Abstract
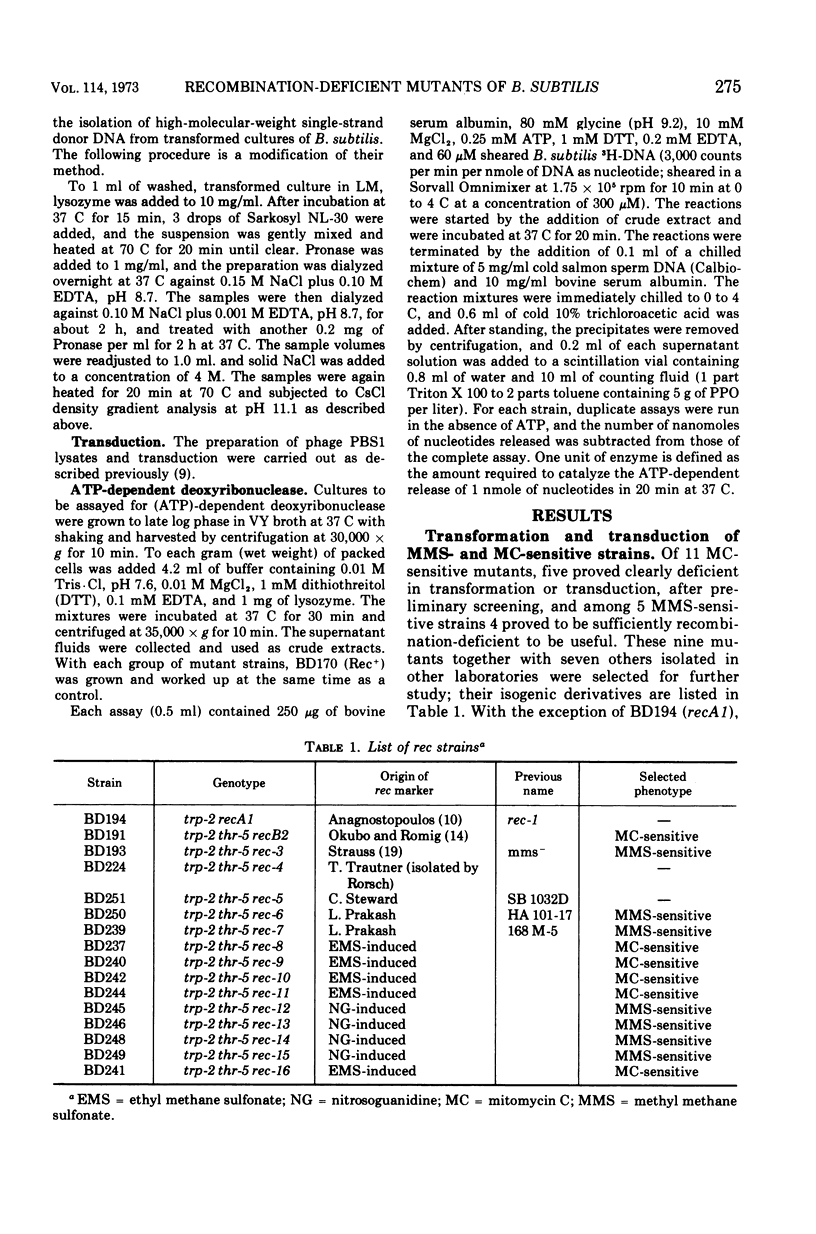
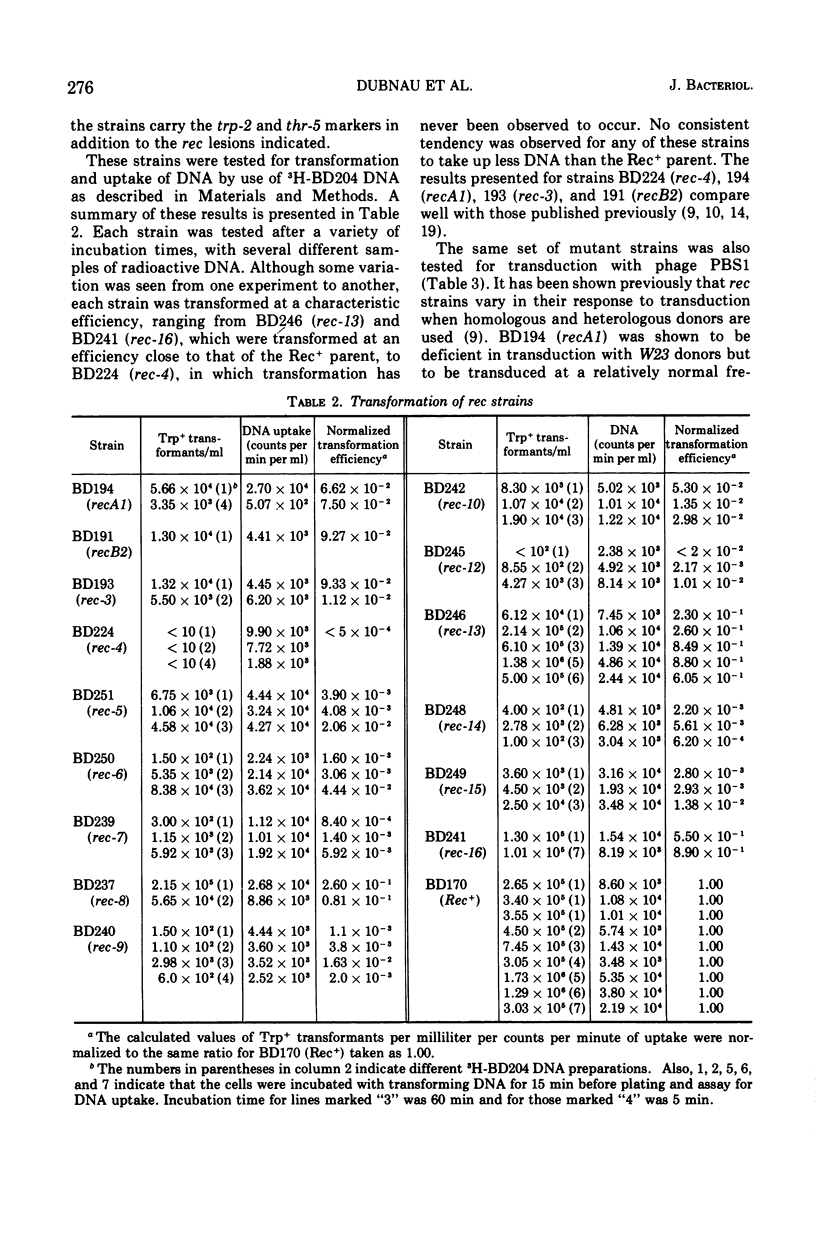
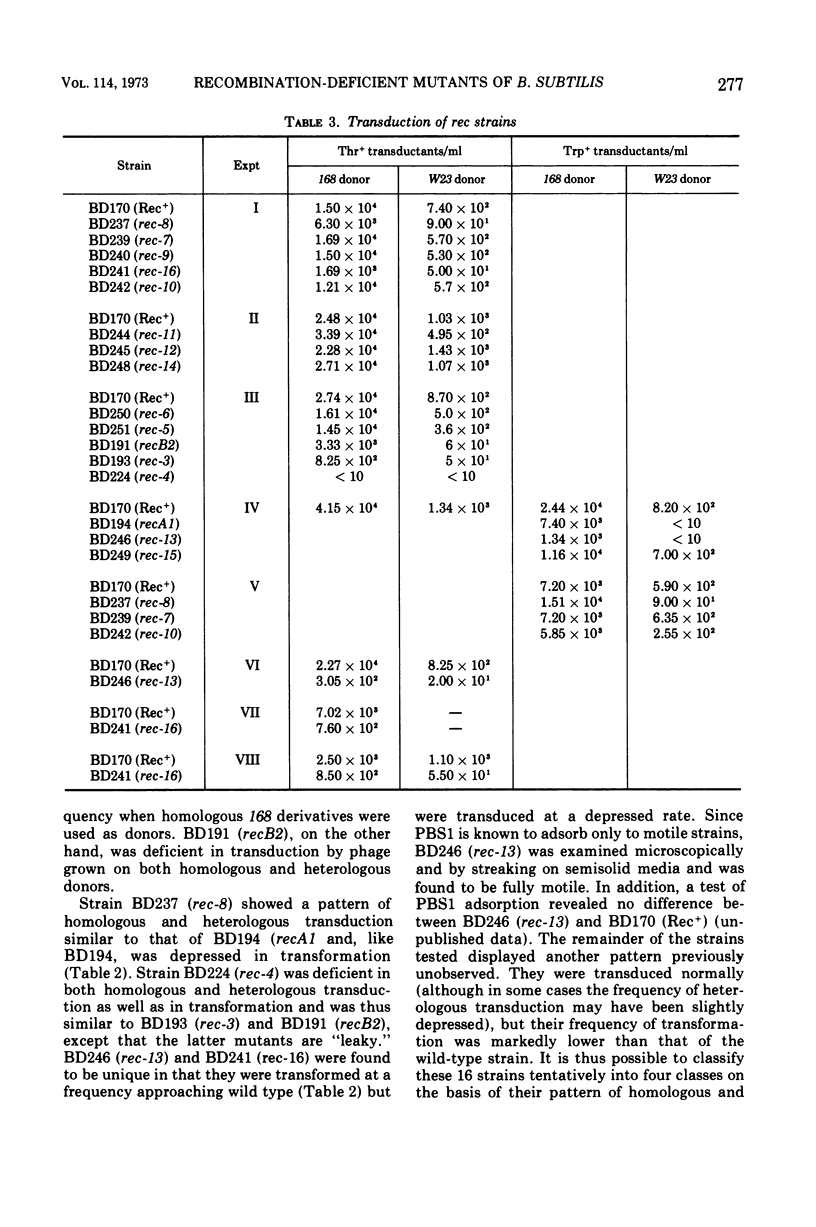
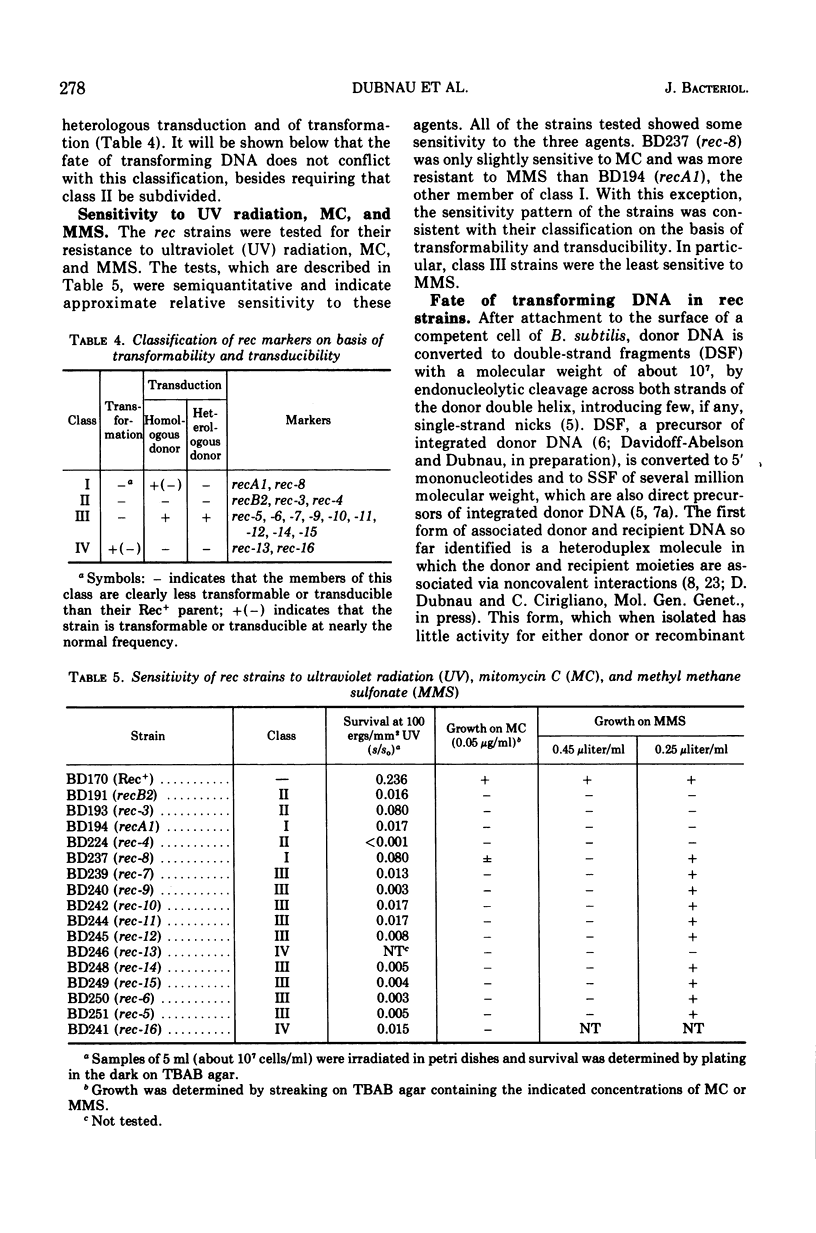
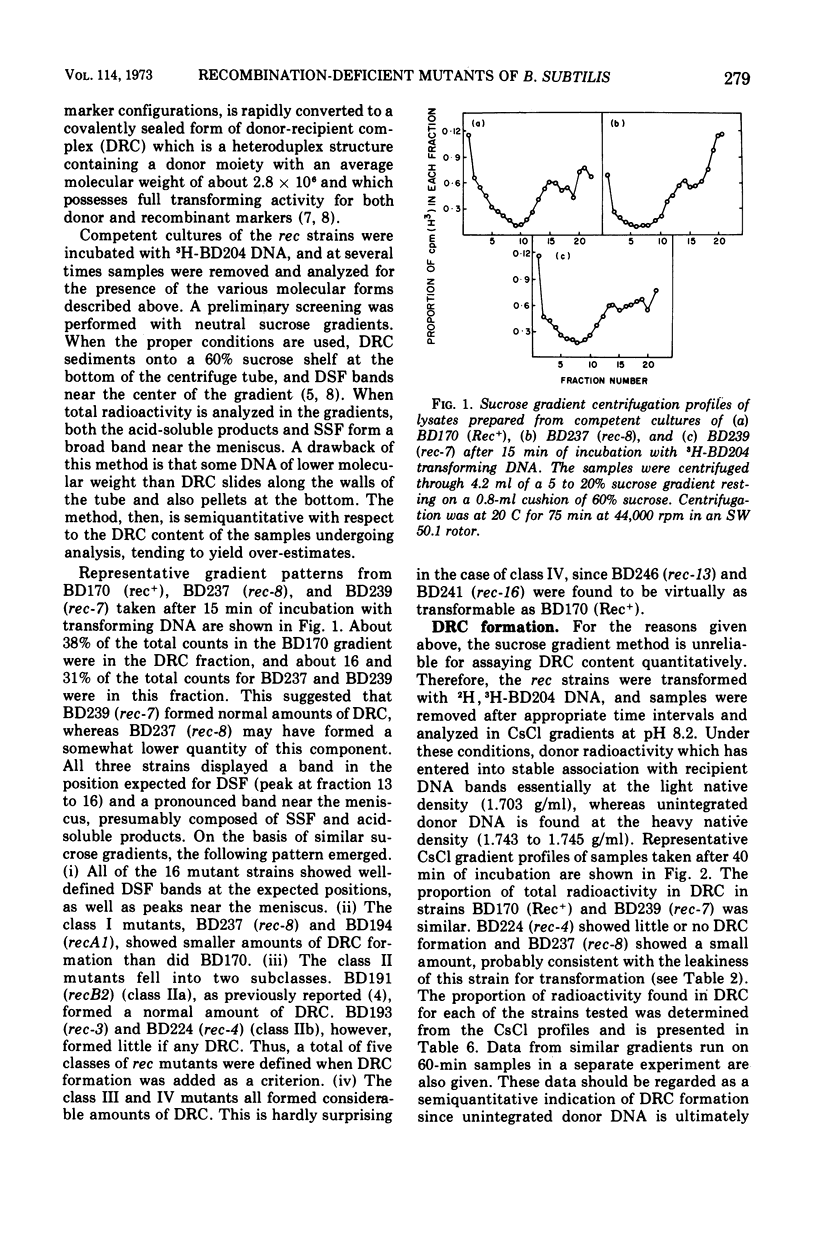
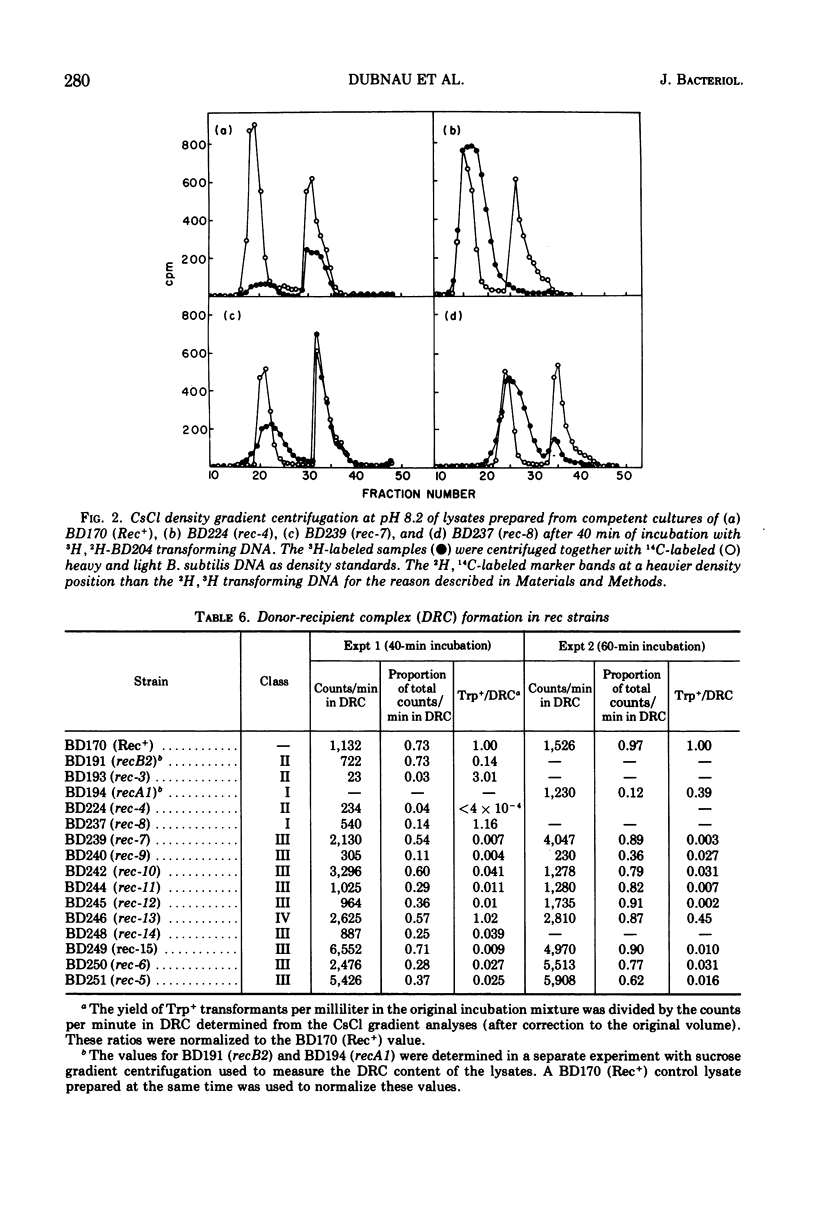
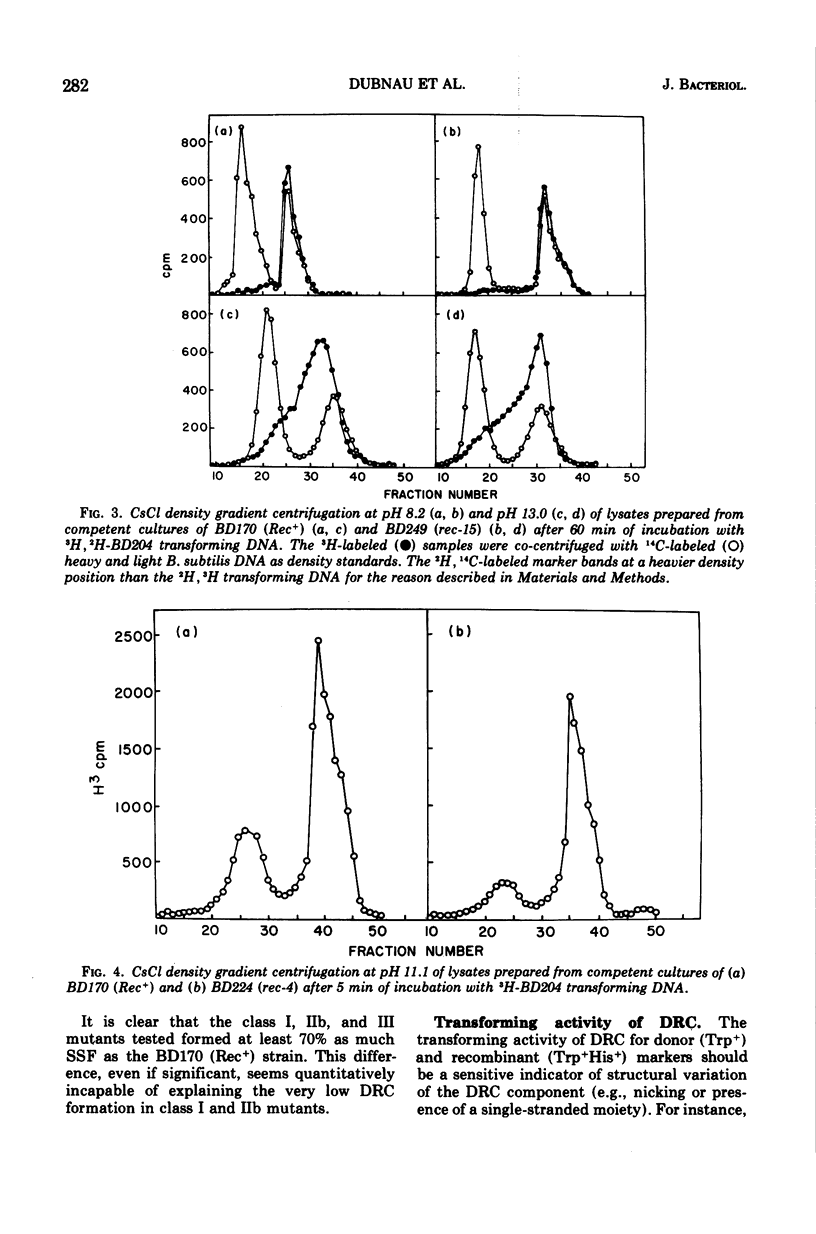
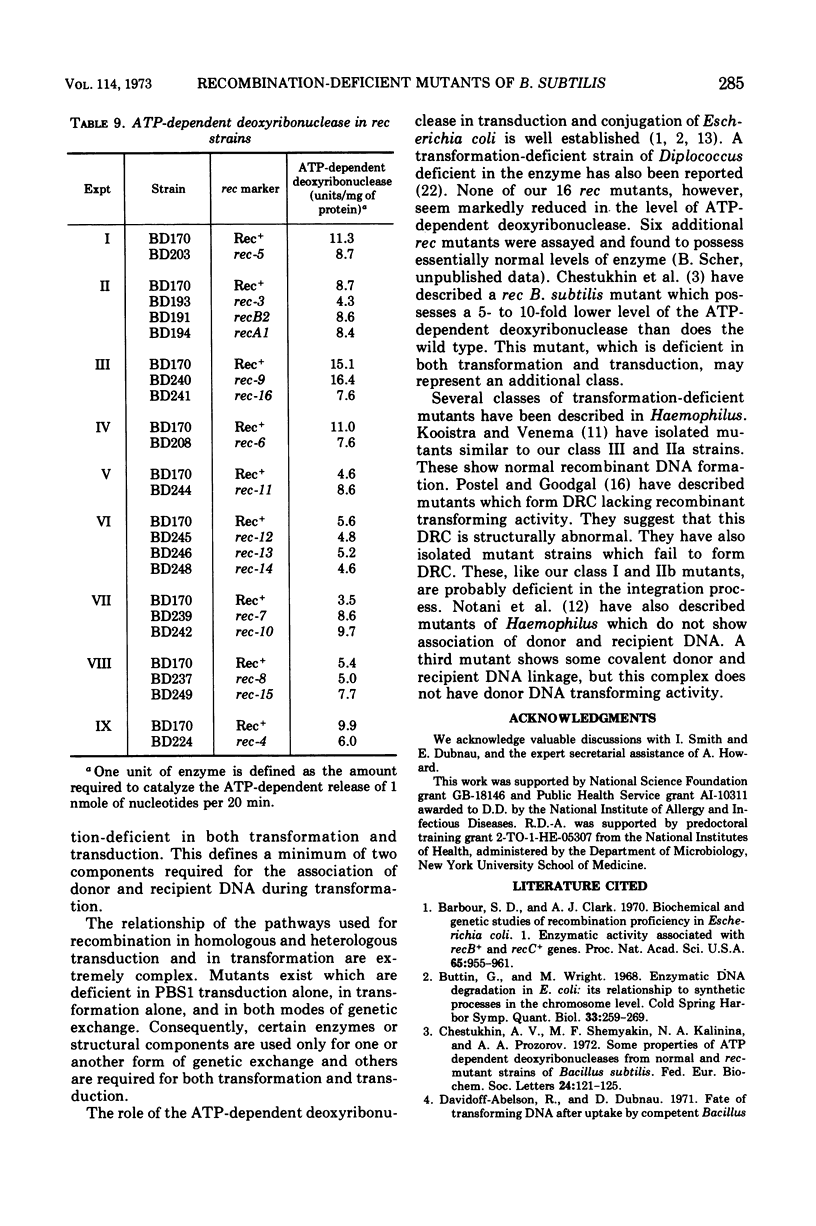
A collection of 16 isogenic recombination-deficient strains of Bacillus subtilis isolated on the basis of sensitivity to methyl methane sulfonate (MMS) or mitomycin C (MC) were characterized phenotypically. All were found to be somewhat