Abstract
The female reproductive organs (ovary, uterus, and placenta) are some of the few adult tissues that exhibit regular intervals of rapid growth. They also are highly vascular and have high rates of blood flow. Angiogenesis, or vascular growth, is therefore an important component of the growth and function of these tissues. As with many other tissues, vascular endothelial growth factors (VEGFs) and fibroblast growth factors (FGFs) appear to be major angiogenic factors in the female reproductive organs. A variety of pathologies of the female reproductive organs are associated with disturbances of the angiogenic process, including dysfunctional uterine bleeding, endometrial hyperplasia and carcinoma, endometriosis, failed implantation and subnormal foetal growth, myometrial fibroids (uterine leiomyomas) and adenomyosis, ovarian hyperstimulation syndrome, ovarian carcinoma, and polycystic ovary syndrome. These pathologies are also associated with altered expression of VEGFs and/or FGFs. In the near future, angiogenic or antiangiogenic compounds may prove to be effective therapeutic agents for treating these pathologies. In addition, monitoring of angiogenesis or angiogenic factor expression may provide a means of assessing the efficacy of these therapies.
In the adult, angiogenesis occurs infrequently. Exceptions are found in the female reproductive system … Normal angiogenesis also occurs as part of the body's repair process, such as in the healing of wounds and fractures. On the other hand, uncontrolled angiogenesis contributes to a wide variety of serious diseases.
As recognized by D'Amore and Klagsbrun, capillary growth, or angiogenesis, in normal adult tissues is indeed an infrequent event, and the endothelium of most tissues represents an extremely stable population of cells with a low mitotic rate (Denekamp 1984; Hudlicka 1984). This observation is not surprising, because in adults physiological angiogenesis is normally associated only with tissue growth or repair, and most adult tissues are relatively stable (Hudlicka 1984; D'Amore & Klagsbrun 1989).
On the other hand, rampant or persistent capillary growth is associated with numerous pathological conditions, including tumour growth, retinopathies, haemangiomas, fibroses and rheumatoid arthritis (Folkman & Klagsbrun 1987; D'Amore & Klagsbrun 1989). Folkman and coworkers were the first to demonstrate that recruitment of a blood supply is requisite for sustained growth of tumours (Folkman & Klagsbrun 1987) and vascular endothelial cells of growing tumours exhibit an extremely high mitotic rate compared with endothelial cells of most normal tissues (Denekamp 1984). Conversely, insufficient capillary growth occurs in several disease states, including delayed wound healing, non-healing fractures and chronic varicose ulcers (Folkman & Klagsbrun 1987).
In contrast, the tissues of the adult female reproductive system, including the ovary, uterus, and placenta, grow extremely rapidly and at regular intervals (Redmer & Reynolds 1996; Reynolds et al. 2000; Reynolds & Redmer 2001). For example, during its growth phase, which lasts about 8–10 days in large mammals (including ruminants and primates) the ovarian corpus luteum, which is the primary source of the pro-gestational hormone progesterone, doubles in size and cell numbers every 60–70 h (Reynolds et al. 1994). This phenomenal growth rate is associated with an extremely high rate of cell proliferation, and is equaled only by the fastest growing tumours (Reynolds et al. 1994). Unlike that of tumours, however, growth of the female reproductive tissues is normally a self-limiting and highly ordered process (Reynolds et al. 1992, 1994).
To support this phenomenal rate of tissue growth, microvascular growth and development also are extremely rapid in the female reproductive organs, and these tissues are highly vascular when mature (Reynolds et al. 1992; Redmer & Reynolds 1996; Reynolds et al. 2000; Reynolds & Redmer 2001). For example, most of the cell proliferation (approximately 50–85%) that occurs during the extremely rapid growth of the corpus luteum is in the microvascular compartment (Reynolds et al. 1994, 2000; Christenson & Stouffer 1996; Redmer et al. 2001). As a result, in the mature corpus luteum microvascular pericytes and endothelial cells comprise about 40–70% of the total cell population (Reynolds et al. 2000; Redmer et al. 2001). In association with their high vascularity, tissues of the female reproductive organs also receive some of the greatest rates of blood flow, per unit of tissue, of any adult organ and exhibit a high metabolic rate (Reynolds 1986; Adair et al. 1990; Reynolds et al. 1990; Reynolds & Redmer 1995; Redmer & Reynolds 1996).
Due to the regular intervals of dramatic tissue growth and angiogenesis, several investigators have proposed that the tissues of the female reproductive organs can serve as a model to study not only reproductive function but also tissue growth and angiogenesis in general (Augustin 2000; Plendl 2000; Reynolds et al. 2000; Reynolds & Redmer 2001). If we can understand regulation of the angiogenic process in these tissues, we should gain a better understanding of angiogenesis that occurs during normal tissue growth as well as abnormalities of the angiogenic process that occur in various pathological processes.
Importance of angiogenesis in pathological conditions of the female reproductive organs
In the female reproductive system, pathologies that are associated with disturbances of the angiogenic process include dysfunctional uterine bleeding, endometrial hyperplasia and carcinoma, endometriosis, failed implantation and subnormal foetal growth, myometrial fibroids (uterine leiomyomas) and adenomyosis, ovarian hyperstimulation syndrome, ovarian carcinoma, and polycystic ovary syndrome (Abulafia & Sherer 2000; Fraser & Lunn 2000; Jaffe 2000; Fraser & Lunn 2001; Hickey & Fraser 2001; Kohn & Libutti 2001; Mueller & Taylor 2001; Reynolds & Redmer 2001).
These pathologies of the female reproductive organs represent major socioeconomic problems. For example, ovarian carcinoma often shows a poor prognosis and low survival rate and therefore is recognized as one of the most dangerous cancers in female patients (Kohn 1997; Schiffenbauer et al. 1997; Abulafia & Sherer 2000). Ovarian, uterine, and cervical cancers represent approximately 13% of new cases of cancer and 10% of cancer deaths in the USA, making them the 4th leading cause of deaths due to cancer among women (Table 1).
Table 1.
Cancer incidence and deaths, by rank, for females in the US 2000 projected*
| Incidence | Deaths |
|---|---|
| Breast (31%)† | Respiratory (25%) |
| Digestive (18%) | Digestive (23%) |
| Respiratory (13%) | Breast (15%) |
| Genital and Genital Tract (13%) | Genital and Genital Tract (10%) |
| Urinary (5%) | Lymphoma (5%) |
| Lymphoma (4%) | Leukaemia (4%) |
| Leukaemia (2%) | Urinary (3%) |
| All others (14%) | All others (15%) |
Total projected for all cancer cases in females: incidence 600400 and deaths 268100 (Amer. Cancer Soc.<http://www.cancer.org>). These projections are based on data from 1979 to 1996 from the National Cancer Institute's SEER (Surveillance, Epidemiology, and End Results) program (<http://seer.cancer.gov/Publications/#statpubs>).
