Abstract
CD8+ T lymphocytes are considered an important cell population involved in the control of parasitaemia and mortality after Trypanosoma cruzi infection. However, despite recent developments in this field, the mechanism whereby this control is exerted is still not completely understood. Here we have used perforin knockout (−/−) mice infected with Y strain T. cruzi in order to evaluate specifically the participation of the perforin-based cytotoxic pathway in the destruction of cardiomyocytes, cellular inflammatory infiltration, and control of parasitaemia and mortality. We observed that although parasitaemia was equivalent in perforin (+/+) and (−/−) groups, survival rate and spontaneous physical performance were significantly lower in the perforin deficient mice. The cardiac inflammatory cell infiltration, mostly composed of CD8+ cells, was more evident in perforin (−/−) mice. Ultrastructural and immunofluorescence analysis, as well as plasma creatine kinase activity, revealed cardiomyocyte damage and necrosis, more evident in perforin (−/−) mice. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assays performed in heart samples revealed similar and modest levels of apoptosis in both perforin (+/+) and (−/−) mice. These results indicate that perforin does not play a pivotal role in the control of parasitaemia and direct lysis of cardiomyocytes, but seems to be an important molecule involved in the control of cardiac inflammation and pathology induced by a highly virulent strain of T. cruzi.
Keywords: perforin, Trypanosoma cruzi, immune regulation, inflammation, myocarditis, infection and cell death
Introduction
Trypanosoma cruzi is the aetiological agent of Chagas' disease, an important infection that affects 16–18 million people in Latin America (TDR, Thirteenth Program Report). Acute infection develops with intracellular proliferation of amastigote forms of T. cruzi in different tissues and circulation of free infective trypomastigote forms in the blood. During experimental infection, foci of myocardial cytolysis are detected, with inflammatory cells surrounding both infected and non-infected fibres (Rossi & Bestetti 1995), an observation consistent with what is observed in chagasic patients (Morris et al. 1990).
The immunological control T. cruzi infection is believed to be mediated by different cell populations, including B lymphocytes (Kumar & Tarleton 1998), T lymphocytes (Da Costa et al. 1991), and NK cells (Cardillo et al. 1996). Regarding T lymphocytes, mice bearing the disrupted gene of β2-microglobulin (−/−) show significantly higher levels of parasitaemia and mortality when compared to infected (+/–) and (+/+) controls (Tarleton et al. 1992). These results are consistent with data obtained with infected mice treated by in vivo administration of anti-CD8+ (Tarleton 1990) or anti-CD4+ mAb (Russo et al. 1988), mice lacking CD4 and/or CD8 gene expression (Rottenberg et al. 1995), thymectomized mice (Schmunis et al. 1971) and nude mice (nu/nu) (Da Costa et al. 1991). In all approaches used, the mice lacked either peripheral CD8+ or CD4+ T cells, or both, which are believed to play central roles in cytotoxic events and production of pleiotropic interleukins, such as IFN-γ and IL-2.
Recent studies used perforin knockout mice to evaluate the role of CD8+ T cells as effector cells in protection against intracellular protozoa, such as Toxoplasma gondii (Denkers et al. 1997) and Trypanosoma cruzi (Henriques-Pons et al. 1998; Kumar & Tarleton 1998; Nickell & Sharma 2000). These papers indicated that perforin-dependent pathways play a limited role in host resistance to these protozoa and suggested that production of IFN-γ is one of the most important elements in this protection.
Perforin is one of the major cytolytic molecules employed by killer cells, such as CD8+ T lymphocytes and NK cells (Young et al. 1986). It is packaged into secretory cytoplasmic granules that also contain granzymes and a number of other molecules (Liu et al. 1995). Upon contact and specific recognition of target cells, the content of the cytotoxic granules is released into the intercellular space. Perforin then aggregates into large pores inserted into target cell plasma membrane while granzyme B is apparently taken up by receptor-mediated endocytosis (Motyka et al. 2001). Although purified perforin can only induce necrosis in vitro (Duke et al. 1989), the presence of both molecules is required to induce apoptosis of the target cell (Nakajima & Henkart 1994; Trapani et al. 1998). Perforin deficient mice specifically lack the perforin-based cytotoxic pathway, maintaining secretory functions, normal distribution of CD4+ and CD8+ T cell subsets, Fas-mediated cytotoxicity and normal patterns of activation and proliferation in response to different pathogens (Cooper et al. 1997; Denkers et al. 1997; Franco et al. 1997).
The study of perforin (−/−) mice infected with a number of different pathogens has revealed that, besides its direct role in target cell killing, perforin is also important in the regulation of peripheral tolerance and autoimmunity (Stepp et al. 2000). The lack of a perforin-dependent negative feedback mechanism in the immune response usually correlates with expansion and persistence of activated CD8+ effector T cells in vivo and increased susceptibility to infection (Kägi et al. 1999; Matloubian et al. 1999; Spaner et al. 1999).
In the present work, we re-assessed the importance of perforin in the host susceptibility to experimental infection with T. cruzi using a highly virulent strain of the parasite. We observed a significantly higher mortality rate, necrotic cardiomyocyte destruction, and more abundant cardiac inflammatory foci, mostly composed by CD8+ T cells, in perforin (−/−) mice. The data indicates that, although perforin may not be directly involved in cardiomyocytes death in vivo, it may play an important role in the control of the cellular cardiac inflammatory response, modulating the gathering of inflammatory CD8+ T cells in the cardiac tissue in our findings, suggesting a broader regulatory function for perforin in the immune system.
Materials and methods
Mice
C57BL /6 mice with disrupted perforin gene (perforin (−/−)) (Walsh et al. 1994), were bred at the animal facilities of FIOCRUZ. The genotype of the animals was determined by PCR analysis of the perforin gene using DNA isolated from tail snips as described (Walsh et al. 1994). The experiments were carried out with 8-week-old male perforin (−/−) mice and their counterpart (+/+) controls. The FIOCRUZ Committee of Ethics in Research approved the experiments described here, according to resolution 196/96 of the National Health Council of the Brazilian Ministry of Health.
