Abstract
Intratracheal instillation (IT) of bleomycin is a widely used experimental model for lung fibrosis. In this study we describe the time-course of bleomycin-induced lung fibrosis in mice using computer-assisted morphometry. C57Bl/6J mice were treated with a single IT dose of bleomycin or control saline. Animals were killed 3, 6, 14 and 21 days post-IT. Lung injury was evaluated by analysis of bronchoalveolar lavage (BAL) fluid, hydroxyproline concentration in the lung, routine light microscopic examination resulting in a semiquantitative morphological index (SMI) of lung injury, and quantitative morphological measurements (fibrosis fraction and alveolar wall area fraction) aided by optimas image analysis software. Changes in BAL fluid attributed to bleomycin treatment include increased total cell count (days 14 and 21), and increased percentage of neutrophils (days 3 and 6) followed by a sustained increase in lymphocytes (days 6, 14 and 21). Hydroxyproline levels increased in bleomycin-treated mice on days 14 and 21. Median SMI grades were significantly elevated on days 3, 14 and 21. Computer-assisted morphometry demonstrated a 3-fold increase in fibrosis fraction and a 1.3-fold increase in wall area fraction in bleomycin-treated mice on day 14, with no further increase on day 21. These data also demonstrate that the most suitable time point for assessing lung fibrosis in this model is 14 days after IT instillation of bleomycin, based on the observation that at 14 days the animals developed extensive fibrosis, but had less variability in the fibrotic response and lower mortality than later at 21 days. Computer-assisted morphometry provides objective and quantitative measurements that are a useful tool for the evaluation of bleomycin-induced lung injury.
Keywords: bleomycin, computer-assisted morphometry, fibrosis, interstitial lung disease, lung, mice
Introduction
Idiopathic interstitial pneumonias have recently been reclassified based on pathologically distinct entities: usual interstitial pneumonia (UIP), desquamative interstitial pneumonia (DIP), non-specific interstitial pneumonia (NSIP), respiratory bronchiolitis interstitial lung disease (RBILD), and acute interstitial pneumonia (AIP) (Katzenstein & Myers 1998; Gross & Hunninhake 2001). The intratracheal instillation (IT) of bleomycin into rodents is widely used as an in vivo experimental model (Snider et al. 1978; Phan et al. 1981) to study inflammatory and fibrotic changes in the lung interstitium that are present to various extents in interstitial pneumonias, especially in UIP, DIP and NSIP.
In this study we characterize in detail the time-course of bleomycin-induced lung fibrosis in C57Bl/6J mice. In addition to traditional pathological and biochemical methods, a novel computer-assisted morphometric image analysis is introduced.
Materials and methods
Animals
C57Bl/6J male mice, 11–12 weeks old, weighing 25–30 g, were used. All procedures involving animals were approved by the Institutional Committee of Animal Care. Mice were housed in plastic cages on hardwood shavings. A 12-h light/dark cycle was maintained and mice had access to water and rodent laboratory chow ad libitum. Mice were acclimated to these conditions at least 1 week before receiving IT treatment.
Experimental design
Bleomycin sulphate (Bristol Laboratories, Syracuse, NY) was dissolved in sterile 0.9% saline and administered as a single dose of 0.06 mg in 0.1 mL saline solution per animal. Control animals received 0.1 mL saline alone. All animals received IT instillations of either bleomycin or saline on day 0 as previously described (Kremer et al. 1999; Laxer et al. 1999; Berkman et al. 2001).
Mice were randomly assigned to five weight-matched experimental groups: (1) IT bleomycin, day 3, 5 animals; (2) IT bleomycin, day 6, 5 animals; (3) IT bleomycin, day 14, 10 animals; (4) IT bleomycin, day 21, 11 animals; and (5) IT saline, 20 animals, 5 at each time point.
On days 3, 6, 14, and 21 following IT bleomycin or saline instillation, animals were sacrificed by transsection of the abdominal aorta while under an overdose of pentobarbital (80 mg/kg, IP). Lung injury was evaluated by analysis of bronchoalveolar lavage (BAL) fluid, measurement of hydroxyproline concentration in the lung, routine light microscopic examination resulting in a semiquantitative index (SMI) of lung injury, and by quantitative morphological examinations as follows.
Bronchoalveolar lavage (BAL) analysis
A polyethylene cannula (PE 205, Clay Adams, Parsippany, NZ) was placed into the trachea. BAL was carried out as previously described with a standard volume of 4 mL saline (Kremer et al. 1999; Laxer et al. 1999; Berkman et al. 2001). The total number of cells was counted, and a differential count was performed on 200 cells per animal, and expressed as a percentage of total cells recovered.