sensitive to ultraviolet irradiation, MC, and MMS. The mutants were all blocked in “late” steps in the transformation process and were provisionally grouped into four categories on the basis of the various properties examined. Class I mutants were deficient in transformation and heterologous transduction with phage PBS1 but were transducible with homologous donors at nearly the wild-type frequency. They were blocked in donor-recipient complex (DRC) formation but formed essentially normal amounts of double-strand fragments (DSF) and single-strand fragments (SSF). The class IIa strain was deficient in transformation and PBS1 transduction, and formed DRC which was normal by all available physical and biological criteria. Class IIb mutants were deficient in transformation and PBS1 transduction, and failed to form DRC. They did produce DSF and SSF. Class III mutants were deficient in transformation, were normal in PBS1 transduction, and formed DRC which was physically indistinguishable from that of the Rec+ parent although with slightly lowered donor-type transforming activity. Class IV strains were deficient in PBS1 transduction but were transformed at nearly the wild-type efficiency. None of the mutant strains was deficient in the adenosine triphosphate-dependent deoxyribonuclease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Chestukhin A. V., Shemyakin M. F., Kalinina N. A., Prozorov A. A. Some properties of ATP dependent deoxyribonucleases from normal and rec-mutant strains of Bacillus subtilis. FEBS Lett. 1972 Jul 15;24(1):121–125. doi: 10.1016/0014-5793(72)80841-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. IV. The endwise attachment and uptake of transforming DNA. J Mol Biol. 1972 Feb 28;64(1):31–46. doi: 10.1016/0022-2836(72)90319-1. