Abstract
Three isoforms of nitric oxide synthase (NOS) have been identified: endothelial NOS, neuronal NOS, and inducible NOS (iNOS). The enhanced expression of iNOS at the protein level using immunohistochemical technique has been reported previously in chemically induced oral carcinomas in hamster buccal-pouch mucosa. However, the corresponding expression of iNOS at the mRNA level has not yet been demonstrated using in situ reverse transcription-polymerase chain reaction (IS RT-PCR). The purpose of the present study is to assess the iNOS mRNA expression level in 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal-pouch carcinomas using IS RT-PCR. Thirty outbred, young (6-weeks old), male, Syrian golden hamsters (Mesocricatus auratus) were randomly divided into one experimental group (10 animals), and two control groups (10 animals each). The pouches of a group of 10 animals of the experimental group were painted bilaterally with a 0.5% DMBA solution three times a week for 15 weeks. Each animal of one of the control groups was similarly treated with only mineral oil. Another control group of 10 animals remained untreated throughout the experiment. Invasive squamous-cell carcinomas with a 100% tumour incidence developed in all of the DMBA-treated buccal pouches. The mineral oil-treated and untreated pouches revealed no obvious changes. Inducible NOS mRNA was demonstrated amongst all the 15-week DMBA-treated hamster buccal-pouch mucosa animals, but not in the untreated animals and not in the animals for which the buccal-pouch was treated with mineral oil. Further study is necessary to evaluate the mechanism(s) which contribute to the increased iNOS mRNA expression for experimentally induced oral carcinogenesis.
Keywords: DMBA-carcinogenesis, hamster, inducible nitric oxide synthase, mRNA, in situ reverse transcription-polymerase chain reaction
Introduction
Nitric oxide (NO), an important mediator in various physiological and pathological activities including inflammatory process and cancer formation, is produced when l-arginine is metabolized to l-citrulline by nitric oxide synthase (NOS; E.C.1.14.13.39). At least three isoenzymes have been identified, i.e. calcium-dependent neuronal and endothelial isoforms that are constitutively expressed, and a calcium-independent (iNOS) isoform that is inducible (Ambs et al. 1998; Thomsen & Miles 1998; Reveneau et al. 1999). Neuronal and endothelial NOS usually produce small amounts of NO that last a short time (minutes), whereas iNOS generates large amounts of NO that last up to several days (Ambs et al. 1998; Thomsen & Miles 1998; Reveneau et al. 1999).
Nitric oxide has been postulated to have play a multifaceted role in cancer (Wink et al. 1998). It has been implicated in a positive role, in permitting tumour growth, including mutagenicity, angiogenesis and metastasis, but has also been involved in the cytotoxicity of macrophages toward tumour cells (Hevel et al. 1991). Additionally, NO reacts with the superoxide anion to form a per oxynitrite anion, a highly toxic molecule causing both DNA damage and protein modifications (Nguyen et al. 1992).
The hamster buccal-pouch mucosa provides one of the most widely accepted experimental models for oral carcinogenesis (Gimenez–Conti & Slaga 1993). Despite anatomical and histological variations between (hamster) pouch mucosa and human buccal tissue, experimental carcinogenesis protocols for the former induce premalignant changes and carcinomas that are similar to the development of premalignancy and malignancy in human oral mucosa (Morris 1961). To date the role of NO in experimental-induced oral carcinogenesis remains to be fully elucidated. The aim of this study was to investigate the mRNA expression of iNOS in 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal-pouch carcinomas.
Materials and methods
Animals
Thirty outbred, young (6-weeks old), male, Syrian golden hamsters (Mesocricatus auratus) (purchased from National Science Council Animal Breeding Centre, ROC), weighing about 100 g each at the commencement of the experiment, were randomly divided into one experimental group (10 animals), and two control groups (10 animals each). The animals were housed under constant conditions (22 °C, 12-h light/dark cycle) and fed with tap water and standard Purina laboratory chow ad libitum. The animal-handling protocol ensured humane practices throughout the experimental process. Subsequent to allowing the animals 1 week of acclimatization to their new surroundings, both pouches of each animal in the experimental group of 10 animals were painted with a 0.5% DMBA solution (Sigma, purity: approx. 95%) was dissolved in mineral oil (Sigma, purity: 100%) at 9 a.m. on Monday, Wednesday and Friday of each week, using a no. 4 sable-hair brush. Both pouches of each animal of one of the control groups of 10 animals were similarly treated with mineral oil (Sigma, purity: 100%). Approximately 0.2 mL of the respective solution was applied topically to the medial walls of both pouches at each painting. Another control group of 10 animals remained untreated throughout the experiment.
