Abstract
Thalidomide could have therapeutic applications in neoplasms and in other diseases, particularly those of autoimmune origin. The objective of this study was to investigate the effect of various doses of thalidomide on the growth of C6 glioma in rats, and to determine its effects on parameters of cell proliferation and angiogenesis. Additionally, we investigated a potential enhancement of the antitumoral action of thalidomide when combined with a low dose of the antineoplastic carmustine. C6 glioma cells were implanted subcutaneously in Wistar rats. A highly malignant glioma developed in 80% of animals. When the tumour reached 2.0 cm diameter thalidomide was administered at doses of 100, 200 or 400 mg/kg/day. When given at a dose of 400 mg/kg/day thalidomide significantly reduced the tumour volume, the mitotic index and cell proliferation but not the vascular density. The combination of thalidomide plus carmustine increased the inhibitory effect on tumoral growth. Our results indicate that thalidomide is effective against malignant glioma; apparently by an antiproliferative effect, rather than by inhibition of angiogenesis; when combined with carmustine it could increase the response of glioma to antineoplastic treatment.
Keywords: thalidomide, glioblastoma, angiogenesis, cancer therapy, carmustine
Introduction
Survival of patients with glioblastoma has not changed significantly over the past three decades (Forsyth & Cairncross 1995; Avgeropoulos & Batchelor 1999). Thalidomide, a derivate of glutamic acid, is experiencing a renaissance as an experimental drug for a wide variety of diseases (Calabrese & Fleischer 2000). This drug has proven anti-inflammatory, immunomodulatory and antiangiogenic effects (Rowland et al. 1998; Arrieta et al. 1999; Marriot et al. 1999). Thalidomide has multiple effects on the immune system, for instance, it reduces phagocytosis by leucocytes, inhibits the production of tumour necrosis factor (TNF)-α by decreasing its half-life, inhibits interferon-γ and interleukin-12 production and decreases the expression of beta integrin subunits produced by leucocytes. Angiogenesis is a fundamental process of tumoral growth that occurs through various mechanisms, including overexpression and mobilization of angiogenic proteins from the extracellular matrix as well as through recruitment of host cells such as macrophages, which in turn produce additional angiogenic proteins as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and platelet-derived growth factor (Battegay 1995; Folkman 1995; Rosen 2000; Plate & Risau 1995). Thalidomide inhibits in vitro the production of new blood vessels induced by bFGF and VEGF (D'Amato et al. 1994; Kruse et al. 1998). Thalidomide also inhibits angiogenesis via the generation of toxic hydroxyl radicals (Parman et al. 1999; Sauer et al. 2000). Other possible effects, as mutagenic and antiproliferative agents, remain controversial (Hatfill et al. 1991; Kida 1994; Santos-Mendoza et al. 1996).
In vitro an analogue of thalidomide (cc-1069) inhibits endothelial cell proliferation without effect on the proliferation of glioma cells (Moreira et al. 1999). Anti-tumoral effects of thalidomide have been mainly attributed to the increased degradation of mRNA for both FGF and VEGF, and a decrease of the expression of adhesion receptors (Stephens et al. 2000). A favourable therapeutic effect of thalidomide in Kaposi's sarcoma has been demonstrated (Grabstad & Golbey 1965; Fife et al. 1998); as well as in the treatment of refractory multiple myeloma (Singhal et al. 1999). There are also promising reports of thalidomide in the treatment of several neoplasms including breast, renal, ovarian and prostatic cancer (Baidas et al. 2000; Eisen et al. 2000). Thalidomide reduces the sensitivity of Lewis tumours in the lung to radiotherapy and increases their sensitivity to chemotherapy. Recent studies show that high doses of thalidomide have positive effects on survival in patients with highly malignant glioma (Fine et al. 2000). Thalidomide is currently under extensive research as antineoplastic drug. Furthermore, thalidomide may also have positive effects in the management of physical and psychosocial symptoms in patients with cancer (Bruera et al. 1999; Ribeiro et al. 2000).
Thalidomide is a potential chemotherapeutic agent for malignant brain tumours. We studied the effects of various doses of thalidomide on the growth and histological characteristics of C6 glioma in the rat as well as its potential profile as a synergistic agent with an antineoplastic drug.
Methods
Thalidomide was synthesized in the Laboratory of Organic Chemistry at the Instituto Politecnico Nacional, Mexico.