Genital and genital tract includes primarily uterus, cervix, and ovary (46, 16, and 30%, respectively); digestive primarily colon and rectum (>50%); respiratory primarily lung and bronchus (>88%); urinary primarily bladder (>50%) and kidney/renal pelvis (>30%); lymphoma primarily non-Hodgkin's (>87%); All Others includes: Skin (primarily melanoma), endocrine system, eye and orbit, brain and nervous system, oral cavity and pharynx, bones and joints, multiple myeloma, soft tissues incluyding heart, and unspecified.
As another example, approximately 30% of fertilized ova in most mammalian species and perhaps over 50% of fertilized ova in humans do not result in a successful pregnancy but rather die as early embryos (Edey 1969; Short 1984). Early pregnancy is a critical period of gestation because of the major developmental events that take place, including embryonic organogenesis as well as formation of the placenta, a process known as placentation (Reynolds & Redmer 1995, 2001). Placentation includes extensive placental angiogenesis, which is accompanied by a marked increase in uterine blood flow (Reynolds & Redmer 1995, 2001). It has been suggested that insufficient vascularization of the placenta is a major contributor to embryonic death during early pregnancy, and indeed reduced placental vascular development and increased vascular resistance have been associated with early embryonic mortality (Reynolds & Redmer 1995, 2001).
Additionally, it has been shown that the large increase in transplacental exchange of nutrients, respiratory gases, and wastes that occurs during the last half of gestation, and which supports the exponential increase in foetal growth, depends primarily on the dramatic growth of the placental vascular beds and the resultant large increases in uterine and umbilical blood flows (Reynolds & Redmer 1995, 2001). Factors that affect foetal growth, such as maternal genotype, increased numbers of foetuses, maternal undernutrition, maternal age, parity, or environmental heat or cold stress, typically have similar effects on placental size, and also are associated with reduced rates of foetal oxygen and nutrient uptakes, as well as reduced placental angiogenesis and blood flow (Reynolds & Redmer 1995, 2001). In fact, increased uterine vascular resistance and reduced uterine blood flow can be used as predictors of high-risk pregnancies and are associated with foetal growth retardation (Trudinger et al. 1985; North et al. 1994). Thus, factors that influence placental vascular development will have a dramatic impact on foetal growth and development and subsequent neonatal survival and growth (Reynolds & Redmer 1995, 2001).
In the last two decades, the importance of vascular growth in normal and abnormal function of organs, including those of the female reproductive system, has become increasingly recognized. For example, only 14 articles per year were published on angiogenesis in female reproductive organs during the 6-year interval from 1985 to 1990, whereas during 1996–2001 more than 100 articles per year were published (WinSPIRS 2001). Because the total number of articles concerning the female reproductive organs was similar during these same intervals (approximately 58 000 vs. 50 000), articles on angiogenesis represented 1.05% compared with only 0.17% of all articles published on the ovary, uterus, or placenta in 1996–2001 vs. 1985–90 (WinSPIRS 2001). This dramatic, seven-fold increase in the number of scientific articles published on angiogenesis in the female reproductive organs is similar in magnitude to the increase in the total number of articles on angiogenesis.
In this review, we hope to provide not only an overview of angiogenesis research in the female reproductive organs but also some indication of the role of vascular growth in the genesis and treatment of diseases of these organs. To accomplish this purpose, we first will review the current state of knowledge concerning angiogenesis and its regulation in female reproductive organs and then will discuss the implications of this research for reproductive pathologies. As Claude Bernard, considered by many to be the first modern biomedical researcher, stated in his classic text Introduction to the Study of Experimental Medicine (Bernard 1865, ‘… knowledge of pathological or abnormal conditions cannot be gained without previous knowledge of normal states …’.
Angiogenesis and its regulation in normal and pathological conditions of the female reproductive organs
Ovary
During the first half of the twentieth century, numerous investigators noticed the high degree of vascularity of the ovarian tissues, and concluded that angiogenesis must be a critical component of follicular and luteal function (Redmer & Reynolds 1996). More recent work has supported the concept that maintenance of the follicular vasculature is important for maintaining follicular health. For example, the thecal layer (which is the stromal compartment of the ovarian follicle and surrounds the granulosa [epithelial] layer and the oocyte) of healthy, preovulatory follicles of sheep and monkeys is not only more vascular but also exhibits increased uptake of serum gonadotropins compared with atretic (regressing) follicles (Redmer & Reynolds 1996 and Reynolds et al. 2000).
Moor and coworkers observed that early atretic follicles of sheep regenerate when placed in vitro and supplied with gonadotropins (Moor & Seamark 1986). These investigators thus concluded that, in vivo, atresia was caused by decreased vascularity, which resulted in limited access of the follicles to nutrients, substrates, and tropic hormones. In addition, Greenwald (1989) found that one of the earliest signs of follicular atresia in hamsters was reduced DNA synthesis of thecal endothelial cells, which was associated with reduced follicular vascularity. Similarly, we have observed a cessation in proliferation of thecal endothelial cells, associated with a decrease in thecal vascularity, soon after the onset of atresia in bovine, ovine and porcine follicles (Reynolds et al. 2000).
Thus, increased thecal vascularity may be a primary determinant of follicular health and, conversely, reduced thecal vascularity appears to be an important component of follicular atresia. In support of these concepts, although thecal tissues of all antral follicles in sheep and cows produce angiogenic activity, the granulosa cells produce angiogenic factors only in healthy, mature (preovulatory) but not in atretic follicles (Taraska et al. 1989; Redmer et al. 1991). Based on these observations, we concluded that production of angiogenic factors by the avascular granulosa cells probably contributes to maintaining follicular vascularity and health (Taraska et al. 1989; Redmer et al. 1991).
Microvascular development of the follicular wall becomes even more extensive after ovulation, in association with vascularization of the corpus luteum, which as mentioned previously is the primary source of the pro-gestational hormone progesterone (Redmer & Reynolds 1996; Reynolds & Redmer 1998). The corpus luteum becomes so vascular that the majority of the parenchymal (steroidogenic) cells of the mature corpus luteum are in contact with one or more capillaries (Dharmarajan et al. 1985; Redmer et al. 2001). In addition, the mature corpus luteum also receives most of the ovarian blood supply, and ovarian blood flow is highly correlated with the rate of progesterone secretion (Reynolds 1986; Niswender & Nett 1988; Reynolds et al. 1994).