Hydroxyproline concentration in lung
The right lung was ligated and cut at the hilum, freed of extraneous tissue, and homogenized in 3 mL PBS (Polytron, Kinematica, Lucerne, Switzerland). As previously described (Lossos et al. 2000), an aliquot was hydrolyzed in 6 N HCl for 24 h at 106 °C, and analysed on an amino acid analyser (Beckman 6300). The hydroxyproline results are expressed as nanomoles per lung.
Morphological examination
The left lung was fixed by IT infusion through the cannula of 4% formalin and 1% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.4), maintained at 25 cm hydrostatic pressure for 5 min and then immersed in fixative for an additional 24 h. Only lungs that were well inflated by the fixative were analysed. Three 0.3 cm thick transverse sections were embedded in paraffin, and sequential 4–6 µm sections were stained with haematoxylin–eosin (H&E) and modified Masson's trichrome. Fibrotic lung injury was assessed morphologically by semiquantitative and quantitative parameters as follows.
Semiquantitative Morphological Index (SMI)
Morphological changes in lung sections were graded semiquantitatively, on three lung sections from each bleomycin-treated animal and on 10 representative saline-treated mice without knowledge of treatment groups using a grading scheme as previously published (Kremer et al. 1999; Laxer et al. 1999; Lossos et al. 2000; Berkman et al. 2001); 0, normal lung; 1, minimal areas of inflammation, epithelial hyperplasia and fibrosis, usually limited to subpleural foci in just 1 or 2 sections; 2, more frequent lesions; 3, all three sections exhibit lung lesions which are not limited to subpleural foci; 4, extensive lesions in at least 2 of 3 sections; and 5, majority of each of three lung sections affected by inflammation and fibrosis.
Quantitative Image Analysis (QIA)
(a) Fibrosis fraction. The degree of fibrosis was quantified by the optimas image analysis computer program (Optimas Corp., Bothell, WA, USA) by analysing slides that were stained with a modified trichrome stain, as previously described (Kremer et al. 1999; Laxer et al. 1999; Berkman et al. 2001), to enhance the blue-stained collagen. Using a 40× objective lens, 20 randomly selected fields per slide were selected by a video camera (Javelin Chromachip II) and displayed on a colour monitor (Sony Trinitron). By adjusting image contrast, brightness, and colour threshold settings, the image analysis program was configured to detect areas of blue-stained collagen within each field. The fraction of blue-stained collagen areas for each field, a constant 135 × 95 µm, was averaged for each animal. The area fraction of fibrosis is presented as a percentage.
(b) Alveolar wall area fraction. Alveolar wall thickness may increase due to either fibrosis or interstitial oedema. Alveolar wall area fraction was quantified by configuring the Optimas system to determine, in sections of H&E-stained slides, the area fraction of alveolar wall tissue. Using a 10× objective lens, 10 randomly selected fields lacking visible blood vessels and airways were selected using the video camera and displayed on the monitor. Each field of interest measured 455 × 345 µm. The image analysis software was programmed to measure the area of all stained tissue and divide it by the constant field of interest area, thereby calculating an alveolar wall area fraction value for 10 fields, which were averaged for each animal. Data are presented as a percentage.
Extraction of cells from lung parenchyma
Mice were exsanguinated as above. The lungs were perfused with normal saline through the right ventricle. Bronchoalveolar lavage (BAL) was performed to eliminate airway and alveolar space cells. Lung cells (LC) were extracted as follows: lungs were removed, minced, and incubated (37 °C, 5% CO2 air) for 45 min with RPMI-1640 containing 1 mg/mL collagenase (Sigma Chemical Co., St. Louis, MO). After enzyme treatment, lung tissue was gently passed through a cell dissociation sieve (Sigma). Cells were washed twice in cold PBS, fixed in 4% paraformaldehyde, prewarmed to 37 °C for 10 min at room temperature and then washed twice in PBS and stored at 4 °C (5 × 106 cells/mL).
Flow cytometry
After 2 washes in FACS buffer (3% FCS in PBS) LC were stained for 45 min in 100 mL FACS buffer containing 1 mg R(PE)-conjugated rat anti-mouse CD4 (Pharmingen, 09425 A), CD8 (Serotec, MCA609PE), NK1.1 (Pharmingen, 01295 A) or B220 (Pharmingen, 01125 A) mAbs. Fluorescence was determined using a FACScan flow cytometer (Beckton-Dickinson).
Statistical analysis
BAL cell counts, hydroxyproline levels and QIA parameters were analysed using the anova and Bonferonni analyses (Snedecor & Cochran 1967). SMI was analysed by the nonparametric Kruskal–Wallis test (Snedecor & Cochran 1967). Probability values of <0.05 were considered statistically significant.