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. VI. Non-covalent association of donor and recipient DNA. Mol Gen Genet. 1973 Jan 24;120(2):101–106. doi: 10.1007/BF00267237. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: size and distribution of the integrated donor segments. J Bacteriol. 1972 Aug;111(2):488–494. doi: 10.1128/jb.111.2.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., Venema G. Fate of donor DNA in some poorly transformable strains of Haemophilus influenzae. Mutat Res. 1970 Mar;9(3):245–253. doi: 10.1016/0027-5107(70)90126-0. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Impaired transformability of Bacillus subtilis mutant sensitive to mitomycin C and ultraviolet radiation. J Mol Biol. 1966 Feb;15(2):440–454. doi: 10.1016/s0022-2836(66)80120-1. [DOI] [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Goodgal S. H. Competence mutants. 3. Responses to radiations. J Bacteriol. 1972 Jan;109(1):298–306. doi: 10.1128/jb.109.1.298-306.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Goodgal S. H. Competence mutants. II. Physical and biological fate of donor transforming deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):292–297. doi: 10.1128/jb.109.1.292-297.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searashi T., Strauss B. Relation of the repair of damage induced by a monofunctional alkylating agent to the repair of damage induced by ultraviolet light in Bacillus subtilis. Biochem Biophys Res Commun. 1965 Sep 22;20(6):680–687. doi: 10.1016/0006-291x(65)90069-0. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., MORRIS J., DAVIDSON N., DOVE W. F., Jr The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc Natl Acad Sci U S A. 1963 Jan 15;49:12–17. doi: 10.1073/pnas.49.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovis G. F., Buttin G. An ATP-dependent deoxyribonuclease from Diplococcus pneumoniae. II. Evidence for its involvement in bacterial recombination. Biochim Biophys Acta. 1970 Nov 12;224(1):42–54. [PubMed] [Google Scholar]
- Zadrazil S., Fucík V. Fate of transforming DNA in Bacillus subtilis strain sensitive to methyl methanesulfonate. Biochem Biophys Res Commun. 1971 Feb 19;42(4):676–683. doi: 10.1016/0006-291x(71)90541-9. [DOI] [PubMed] [Google Scholar]