C6 Glioma induction
C6 glioma cells (Benda et al. 1968), obtained from the American Tissue Culture Collection (Rockville, MD) were cultured under sterile conditions at 37 °C in a humid environment with 5% of CO2 in Ham F-10 medium supplemented with bovine foetal serum (2.5%) and horse serum (15%). After the cultures became confluent, the cells were washed with saline solution, harvested and counted; 1 × 107 C6 cells were inoculated intraperitoneally in a male Wistar rat. Twenty days later, a large peritoneal tumour developed. The tumour was mechanically dissociated at 4 °C and 1 × 107 cells, suspended in 500 µL of saline solution, were inoculated subcutaneously into the left thighs of 12-week-old Wistar rats. A subcutaneous tumour developed in 80% of animals (Arrieta et al. 1998; Guevara & Sotelo 1999). Initial in vitro proliferation of C6 cells within the peritoneum greatly increases the rate of tumour development in subcutaneous tissue (Guevara & Sotelo 1999).
Administration of thalidomide
When the rats developed a noticeable tumour of approximately 2 cm diameter, which occurred approximately 15 days after the inoculation of C6 glioblastoma cells, they were randomly allocated to one of four groups: Group A (n = 13) was followed as control; animals from groups B (n = 10), C (n = 10) and D (n = 10), were treated with 100, 200 or 400 mg/kg/day of thalidomide, respectively. Thalidomide was mixed with corn oil and administered daily through an oral catheter. Controls received the vehicle (corn oil). Treatment was given from day 15–45 after cell inoculation.
Evaluation of antitumoral effect
Thirty days after commencing thalidomide treatment (45 days after cell inoculation) animals from all groups were anaesthetized and perfused by intracardiac route with a solution of 10% formaldehyde in saline solution. Tumours were dissected and the volume was determined by fluid displacement.
Drug toxicity on haematic and biochemical parameters
For studies of haematic biometry and blood chemistry (glucose, BUN, creatinine and liver function tests), five rats from each group were anaesthetized and blood samples were obtained by intracardiac puncture prior to intracardiac perfusion. The same parameters were determined in five healthy rats and were taken as control values.
Histological analysis, vascular density and cell proliferation study
The tumours were extracted and cut in the middle into eight equal parts. They were included in paraffin wax and sections were stained by Hematoxiline-Eosine method for microscopical study. Mitotic index was determined by the mean number of mitoses per microscopic field of 10 separate observations in two different slices at 40× magnification. Tumours from animals in groups A and D were studied by immunohistochemistry; mouse antibodies to Von Willebrand factor VIII (Dako Corporation, Code M0616, Denmark) were used as markers for endothelial vascular cells (Harris 1997). To determine vascular density 20 tissue segments from each group were used. 5 µm sections were treated with boiling 10 mm citrate buffer pH 6.0 for 10 min and washed; afterwards, then incubated with the antiserum for one hour at 37 °C and washed. The avidin-biotin complex immunolabelling method was then used (BioGenex). As malignant glioma is characterized by vast areas of necrosis in the centre of the tumour, histological analysis of vascular neoformation was made on the periphery of the tumour. The capillary lumen was observed in areas of maximal vascular density in three fields at 16× magnification and the mean number of vessels was obtained. For studies of cell proliferation histological sections were stained by immunohistochemistry with monoclonal antibodies against the nuclear cell proliferation antigen (BioGenex) (Hoyt et al. 1995). The cell proliferation index was obtained by the mean number of positive cells in 10 different microscopic fields at 40× magnification. All histological observations (Fig. 1) were made by two independent observers (OA and DR) without previous knowledge of the group source of the specimen.
Figure 1.
Formalin-fixed paraffin sections of rat C6 glioma, immunostained for PCNA and Von Willebrand factor VIII by the peroxidase antiperoxidase method (positive cells are stained brown, see arrows). (a) PCNA in C6 cells from a control rat (40×) (b) PCNA in C6 cells from a rat treated with thalidomide: a marked decrease in PCNA is observed (40×). (c) Factor VIII in C6 cells from a control rat (20×) (d) Factor VIII in C6 cells from a rat treated with thalidomide. No significant differences on the number of blood vessels are seen (20×).