Conversely, inadequate or abnormal luteal function, which can occur in animals during various physiological states, including puberty, resumption of ovarian cyclicity postpartum, the beginning of the breeding season, and after induced ovulation, has been suggested to result from inadequate luteal vascularization (Redmer & Reynolds 1996 and Reynolds et al. 2000). Similarly, inadequate luteal function in humans, which occurs relatively frequently and is termed luteal phase defect or deficiency, is associated with reduced vascularity of the corpus luteum (Reynolds et al. 2000).
The corpus luteum is one of the most angiogenic tissues known, and stimulates angiogenesis in a variety of in vitro and in vivo assays (Fig. 1; Redmer et al. 1985, 1988; Reynolds et al. 2000). As for other tissues (Ballara et al. 1999), a bewildering array of potential angiogenic growth factors and their receptors are present in the corpus luteum, including angiopoietins, epidermal growth factor, fibroblast growth factors, insulin-like growth factors, nerve growth factor, transforming growth factors, tumour necrosis factors, and vascular endothelial growth factors (Reynolds et al. 1994; Redmer & Reynolds 1996; Goede et al. 1998; Neufeld et al. 1999; Grazul-Bilska et al. 2001). However, based on numerous studies, it has been suggested that the major ovarian angiogenic factors belong to the fibroblast growth factor (FGF) or the vascular endothelial growth factor (VEGF) families of proteins (Redmer & Reynolds 1996; Augustin 2000; Plendl 2000; Grazul-Bilska et al. 2001; Reynolds & Redmer 2001). This suggestion is consistent with the recent observation that the FGFs and VEGFs are probably key mediators of the angiogenic process in a variety of tissues (D'Amore & Klagsbrun 1989; Ferrara & Davis-Smyth 1997; Neufeld et al. 1999).
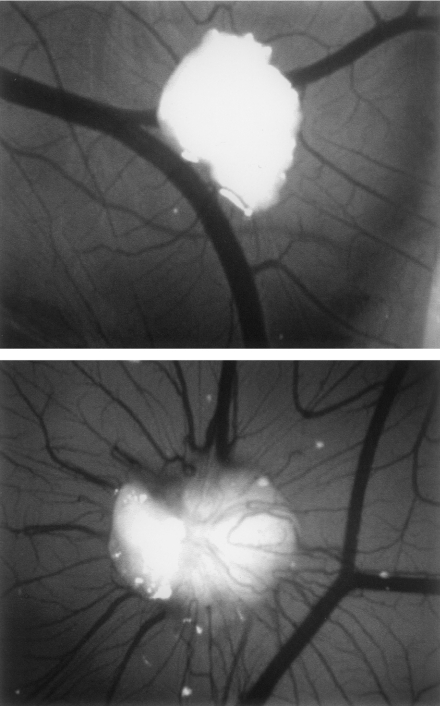
Figure 1.
Vascular response of the chicken chorioallantoic membrane (CAM) to bovine foetal muscle (top micrograph) and corpus luteum (bottom micrograph) tissue implants. Note that luteal tissue stimulates the classic spoke-wheel pattern of vascular invasion, indicating production of angiogenic activity, whereas the foetal muscle does not (reproduced with permission from Redmer et al. 1988).
For example, we and others have shown in several studies that FGF-1 and FGF-2 proteins are present in bovine and ovine corpora lutea (Reynolds et al. 2000). We also have shown that these FGFs stimulate proliferation of ovine luteal cells from several stages of the oestrous cycle (Grazul-Bilska et al. 1995). Stirling et al. (1991) have shown that the pattern of expression of FGF-2 mRNA by bovine corpora lutea closely follows that of angiogenic activity production. In addition, both luteal angiogenic activity and FGF-2 mRNA levels are stimulated by luteinizing hormone (LH), which is the major luteotropic hormone in most mammals (Redmer et al. 1987, 1988; Stirling et al. 1991). Moreover, antibodies against FGF-2 will immunoneutralize about 80% of the angiogenic activity produced by the cow, pig, and sheep corpora lutea (Reynolds et al. 2000).
We also have suggested the FGFs are probably involved not only in luteal angiogenesis, which occurs primarily early in the oestrous cycle, but also in other aspects of luteal function as well. For example, we and others have shown that FGF affects luteal progesterone production (Reynolds et al. 2000; Grazul-Bilska et al. 2001). In addition, the FGFs have been shown to inhibit cell death in several cell types (Redmer & Reynolds 1996; Reynolds & Redmer 1998). Interestingly, many of the larger luteal microvessels are maintained during luteal regression, and we observed an increase in FGF receptors in these vessels, which could explain how they selectively avoid cell death while the remaining luteal tissue is resorbed (Redmer & Reynolds 1996; Doraiswamy et al. 1998; Reynolds & Redmer 1998). We have therefore suggested that FGF may affect not only luteal cell proliferation but also luteal cell function and vascular maintenance (Redmer & Reynolds 1996; Reynolds et al. 2000).
Luteal levels of VEGF mRNA are greatest early in the estrous cycle when the majority of luteal growth and vascularization is occurring (Redmer et al. 1996; Reynolds et al. 2000; Redmer et al. 2001). In addition, an antibody against VEGF will neutralize approximately 65% of the endothelial chemotactic activity produced by ovine corpora lutea (Reynolds et al. 2000). Moreover, in monkey corpus luteum VEGF mRNA is present in greatest amounts in the early luteal phase and is reduced when serum gonadotropin levels are decreased experimentally (Ravindranath et al. 1992). Consistent with these studies, expression of VEGF protein is greatest during early luteal development and least late in the estrous cycle, during luteal regression (Redmer et al. 2001). In addition, VEGF protein is present in luteal connective tissue cells, luteal vascular smooth muscle, and, most interestingly, luteal capillary pericytes.
Several recent studies have shown that the thecal-derived microvasculature is responsible for vascularizing the developing corpus luteum, and that both capillary endothelial cells and capillary pericytes are involved in this process (Goede et al. 1998; Amselgruber et al. 1999; Redmer et al. 2001). These observations are especially interesting because in the last decade numerous studies have indicated that the primary role of perivascular cells, including vascular smooth muscle cells and capillary pericytes, is to regulate endothelial cell function and angiogenesis (Reynolds & Redmer 1999; Reynolds et al. 2000; Redmer et al. 2001). In addition, vascular smooth muscle cells and pericytes have been shown to express VEGF and to increase their VEGF expression during hypoxia (Reynolds et al. 2000; Redmer et al. 2001).