At the end of 15 weeks of such treatment (three days following the last treatment), in order to avoid the potential influence of any diurnal variation (Lin & Chen 1997), all of the animals from each group were simultaneously killed at 9 a.m. by inhaling a lethal dose of diethyl ether. The animals' pouches were exposed by dissection, and cut from their oral opening to their caudal ends along the middle of their lateral walls and examined grossly. Both pouches were then excised and fixed in 10% neutral-buffered formalin solution for about 24 h, dehydrated in ascending alcohols, cleared in xylene, and embedded in paraffin for light microscopy. Two serial sections of each specimen were cut at 4-µm-thickness. One of the sections was prepared for IS RT-PCR study while another was used for haematoxylin–eosin staining. In order to test the specificity of the iNOS primers, a portion of selective samples of DMBA-treated pouch tissues was immediately frozen in liquid nitrogen for subsequent RNA extraction and routine RT-PCR reaction.
Preparation of tissue sections
RNase-free conditions were used throughout the slide preparation and the IS RT-PCR procedure. After deparaffinization and dehydration, the section was pretreated with 10 µg/mL proteinase K (Sigma, St. Louis, MO) for 1 min at room temperature at which time enzyme activity was prevented with 0.1 m glycine in PBS.
Reverse transcription and amplification
The Titan one-tube RT-PCR system (Boehringer Mannheim, Indianapolis, IN) was used to perform this IS RT-PCR reaction. The final concentration for the RT-PCR reaction mixture was as follows: 200 µm each of deoxyribonucleotide (dATP, dCTP, dGTP), 180 µm dTTP, 40 µm Dig-11-DTP, 0.4 µm downstream primer, 0.4 µm upstream primer, 5 mm dithiothreitol solution (DTT), 1.5 mm MgCl2 and 1 µL Expanol high-fidelity enzyme mix (all of the reagents were obtained from Boehringer Mannheim, with the exception of primers). The oligonucleotide primers specific for iNOS were purchased from Genset Corp. (La Jolla, CA) (Table 1). The primer pairs were chosen from the published cDNA sequences of iNOS (GenBank accession no. D14051). The specificity of the primers has been tested with selective fresh tissues of the DMBA-induced hamster buccal-pouch carcinomas using the standard procedures for routine RT-PCR. Hybaid Sure-Seal (Hybaid Instruments, Holbrook, NY) was placed around the sample on the slides. The reaction mixture was then carefully pipetted onto each tissue section. After coverslips were applied, slides were placed on the block of a thermal cycler (TaKaRa MP, Tokyo, Japan). Reverse transcription was carried out at 50 °C for 30 min. PCR amplification was carried out with an initial denaturing step at 94 °C for 2 min and then 20 cycles of amplification with denaturing at 94 °C for 30 s; annealing at 55 °C for 30 s and elongation at 68 °C for 2 min; and a final extension of 68 °C for 7 min.
Table 1.
Oligonucleotide primers used to amplify iNOS cDNAs
| Oligonucleotide primers | cDNA positions | Sequences | PCR products |
|---|---|---|---|
| iNOS sense | 1425–1441 | 5′-GCC TCG CTC TGG AAA GA-3′ | 499bp |
| iNOS antisense | 1908–1924 | 5′-TCC ATG CAG ACA ACC TT-3′ |
Immunohistochemical detection of digoxigenin
We used the DIG nucleic acid detection kit (Boehringer Mannheim) to detect the digoxigenin tagged IS RT-PCR amplified products. The slides, after rinsing with PBS, were incubated in anti-digoxigenin antibody conjugated with alkaline phosphatase diluted at 1: 500 for 2 h. A subsequent enzyme-catalysed colour reaction solution with 5-bromo-4-chloryl-3-indolyl phosphate and nitrobluetetrazolium salt was applied on the slides to produce an insoluble purple coloration. Colour development, checked under the microscope, was terminated by washing the slides with Tris buffer containing EDTA within approximately 10 min or as soon as the purple colour was observed in any of the slides. Slides were then counterstained with nuclear Fast Red, and cover-slipped with a water-soluble mounting medium. The positivity of iNOS mRNA of each section was observed using a light microscope.