Effect of thalidomide plus carmustine
After the results of experiments using various doses of thalidomide were analysed, the effect of thalidomide plus carmustine was studied in two new groups of rats. A low single dose of carmustine, 7 mg/kg intraperitoneally, was given to rats from group E (n = 12) with a tumour of 2 cm diameter (15 days after C6 cell inoculation), corresponding to 30% of the optimal antineoplastic dose of carmustine reported for this animal model (Barker et al. 1973). To study the eventual cumulative effect of thalidomide on the antineoplastic action of carmustine, rats from group F (n = 11) were treated with a single dose of carmustine (as with group E) plus thalidomide (400 mg/kg daily for 30 days).
Statistical analysis
Comparison between groups was made by the ANOVA analysis and Tukey test for independent values.
Results
Effect of thalidomide on tumoral growth
When compared with the tumour size of controls (50 ± 8 cm3), a significant growth inhibition was observed in animals treated with 400 mg/kg/day of thalidomide (25.4 ± 8 cm3, p < 0.05). The mean volume of tumours in rats treated with 100 and 200 mg/kg was 45 ± 6 and 48 ± 7 cm3, respectively, non-significant when compared with controls.
Histological analysis, mitotic index, cell proliferation and vascular density
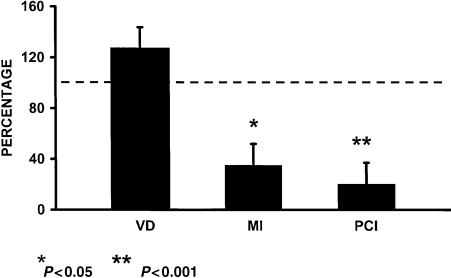
Microscopic analysis showed ample areas of tumour necrosis in all animals. However, these areas were more evident in animals treated with thalidomide and appeared to be dose-dependent. Mitotic index (Fig. 2) was lower in viable areas of tumour from animals treated with thalidomide (400 mg/kg); 3.2 ± 3 per microscopic field, vs. 9.2 ± 4 in controls (P < 0.05) with value of kappa of 0.87 and 0.80 for intrarater and interrater reliability. The cell proliferation index (Fig. 2) was lower in tumours from animals treated with thalidomide (400 mg/kg), 4.6 ± 4 vs. 23 ± 6 with value of kappa 0.84 and 0.94 for intrarater and interrater reliability. In controls (P < 0.01) mean number of capillaries in tumours from animals treated with thalidomide (400 mg/kg) showed no statistical difference when compared with controls (11.9 ± 5 vs. 9.4 ± 7, respectively) (Fig. 2), with value of kappa of 0.83 and 0.90 for intrarater and interrater reliability.
Figure 2.
Percent of variability on vascular density (VD), mitotic index (MI) and proliferation cellular index (PCI) ±ΣE, in animals with C6 glioma treated with thalidomide as compared with values obtained in controls (100%).
Effect of thalidomide plus carmustine
The mean volume of tumours from rats treated with a low dose of carmustine plus thalidomide was 15 ± 4 cm3, whereas in controls it was 50 ± 8 cm3. In animals treated only with the low dose of carmustine the volume was 38.5 ± 7 cm3 (NS when compared with controls and P < 0.05 when compared with carmustine plus thalidomide) (Fig. 3). However, the difference between animals treated with thalidomide and those treated with thalidomide-carmustine was non-significant.
Figure 3.
Mean tumour size of C6 glioma in rats treated either with thalidomide or with carmustine or with thalidomide/carmustine.
Drug toxicity
There was no mortality in any group during thalidomide treatment. The mean values of laboratory blood tests were similar among the groups and no signs of drug-induced toxicity were observed.
Discussion
Thalidomide is known to have intense antiangiogenic activity in different animal models (D'Amato et al. 1994; Battegay 1995). The notorious teratogenic effects of thalidomide observed in the early 1960s when administrated to women in the first trimester of pregnancy (McBride 1961) have been attributed, among other factors, to inhibition of blood vessel growth in the developing foetal limb bud and oxidative DNA damage mediated by free radicals (Parman et al. 1999).