Parasites and infection
Y strain T. cruzi was maintained in vivo by passage in syngeneic C57BL /6 mice. Blood was collected and trypomastigote forms were isolated as previously described (Araújo-Jorge et al. 1989). Thereafter, the parasites were diluted in phosphate-buffered saline (PBS) (Sigma, St Louis, USA), counted in a haemacytometer and the inocula (1 × 104 parasites in 200 µL) then applied by intraperitoneal (ip) injection.
Parasitaemia and mortality
Parasitaemia, expressed as parasites/mL, was evaluated in 5 µL of blood collected from tail snips at the indicated time points, and mortality rate was scored daily. Plasma samples were obtained by centrifugation of 100 µL of blood collected from tail snips in heparinized capillaries, separated by centrifugation, and kept frozen until use.
Spontaneous physical activity
An assay for physical performance was set up using activity wheels (Abelmann 1969). Briefly, mice were adapted to run during three alternate days in periods of 2 h per day. One day before infection, they were allowed to run spontaneously during a 30-min session in order to record the baseline performance of individual mice, expressed in revolutions per session of 30 min (rp 30 min). Each animal was then submitted to the same test on the 8th, 15th, 22nd, 28th, and 35th day post-infection (dpi).
Histopathological and immunohistochemical analysis
Mice were sacrificed 8, 15 and 21/22 days post-infection and processed as described elsewhere (Andrade 1990). Hearts were collected and immediately cryopreserved or fixed in 10% formalin diluted in PBS. Fixed slices were embedded in paraffin and 5 µm thick sections were stained with haematoxylin-eosin. The number of inflammatory foci (composed by ≥10 inflammatory cells) and the number of inflammatory cells per focus were determined by scanning the stained tissue slice starting at least 0.5 mm from the tissue edge. The pericardial region was excluded due to difficulties imposed by the excess of inflammatory cells and tissue destruction. The number of parasite nests was determined in the whole tissue and expressed in nests/mm2. Quantitative analysis was always performed in 10 microscopic fields in each of 2 different slides of heart sections obtained from 3 different mice in each group and time point (a total of 60 individual microscopic fields examined). For phenotypic analysis, 5 µm cryopreserved heart slices incubated with 10% normal goat serum and the samples were stained with anti-CD4 or -CD8 FITC-labelled mAb either on the 21st or 22nd dpi. Actin staining was carried out with 10% phalloidin-FITC (Sigma) diluted in PBS on the 15th dpi. In situ apoptosis was accessed on cryopreserved sections collected on the 21st dpi using a FITC-labelled TUNEL assay (Mannhein, Lewes, UK) performed according to the manufacturer's instructions. For in situ IFN-γ detection, paraffin was removed from the cardiac sections by heating the slides for 30 min at 60 °C, followed by treatment with xylol and ethanol. The hydrated sections were then washed with PBS for 10 min, incubated with 3% urea for 5 min, and 10% normal goat serum for 30 min. The slides were then washed with PBS for 10 min and incubated for 48 h with anti-IFN-γ (Caltag, San Francisco, USA) at room temperature. After washing overnight, the secondary goat antirat-FITC (Caltag) was added for an additional 1 h and washed with PBS for one hour. Slices were also labelled with the DNA-specific fluorescent dye 4,6-diamidino-2-phenylindole (DAPI) (Sigma) for identification of the nucleus of inflammatory cells.
Ultrastructural analysis
Hearts from normal and infected mice were collected on the 21st dpi and then fixed for 2 h at room temperature with 2.5% glutaraldehyde in 0.1 m sodium cacodylate buffer, pH 7.2. The samples were postfixed for 30 min in a solution containing 1% osmium tetroxide (Merck, Darmstadt, Germany), 0.8% potassium ferricyanide (Sigma), and 5 mm CaCl2 in 0.1 m cacodylate buffer (Sigma), dehydrated in acetone, and embedded in epon (Merck). Ultra-thin sections were recovered in copper grids, stained with 2% aqueous uranyl acetate, and observed under a Zeiss EM-10 (Zeiss, Germany) transmission electron microscope.
IFN-γ quantification
IFN-γ was quantified as previously described (Minoprio et al. 1991). Briefly, plates were coated overnight at 4 °C with R46A2 mAb (originally obtained from DNAX Corp. Palo Alto, CA, USA) and exposed to serially diluted serum samples. Standard curves were simultaneously prepared with known amounts of recombinant IFN-γ (Pharmingen, San Diego, CA, USA). Second step reactions were carried out by incubating the plates with biotinylated AN-18 antibodies and revealed by further incubation with peroxidase-labelled streptavidin. Optical density (od) was scored at 650 nm wavelength in a multi-well plate spectrophotometer (Bio Tek Instruments, Burlington, Vt, USA).
Creatine kinase MB (CK-MB) activity
Heparinized plasma samples collected one day before and on the 8th, 15th and 22nd dpi were analysed for creatine kinase MB (the cardiac isoform of CK) activity using a commercially available kit (Merck), as described elsewhere (Souza et al. 2000). This quantitative assay was used as a marker of cardiomyocytes necrotic damage, and the results were expressed as the rate of increase in NADPH (Delta E/min) after 6 sequential readings at 1min intervals at 340 nm in a multiwheel spectrophotometer (BioTek Instruments) at 340 nm.
Statistical analysis
Parasitaemia, spontaneous physical activity and CK-MB levels were compared using the Wilcox non-parametric test. Survival analysis was performed using the Cox-Mantel, Cox-F or Log rank tests. Student's t-test was applied for comparing the other parameters. Significant differences were considered when p < 0.05.
