of metastatic PC deposits within the bone.
The c-kit and SCF protein expression profiles observed by us in vitro initially suggested that PC cells would be able to contribute SCF to the bone microenvironment but would not be responsive to juxtacrine (mSCF) or autocrine/paracrine (sSCF) stimulation, because they lack the c-kit receptor or they express it at very low levels. Surprisingly, we found that c-kit was strongly expressed by PC3 bone tumors, but not by PC3 subcutaneous tumors, suggesting that the bone microenvironment up-regulates c-kit expression in PC3 cells. Therefore, it is reasonable to assume that PC3 cells become responsive to SCF produced by different resident cells present within the bone and further contributed by the PC3 cells themselves. The potential functional significance of this induced c-kit protein expression may prove challenging because PC3 cells do not show detectable levels of c-kit on the cell surface in vitro. Therefore, the use of PC3 cells with ectopic expression of c-kit may be necessary to mimic the in vivo up-regulation of c-kit shown by PC3 cells within the bone microenvironment.
Our immunohistochemical analysis of human specimens revealed an increased incidence in c-kit expression in the epithelial compartment of PC bone metastasis, compared to BPH and primary PC, strengthening the data found by us in the SCID-human model and suggesting the relevance of PC-associated c-kit to bone metastasis. As opposed to our study and that of Di Lorenzo et al. [42], Simak et al. [30] reported a higher frequency of c-kit expression in epithelial cells in BPH than in primary PC. This difference could be due to different techniques used in the studies. As for SCF, we found that most BPH, primary PC, and PC bone metastasis specimens expressed it in the epithelial compartment. Our results reveal an association between coexpression of SCF and c-kit in PC cells and bone metastasis, suggesting that both ligand and receptor should be expressed by PC cells for intraosseous development and therefore indicating the existence of an autocrine loop. However, paracrine and juxtacrine mechanisms exerted by tumor-derived and bone-derived SCF cannot be excluded and deserve to be investigated in future studies.
The increased frequency of c-kit shown by PC skeletal metastasis could be the result of a selective dissemination and/or growth into the bone of c-kit-positive cells already present in PC primary tumors, which are known to be heterogeneous [43]. These c-kit-positive cells could also be “stem-like” cancer cells [44,45], which were reported to be present in some human metastatic lesions [46]. However, our data obtained with the experimental bone metastasis model suggest an up-regulation of PC c-kit expression by the bone microenvironment. On the basis of our findings, we propose that the SCF/c-kit axis may contribute to the pathophysiology underlying PC skeletal metastasis. Further studies on signaling pathways and cell functions triggered by the activation of c-kit expressed by PC cells are needed to clarify the role and therapeutic value of this RTK and its ligand in PC bone metastasis.
Acknowledgments
The authors thank Zhong Dong for the critical reading of the manuscript and helpful comments and Allen Saliganan for his excellent technical assistance.
Abbreviations
- BPH
benign prostatic hyperplasia
- BMS
bone marrow stromal
- CM
conditioned medium
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde phosphate dehydrogenase
- KL
kit ligand
- mSCF
membrane-bound SCF
- MW
molecular weight
- PBS
phosphate-buffered saline
- PC
prostate cancer
- RTK
receptor tyrosine kinase
- PCR
polymerase chain reaction
- SCF
stem cell factor
- SCID
severe combined immunodeficient
- sSCF
soluble SCF
Footnotes
This research was supported by a grant from The Fund for Cancer Research. C. Wiesner was supported by Deutsche Forschungsgemeinschaft.
References
- 1.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 2.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8:161–173. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]
- 3.Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besmer P, Murphy JE, George PC, Qiu FH, Bergold PJ, Lederman L, Snyder HW, Jr, Brodeur D, Zuckerman EE, Hardy WD. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986;320:415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- 5.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 6.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, March CJ, Boswell HS, Gimpel SD, Cosman D, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 8.Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, Wellner D, Leder P, Besmer P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 9.Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327–340. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 11.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaguchi N, Horiuchi K, Becherer JD, Toyama Y, Besmer P, Blobel CP. Different ADAMs have distinct influences on Kit ligand processing: phorbol-ester-stimulated ectodomain shedding of Kitl1 by ADAM17 is reduced by ADAM19. J Cell Sci. 2007;120:943–952. doi: 10.1242/jcs.03403. [DOI] [PubMed] [Google Scholar]
- 13.Longley BJ, Tyrrell L, Ma Y, Williams DA, Halaban R, Langley K, Lu HS, Schechter NM. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc Natl Acad Sci USA. 1997;94:9017–9021. doi: 10.1073/pnas.94.17.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- 15.Lemmon MA, Pinchasi D, Zhou M, Lax I, Schlessinger J. Kit receptor dimerization is driven by bivalent binding of stem cell factor. J Biol Chem. 1997;272:6311–6317. doi: 10.1074/jbc.272.10.6311. [DOI] [PubMed] [Google Scholar]