The glial tumours induced in this experiment are highly vascularized (Arrieta et al. 1998), however, in our study thalidomide had no significant effect on tumour vascular density. Angiogenesis inhibition due to thalidomide and its active metabolites has been associated to its teratological potency (Joussen et al. 1999; Thiel et al. 2000). As thalidomide has no teratogenicity in rats it may be possible that thalidomide also lacks antiangiogenic properties in rats. Bauer et al. (1998) demonstrated that thalidomide inhibits angiogenesis in human aortic endothelial cells, but not in rat aorta cells, suggesting that the antiangiogenic effect of thalidomide is species-related, acting in humans and rabbits, but not in rodents. However Jakkula et al. (2000) have shown inhibition of the alveolarization in the developing rat lung by thalidomide and other inhibitors of angiogenesis, possibly through additional antiproliferative mechanisms. Nevertheless, we found an antitumoral effect with high doses of the drug, which could be explained by additional mechanisms different to those related with inhibition of vascular neoformation.
A diminution on cell proliferation of C6 glioma related to the decrease of tumour size was found. Thalidomide interacts with the DNA of rabbit embryos. This interaction may account for a direct antiproliferative effect (Huang et al. 1999). Its active metabolites specifically bind to GC promoter sites and inhibit the transcription of growth factors such as FGF-2 and insulin-like growth factor (IGF-I) (Stephens & Fillmore 2000; Stephens et al. 2000). Synthesis of IGF plays an important role in the growth of glioma C6 (Trojan et al. 1993; Patti et al. 2000). Its inhibition and that of its receptor may be an explanation of the antiproliferative effect produced by thalidomide on C6 glioma, which has also been observed in vitro in endothelial cells from human glioma (Moreira et al. 1999) and in vivo in endoneurial cells from nerves of rats (Schroder et al. 1995). The antiproliferative effect of thalidomide in non-tumoral cells is controversial. In vitro studies with retinal pigment epithelium have shown inhibition of cell migration and cell proliferation (Spraul et al. 1999), whereas no effect has been observed in human lymphocytes (Santos-Mendoza et al. 1996). To study alternative effects of thalidomide on tumoral growth the measurement of tissular concentrations of various growth factors are currently being undertaken in our laboratory on different experimental tumours.
In conclusion, we found that the antiproliferative effect of a low dose of carmustine (30% of that used as effective) was significantly enhanced by thalidomide, although it was not significantly greater than thalidomide alone. It is important to stress that the absence of a therapeutic effect of carmustine was due to the very low doses used in this experiment, which allowed us to explore a potential effect when combined with thalidomide. As the two therapeutic strategies have different pharmacological mechanisms, thalidomide may prove a useful adjuvant (Rosen 2000) in combination with standard chemotherapy for malignant glial tumours.
Acknowledgments
This work was partly supported by the National Council of Science and Technology of Mexico (CONACyT) grant L0001-M9608.
References
- Arrieta O, Guevara P, Reyes S, Ortiz A, Rembao D, Sotelo J. Protamine inhibits angiogenesis and growth of C6 rat glioma; a synergistic effect when combined with carmustine. Eur. J. Cancer. 1998;34:2101–2106. doi: 10.1016/s0959-8049(98)00244-5. [DOI] [PubMed] [Google Scholar]
- Arrieta O, Ortiz Reyes A, Rembao D, Calvillo M, Rivera E, Sotelo J. Protective effect of pentoxifylline plus thalidomide against septic shock. Int. J. Exp. Path. 1999;80:11–16. doi: 10.1046/j.1365-2613.1999.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgeropoulos NG, Batchelor TT. New treatment strategies for malignat gliomas. Oncologist. 1999;4:209–224. [PubMed] [Google Scholar]
- Baidas SM, Winer EP, Fleming GF, et al. Phase II evaluation of thalidomide in patients with metastatic breast cancer. J. Clin. Oncol. 2000;18:2710–2717. doi: 10.1200/JCO.2000.18.14.2710. [DOI] [PubMed] [Google Scholar]