Results
Course of T. cruzi infection in perforin (−/−) mice
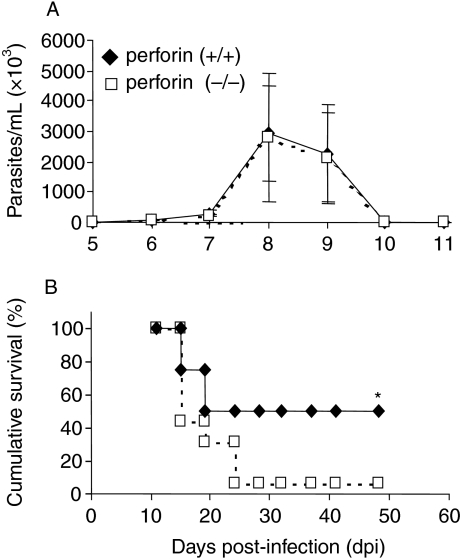
To evaluate whether the lack of perforin affects parasitaemia kinetics and mortality rate after infection, perforin (+/+) and (−/−) mice were infected with 1 × 104 trypomastigote forms of Y strain T. cruzi (Fig. 1). There were no significant differences between the parasitaemia of the two groups of mice, as ascertained by the similar latent period, number of circulating parasites, and ability to control parasitaemia to sublatent levels (Fig. 1a). In addition, we have also observed that when chronic mice (6 months post-infection), previously infected with 1 × 102 Y strain parasites, were re-infected with 5 × 105 parasites, both perforin (+/+) and (−/−) mice showed no detectable circulating parasitaemia (data not shown). However, despite the similarities in parasitaemia, the survival rate of perforin (−/−) mice was significantly lower than the one of perforin (+/+) mice (Fig. 1b). After approximately 25 days of infection with 1 × 104 parasites, the survival of perforin (−/−) mice was only 0–5%, while in their perforin (+/+) counterparts the survival was 50%. This difference was statistically significant (P < 0.01) and represents seven independent experiments with at least 8 mice per group.
Figure 1.
Parasitaemia (a) and survival (b) in perforin (+/+) (closed symbols) and (−/−) (opened symbols) mice infected with 104 trypomastigote forms of Y strain T. cruzi. The data represents mean ± standard deviation obtained from at least 8 mice per group. (*) Indicates statistically significant differences (P < 0.01) of perforin (−/−) mice as compared to perforin (+/+) mice. Data are representative of 15 experiments.
Evaluation of physical activity
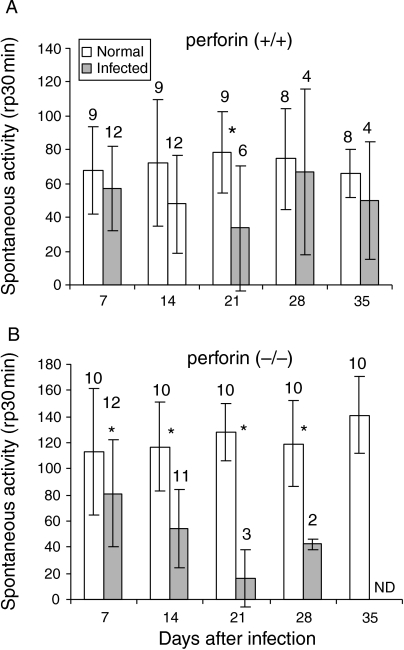
To investigate possible functional impairments in T. cruzi-infected perforin (−/−) mice, an assay for physical activity was carried out by submitting the mice to 30 min sessions of spontaneous exercise on activity wheels (Fig. 2). To perform these experiments, 12-week-old perforin (−/−) mice were used, and the results compared to age-matched non-infected controls. Perforin competent mice showed significantly less spontaneous activity only on the 21st day pi (Fig. 2a), compared to non-infected animals. Surviving mice recovered normal physical activity thereafter. However, perforin deficient mice displayed significantly diminished physical activity after the first week post-infection (Fig. 2b), some time before a decrease in the survival rate could be noticed (Fig. 1b). The P-values for infected perforin (−/−) mice when compared to non-infected (−/−) mice on days 7, 14, 21 and 28 pi were 0.02, 0.001, 0.01 and 0.03, respectively.
Figure 2.
Spontaneous physical activity of normal and infected perforin (+/+) (a) and (−/−) mice (b), infected with 104 trypomastigote forms of Y strain T. cruzi. Data represent mean ± standard deviation. The number of mice analysed on each day post-infection (indicated upon the bars) decreased due to mortality in the course of infection. (*) Indicates statistically significant differences (P < 0.05) of infected mice as compared to non-infected mice.
Histopathological analysis
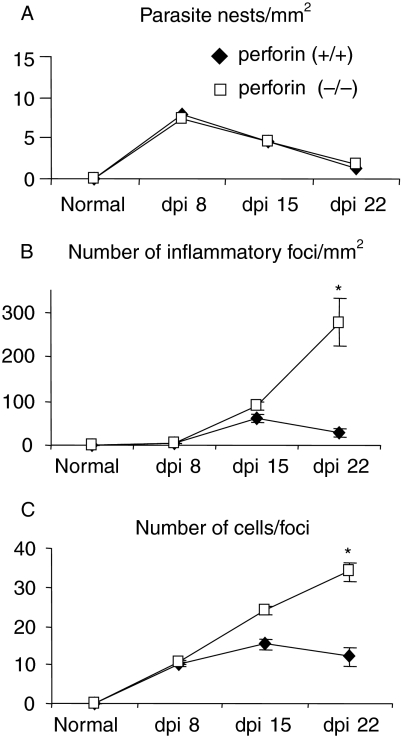
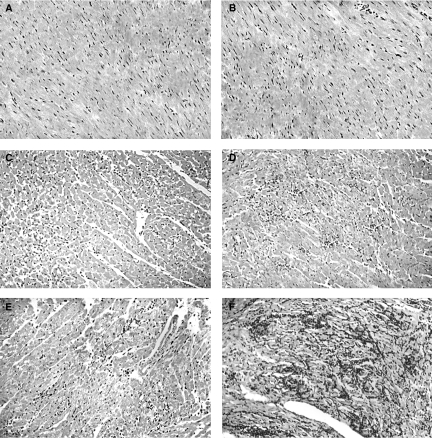
To study the lower survival rate and the demeaning performance further in the activity test, we investigated the cardiac parasite load and the inflammatory response after infection in perforin (−/−) mice. We first analysed cardiac slices collected on the 8th, 15th and 22nd dpi for the density of parasite nests and the number of infiltrating mononuclear cells (Figs 3 and 4). There was no significant difference between the two groups of mice regarding the frequency of parasite nests (Fig. 3a). A higher frequency of parasitized cardiac fibres was observed on the 8th dpi, corresponding to the peak of circulating parasitaemia (compare Fig. 3a with Fig. 1a), and clearly decreased later. With regard to cellular inflammatory response, we did not find any important cellular infiltration in neither group of mice until the 8th dpi (Figs 3b–c and 4a–b). On the 15th dpi, both perforin (+/+) and (−/−) mice showed increased numbers of inflammatory foci and inflammatory cells per focus (Figs 3b–c and 4c–d), but thereafter perforin (+/+) mice bend to control the cardiac cellular inflammatory response (Figs 3b–c and 4e). In contrast, the perforin (−/−) group displayed inflammatory cell gathering and exuberant foci of inflammation distributed throughout the tissue on the 22nd dpi (Figs 3b–c and 4f).