Redmer et al. (2001) found that the VEGF-expressing thecal pericytes invade the granulosa layer within hours after ovulation, before or coincident with the thecal endothelial cells. In addition, capillary pericytes exhibit a high rate of cell proliferation in the early corpus luteum. Interestingly, under hypoxic conditions VEGF itself has been shown to be mitogenic for pericytes (Yamagishi et al. 1999). These observations, taken together with studies showing that VEGF is critical for normal vascular development, are consistent with the hypothesis that thecal-derived perivascular cells may direct vascularization of the developing corpus luteum via production of VEGF (Reynolds et al. 2000; Redmer et al. 2001).
We also have recently reported that VEGF mRNA expression in cultured ovine luteal cells is increased by about 30% with luteinizing hormone (LH) treatment, and by 300% under low oxygen (O2) conditions (Toutges et al. 1999). These studies were based partly on the observation that gonadotropin treatment induces VEGF mRNA expression in preovulatory rat follicles and in cultured bovine luteal cells (Garrido et al. 1993; Koos 1995). If VEGF is the major luteal angiogenic factor, its regulation by LH would make sense because LH is an important luteotropic factor and is critical for normal luteal development and function (Niswender & Nett 1988).
Additionally, O2 is a potent stimulator of VEGF expression across a number of cell and tissue types, which is consistent with the concept that metabolic demand is the primary factor regulating vascular development in all tissues (Adair et al. 1990; Neufeld et al. 1999). Because the developing corpus luteum resembles a healing wound, and thus would be expected to be hypoxic until the luteal parenchymal lobules become well vascularized (Silvester & Luck 1999), it also is logical to expect that O2 levels would be major regulators of luteal VEGF expression, and our data support this contention (Toutges et al. 1999).
Recent work also has shown that nitric oxide (NO), which is primarily an endothelial product and an important local vasodilator, can stimulate VEGF production and angiogenesis (Reynolds et al. 2000; Redmer et al. 2001). Similarly VEGF, which as mentioned previously is present in luteal perivascular cells, can stimulate endothelial nitric oxide synthase (eNOS) expression and thus NO production (Hood et al. 1998; Zheng et al. 1999). We have therefore proposed the existence of a paracrine loop, whereby luteal endothelial cells release NO, which stimulates perivascular VEGF production, which in turn stimulates endothelial expression of eNOS. This paracrine loop would thereby serve as a feed-forward system to maximize vasodilation and angiogenesis during luteal development.
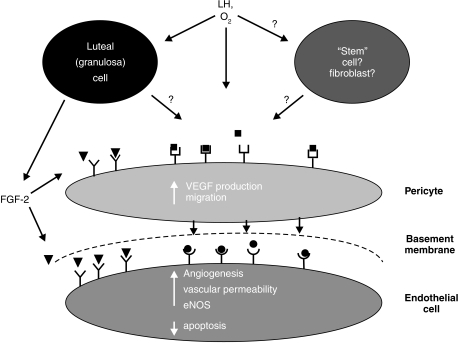
In support of this proposal, we recently have found that eNOS is expressed in endothelial cells of luteal arterioles and capillaries early in the estrous cycle but its expression is greatly reduced by mid-cycle (Reynolds et al. 2000). Anthony et al. (1997) have found that NOS is expressed in the human corpus luteum. We also have shown that luteal eNOS expression is often associated with expression of VEGF by perivascular cells (capillary pericytes and arteriolar smooth muscle; D.A. Arnold, D.A. Redmer, and L.P. Reynolds, unpublished observations). These observations led us to propose a new model for luteal vascularization (Fig. 2; Reynolds et al. 2000).
Figure 2.
Model of luteal vascularization. Basic fibroblast growth factor (FGF-2) and vascular endothelial growth factor (VEGF) seem to be the major luteal angiogenic growth factors. Granulosa cells and the luteal cells derived from them are capable of producing FGF-2 (black triangles), which may regulate endothelial cell survival (via inhibition of cell death) as well as migration of pericytes (based on unpublished data). VEGF derived from the pericytes (black circles) targets endothelial cells to stimulate not only luteal angiogenesis but also luteal vascular permeability and blood flow (via increased nitric oxide synthase [NOS] production). Likewise, NO produced by the luteal endothelial cells can act in a paracrine loop to stimulate relaxation of arteriolar smooth muscle, and thus vasodilation, as well as increased VEGF production by capillary pericytes. Although LH stimulates production of angiogenic factors by luteal tissues, whether this effect involves only pericytes or is partially mediated by luteal parenchymal or other luteal cells is unknown. Oxygen tension seems to be a major regulator of luteal VEGF production; again, whether this effect is directly on perivascular cells or is mediated by luteal parenchymal or other cells is not known. (Reproduced with permission from Reynolds et al. (2000)).
Uterus/placenta
As previously alluded to, the placenta's primary role is to support foetal growth and development by providing for physiological exchange between the foetal and maternal systems (Reynolds & Redmer 1995, 2001). The importance of the placental circulation to placental function has long been recognized and is exemplified by the close relationship between foetal weight, placental size, and uterine and umbilical blood flows during normal pregnancies across mammalian species (for review, see Reynolds & Redmer 1995, 2001). Uterine and umbilical blood flows, which represent the circulation to the maternal and foetal portions of the placenta, respectively, increase exponentially throughout gestation, and thus keep pace with foetal growth (Reynolds & Redmer 1995; Magness 1998).
Numerous studies have indicated that angiogenesis is indeed a major component of the increase in placental blood flow throughout gestation. For example, by day 24 after mating, vascular density of the uterine mucosa, or endometrium, exhibits a two-fold increase (Reynolds & Redmer 1992). Vascular density of endometrial (maternal placental) tissues continues to increase gradually throughout gestation (Reynolds & Redmer 2001). In contrast, vascular density of the foetal placental tissues, or cotyledons, remains relatively constant until midgestation then increases dramatically thereafter. These patterns of placental angiogenesis coincide with the dramatic increases that have been reported for uterine and umbilical blood flows throughout gestation (Reynolds & Redmer 1995, 2001).
As discussed above for the ovary, recent studies have suggested that VEGF and FGF are major angiogenic growth factors of the placenta, which is not surprising because these two families of proteins account for the majority of the angiogenic activity produced by both ovarian (Reynolds & Redmer 1998; Reynolds et al. 2000) and placental (Reynolds & Redmer 2001) tissues. In addition, expression of VEGF is correlated with uterine angiogenesis during the menstrual cycle in humans (Gargett & Rogers 2001). Moreover, VEGF and VEGFR mRNA and protein are present in foetal and placental tissues throughout gestation and their expression coincides with the angiogenesis that occurs during development of placental tissues in late pregnancy (Hildebrandt et al. 2001; Reynolds & Redmer 2001).