Results
Gross observations and histopathology
Gross and histopathological changes in the DMBAtreated pouches were similar to those described in our previous study (Chen & Lin 1996). Invasive squamous cell carcinomas with a 100% tumour incidence developed in all of the DMBA-treated pouches. The mineral oil-treated and untreated pouches revealed no obvious changes.
In situ reverse transcription-polymerase chain reaction
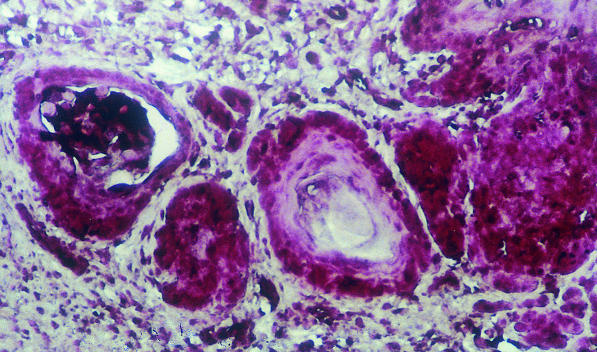
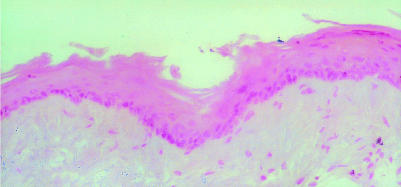
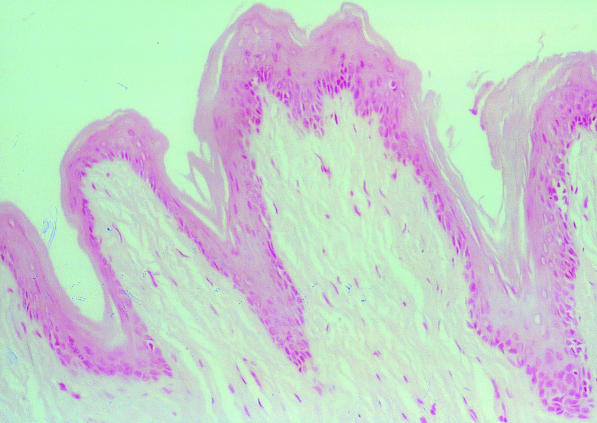
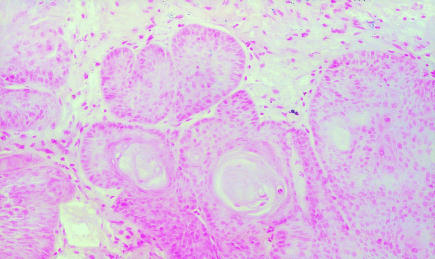
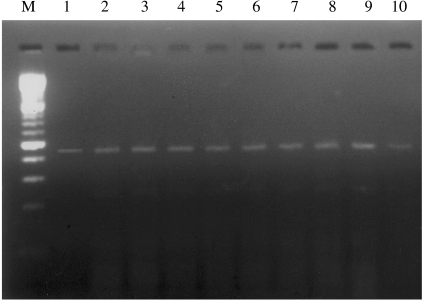
The iNOS mRNA was detected in all of the samples of hamster buccal-pouch tissue specimens treated with DMBA for 15 weeks (Figure 1). The iNOS mRNA staining was also present in cells of the tumour stroma, presumed to be macrophages (Figure 1). Consistent with our previous study using immunohistochemistry (Chen & Lin 2000), a purple staining for the detection of iNOS mRNA using IS RT-PCR was noted in comparable sites (cytoplasm and nuclei of the tumour islands). Inducible NOS mRNA activity could not be found in the untreated (Figure 2) and the mineral oil-treated (Figure 3) pouches. Omission of the primers in control sections showed negative findings for iNOS mRNA activity in all specimens (Figure 4). A band corresponding to 499-bp was observed for all the selected samples of hamster buccal-pouch tissue specimens treated with DMBA for 15 weeks (Figure 5).