Figure 3.
Quantitative analysis of cardiac inflammatory response in perforin (+/+) (closed symbols) and (−/−) (opened symbols) mice infected with 104 trypomastigote forms of Y strain T. cruzi. The panels represent (a) parasite nests per mm2 (b) number of foci per mm2 and (c) number of inflammatory cells per foci. Data are mean ± standard deviation obtained by counting 60 microscopic fields of haematoxilin/eosin-stained heart sections per group and time point. (*) Indicates statistically significant differences (P < 0.01) of perforin (−/−) mice as compared to perforin (+/+) mice.
Figure 4.
Histopathological analysis of heart in perforin (+/+) (a, c and e) and (−/−) mice (b, d and f). Hematoxilin/eosin-stained heart sections were taken after 8 (a and b), 15 (c and d), and 22 (e and f) days post infection with Y strain T. cruzi. The original magnification was 160×.
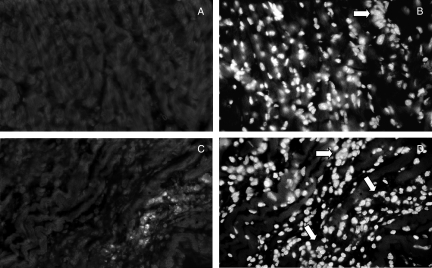
The phenotypic analysis of cardiac inflammatory cells performed with anti-CD4 or -CD8 mAb indicated an early and modest migration of CD4+ cells to the heart from the 8th to the 15th dpi, in both groups of infected mice (data not shown). However, from the second to the third week of infection, those cells were replaced by CD8+ cells, which composed most of the inflammatory foci thereafter in both perforin (+/+) and (−/−) mice (Figs 5a–c).
Figure 5.
Immunofluorescence labelling of CD8+ cells (a and c) and DNA staining with DAPI (b and d) in cardiac inflammatory foci of perforin (+/+) (a and b) and (−/−) mice (c and d) after 22 days of infection with Y strain T. cruzi. The original magnification was 630×.
IFN-γ production
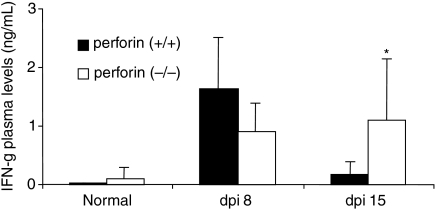
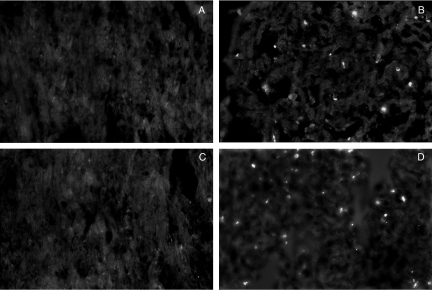
CD8+ T cells are important producers of IFN-γ, and this cytokine plays a pivotal role in the resistance of T. cruzi infection, inducing macrophages activation and destruction of intracellular parasites (Cardillo et al. 1996). However, at higher levels, this cytokine can also induce cardiomyocytes death (Shimojo et al. 1999; Melino et al. 2000). Therefore we decided to evaluate the plasma levels IFN-γ during the first two weeks of infection (Fig. 6) and the presence of IFN-γ-producing cells in the heart on the 21st dpi (Fig. 7). Perforin (+/+) infected mice displayed a peak of plasma IFN-γ on the 8th dpi that returned to normal levels on the 15th dpi (Fig. 6), and IFN-γ-producing cells were rarely found in the myocardial infiltrations of these mice on the 21st dpi (Fig. 7a–b). Perforin (−/−) infected mice also displayed increased levels of plasma IFN-γ on the 8th dpi, but, in contrast to the perforin (+/+) group, this value remained high on the 15th dpi (Fig. 6). In addition, high numbers of IFN-γ-producing inflammatory cells were observed in the myocardial tissue on the 21st dpi (Fig. 7c–d).
Figure 6.
Quantification of plasma levels of IFN–γ in perforin (+/+) (closed bars) and (−/−) (opened bars) mice before infection and after the 8th and 15th dpi with Y strain T. cruzi. (*) Indicates statistically significant differences (P < 0.05) of perforin (−/−) mice as compared to perforin (+/+) mice.
Figure 7.
Immunofluorescence detection of inflammatory IFN-γ-producing cells in paraffin-embedded heart sections taken from perforin (+/+) (a) and (−/−) (c) mice after 21 days of infection with Y strain T. cruzi. Nuclear labelling with DAPI corresponding to the same microscopic fields are also shown in (b) and (d), respectively. Arrows indicate inflammatory infiltrates. The original magnification was 630×.