- Barker M, Hoshimo T, Gurcay O, Wilson CB, Nielsen SL, Downie R. Development of an animal brain tumor model and its response to therapy with 1,3 (2-chloroethyl)-1- nitrosourea. Cancer Res. 1973;33:976–986. [PubMed] [Google Scholar]
- Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J. Mol. Med. 1995;73:333–346. doi: 10.1007/BF00192885. [DOI] [PubMed] [Google Scholar]
- Bauer KS, Dixon SC, Figg WD. Inhibition of angiogenesis by thalidomide requires metabolic activation, which is species-dependent. Biochem. Pharmacol. 1998;55:1827–1834. doi: 10.1016/s0006-2952(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Benda P, Lightbody J, Sato G, Levine L, Sweet W. Differentiated Rat Glial Cell Strain in Tissue Culture. Science. 1968;161:370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Bruera E, Neumann CM, Pituskin E, Calder K, Ball G, Hanson J. Thalidomide in patients with cachexia due to terminal cancer: preliminary report. Ann. Oncol. 1999;10:857–859. doi: 10.1023/a:1008329821941. [DOI] [PubMed] [Google Scholar]
- Bruera E, Neummann CM, Pituskin E, Calder K, Ball G, Hanson J. Thalidomide in patients with cachexia due to terminal cancer: preliminary report. Ann. Oncol. 1999;10:857–859. doi: 10.1023/a:1008329821941. [DOI] [PubMed] [Google Scholar]
- Calabrese L, Fleischer AB. Thalidomide: Current and Potential Clinical Applications. Am. J. Med. 2000;108:487–495. doi: 10.1016/s0002-9343(99)00408-8. [DOI] [PubMed] [Google Scholar]
- D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen T, Boshoff C, Mak I, et al. Continuos low dose thalidomide: a phase II study in advanced melanoma, renal cell, ovarian and breast cancer. Br. J. Cancer. 2000;82:812–817. doi: 10.1054/bjoc.1999.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife K, Howard MR, Gracie F, Phillips RM, Bower M. Activity of thalidomide in AIDS related Kaposi's sarcoma and correlation with HHV8 titre. Int. J. STD AIDS. 1998;9:751–755. doi: 10.1258/0956462981921512. [DOI] [PubMed] [Google Scholar]
- Fine HA, Figg WD, Jaeckle K, et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J. Clin. Oncol. 2000;18:708. doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- Folkman J. Clinical Applications of Research on Angiogenesis. N. Engl. J. Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Forsyth PAJ, Cairncross JG. Treatment of malignant glioma in adults. Curr. Opin. Neurol. 1995;8:414–418. doi: 10.1097/00019052-199512000-00002. [DOI] [PubMed] [Google Scholar]
- Grabstad H, Golbey R. Clinical experience with thalidomide in patients with cancer. Clin. Pharmacol. Ther. 1965;6:298–302. doi: 10.1002/cpt196563298. [DOI] [PubMed] [Google Scholar]
- Guevara P, Sotelo J. C6 rat glioma grown into the peritoneal cavity, a large source of tumoral cells for subcutaneous transplant of glioma. J. Neuro-Oncol. 1999;44:91–92. doi: 10.1023/a:1006112422132. [DOI] [PubMed] [Google Scholar]
- Harris AL. Antiangiogenesis for cancer therapy. Lancet. 1997;349(Suppl. II):13–15. doi: 10.1016/s0140-6736(97)90014-3. [DOI] [PubMed] [Google Scholar]
- Hatfill SJ, Fester E, De Beer DP, Bohm L. Induction of morphological diferentiation in the leukemic cell line K 562 by exposure to thalidomide metabolites. Leuk. Res. 1991;15:129. doi: 10.1016/0145-2126(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Hoyt J, Gown AM, Kim K, Berger MS. Analysis of proliferative grade in a glial neoplasm using antibodies to the Ki-67 defined and PCNA in formalin fixed deparaffinized tissues. J. Neuro-Oncol. 1995;24:163–169. doi: 10.1007/BF01078486. [DOI] [PubMed] [Google Scholar]
- Huang PH, McBride WG, Tuman WG. Interaction of thalidomide with DNA of rabbit embryos: a possible explication for its immunosuppressant and teratogenic effects. Pharmacol. Toxicol. 1999;85:103–104. doi: 10.1111/j.1600-0773.1999.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis decreases alveolarization in the development rat lung. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Germann T, Kirchhof B. Effect of thalidomide and structurally related compounds on corneal angiogenesis is comparable to their teratological potency. Graefes Arch. Clin. Exp. Ophthalmol. 1999;237:952–961. doi: 10.1007/s004170050330. [DOI] [PubMed] [Google Scholar]