Figure 1.
Inducible NOS mRNA as shown by the purple coloration was identified in the invasive tumour islands of a representative sample of hamster buccal-pouch tissue specimen treated with DMBA for 15 weeks. Note that iNOS mRNA staining was also present in cells of the tumour stroma, presumed to be macrophages (×100).
Figure 2.
Inducible NOS mRNA activity could not be found in a representative sample of non-treated pouch tissue specimen (×100).
Figure 3.
Inducible NOS mRNA activity could not be detected in a representative sample of mineral oil-treated pouch tissue specimen (×100).
Figure 4.
Omission of the primers in control sections showed negative finding for iNOS mRNA activity (×100).
Figure 5.
A band corresponding to 499 bp was observed for all the selected samples of hamster buccal-pouch tissue specimens treated with DMBA for 15 weeks (lanes 1–10). Lane M is the DNA molecular-weight marker.
Discussion
Using immunohistochemical techniques, we have previously demonstrated an enhanced expression of iNOS at the protein level in chemically induced oral carcinomas in hamster buccal-pouch mucosa (Chen & Lin 2000). However, the corresponding expression of iNOS at the mRNA level using IS RT-PCR has not, to the best of our knowledge, been reported previously for hamster buccal-pouch carcinomas. Using the same experimental model system, we now report the presence of iNOS mRNA in DMBA-induced hamster buccal-pouch carcinomas with IS RT-PCR. Therefore, it appears reasonable to suggest that the escalation in activity of iNOS mRNA may be closely related to chemically induced oral carcinogenesis.
Compared with the demonstration of iNOS mRNA by RT-PCR, this method (IS RT-PCR) provides important additional information about the cellular localization of mRNA. Furthermore, the IS RT-PCR technique can use archival tissues and is not constrained by methods of tissue fixation. Compared to immunohistochemical approaches, the preservation of tissue morphology was less than ideal, but the general outlines of tissue architecture, especially the epithelial vs. stromal boundaries, were clearly distinguishable.
This study shows that iNOS mRNA is over-expressed in the hamster buccal-pouch carcinomas that develop from DMBA treatment. It does not demonstrate whether or not over-expression of iNOS mRNA in the early stages of DMBA treatment is a risk factor for the development of carcinomas. Further study on the sequential expression of iNOS mRNA during DMBA-induced hamster buccal-pouch carcinogenesis is required to understand a role (e.g. in initiation or promotion) of iNOS on oral carcinogenesis.
The exposure of normal human hepatocytes to a high level of NO could initiate a series of events that rapidly leads to damage of both nuclear and mitochondrial DNA, such damage subsequently leading to cell-cycle arrest and mitochondrial dysfunction, and ultimately resulting in the pathological events associated with the development of hepatocellular carcinoma (D'Ambrosio et al. 2001).
Recently, iNOS expression has been correlated with p53 for human oral epithelial dysplasia (Brennan et al. 2000a) and oral squamous-cell carcinoma (Brennan et al. 2000b). Thus it appears worthwhile to attempt to verify whether or not a similar correlation between NOS and p53 exist in cases of DMBA-induced hamster buccal-pouch carcinogenesis. Subsequent experiments focusing upon the interaction between iNOS and p53 in DMBA-induced hamster buccal-pouch carcinogenesis may provide a new insight to the mechanism of chemically induced carcinogenesis in the oral cavity.
Inducible NOS protein has been suggested as an immunohistochemical marker for malignant neoplasia in prostate tissue (Klotz et al. 1998). Assessed at the mRNA level in the present study, we have demonstrated that iNOS may also be a potential marker at the mRNA level for DMBA-induced hamster buccal-pouch carcinomas. In addition, high expression of iNOS mRNA in DMBA-induced hamster buccal-pouch carcinomas as shown in this study may suggest a possible therapeutic approach through the inhibition of tumour growth by a novel inhibitor of iNOS such as N-(3-(aminomethyl)benzyl)acetmidine (1400 W) (Thomsen et al. 1997).