Myocardial destruction
We then evaluated the pattern of cardiomyocyte death and whether the destruction of these cells was involved in the higher susceptibility of perforin (−/−) mice to the infection. TUNEL assay performed on the 21st dpi showed that both perforin (+/+) and (−/−) mice displayed small and similar pattern of apoptosis (Fig. 8). Plasma CK-MB activity, a myocardial marker of cell damage (Souza et al. 2000), was measured to quantify cardiomyocyte cell death through necrotic pathways (Fig. 9). Significantly higher levels of CK-MB activity was observed in perforin (−/−) infected mice on the 15th dpi when compared to perforin (+/+) mice on the same time-point (P < 0.05) (Fig. 9). These results correlate with the levels of cardiac inflammation observed in Fig. 3 and indicate that there is an increase in cardiomyocyte necrotic cell death in the course of the inflammatory process in perforin deficient mice.
Figure 8.
Apoptosis detection by TUNEL-FITC in cryopreserved heart sections taken from normal (a) or infected (b) perforin (+/+) mice and from normal (c) or infected (d) perforin (−/−) mice on the 21st dpi. The original magnification was 630×.
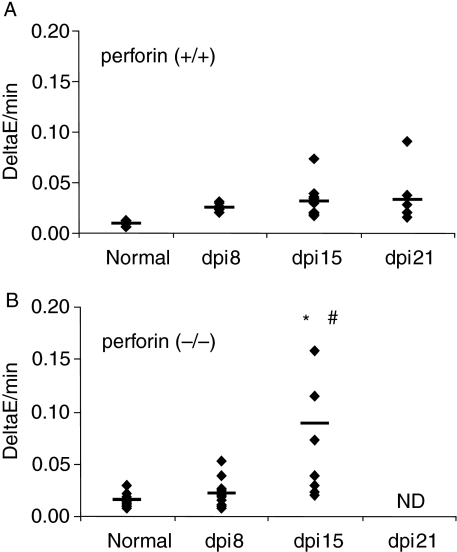
Figure 9.
Quantification of CK-MB activity in plasma taken from perforin (+/+) (a) and (−/−) (b) mice before and after 8, 15 and 22 days of infection. Solid lines represent the median of individual groups and the dots indicate individual mice values. (ND) The corresponding CK-MB dosages were not included because most of the perforin (−/−) mice died before the 21st dpi. (*) Indicates statistically significant differences (P < 0.05) of perforin (−/−) mice as compared to perforin (+/+) mice. (#) Indicates statistically significant differences (P < 0.05) of infected perforin (−/−) mice on the 15th dpi as compared with control (−/−) mice.
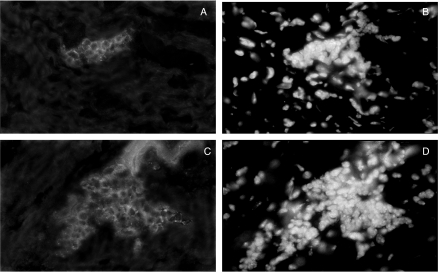
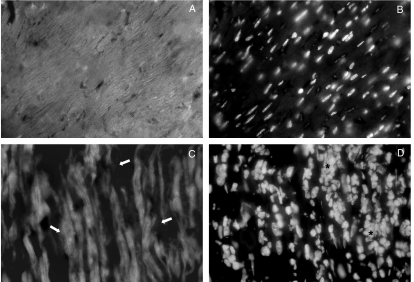
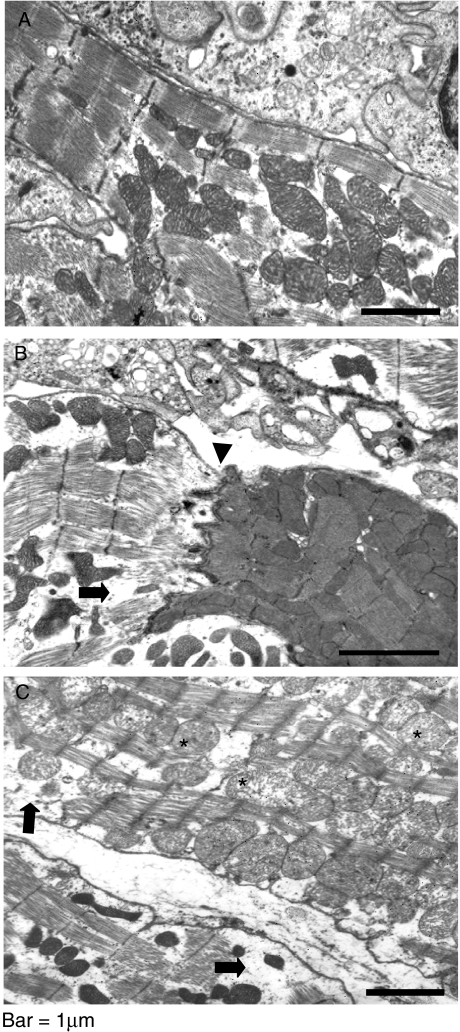
To further evaluate cardiomyocyte damage, we labelled heart slices with the actin-specific fluorescent dye phalloidin-FITC (Fig. 10). Cytoskeletal disarrange and loss of cardiomyofibres integrity surrounded by inflammatory foci was clearly more evident in perforin (−/−) mice (Fig. 10c–d) when compared with perforin (+/+) mice (Fig. 10a–b). To better characterize the pattern of destruction of cardiomyocyte, transmission electron microscopy analysis was performed in cardiac tissues of perforin (−/−) mice on the 21st dpi (Fig. 11). We observed extensive areas with apparent destruction of cytoskeleton and loss of organization of the highly structured myofibrils typical of normal myocardium. In addition, we have also observed swollen and collapsed patterns of mitochondria morphology. We found no direct association between myofibrils derangement and abnormal mitochondria.
Figure 10.
Actin labelling with phalloidin-FITC in cryopreserved heart sections taken from normal (a–b) or infected perforin (−/−) mice (c–d) on the 15th dpi. Nuclear labelling with DAPI corresponding to the same microscopic fields of (a) and (c) are shown in (b) and (d), respectively. The original magnification was 630×. Arrows indicate some points of cardiomyocytes destruction and (*) indicate inflammatory infiltration.
Figure 11.