Recently, gene knockout studies have provided convincing evidence for a central role of VEGF in foetal and placental angiogenesis. In mice, homozygous knockouts of the genes for VEGFR-1 (also known as flt-1) or VEGFR-2 (KDR or flk-1) led to defects in foetal and placental angiogenesis resulting in embryonic death by about day 8 of pregnancy (length of pregnancy approximately 20 days; Fong et al. 1995; Shalaby et al. 1995). Similarly, homozygous gene knockouts for VEGF were lethal by about day 11 of pregnancy, and these embryos exhibited dramatic cardiovascular defects, such as delayed or abnormal development of the heart, aorta, major vessels, and extraembryonic vasculature, including the yolk sac and placenta (Carmeliet et al. 1996; Ferrara et al. 1996). Surprisingly, heterozygous VEGF-gene knockout embryos, which still expressed VEGF but at reduced levels, exhibited similar defects in foetal and placental angiogenesis, and also died by about day 11–12 of gestation (Carmeliet et al. 1996; Ferrara et al. 1996). These authors therefore concluded that not only was foetal and placental angiogenesis dependent on VEGF, but that threshold levels of VEGF must be achieved for normal vascular development to occur.
The FGFs are especially interesting because they are unique among the major angiogenic growth factors in that they are pleiotropic and influence not only angiogenesis but also various other developmental and differentiated functions (Gospodarowicz 1991). We already have mentioned FGF-2′s potential role in regulating follicular and luteal cell growth, cell survival, and steroidogenesis. In addition, the FGFs have been shown to stimulate differentiation of the embryonic germ layers, especially embryonic mesoderm (Slack et al. 1987; Klein & Melton 1994). FGF-2-gene knockout mice are fertile and display only mild defects associated with brain development, blood pressure regulation, and wound healing (Ortega et al. 1998; Miller et al. 2000). However, mice defective in FGFR-2 die in utero by about day 4.5 of gestation and the lethal defect is associated with abnormal differentiation of the embryonic germ layers (Arman et al. 1998), suggesting that FGF signalling is critical for normal embryonic differentiation.
We and others have shown that FGF-2 is produced by foetal and maternal placental tissues throughout gestation (Rider & Piva 1998; Reynolds & Redmer 2001). These observations have led us to propose a role for FGF-2 in amplifying the angiogenic response of the endometrium to the presence of the embryonic tissues as well as in the differentiation of vascular and non-vascular tissues derived from mesoderm (Reynolds & Redmer 2001).
The sex steroids oestrogen and progesterone appear to be the primary regulators of uterine vascular function, including angiogenesis, in all mammalian species (Magness 1998; Hyder & Stancel 2000; Reynolds & Redmer 2001). For example, uterine expression of VEGF and FGF-2 mRNA is up-regulated by three- to ten-fold in ovariectomized ewes within a few hours after oestrogen treatment (Reynolds et al. 1998a,b). This strong up-regulation of VEGF and FGF-2 mRNA is associated with increased endometrial VEGF and FGF-2 protein expression as well as with dramatic increases (five- to ten-fold) in uterine angiogenesis and blood flow (Magness 1998; Reynolds et al. 1998a,b). In addition to their effects on the non-pregnant or ovariectomized uterus, it appears that oestrogens also may play a central role in regulating placental angiogenesis and angiogenic factor expression throughout pregnancy (Hildebrandt et al. 2001; Reynolds & Redmer 2001).
The uterine blood flow response to oestrogen is probably mediated largely by NO, which can stimulate production of VEGF and FGF-2 (Magness 1998). As discussed previously, both VEGF and FGF-2 have been implicated in stimulating endothelial production of NO (Hood et al. 1998; Zheng et al. 1999). Moreover, VEGF is present primarily in uterine vascular smooth muscle, which is consistent with its localization to peri-endothelial cells in ovarian tissues (Reynolds & Redmer 1998; Reynolds et al. 1998b; Reynolds et al. 2000). Because endometrial vascular oestrogen receptors (ER) also localize to the vascular smooth muscle (Rogers et al. 1996; Wang et al. 2000; Reynolds & Redmer 2001), we recently investigated the expression of ER protein in endometrial blood vessels in response steroid treatment and also to early pregnancy.
Estrogen receptor protein was up-regulated in endometrial vascular smooth muscle at estrus, by oestrogen treatment in ovariectomized ewes, and during early pregnancy (Reynolds & Redmer 2001). In these studies, the vascular ER localized primarily to the vascular smooth muscle of the endometrial arterioles. Although the steroid hormones oestrogen and progesterone also regulate uterine growth and blood flow in primates (Ferenczy & Bergeron 1989; King & Arcangues 1992), because of discrepant reports concerning steroid receptor localization, it remains controversial whether their effects on the endometrial vasculature during the normal menstrual cycle or in endometrial pathologies are direct or indirect (Hickey & Fraser 2001; Mueller & Taylor 2001; Rogers & Gargett 2001). Recently, we also have shown that eNOS localizes exclusively to endothelial cells of endometrial arterioles and capillaries in ovariectomized, steroid-treated sheep and also in early pregnant sheep (Reynolds & Redmer 2001).
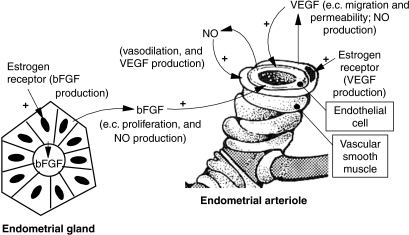
Thus, based on these and other observations across a variety of mammalian species, we developed a model for regulation of uterine/placental angiogenesis and blood flow by oestrogen (Fig. 3). An important aspect of this model is the paracrine interaction between uterine endothelial cells, which express eNOS, and peri-endothelial cells (vascular smooth muscle and capillary pericytes), which express VEGF and also contain the oestrogen receptors. This paracrine interaction would provide a positive feedback loop to stimulate uterine angiogenesis and blood flow, thereby ensuring adequate placental vascularization and perfusion. In addition to the factors presented in this model, it appears that oxygen levels may be important regulators of uterine and placental angiogenic factor expression (Charnock-Jones & Smith 2001), which emphasizes that multiple factors are probably involved in placental angiogenesis.
Figure 3.
Model for regulation of uterine/placental angiogenesis and blood flow by oestrogen. This model is based on numerous studies from our laboratories and those of others in which the major players have been not only quantified but also localized to specific tissues and cell types. Note that a major feature of the model involves a positive feedback loop between endothelial cells and peri-endothelial cells (vascular smooth muscle and capillary pericytes). This figure is taken from Reynolds & Redmer (2001), with permission.