Results
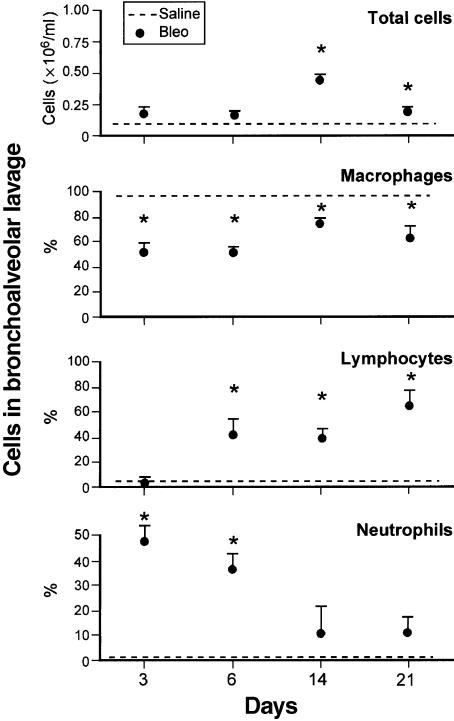
Total and differential BAL fluid cell counts of each day are presented in Figure 1. Total cell count was higher in bleomycin- compared to saline-treated mice at 14 days (P < 0.001) and 21 days (P < 0.05). The percentage of neutrophils was higher (P < 0.001) in bleomycin- compared to saline-treated mice at 3 days and 6 days. A sustained increase in lymphocytes was observed from 6 days (P < 0.05) through 21 days (P < 0.001). The percentage of macrophages was significantly decreased in bleomycin-treated mice when compared to saline, at all time points studied (P < 0.001). However, the absolute number of macrophages increased significantly at 14 days (322 622 ± 38 763, mean ± SE) but not at 3 days (102 400 ± 29 173), 6 days (74 650 ± 10 901) or 21 days (159 982 ± 43 214) as compared with saline (97 865 ± 56 181).
Figure 1.
Total and differential cell count in BAL fluid of mice treated by intratracheal instillation of Bleo (circle) or Sal (hatched line), and sacrificed on different days. Values are mean ± SE; n = 20 for Saline-treated animals and 5, 5, 10 and 11 for bleomycin-treated animals at 3, 6, 14 and 21 days, respectively. *P < 0.05 compared to Sal.
Flow cytometry studies of cells extracted from lung tissue (LC) showed a progressive increase in CD4 cells in bleomycin-treated animals, from 13% at 3 days to 21% at 14 days; and in B cells from 28% at 3 days to 35% at 14 days. There were no significant changes over time in NK and CD8 cells. The CD4: CD8 ratio shifted from 1: 1 to 2: 1.
Hydroxyproline data are presented in Figure 2. Hydroxyproline levels increased in bleomycin-treated mice as compared to saline, at 14 days (P < 0.05) and 21 days (P < 0.001).
Figure 2.
Hydroxyproline levels in Bleo and Sal-treated mice, at different time-points. Data are presented as mean ± SE, n = 20 for Saline-treated animals and 5, 5, 10 and 11 for bleomycin-treated animals at 3, 6, 14 and 21 days, respectively. *P < 0.05 compared to Sal.
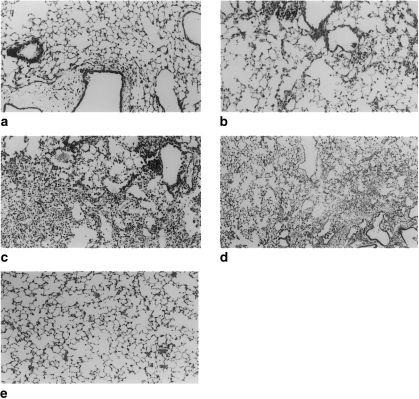
Lungs were examined histologically at 3, 6, 14 and 21 days after bleomycin and saline instillations. Representative photomicrographs are presented in Figure 3. Histopathological assessment showed that there were slight increases in the number of alveolar macrophages and increased cellularity of alveolar septa as early as 3 days post-IT bleomycin. Vascular margination and tissue infiltration of neutrophils and macrophages, and increased numbers of perivascular and peribronchiolar lymphocytes were also seen (Figure 3a). The severity of the changes was generally very slight. These changes were absent in lungs from saline-treated mice (Figure 3e), and thus are considered specific early changes in response to bleomycin. Changes observed at 6 days were similar to those observed at 3 days except that focal subcapsular fibrosis was also present (Figure 3b). At 14 days most mice had multifocal or diffuse changes consisting of some combination of thickened alveolar septa, intra-alveolar fibrosis with myofibroblasts within the lumen, occasional foci of dense fibrosis, increased alveolar macrophages, and focal dilatation of respiratory bronchioles and alveolar ducts (Figure 3c). Some animals exhibited epithelial hyperplasia in alveolar ducts. The severity of these changes varied somewhat from mouse to mouse, ranging from slight to moderate. At 21 days, the severity of the changes varied greatly, from nearly normal to severe (Figure 3d). In the more severely affected animals, the changes were diffuse and included intra-alveolar fibrosis, focally dense fibrosis, often subpleural, and epithelial hyperplasia in alveolar ducts.