In conclusion, enhanced expression of iNOS mRNA in DMBA-induced hamster buccal-pouch carcinomas compared with the untreated and mineral oil-treated counterparts in the current study, is consistent with our previous work at the protein level using immunohistochemical techniques (Chen & Lin 2000), suggesting that iNOS gene expression may be regulated at the level of mRNA. However, further work remains to be done to unravel the molecular mechanism(s) that modulate the increased iNOS mRNA expression in experimentally induced oral carcinogenesis.
Acknowledgments
We wish to acknowledge the technical assistance of L.L. Chang A.P. (Department of Microbiology, Kaohsiung Medical University), and Ms N.Y. Dai. This research was supported by a grant from the National Science Council, ROC (N.S.C. 89-2314-B-037-046).
References
- Ambs S, Merriam WG, Bennett WP, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- Brennan PA, Conroy B, Sinclair D, Spedding AV. Expression of inducible nitric oxide synthase and p53 in oral epithelial dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000b;90:624–629. doi: 10.1067/moe.2000.108800. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Palacios-Callender M, Sinclair D, Spedding AV, Zaki GA. Correlation between type II nitric oxide synthase and p53 expression in oral squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 2000a;38:627–632. doi: 10.1054/bjom.2000.0540. [DOI] [PubMed] [Google Scholar]
- Chen YK, Lin LM. Sequential expression of placental glutathione S-transferase isoenzyme (GST-P) during DMBA-induced hamster buccal pouch squamous cell carcinogenesis. J. Oral Pathol. Med. 1996;25:388–394. doi: 10.1111/j.1600-0714.1996.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Chen YK, Lin LM. Immunohistochemical expression of inducible nitric oxide synthase in DMBA-induced hamster buccal pouch carcinogenesis. Oral Oncol. 2000;36:221–224. doi: 10.1016/s1368-8375(99)00081-0. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio SM, Gibson-D'Ambrosio RT, Brady T, Oberyszyn AS, Robertson FM. Mechanisms of nitric oxide-induced cytotoxicity in normal human hepatocytes. Environ. Mol. Mutagen. 2001;37:46–54. doi: 10.1002/1098-2280(2001)37:1<46::aid-em1005>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gimenez-Conti IB, Slaga TJ. The hamster cheek pouch carcinogenesis model. J. Cell. Biochem. 1993;17F(Suppl):83–90. doi: 10.1002/jcb.240531012. [DOI] [PubMed] [Google Scholar]
- Hevel JM, White KA, Marletta MA. Purification of the inducible murine macrophage nitric oxide synthase. J. Biol. Chem. 1991;264:22789–22791. [PubMed] [Google Scholar]
- Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–1903. [PubMed] [Google Scholar]
- Lin LM, Chen YK. Diurnal variation of γ-glutamyl transpeptidase activity during DMBA-induced hamster buccal pouch carcinogenesis. Oral Dis. 1997;3:153–156. doi: 10.1111/j.1601-0825.1997.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Morris AL. Factors influencing experimental carcinogenesis in the hamster cheek pouch. J. Dent. Res. 1961;40:3–15. doi: 10.1177/00220345610400012001. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc. Nat. Acad. Sci. USA. 1992;89:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveneau S, Arnould L, Jolimoy G, et al. Nitric oxide synthase in human breast cancer is associated with tumor grade, proliferation rate, and expression of progesterone receptors. Laboratory Invest. 1999;79:1215–1225. [PubMed] [Google Scholar]
- Thomsen LL, Miles D. Roles of nitric oxide in tumor progression: lesions from human tumors. Cancer Metastasis Rev. 1998;17:107–118. doi: 10.1023/a:1005912906436. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Scott JM, Topley P, Knowles RG, Keerie AJ, Frend AF. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. [PubMed] [Google Scholar]
- Wink DA, Vodovotz Y, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]