Electron microscopic analysis of cardiac tissue samples performed in infected perforin (−/−) mice collected on the 21st dpi. (a) Normal morphology; (b–c) Some damaged mitochondria (*), disarranged cytoskeleton (arrow). The arrowhead in (b) indicate, and an intercalar disc between a damaged (left) and an apparently normal fibre (right).
Discussion
It has already been shown that T lymphocytes, and particularly CD8+ T cells, play an important role in the control of T. cruzi infection (Schmunis et al. 1971; Da Costa et al. 1991; Rottenberg et al. 1995; Tarleton 1990). However, since most of the previous studies were based in the depletion of peripheral T cell subsets, the participation of perforin in the destruction of cardiomyocytes and morbidity was not clear. The use of perforin (−/−) mice allowed us and others investigate specifically the perforin-based cytotoxic pathway (Kumar & Tarleton 1998; Walsh et al. 1994; Nickel & Sharma 2000). In this regard, it is worth noting that besides target cell lysis, perforin has also been involved in the control of activated CD8+ T cell population by an unknown mechanism (Stepp et al. 2000; Sambhara et al. 1998; Spielman et al. 1998; Matloubian et al. 1999; Spaner et al. 1999). Moreover, despite the clear participation of perforin-mediated cytotoxicity in many important events (Van den Broek et al. 1996; Kägi et al. 1994), some cases were described in which perforin is not apparently required to control infection, such as mycobacterial infection (Cooper et al. 1997), rotaviruses (Franco et al. 1997), primary rejection of some allografts (Walsh et al. 1996) and some cytophatic and non-cytophatic viruses (Kägi et al. 1995). In addition, a direct role for perforin in parasite destruction does not seem to be the case, since the purified protein is unable to form pores and kill T. cruzi in in vitro assays (Bisaggio et al. 1997).
Therefore, the participation of CD8+ T cells in the control of the infection could be conveyed by at least three basic mechanisms: (1) direct destruction of infected cells by either a perforin-dependent or independent mechanism such as fas/fas–L interaction; (2) secretion of pleiotropic cytokines; and/or (3) perforin-dependent negative feedback on activated CD8+ T cells.
Our results show that, in regard to parasite load, perforin (−/−) mice did not differ from their (+/+) counterpart in the ability to control circulating parasitaemia after infection with Y strain T. cruzi, as originally described for the Brazil strain of the parasite (Kumar & Tarleton 1998). Furthermore, the time course of the control of parasitaemia suggests that cognate immune response is also not involved during this stage of infection as studies performed with RAG (−/−) mice showed that the contribution of this type of immune response becomes apparent only by day 14 of infection (Abrahamson & Coffman 1996). In accordance with this hypothesis, we have not detected significant differences between perforin (+/+) and (−/−) mice when they were infected with the clone Dm28c, a model in which detectable parasitaemia develops only after two weeks of infection (Henriques-Pons et al. 1998). These results demonstrate that perforin is not a central molecule employed either by innate or by specific immune responses to control the parasite load of the acute phase. These findings called our attention to the importance of cytokines production by CD8+ T cells in the control of parasitaemia. In addition, Denkers and collaborators (Denkers et al. 1997) found that vaccinated perforin (−/−) mice are completely resistant to challenge with a virulent strain of T. gondii. These authors attributed this result to IFN-γ production, which remained unimpaired in the perforin (−/−) mice.
The infection with Y strain T. cruzi displayed a higher rate of mortality in perforin (−/−) when compared to (+/+) mice, although another group has observed no differences in the susceptibility after infection with the Brazil strain of T. cruzi (Kumar & Tarleton. 1998). The data illustrates the complex variation of resistance and immunological interactions of the different models used to study parasite infections.
The histopathological analysis showed that both groups of infected mice had intense cardiac damage, mainly through non-apoptotic pathways, as ascertained by plasma CK-MB activity, cytoskeletal labelling, TUNEL assays, and ultrastructural analysis. The data indicates that although CD8+ T cells represent most of the inflammatory infiltrates, perforin does not directly induce destruction of myocardial fibres. We also found a much stronger cardiac inflammatory response, with more abundant and exuberant inflammatory foci in the perforin-deficient mice from the 15th dpi on. This suggests that perforin may be somehow involved in the restrain of the cellular cardiac inflammatory response after T. cruzi infection. This hypothesis is in agreement with recently connoted functions of perforin in the regulation of the immune response (Sambhara et al. 1998; Spielman et al. 1998; Matloubian et al. 1999; Spaner et al. 1999; Stepp et al. 2000). These include down-regulation of Ig production, regulation of autoimmunity and cytotoxic T lymphocyte responses to some viral infections, and, possibly, activation-induced cell death (AICD) of cytotoxic T lymphocytes. It could be possible that in T. cruzi-infected perforin-competent mice, perforin-producing cytotoxic cells were recognizing and destroying infected inflammatory-recruiting cells in the cardiac tissue, such as macrophages, thus reducing the migration of new inflammatory cells after two weeks of infection. In the absence of perforin, these recruiting cells would still be present and stimulating the inflammation. A similar hypothesis has been suggested in other situations (Stepp et al. 2000), and is under investigation in our laboratory.
Our results demonstrated cardiomyocytes damage determined by perforin-alternative pathway(s). It is possible that IFN-γ, locally secreted by the abundant cardiac inflammatory cells observed in infected perforin (−/−) mice were inducing cardiomyocytes necrosis (Pulkki 1997). In accordance with this hypothesis, the infected perforin-deficient mice displayed higher plasma levels of IFN-γ after the 15th dpi and also more IFN-γ-producing cells in the cardiac inflammatory foci on the 21st dpi, when compared to the infected (+/+) counterparts. In spite of the clear role of IFN-γ as a protective cytokine in different pathologies, such as Leishmania major (Scharton-Kersten & Scott 1995), T. gondii (Walker et al. 1997) and T. cruzi (Cardillo et al. 1996), it is possible that overproduction of this cytokine can induce the death of cardiomyocytes (Reed 1998; Shimojo et al. 1999). Moreover, inflammatory cells gathered in the cardiac tissue of deficient mice could inflict direct damage to the cardiomyocytes. Cardiomyocytes have altered plasma membranes and remarkably increased permeability at the site of contact or close opposition with macrophages in experimental T. cruzi myocarditis (Rossi & Silva 1990).