Angiogenesis in pathologies of the female reproductive organs: clinical relevance
Ovary
As mentioned previously, luteal insufficiency or luteal phase defect is a luteal dysfunction that may result from inadequate luteal angiogenesis (for review, see Redmer & Reynolds 1996 and Reynolds et al. 2000). In this pathology, insufficient progesterone secretion is unable to maintain the appropriate length of the luteal phase during the oestrous cycle or pregnancy. For example, in cows inadequate progesterone production has been associated with infertility and early embryonic death especially early in gestation (Lamming et al. 1989; Short et al. 1990). In humans, luteal phase defect is a factor in as high as 20% of infertility cases and 60% of repeated first-trimester abortions (Redmer & Reynolds 1996 and Reynolds et al. 2000). Whether reduced luteal vascularity is secondary to luteal insufficiency or, conversely, whether inadequate luteal vascularization is a cause of luteal dysfunction is not known.
However, while cause and effect are difficult to establish, recent studies have shown that inhibition of VEGF action in the developing corpus luteum can lead to reduced luteal vascularity resulting in reduced luteal progesterone production (Ferrara et al. 1998; Fraser et al. 2000; Wulff et al. 2001). These investigators concluded that VEGF expression plays a critical role in luteal vascularization and thereby in luteal function. In addition, it has been shown recently that treatment with an antibody against VEGF will delay follicular development in cyclic Rhesus monkeys (Zimmerman et al. 2001). Thus, it seems reasonable to suggest that inappropriate follicular or luteal VEGF expression may contribute to ovarian dysfunction and thereby be an important cause of infertility.
Several other ovarian pathologies also may involve abnormal angiogenesis, including ovarian carcinoma, ovarian hyperstimulation syndrome, and polycystic ovary syndrome. In ovarian carcinoma, increased vascular density has been correlated with an increased incidence of metastasis as well as decreased patient survival rates (Hata et al. 1998; Abulafia & Sherer 2000; Brown et al. 2000; Kohn & Libutti 2001). In these studies, vascular density was quantified by using standard quantitative morphometry or computerized image analysis along with histochemical or immunohistochemical markers, including CD34, Factor VIII (von Willebrand factor), and various lectins, to identify endothelial cells. Angiogenesis in ovarian tumours also has been evaluated using minimally invasive vascular imaging techniques (Hata et al. 1998; Kohn & Libutti 2001). In addition, levels of FGF-2 and VEGF in body fluids (serum, urine, etc.) appear to be elevated in patients with ovarian cancer (Abulafia & Sherer 2000; Brown et al. 2000).
However, not all studies have shown correlations with patient outcome, and some investigators have therefore questioned the clinical usefulness of these observations (Abulafia & Sherer 2000; Brown et al. 2000). This is similar to the situation with breast cancer, in which significant correlations have been shown between a variety of measures of tumour angiogenesis and patient outcome but the clinical usefulness of these measures has been limited by the need for more reproducible techniques (Fox 2001). Thus, before measurements such as vascular density or angiogenic factor levels can become useful in the prognosis of ovarian cancer, they will probably need to be examined in larger cohorts of patients using standardized procedures.
Not only is ovarian carcinoma associated with abnormal angiogenesis, but both ovarian hyperstimulation syndrome, which is a serious consequence of induced ovulation in women, and polycystic ovary syndrome (also known as Stein–Leventhal syndrome), which is associated with hyperandrogenism, anovulation, and infertility, also exhibit increased follicular vascularity and vascular permeability, as well as elevated follicular and serum levels of VEGF (Abulafia & Sherer 2000; Geva & Jaffe 2000). Thus, it seems that angiogenesis and angiogenic factors such as VEGF and FGF also are involved in these ovarian pathologies.
As with infertility resulting from ovarian dysfunction, ovarian pathologies such as those mentioned above may be amenable to treatment with regulators of angiogenesis. For example, antibodies to VEGF have been shown to be relatively effective in treating ovarian hyperstimulation syndrome as well as ovarian cancer in animal models (Geva & Jaffe 2000). In addition, several of the antiangiogenic factors that have been identified are currently in clinical trials for treatment of ovarian cancer (Brown et al. 2000; Kohn & Libutti 2001). An adverse side-effect of these compounds is that they seem to also affect the vasculature of tissues other than those being targeted, which may indicate that angiogenic factors are important in maintenance of the vasculature even in tissues exhibiting no active vascular growth (Fraser & Lunn 2000). Thus, their usefulness as therapeutic agents in the treatment of ovarian pathologies remains to be proven. Nevertheless, the vascular imaging techniques mentioned above (Hata et al. 1998; Kohn & Libutti 2001) will likely be important in evaluating the efficacy of these compounds in treating ovarian diseases.
Uterus/placenta
The likely role of reduced placental angiogenesis in failed implantation and subnormal foetal growth has already been discussed. Although it has been suggested that stimulators of angiogenesis could be clinically useful in treating these dysfunctional pregnancies (Fraser & Lunn 2000), such studies have, to our knowledge, not yet been undertaken in any animal model. Several other pathologies of the endometrium and placenta are associated with abnormal angiogenesis, including dysfunctional uterine bleeding, endometrial hyperplasia and carcinoma, and endometriosis. They also include myometrial pathologies such as fibroids (leiomyomas) and adenomyosis. We will confine our concluding comments to these uterine pathologies.
Dysfunctional uterine bleeding is associated with increased endometrial vascular density and blood flow, as well as changes in vascular morphology (Hickey & Fraser 2001). Development and progression of endometrial hyperplasia and carcinoma also are associated with increased endometrial microvascular density, as are increased recurrence of endometrial cancer and decreased patient survival rate (Hyder & Stancel 2000). Similarly, expression of VEGF and VEGF receptors is elevated in endometrial carcinoma (Doldi et al. 1996; Guidi et al. 1996). Endometriosis, or ectopic endometrial growth, also is associated with elevated endometrial and peritoneal fluid VEGF levels (Mueller & Taylor 2001).
Uterine fibroids, or leiomyomas, which are formed by hyperproliferation of myometrial smooth muscle cells, are associated with menstrual disorders including menorrhagia (excessive menstrual bleeding; Hickey & Fraser 2001). Uterine fibroids also are highly vascular, and it has been suggested that their vasculature could therefore be a target for antiangiogenic therapy (Hickey & Fraser 2001). Similarly, endometrial and myometrial vascular density are elevated in adenomyosis, which is a pathology in which endometrial glands and stroma are located deep within the myometrium, and which is associated with menorrhagia, dysmenorrhea, and uterine fibroids (Hickey & Fraser 2001).