Figure 3.
Representative photomicrographs of intratracheal bleomycin and saline-treated animals. (a) At 3 days following bleomycin instillation there is an increased number of perivascular and peribronchial lymphocytes. (b) At 6 days these changes are accompanied by focal subcapsular fibrosis. (c) At 14 days, there are increased alveolar macrophages, thickened alveolar septa and foci of dense fibrosis. (d) At 21 days, the severity of the lesion varies greatly, with some areas demonstrating dense fibrosis and normal lung adjacently. (e) Saline-treated animals, at all time points, show normal lung architecture. (Paraffin blocks, haematoxylin–eosin, original magnification: 100×).
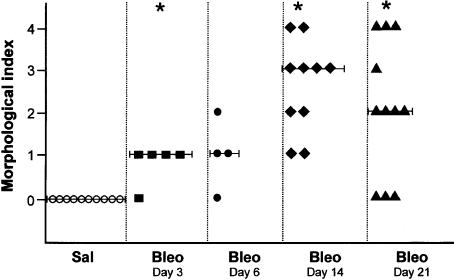
Pulmonary morphological changes as assessed by the semiquantitative morphological index (SMI) are presented in Figure 4. Median SMI grades were 1, 1, 3 and 2 for bleomycin-treated mice at 3 days, 6 days, 14 days and 21 days, respectively. Saline-treated mice were uniformly grade 0. The difference between bleomycin- and saline-treated mice was significant on days 3 (P < 0.05), 14 (P < 0.001) and 21 (P < 0.05).
Figure 4.
Semiquantitative morphological index (SMI) in bleomycin- and saline-treated mice at different time-points. Each point represents SMI of an individual mouse. Median value is presented as a line. Details of SMI are specified in the Methods section. *P < 0.05 compared to Sal.
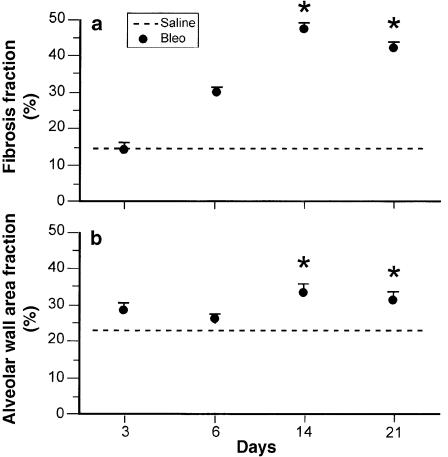
Fibrosis fraction and alveolar wall area fraction as assessed by QIA are presented in Figure 5(a) and Figure 5(b), respectively. At 14 days, bleomycin- as compared to saline-treated animals, showed a 3-fold increase in fibrosis fraction and a 1.3-fold increase in wall thickness. There was no further increase in morphometric parameters after day 14 in bleomycin-treated animals. Moreover, the statistical significance of the difference in the QIA parameters between bleomycin and saline was weaker at 21 days (P < 0.05 vs. P < 0.001 at 14 days).
Figure 5.
(a) Fibrosis fraction and (b) alveolar wall area fraction were measured at different time-points by quantitative image analysis, using the optimas image analysis program. Data are presented as mean ± SE, n = 20 for saline-treated animals and 5, 5, 10 and 11 for bleomycin-treated animals at 3, 6, 14 and 21 days respectively. *P < 0.05 compared to Sal.
Discussion
In this study we present a computer-aided morphometric analysis of bleomycin injury (QIA). This measurement is objective and quantitative and therefore a more precise and reproducible tool than the use of semiquantitative pathological scores. When compared to measurements of tissue collagen such as hydroxyproline, the QIA has the advantage of evaluating a more comprehensive morphological parameter rather than the limited focus on a biochemical measurement. In addition, the variability in the QIA measurements of fibrosis fraction was smaller than that in hydroxyproline measurements. We therefore believe that quantitative computer-based morphometric methods are an important advance in the quantitative analysis of experimental bleomycin-induced fibrosis. We show that an off-the-shelf system can be readily applied to this model. Furthermore, our results show that after a single instillation of bleomycin, 14 days is the best time point to measure lung fibrosis parameters. At this time, the animals developed extensive fibrosis and had a lower mortality than later (day 21), and the QIA parameters demonstrated less variability at 14 days compared to 21 days, as reflected by the lower P-values for the comparisons between bleomycin- and saline-treated animals.