In conclusion, we demonstrated that the perforin-based cytotoxic pathway can modulate the cellular inflammatory response in the cardiac tissue after T. cruzi infection. However, perforin does not seem to be involved in the destruction of cardiomyocytes or in the control of circulating and cardiac parasite load observed in the acute phase of the infection.
Acknowledgments
We are grateful to Joel Majerowiks for helping in the establishment of the knockout mice colony. We also would like to thank Solange Lisboa de Castro for critical reviews of the manuscripts, Paola Minoprio and Alain Cosson for helpful support in cytokine quantification, and Bianca Perdigão Olivieri and Cleber da Silva Marques for helpful support during the experiments.
Funding
This work was financed by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brazil (CNPq), Programa de Núcleos de Excelência (Pronex), Financiadora de Estudos e Projetos (FINEP), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação Oswaldo Cruz (Papes-FIOCRUZ), and Fundação Universitária José Bonifácio (FUJB/UFRJ).
References
- Abelmann WH. Experimental infection with Trypanosoma cruzi (Chagas' disease): a model of acute and chronic myocardiopathy. Ann. N.Y. Acad. Sci. 1969;156:137–151. doi: 10.1111/j.1749-6632.1969.tb16723.x. [DOI] [PubMed] [Google Scholar]
- Abrahamson IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-γ, and IL-12 regulate innate and acquired immunity to infection. Exp. Parasitol. 1996;84:231–244. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- Andrade S. Influence of Trypanosoma cruzi strain on the pathogenesis of chronic myocardiopathy in mice. Mem. Inst Oswaldo Cruz. 1990;85:17–27. doi: 10.1590/s0074-02761990000100003. [DOI] [PubMed] [Google Scholar]
- Araújo-Jorge TC, Sampaio EP, De Souza W, Meirelles MNL. Trypanosoma cruzi: The effect of variations in the experimental conditions on the levels of macrophage infection in vitro. Parasitol. Res. 1989;75:257–263. doi: 10.1007/BF00931809. [DOI] [PubMed] [Google Scholar]
- Bisaggio RC, Castro SL, Barbosa HS, Brandão CA, Persechini PM. Trypanosoma cruzi: Resistance to the pore-forming protein of cytotoxic lymphocytes – perforin. Exp. Parasitol. 1997;86:144–154. doi: 10.1006/expr.1997.4172. [DOI] [PubMed] [Google Scholar]
- Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect. Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AM, D'Souza C, Frank AA, Orme IM. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzime-mediated cytolytic mechanisms. Infect. Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers EY, Yap G, Scharton-Kersten T, et al. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 1997;159:1903–1908. [PubMed] [Google Scholar]
- Duke RC, Persechini PM, Chang S, Liu C-C, Cohen JJ, Young JDE. Purified perforin induces target cell lysis but not DNA fragmentation. J. Exp. Med. 1989;170:1451–1456. doi: 10.1084/jem.170.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco MA, Tin C, Rott LS, Vancott JL, McGhee JR, Greenberg HB. Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, Fas, and gamma interferon. J. Virol. 1997;71:479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa SCG, Calabrese KS, Bauer PG, Savino W, Lagrange PH. Studies of the thymus in Chagas' disease III. Colonization of the thymus and other lymphoid organs of adult and newborn mice by Trypanosoma cruzi. Path. Biol. 1991;39:91–97. [PubMed] [Google Scholar]
- Henriques-Pons A, Correa AFS, Batista MM, et al. Perforin knock-out mice infected with a highly virulent strain of Trypanosoma cruzi have normal patterns of parasitaemia but higher mortality rates and myocarditis. In: Talwar GP, Nath I, Ganguly NK, Rao KVS, editors. 10th International Cong. Immunology, New Delhi, India. Vol. 2. Bologna: Monduzzi Ed.; 1998. pp. 1007–1010. [Google Scholar]
- Kägi D, Ledermann B, Bürki K, Hengartner H, Zinkernagel RM. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur. J. Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- Kägi D, Odermatt B, Mak TW. Homeostatic regulation of CD8+ T cells by perforin. Eur. J. Immunol. 1999;29:3266–3272. doi: 10.1002/(SICI)1521-4141(199910)29:10<3262::AID-IMMU3262>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kägi D, Seiler P, Pavlovic J, et al. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur. J. Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tarleton RL. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 1998;20:207–216. doi: 10.1046/j.1365-3024.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- Liu C-C, Walsh CM, Young JD-E. Perforin: structure and function. Immunol. Today. 1995;16:194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Sureesh M, Glass A, et al. A role for perforin in downregulating T-cell responses during chronic viral infection. J. Virol. 1999;73:2527–2536. doi: 10.1128/jvi.73.3.2527-2536.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino G, Catani MV, Guerrieri P, Bernassola F. Nitric Oxide can inhibit apoptosis or switch it into necrosis. Cell. Mol. Life Sci. 2000;57:612–622. doi: 10.1007/PL00000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoprio PM, Eisen H, Forni L, Lima MRD, Joskowicz M, Coutinho A. Polyclonal lymphocyte responses to murine Trypanosoma cruzi infection. I. Quantification of both T- and B-cell responses. Scand. J. Immunol. 1991;24:661–668. doi: 10.1111/j.1365-3083.1986.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Morris SA, Tanowitz HB, Wittner M, Bilezikian JP. Pathophysiological insights into the cardiomyopathy of Chagas' disease. Circulation. 1990;82:1990–1909. doi: 10.1161/01.cir.82.6.1900. [DOI] [PubMed] [Google Scholar]