Thus, numerous pathologies of the uterus and placenta are associated with aberrant angiogenesis. As with the various ovarian pathologies, it has been suggested that treatment with angiogenic or antiangiogenic agents could be used therapeutically to treat these uterine diseases (Fraser & Lunn 2000; Hyder & Stancel 2000; Mueller & Taylor 2001). Because oestrogen is such a strong stimulator of uterine angiogenesis and angiogenic factor expression, it also has been suggested that treatment with antiestrogenic compounds, such as ICI 182,780, could provide effective therapy for these diseases (Hyder & Stancel 2000). It also seems likely that the vascular imaging techniques mentioned above (Hata et al. 1998; Kohn & Libutti 2001) will be important in evaluating the efficacy of these compounds in treating these uterine and placental diseases. Although the clinical studies are at an early stage, the recent spate of work on regulators of angiogenesis (Barinaga 1997; Ballara et al. 1999) leads us to believe that regulation of angiogenesis in the female reproductive organs will eventually become a powerful method not only for treating diseases of these organs but also for regulating fertility.
Acknowledgments
We gratefully acknowledge the contributions of our collaborators (Dr Russell Anthony, Dr Stephen Ford, Dr Derek Killilea, Dr Ronald Magness, and Dr Robert Moor), laboratory technicians (Dr Jerzy Bilski, Mr James Kirsch, and Mr Kim Kraft), and former students (Mr Daniel Arnold, Dr Vinayak Doraiswamy, Dr Paul Fricke, Dr Albina Jablonka-Shariff, Dr Mary Lynn Johnson, Dr William Ricke, and Dr Jing Zheng), each of whom has made invaluable contributions to our work in this area. We also gratefully acknowledge the financial support of the North Dakota Agricultural Experiment Station as well as the United States Department of Agriculture, National Institutes of Health, and National Science Foundation.
References
- Abulafia O, Sherer DM. Angiogenesis of the ovary. Am. J. Obstet. Gynecol. 2000;182:240–246. doi: 10.1016/s0002-9378(00)70519-9. [DOI] [PubMed] [Google Scholar]
- Adair TH, Gay WJ, Montani J-P. Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am. J. Physiol. 1990;259:R393–R404. doi: 10.1152/ajpregu.1990.259.3.R393. [DOI] [PubMed] [Google Scholar]
- Amselgruber WM, Schafer M, Sinowatz F. Angiogenesis in the bovine corpus luteum: An immunocytochemical and ultrastructural study. Anat. Histol. Embryol. 1999;28:157–166. doi: 10.1046/j.1439-0264.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- Anthony FW, Gornall RJ, Lincoln J, Richardson MC, Hitchcock A, Stones RW. Expression of endothelial nitric oxide synthase in the human corpus luteum. J. Reprod. Fertil. Abstract. Series. 1997;19:63. [Google Scholar]
- Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc. Natl. Acad. Sci. USA. 1998;95:5082–5087. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin HG. Vascular morphogenesis in the ovary. Bailliere's Clin. Obstet. Gynecol. 2000;14:867–882. doi: 10.1053/beog.2000.0132. [DOI] [PubMed] [Google Scholar]
- Ballara SC, Miotla JM, Paleolog EM. New vessels, new approaches: Angiogenesis as a therapeutic target in musculoskeletal disorders. Int. J. Exp. Pathol. 1999;80:235–250. doi: 10.1046/j.1365-2613.1999.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinaga M. Cancer Research: Designing therapies that target tumor blood vessels. Science. 1997;275:482–484. doi: 10.1126/science.275.5299.482. [DOI] [PubMed] [Google Scholar]
- Bernard C. Translated by H.C. Greene (Copyright 1927) New York: Schuman; 1865. An Introduction to the Study of Experimental Medicine. [Google Scholar]
- Brown MR, Blanchette JO, Kohn EC. Angiogenesis in ovarian cancer. Bailliere's Clin. Obstet. Gynecol. 2000;14:901–918. doi: 10.1053/beog.2000.0134. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones SD, Smith SK. Placental vascular morphogenesis: Introduction and overview. In: Augustin HG, Iruela-Arispe ML, Rogers PAW, Smith SK, editors. Vascular Morphogenesis in the Female Reproductive System. Boston: Birkhauser; 2001. pp. 273–284. [Google Scholar]
- Christenson LK, Stouffer RL. Proliferation of microvascular endothelial cells in the primate corpus luteum during the menstrual cycle and simulated early pregnancy. Endocrinology. 1996;137:367–374. doi: 10.1210/endo.137.1.8536637. [DOI] [PubMed] [Google Scholar]
- D'Amore PA, Klagsbrun M. The Pathobiology of Neoplasia. New York: Plenum; 1989. Angiogenesis: Factors and mechanisms; pp. 513–531. [Google Scholar]
- Denekamp J. Progress in Applied Microcirculation. Vol. 4. Basel: Karger; 1984. Vasculature as a target for tumour therapy; pp. 28–38. [Google Scholar]
- Dharmarajan AM, Bruce NW, Meyer GT. Quantitative ultrastructural characteristics relating to transport between luteal cell cytoplasm and blood in the corpus luteum of the pregnant rat. Am. J. Anat. 1985;172:87–99. doi: 10.1002/aja.1001720107. [DOI] [PubMed] [Google Scholar]
- Doldi N, Bassau M, Gulisano M, Broccoli V, Boucinelli E, Ferrari A. Vascular endothelial growth factor messenger ribonucleic acid expression in human ovarian and endometrial cancer. Gynecol. Endocrinol. 1996;10:375–382. doi: 10.3109/09513599609023600. [DOI] [PubMed] [Google Scholar]
- Doraiswamy V, Knutson DL, Grazul-Bilska AT, Redmer DA, Reynolds LP. Fibroblast growth factor receptor (FGFR) -1 and -2 in the ovine corpus luteum throughout the estrous cycle. Growth Factors. 1998;16:125–135. doi: 10.3109/08977199809002123. [DOI] [PubMed] [Google Scholar]
- Edey TN. Prenatal mortality in sheep: a review. Anim Breeders Abstract. 1969;37:173–190. [Google Scholar]
- Ferenczy A, Bergeron C. Endometrial hyperplasia and neoplasia. In: Wynn RM, Jollie WP, editors. Biology of the Uterus. 4. New York: Plenum; 1989. pp. 333–353. [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisolm V, Hillan KJ, Schwall RH. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nature Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocrine Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;233:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Fox SB. Angiogenesis in mammary tumors: Quantitation and relation to prognosis. In: Augustin HG, Iruela-Arispe ML, Rogers PAW, Smith SK, editors. Vascular Morphogenesis in the Female Reproductive System. Boston: Birkhauser; 2001. pp. 63–85. [Google Scholar]