- 16.Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova RF. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl. 1993:125–137. [PubMed] [Google Scholar]
- 17.Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–1425. doi: 10.1177/42.11.7523489. [DOI] [PubMed] [Google Scholar]
- 19.Ning ZQ, Li J, McGuinness M, Arceci RJ. STAT3 activation is required for Asp(816) mutant c-Kit induced tumorigenicity. Oncogene. 2001;20:4528–4536. doi: 10.1038/sj.onc.1204590. [DOI] [PubMed] [Google Scholar]
- 20.Tian Q, Frierson HF, Jr, Krystal GW, Moskaluk CA. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol. 1999;154:1643–1647. doi: 10.1016/S0002-9440(10)65419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 22.Webster JD, Yuzbasiyan-Gurkan V, Kaneene JB, Miller R, Resau JH, Kiupel M. The role of c-KIT in tumorigenesis: evaluation in canine cutaneous mast cell tumors. Neoplasia. 2006;8:104–111. doi: 10.1593/neo.05622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takaoka A, Toyota M, Hinoda Y, Itoh F, Mita H, Kakiuchi H, Adachi M, Imai K. Expression and identification of aberrant c-kit transcripts in human cancer cells. Cancer Lett. 1997;115:257–261. doi: 10.1016/s0304-3835(97)04746-0. [DOI] [PubMed] [Google Scholar]
- 24.Paronetto MP, Farini D, Sammarco I, Maturo G, Vespasiani G, Geremia R, Rossi P, Sette C. Expression of a truncated form of the c-Kit tyrosine kinase receptor and activation of Src kinase in human prostatic cancer. Am J Pathol. 2004;164:1243–1251. doi: 10.1016/S0002-9440(10)63212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krystal GW, Hines SJ, Organ CP. Autocrine growth of small cell lung cancer mediated by coexpression of c-kit and stem cell factor. Cancer Res. 1996;56:370–376. [PubMed] [Google Scholar]
- 26.Hines SJ, Litz JS, Krystal GW. Coexpression of c-kit and stem cell factor in breast cancer results in enhanced sensitivity to members of the EGF family of growth factors. Breast Cancer Res Treat. 1999;58:1–10. doi: 10.1023/a:1006272527435. [DOI] [PubMed] [Google Scholar]
- 27.Bellone G, Silvestri S, Artusio E, Tibaudi D, Turletti A, Geuna M, Giachino C, Valente G, Emanuelli G, Rodeck U. Growth stimulation of colorectal carcinoma cells via the c-kit receptor is inhibited by TGF-beta 1. J Cell Physiol. 1997;172:1–11. doi: 10.1002/(SICI)1097-4652(199707)172:1<1::AID-JCP1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 28.Inoue M, Kyo S, Fujita M, Enomoto T, Kondoh G. Coexpression of the c-kit receptor and the stem cell factor in gynecological tumors. Cancer Res. 1994;54:3049–3053. [PubMed] [Google Scholar]
- 29.Bokemeyer C, Kuczyk MA, Dunn T, Serth J, Hartmann K, Jonasson J, Pietsch T, Jonas U, Schmoll HJ. Expression of stem-cell factor and its receptor c-kit protein in normal testicular tissue and malignant germ-cell tumours. J Cancer Res Clin Oncol. 1996;122:301–306. doi: 10.1007/BF01261407. [DOI] [PubMed] [Google Scholar]
- 30.Simak R, Capodieci P, Cohen DW, Fair WR, Scher H, Melamed J, Drobnjak M, Heston WD, Stix U, Steiner G, et al. Expression of c-kit and kit-ligand in benign and malignant prostatic tissues. Histol Histopathol. 2000;15:365–374. doi: 10.14670/HH-15.365. [DOI] [PubMed] [Google Scholar]
- 31.Savarese DM, Valinski H, Quesenberry P, Savarese T. Expression and function of colony-stimulating factors and their receptors in human prostate carcinoma cell lines. Prostate. 1998;34:80–91. doi: 10.1002/(sici)1097-0045(19980201)34:2<80::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 32.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 33.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 34.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 35.Dong Z, Nemeth JA, Cher ML, Palmer KC, Bright RC, Fridman R. Differential regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase- 1 (TIMP-1) and TIMP-2 expression in co-cultures of prostate cancer and stromal cells. Int J Cancer. 2001;93:507–515. doi: 10.1002/ijc.1358. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Nemeth JA, Harb JF, Barroso U, Jr, He Z, Grignon DJ, Cher ML. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999;59:1987–1993. [PubMed] [Google Scholar]
- 38.Roudier MP, Vesselle H, True LD, Higano CS, Ott SM, King SH, Vessella RL. Bone histology at autopsy and matched bone scintigraphy findings in patients with hormone refractory prostate cancer: the effect of bisphosphonate therapy on bone scintigraphy results. Clin Exp Metastasis. 2003;20:171–180. doi: 10.1023/a:1022627421000. [DOI] [PubMed] [Google Scholar]
- 39.Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 41.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–2548. doi: 10.1007/s00018-004-4189-6. [DOI] [PubMed] [Google Scholar]
- 42.Di Lorenzo G, Autorino R, D'Armiento FP, Mignogna C, De Laurentiis M, De Sio M, D'Armiento M, Damiano R, Vecchio G, De Placido S. Expression of proto-oncogene c-kit in high risk prostate cancer. Eur J Surg Oncol. 2004;30:987–992. doi: 10.1016/j.ejso.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 44.Ceder JA, Jansson L, Ehrnstrom RA, Ronnstrand L, Abrahamsson PA. The characterization of epithelial and stromal subsets of candidate stem/progenitor cells in the human adult prostate. Eur Urol. 2008;53:524–531. doi: 10.1016/j.eururo.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Chen X, Calhoun-Davis T, Claypool K, Tang DG. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 2008;68:1820–1825. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]
- 46.Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, Berman DM. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007;67:9199–9206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]