Our results confirm that the inflammatory response to intratracheal bleomycin starts with an acute neutrophilic infiltrate, followed by a transition to a lymphocyte predominant chronic inflammation. Neutrophils isolated from the BAL of animals 3 days post-bleomycin instillation are activated, since they have a greater capacity to produce superoxide anion than neutrophils isolated from the blood of these animals (Tarnell et al. 1992). This response was shown to be transient. Since neutrophils decrease to control levels before significant fibrosis occurs, those cells are unlikely to affect fibrogenesis directly, but may have a role in the initiation of the inflammatory cascade that leads to lung fibrosis.
It is as yet unclear whether the chronic lymphocytic inflammation observed in our model is key to the evolution of the fibrotic response. T-lymphocytes are capable of generating fibroblast chemotactic factors and factors that influence both fibroblast proliferation and collagen synthesis (Johnson & Ziff 1976; Hibbs et al. 1983; Postlethwaite 1983; Wahl & Gately 1983; Postlethwaite et al. 1984). It has also been established that lymphocytes infiltrate the lungs of animals with bleomycin-induced lung injury (Chandler et al. 1983; Thrall & Barton 1984; Janick-Buckner et al. 1986) and that they are activated (Karpel et al. 1989). Increases in both B- and T- lymphocytes were demonstrated, as well as shifts in helper and suppressor T-cell populations in lung tissue post-bleomycin injury; as confirmed in our study, the CD4: CD8 ratio of 1: 1 was shown to be shifted to 2: 1 at 14 days post-bleomycin (Kumar 1984; Thrall & Barton 1984). The increase in helper T-cells occurs when collagen synthesis is very active, and it has also been shown that there is an increase in suppressor T-cells when collagen synthesis decreases in the later stages of the disease process (Kumar 1984; Thrall & Barton 1984). Taken together, these results suggest that T-lymphocytes are likely to play a role in the pathogenesis of the fibrogenic process.
However, several conflicting studies either emphasize or diminish the importance of T-lymphocytes in the model, suggesting that there may be a diverse role for these cells in fibrotic lung injury. Studies using athymic nude mice showed that development of pulmonary fibrosis in these animals was reduced in response to bleomycin (Schrier et al. 1983) or peplomycin (Ekimoto et al. 1985), an analogue of bleomycin. Treatment of animals with anti-CD3 monoclonal antibody (Sharma et al. 1996), or with immunosuppressive agents such as anti-lymphocyte globulin (Thrall et al. 1979) or by thymectomy (Thrall et al. 1980) resulted in decreased collagen deposition in bleomycin-exposed animals. In contrast, another study found no difference in the fibrotic response to bleomycin in nude mice compared to their congenic euthymic counterparts (Szapiel et al. 1979). In addition, experiments in which mice were depleted of Thy1.2+ cells (T-lymphocytes and a subset of NK cells), L3T4+ cells (a marker of MHC-II restricted functions), or Lyt-2+ cells (suppressor and cytotoxic T-lymphocytes) had no effect on the development of bleomycin-induced pulmonary fibrosis in C57Bl/6 mice (Janick-Buckner et al. 1989).
Our finding of a significant increase in the number of B-cells infiltrating the lungs of bleomycin-treated mice is intriguing. Very little is known about B-cells and lung fibrosis. Thrall and colleagues (Thrall & Barton 1984) found an increase in the number of B-cells in lung tissue of rats after instillation of bleomycin. More recently aggregates of B-lymphocytes were found in open lung biopsies from 37/38 patients with idiopathic pulmonary fibrosis (IPF) (Wallace et al. 1996).
Do B-lymphocytes play a role in the pathogenesis of lung fibrosis? To the best of our knowledge, no studies have been published in which B-cells were specifically eliminated in an animal model of lung fibrosis. However, circumstantial evidence abounds. BAL fluid from IPF patients contains elevated levels of IgG compared to normal controls, despite a paucity of lymphocytes in the same samples (Reynolds et al. 1977). Circulating immune complexes are present in the blood (Haslam et al. 1979) and BAL fluid (Dall'Aglio et al. 1988) of patients with IPF, and not just those with fibrotic lung disease associated with autoimmune disorders. Circulating immune complexes clearly have the potential to cause lung injury, and indeed immune-complex induced lung injury has been used as a model of human interstitial lung disease (Brentjens et al. 1974). However, the actual importance of immune complexes in IPF has been questioned by studies of lung biopsy tissues from IPF patients, which failed to demonstrate in situ immune complexes (Eisenberg et al. 1979; Coalson 1982; Endo et al. 1990). Others have managed to demonstrate immune deposits in lung tissue (Schwarz et al. 1978; Gelb et al. 1983).