- Motyka B, Korbutt G, Pinkoski MJ, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2001;103:491–500. doi: 10.1016/s0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Henkart PA. Cytotoxic lymphocyte granzymes trigger a target cell internal disintegration pathway leading to cytolysis and DNA breakdown. J. Immunol. 1994;152:1057–1063. [PubMed] [Google Scholar]
- Nickell SP, Sharma D. Trypanosoma cruzi: roles for perforin-dependent and perforin-independent immune mechanisms in acute resistance. Exp. Parasitol. 2000;94:207–216. doi: 10.1006/expr.2000.4498. [DOI] [PubMed] [Google Scholar]
- Pinkoski MJ, Hobman M, Heibein JA, et al. Entry and trafficking of granzyme B., in target cells during granzyme B. and -perforin-mediated apoptosis. Blood. 92:1044–1054. [PubMed] [Google Scholar]
- Pulkki KJ. Cytokines and cardiomyocyte death. Ann. Med. 1997;29:339–343. doi: 10.3109/07853899708999358. [DOI] [PubMed] [Google Scholar]
- Reed SG. Immunology of Trypanosoma cruzi infections. Chem. Immunol. Basel. Karger. 1998;70:124–143. [PubMed] [Google Scholar]
- Rossi MA, Bestetti RB. The challenge of chagasic cardiomyopathy. Cardiology. 1995;86:1–7. doi: 10.1159/000176822. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Silva JS. Permeability alteration of the sarcolemmal membrane, particularly at the site of macrophage contact, in experimental chronic Trypanosoma cruzi myocarditis in mice. Inst J. Exp. Pathol. 1990;71:545–555. [PMC free article] [PubMed] [Google Scholar]
- Rottenberg ME, Riarte A, Sporrong L, et al. Outcome of infection with different strains of Trypanosoma cruzi in mice lacking CD4 and/or CD8. Immunol. Lett. 1995;45:53–60. doi: 10.1016/0165-2478(94)00221-c. [DOI] [PubMed] [Google Scholar]
- Russo M, Starobinas N, Minoprio P, et al. Parasitic load increases and myocardial inflammation decreases in Trypanosoma cruzi-infected mice after inactivation of helper T cells. Ann. Inst Pasteur Immunol. 1988;139:225–236. doi: 10.1016/0769-2625(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Sambhara S, Switzer I, Kurichh A, et al. Enhanced antibody and cytokine responses to influenza viral antigens in perforin-deficient mice. Cell. Immunol. 1998;187:13–18. doi: 10.1006/cimm.1998.1314. 10.1006/cimm.1998.1314. [DOI] [PubMed] [Google Scholar]
- Scharton-Kersten T, Scott P. The role of the innate immune response in Th1 cell development following Leishmania major infection. J. Leuk. Biol. 1995;57:515–522. doi: 10.1002/jlb.57.4.515. [DOI] [PubMed] [Google Scholar]
- Schmunis GA, Cappa SMG, Traversa OC, Janovsky JF. The effect of immuno-depression due to neonatal thymectomy on infections with Trypanosoma cruzi in mice. Trans. R. Soc. Trop. Med. Hyg. 1971;65:89–94. doi: 10.1016/0035-9203(71)90190-8. [DOI] [PubMed] [Google Scholar]
- Shimojo T, Hiroe M, Ishiyama S, Ito H, Nishikawa T, Marumo F. Nitric oxide induces apoptotic death of cardiomyocytes via a cyclic-GMP-dependent pathway. Exp. Cell. Res. 1999;247:38–47. doi: 10.1006/excr.1998.4310. [DOI] [PubMed] [Google Scholar]
- Souza AP, Olivieri BP, De Castro SL, Araújo-Jorge TC. Enzymatic markers of heart lesions in mice infected with Trypanosoma cruzi and submitted to benznidazole chemotherapy. Parasitol. Res. 2000;86:800–808. doi: 10.1007/s004360000262. [DOI] [PubMed] [Google Scholar]
- Spaner D, Kaliannan R, Rabinovich B, Miller RG. A role for perforin in activation-induced T cell death in vivo: increased expansion of allogeneic perforin-deficient T cells in SCID mice. J. Immunol. 1999;162:1192–1199. [PubMed] [Google Scholar]
- Spielman J, Lee RK, Podack ER. Perforin/Fas-Ligand double deficiency is associated with macrophage expansion and severe pancreatitis. J. Immunol. 1998;161:7063–7070. [PubMed] [Google Scholar]
- Stepp SE, Mathew AP, Benett M, Saint Basile G, Kumar V. Perforin: more than just an effector molecule. Immunol. Today. 2000;21:254–256. doi: 10.1016/s0167-5699(00)01622-4. [DOI] [PubMed] [Google Scholar]
- Tarleton RL. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J. Immunol. 1990;144:717–724. [PubMed] [Google Scholar]
- Tarleton RL, Koller BH, Latour A, Postan M. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356:338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- Trapani JA, Jans DA, Jans PJ, Smyth MJ, Browne KA, Sutton VR. Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J. Biol. Chem. 1998;273:27934–27938. doi: 10.1074/jbc.273.43.27934. [DOI] [PubMed] [Google Scholar]
- UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease. Geneva: UNDP/World Bank/WHO;; 1997. Tropical Disease Research (TDR), Thirteenth Programme Report; pp. 113–123. [Google Scholar]
- Van den Broek MF, Kägi D, Ossendorp F, et al. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W, Roberts CW, Ferguson DJP, Jebbari H, Alexander J. Innate immunity to Toxoplasma gondii is influenced by Gender and is associated with differences in interleukin-12 and gamma interferon production. Infect. Immun. 1997;65:1119–1121. doi: 10.1128/iai.65.3.1119-1121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Hayashi F, Saffran DC, Ju S, Berke G, Clark WR. Cell-mediated cytotoxicity results from, but may not be critical for, primary allograft rejection. J. Immunol. 1996;156:1436–1441. [PubMed] [Google Scholar]
- Walsh CM, Matloubian M, Liu C-C, et al. Immune function in mice lacking the perforin gene. Proc. Natl. Acad. Sci. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD-E, Cohn ZA, Podack ER. The ninth component of complement and the pore-forming protein (perforin I) from cytotoxic T cells: structural, immunological and functional similarities. Science. 1986;233:184–190. doi: 10.1126/science.2425429. [DOI] [PubMed] [Google Scholar]