- Kida M. Thalidomide may not be a mutagen (letter; comment) Br. Med. J. 1994;309:741. doi: 10.1136/bmj.309.6956.741b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse FE, Janssen AM, Rohrschneider H, Becker MD, Volcker HE. Thalidomide inhibits corneal angiogenesis induced by vascular endothelial growth factor. Graefes. Arch. Clin. Exp. Ophtalmol. 1998;236:461–466. doi: 10.1007/s004170050106. [DOI] [PubMed] [Google Scholar]
- Marriot JB, Muller G, Dalgleish AG. Thalidomide as an emerging immunotherapeutic agent. Immunol. Today. 1999;20:538–540. doi: 10.1016/s0167-5699(99)01531-5. [DOI] [PubMed] [Google Scholar]
- McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2:1358. [Google Scholar]
- Moreira AL, Friedlander DR, Shif B, Kaplan G, Zagzag D. Thalidomide and a thalidomide analogue inhibit endothelial cell proliferation in vitro. J. Neurooncol. 1999;43:109–114. doi: 10.1023/a:1006202700039. [DOI] [PubMed] [Google Scholar]
- Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of Thalidomide teratogenicy. Nat. Med. 1999;5:582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- Patti R, Reddy CD, Geerger B, et al. Autocrine secreted insulin-like growth factor-I stimulates MAP kinase-dependent mitogenic effects in human primitive neuroectodermal tumor/medulloblastoma. Int. J. Oncol. 2000;16:577–584. doi: 10.3892/ijo.16.3.577. [DOI] [PubMed] [Google Scholar]
- Plate K, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15:339–347. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- Ribeiro RA, Vale ML, Ferreira SH, Cunha FQ. Analgesic effect of thalidomide on inflammatory pain. Eur. J. Pharmacol. 2000;391:97–103. doi: 10.1016/s0014-2999(99)00918-8. [DOI] [PubMed] [Google Scholar]
- Rosen L. Antiangiogenic Strategies and agent in clinical Trials. Oncologist. 2000;5(Suppl. 1):20–27. doi: 10.1634/theoncologist.5-suppl_1-20. [DOI] [PubMed] [Google Scholar]
- Rowland TL, McHogh SM, Deighton J, Dearman RL, Evan PW, Kimber I. Differential regulation by thalidomide and dexametasona of cytokine expression in human peripheral blood mononuclear cells. Immunopharmacology. 1998;40:11–20. doi: 10.1016/s0162-3109(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Santos-Mendoza T, Favila-Castillo L, Oltra A, et al. Thalidomide and its metabolites have no effect on human lymphocyte proliferation. Int. Arch. Allergy. Immunol. 1996;111:13–17. doi: 10.1159/000237338. [DOI] [PubMed] [Google Scholar]
- Sauer H, Gunther J, Hescheler J, Wartenberg M. Thalidomide inhibits angiogenesis in embryoid bodies by the generation of hydroxyl radicals. Am. J. Pathol. 2000;156:151–158. doi: 10.1016/S0002-9440(10)64714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder JM, Sellhaus B, Wohrmann T, Kogel B, Zwingenberger K. Inhibitory effects of thalidomide on cellular proliferation, endoneurial edema and myelin phagocytosis during early wallerian degeneration. Acta Neuropathol. 1995;89:415–419. doi: 10.1007/BF00307645. [DOI] [PubMed] [Google Scholar]
- Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 1999;441:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Kaven CK, Kampmeier JK, Lang GK, Lang GE. Effect of thalidomide, octreotide, and prednisolone on the migration and proliferation of RPE cells in vitro. Curr. Eye. Res. 1999;19:483–490. doi: 10.1076/ceyr.19.6.483.5281. [DOI] [PubMed] [Google Scholar]
- Stephens TD, Bunde CJ, Fillmore BJ. Mechanism of action in thalidomide teratogenesis. Biochem. Pharmacol. 2000;59:1489–1499. doi: 10.1016/s0006-2952(99)00388-3. [DOI] [PubMed] [Google Scholar]
- Stephens TD, Fillmore BJ. Hypothesis: thalidomide embryopathy-proposed mechanism of action. Teratology. 2000;61:189–195. doi: 10.1002/(SICI)1096-9926(200003)61:3<189::AID-TERA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Thiel R, Kastner U, Neubert R. Expression of adhesion receptors on rat limb bud cells and results of treatment with a thalidomide derivative. Life Sci. 2000;66:133–141. doi: 10.1016/s0024-3205(99)00571-8. [DOI] [PubMed] [Google Scholar]
- Trojan J, Johnson TR, Rudin SD, Ilan J, Tykocinski ML, Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Sciences. 1993;259:94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]