Some recent studies have shown that the circulating immune complexes found in the sera of IPF patients contain autoantibodies against lung epithelial antigens (Dobashi et al. 2000a; Dobashi et al. 2000b). These reports suggest a new possibility for immune damage to the lung in IPF – specific autoimmune injury, rather than non-specific precipitation of immune complexes within the pulmonary microvasculature. This novel theory would be highly consistent with a pathogenic role for B-lymphocytes present in the lung.
The percentage of macrophages in the BAL fluid of bleomycin-treated animals was consistently decreased compared to saline controls. This should not be interpreted to mean that macrophages are not involved in the bleomycin-induced lung injury since the absolute number of macrophages in BAL fluid increased significantly at 14 days. As seen in patients with IPF (Struhar et al. 1986), the percentage of alveolar macrophages expressing MHC class II molecules is significantly elevated in animals post-bleomycin injury, suggesting activation and involvement in antigen presentation (Struhar et al. 1990). Conditioned media from cultured alveolar macrophages isolated from bleomycin-injured animals have been shown to have a multitude of capabilities, including neutrophil chemotactic activity and stimulation of collagen synthesis. These studies suggest an important role for macrophages in the bleomycin-induced lung injury.
Almost every lung cell and inflammatory cell has been implicated in the pathogenesis of bleomycin-induced pulmonary fibrosis. The complexity of the inflammatory response makes it impossible to single out a certain cell type or mediator as the major regulatory system in the pathogenesis of the fibrotic process. Studying the kinetics of inflammatory cell infiltration of bleomycin-injured lungs contributes to the understanding of the process as a whole.
Three and six days after treatment with a single intratracheal instillation of bleomycin, only a mild increase in the pathological scores was observed. The fibrotic changes were maximally increased at 14 days after bleomycin instillation and there was no further increase in the median pathologic appearance scores (SMI and QIA) from 14 days to 21 days. This is consistent with what has previously been described in a study comparing IT to IV instillation of bleomycin in mice (Lindenschnidt et al. 1986). Different animals have been used as experimental models of lung fibrosis (Fleischman et al. 1971). Regardless of the route of administration, lung injury progressed uniformly with the development of limited pulmonary fibrosis dependent upon the cessation of bleomycin administration. A single IT instillation of bleomycin does not produce a chronic type of lesion. However, continuous bleomycin caused the development of a progressive pulmonary fibrotic lesion (Harrison & Lazo 1987). In a more recent study (Swiderski et al. 1998), pulmonary fibrosis was induced in C57Bl/6 mice by multiple intraperitoneal (IP) injections of bleomycin. Masson trichrome blue staining and hydroxyproline measurements of lung tissue demonstrated fibrosis at 2 and 4 months after treatment. However, fibrosis still appeared to subside in all cases after bleomycin treatment was withdrawn.
In summary, in this study we introduce computerized morphometry as a useful tool in the study of experimental lung fibrosis. Using this and other parameters to study the time course of bleomycin lung injury in mice, we conclude that 14 days after a single intratracheal instillation of bleomycin is the most appropriate time for comparative studies using this model.
Acknowledgments
This study was supported in part by the Nathan Shainberg Fund; the Charlotte and Louis Kaitz Boston University School of Medicine–The Hebrew University-Hadassah Medical School Program; and by the Chief Scientist, Israel Ministry of Health.
References
- Berkman N, Kremer S, Or R, et al. Human recombinant interferon-α2a and interferon-αA/D have different effects on bleomycin-induced lung injury. Respiration. 2001;68:169–177. doi: 10.1159/000050488. [DOI] [PubMed] [Google Scholar]
- Brentjens JR, O'Connell DW, Pawlowski IB, Hsu KC, Andres GA. Experimental immune complex disease of the lung. The pathogenesis of a laboratory model resembling certain human interstitial lung diseases. JExpMed. 1974;140:105–125. doi: 10.1084/jem.140.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DB, Hyde DM, Giri SN. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. AmJPathol. 1983;112:170–177. [PMC free article] [PubMed] [Google Scholar]
- Coalson JJ. The ultrastructure of human fibrosing alveolitis. Virchows ArchAPatholAnatHistol. 1982;395:181–199. doi: 10.1007/BF00429611. [DOI] [PubMed] [Google Scholar]
- Dall'Aglio PP, Pesci A, Bertorelli G, Brianti E, Scarpa S. Study of immune complexes in bronchoalveolar lavage fluids. Respiration. 1988;54(Suppl):136–141. doi: 10.1159/000195495. [DOI] [PubMed] [Google Scholar]
- Dobashi N, Fujita J, Murota M, et al. Elevation of anti-cytokeratin 18 antibody and circulating cytokeratin 18: anti-cytokeratin 18 antibody immune complexes in sera of patients with idiopathic pulmonary fibrosis. Lung. 2000a;178:171–179. doi: 10.1007/s004080000020. [DOI] [PubMed] [Google Scholar]
- Dobashi N, Fujita J, Ohtsuki Y, et al. Circulating cytokeratin 8: anti-cytokeratin 8 antibody immune complexes in sera of patients with pulmonary fibrosis. Respiration. 2000b;67:397–401. doi: 10.1159/000029537. [DOI] [PubMed] [Google Scholar]
- Eisenberg H, Simmons DH, Barnett EV. Diffuse pulmonary interstitial disease: an immunohistologic study. Chest. 1979;75(Suppl):262–264. doi: 10.1378/chest.75.2_supplement.262. [DOI] [PubMed] [Google Scholar]
- Ekimoto H, Aikawa M, Ohnuki T, et al. Immunological involvement in pulmonary fibrosis induced by peplomycin. JAntibiot. 1985;38:94–98. doi: 10.7164/antibiotics.38.94. [DOI] [PubMed] [Google Scholar]
- Endo Y, Matsushita H, Matsuya S, Hara M. A study of human interstitial lung diseases with special reference to immune complexes and hyaline membrane. Acta PatholJpn. 1990;40:239–248. doi: 10.1111/j.1440-1827.1990.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Fleischman RW, Baber JR, Thompson GR, et al. Bleomycin induced interstitial pneumonia in dogs. Thorax. 1971;26:675–682. doi: 10.1136/thx.26.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb AF, Dreisen RB, Epstein JD, et al. Immune complexes, gallium lung scans, and bronchoalveolar lavage in idiopathic interstitial pneumonitis fibrosis. Chest. 1983;84:148–153. doi: 10.1378/chest.84.2.148. [DOI] [PubMed] [Google Scholar]
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. NEnglJMed. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- Harrison JH, Lazo JS. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. JPharmacolExpTher. 1987;243:1185–1194. [PubMed] [Google Scholar]
- Haslam PL, Thompson B, Mohammed I, et al. Circulating immune complexes in patients with cryptogenic fibrosing alveolitis. ClinExpImmunol. 1979;37:381–390. [PMC free article] [PubMed] [Google Scholar]
- Hibbs MS, Postlethwaite AE, Mainardi CL, Seyer JM, Kang AH. Alterations in collagen production in mixed mononuclear leukocyte–fibroblast cultures. JExpMed. 1983;157:47–59. doi: 10.1084/jem.157.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janick-Buckner D, Ranges GE, Hacker MP. T-cell modulation of bleomycin-induced pulmonary fibrosis. Pharmacologist. 1986;28:152. [Google Scholar]
- Janick-Buckner D, Ranges GE, Hacker MP. Effect of cytotoxic monoclonal antibody depletion of T-lymphocyte subpopulations on bleomycin-induced lung damage in C57BL/6J mice. ToxicolApplPharmacol. 1989;100:474–484. doi: 10.1016/0041-008x(89)90295-0. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Ziff M. Lymphokine stimulation of collagen accumulation. JClinInvest. 1976;58:240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpel JP, Aldrich TK, Mitsudo S, Norin AJ. Lung lymphocytes in bleomycin-induced pulmonary disease. Lung. 1989;167:163–172. doi: 10.1007/BF02714945. [DOI] [PubMed] [Google Scholar]
- Katzenstein AA, Myers JL. Idiopathic pulmonary fibrosis. Clinical relevance of pathological classification. AmJRespirCritCare Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- Kremer S, Breuer R, Lossos IS, et al. Effect of immunomodulators on bleomycin-induced lung injury. Respiration. 1999;66:455–462. doi: 10.1159/000029410. [DOI] [PubMed] [Google Scholar]
- Kumar RK. Separation and characterization of lymphocytes from rat lung parenchyma. ExpLung Res. 1984;7:113–122. doi: 10.3109/01902148409069672. [DOI] [PubMed] [Google Scholar]
- Laxer U, Lossos IS, Gillis S, et al. The effect of enoxaparin on bleomycin-induced lung injury in mice. ExpLung Res. 1999;25:531–541. doi: 10.1080/019021499270114. [DOI] [PubMed] [Google Scholar]
- Lindenschnidt RC, Tryka AF, Godfrey GA, Frome AL, Witschi H. Intratracheal versus intravenous administration of bleomycin in mice: acute effects. ToxicolApplPharmacol. 1986;85:69–77. doi: 10.1016/0041-008x(86)90388-1. [DOI] [PubMed] [Google Scholar]
- Lossos IS, Izbicki G, Or R, Goldstein RH, Breuer R. The effect of suramin on bleomycin-induced lung injury. Life Sci. 2000;67:2873–2881. doi: 10.1016/s0024-3205(00)00865-1. [DOI] [PubMed] [Google Scholar]
- Phan SH, Thrall RS, Williams C. Bleomycin-induced pulmonary fibrosis: Effects of steroid on lung collagen metabolism. AmRevRespirDis. 1981;14:428–434. doi: 10.1164/arrd.1981.124.4.428. [DOI] [PubMed] [Google Scholar]
- Postlethwaite AE. Cell–cell interaction in collagen biosynthesis and fibroblast migration. In: weissmann g., editor. Advances in Inflammation Research Vol. 5. New York: Raven Press; 1983. pp. 27–55. [Google Scholar]
- Postlethwaite AE, Smith GH, Mainardi CL. Lymphocyte modulation of fibroblast function in vitro: stimulation and inhibition of collagen production by different effector molecules. JImmunol. 1984;132:2470–2477. [PubMed] [Google Scholar]
- Reynolds HY, Fulmer JD, Kazmierowski JA, Roberts WC, Frank MM, Crystal RG. Analysis of cellular and protein content of broncho–alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. JClinInvest. 1977;59:165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier DJ, Phan SH, McGarry BM. The effects of the nude (nu/nu) mutation on bleomycin-induced pulmonary fibrosis. AmRevRespirDis. 1983;127:614–617. doi: 10.1164/arrd.1983.127.5.614. [DOI] [PubMed] [Google Scholar]
- Schwarz MI, Dreisin RB, Pratt DS, Stanford RE. Immunofluorescent patterns in the idiopathic interstitial pneumonias. JLabClinMed. 1978;91:929–938. [PubMed] [Google Scholar]
- Sharma SK, MacLean JA, Pinto C, Kradin RL. The effect of an anti-CD3 monoclonal antibody on bleomycin-induced lymphokine production and lung injury. AmJRespirCritCare Med. 1996;154:193–200. doi: 10.1164/ajrccm.154.1.8680680. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. 6. Ames, IA: Iowa State University Press;; 1967. pp. 258–298. [Google Scholar]
- Snider GL, Celli RR, Goldstein RH, O'Brien JJ, Lucey EC. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. AmRevRespirDis. 1978;117:289–297. doi: 10.1164/arrd.1978.117.2.289. [DOI] [PubMed] [Google Scholar]
- Struhar D, Harbeck RJ, Horiuchi T, Edelson J, Mason RJ. Class II antigens of the major histocompatibility complex are expressed in lung tissue of bleomycin treated rats and patients with idiopathic pulmonary fibrosis (IPF) AmRevRespirDis. 1986;133:A144. [Google Scholar]
- Struhar D, Greif J, Harbeck RJ. Class II antigens of the major histocompatibility complex are increased in lungs of bleomycin-treated rats. ImmunolLett. 1990;26:197–202. doi: 10.1016/0165-2478(90)90146-h. [DOI] [PubMed] [Google Scholar]
- Swiderski RE, Dencoff JE, Floerchinger CS, Shapiro SD, Hunninghake GW. Differential expression of extracellular matrix remodeling genes in a murine model of bleomycin-induced pulmonary fibrosis. AmJPathol. 1998;152:821–828. [PMC free article] [PubMed] [Google Scholar]
- Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. AmRevRespirDis. 1979;120:893–899. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- Tarnell EB, Oliver BL, Johnson GM, Watts FL, Thrall RS. Superoxide production by rat neutrophils in the bleomycin model of lung injury. Lung. 1992;170:41–50. doi: 10.1007/BF00164754. [DOI] [PubMed] [Google Scholar]
- Thrall RS, McCormick JR, Jack RM, Phan SH, Ward PA. The effect of antilymphocyte globulin on the development of bleomycin induced pulmonary fibrosis in the rat. AmRevRespirDis. 1979;119(S):83. [Google Scholar]
- Thrall RS, Lovely EJ III, Baron RW, McCormick JR, Phan SH, Ward PA. The effect of T-cell depletion on the development of bleomycin induced pulmonary fibrosis in the rat. AmRevRespirDis. 1980;121(S):99. [Google Scholar]
- Thrall RS, Barton RW. A comparison of lymphocyte populations in lung tissue and in bronchoalveolar lavage fluid of rats at various times during the development of bleomycin-induced pulmonary fibrosis. AmRevRespirDis. 1984;129:279–283. [PubMed] [Google Scholar]
- Wahl SM, Gately CL. Modulation of fibroblast growth by a lymphokine of human T cell continuous T cell line origin. JImmunol. 1983;130:1226–1230. [PubMed] [Google Scholar]
- Wallace WA, Howie SE, Krajewski AS, Lamb D. The immunological architecture of B-lymphocyte aggregates in cryptogenic fibrosing alveolitis. JPathol. 1996;178:323–329. doi: 10.1002/(SICI)1096-9896(199603)178:3<323::AID-PATH467>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]