- Fraser H, Dickson SE, Lunn SF, et al. Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology. 2000;141:995–1000. doi: 10.1210/endo.141.3.7369. [DOI] [PubMed] [Google Scholar]
- Fraser H, Lunn SF. Angiogenesis and its control in the female reproductive system. Br. Med. Bull. 2000;56:787–797. doi: 10.1258/0007142001903364. [DOI] [PubMed] [Google Scholar]
- Fraser H, Lunn SF. Regulation and manipulation of angiogenesis in the primate corpus luteum. Reproduction. 2001;121:355–362. doi: 10.1530/rep.0.1210355. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Rogers PAW. Human endometrial angiogenesis. Reproduction. 2001;121:181–186. doi: 10.1530/rep.0.1210181. [DOI] [PubMed] [Google Scholar]
- Garrido C, Saule S, Gospodarowicz D. Transcriptional regulation of vascular endothelial growth factor gene expression in ovarian bovine granulosa cells. Growth Factors. 1993;8:109–117. doi: 10.3109/08977199309046931. [DOI] [PubMed] [Google Scholar]
- Geva E, Jaffe RB. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fert. Steril. 2000;74:429–438. doi: 10.1016/s0015-0282(00)00670-1. [DOI] [PubMed] [Google Scholar]
- Goede V, Schmidt T, Kimmina S, Kozian D, Augustin HG. Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Laboratory Invest. 1998;78:1385–1394. [PubMed] [Google Scholar]
- Gospodarowicz D. Biological activities of fibroblast growth factors. Ann. NY Acad. Sci. 1991;638:1–8. doi: 10.1111/j.1749-6632.1991.tb49012.x. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Redmer DA, Jablonka-Shariff A, Biondini ME, Reynolds LP. Proliferation and progesterone production of ovine luteal cells from several stages of the estrous cycle: Effects of fibroblast growth factors (FGF) and luteinizing hormone (LH) Can. J. Physiol. Pharmacol. 1995;73:491–500. doi: 10.1139/y95-062. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Redmer DA, Reynolds LP. Growth factors during ovarian angiogenesis. In: Augustin HG, Iruela-Arispe ML, Rogers PAW, Smith SK, editors. Vascular Morphogenesis in the Female Reproductive System. Boston: Birkhauser; 2001. pp. 131–147. [Google Scholar]
- Greenwald GS. Temporal and topographic changes in dna synthesis after induced follicular atresia. Biol. Reprod. 1989;41:175–181. doi: 10.1095/biolreprod41.1.175. [DOI] [PubMed] [Google Scholar]
- Guidi AJ, Abu-Jawdeh G, Tognazzi K, Dvorak HF, Brown LF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in endometrial carcinoma. Cancer. 1996;78:454–460. doi: 10.1002/(SICI)1097-0142(19960801)78:3<454::AID-CNCR12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hata K, Nagami H, Iida K, Miyazaki K, Collins WP. Expression of thymidine phosphorylase in malignant ovarian tumors: Correlation with microvessel density and an ultrasound-derived index of angiogenesis. Ultrasound Obstet. Gynecol. 1998;12:201–206. doi: 10.1046/j.1469-0705.1998.12030201.x. [DOI] [PubMed] [Google Scholar]
- Hickey M, Fraser IS. The clinical relevance of disturbances of uterine vascular growth, remodeling, and repair. In: Augustin HG, Iruela-Arispe ML, Rogers PAW, Smith SK, editors. Vascular Morphogenesis in the Female Reproductive System. Boston: Birkhauser; 2001. pp. 223–244. [Google Scholar]
- Hildebrandt VA, Babischkin JS, Koos RD, Pepe GJ, Albrecht ED. Developmental regulation of vascular endothelial growth/permeability factor messenger ribonucleic acid levels in and vascularization of the villous placenta during baboon pregnancy. Endocrinology. 2001;142:2050–2057. doi: 10.1210/endo.142.5.8174. [DOI] [PubMed] [Google Scholar]
- Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- Hudlicka O. Development of microcirculation: Capillary growth and adaptation. In: Renkin EM, Michel CC, editors. Handbook of Physiology. IV. Baltimore: Waverly Press; 1984. pp. 165–216. [Google Scholar]
- Hyder SM, Stancel GM. Regulation of VEGF in the reproductive tract by sex-steroid hormones. Histol. Histopathol. 2000;15:325–334. doi: 10.14670/HH-15.325. [DOI] [PubMed] [Google Scholar]
- Jaffe RB. Importance of angiogenesis in reproductive physiology. Seminars Perinatol. 2000;24:79–81. doi: 10.1016/s0146-0005(00)80062-2. [DOI] [PubMed] [Google Scholar]
- King RJB, d'Arcangues C. Steroid hormone effects on uterine blood vessels. In: Alexender NJ, D'Arcangues C, editors. Steroid Hormones and Uterine Bleeding. Washington DC: AAAS Press; 1992. pp. 25–36. [Google Scholar]
- Klein PS, Melton DA. Hormonal regulation of embryogenesis: the formation of mesoderm on xenopul laevis. Endocrine Rev. 1994;15:326–341. doi: 10.1210/edrv-15-3-326. [DOI] [PubMed] [Google Scholar]
- Kohn EC. Angiogenesis in ovarian carcinoma. Cancer. 1997;80:2219–2221. doi: 10.1002/(sici)1097-0142(19971215)80:12<2219::aid-cncr1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kohn EC, Libutti SK. Angiogenesis, vascular imaging, and therapeutic approaches in ovarian tumors. In: AugustinMLIruela-Arispe hg, Rogers paw, Smith sk., editors. Vascular Morphogenesis in the Female Reproductive System. Boston: Birkhauser; 2001. pp. 187–205. [Google Scholar]
- Koos RD. Increased expression of vascular endothelial growth/permeability factor in the rat ovary following an ovulatory gonadotropin stimulus: potential roles in follicle rupture. Biol. Reprod. 1995;52:1426–1435. doi: 10.1095/biolreprod52.6.1426. [DOI] [PubMed] [Google Scholar]
- Lamming GE, Darwash AO, Back HL. Corpus luteum function in dairy cows and embryo mortality. J. Reprod. Fertil. 1989;37(Supplement):245–252. [PubMed] [Google Scholar]
- Magness RR. Maternal cardiovascular and other physiological responses to the endocrinology of pregnancy. In: Bazer FW, editor. The Endocrinology of Pregnancy. Totowa, NJ: Humana Press; 1998. pp. 507–539. [Google Scholar]
- Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotype defects observed in FGF2 null mice. Mol. Cell. Biol. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor RM, Seamark RF. Cell signaling, permeability, and microvasculatory changes during antral follicle development in mammals. J. Dairy. Sci. 1986;69:927–943. doi: 10.3168/jds.S0022-0302(86)80482-9. [DOI] [PubMed] [Google Scholar]
- Mueller MD, Taylor RN. Angiogenesis in endometriosis. In: Augustin HG, Iruela-Arispe ML, Rogers PAW, Smith SK, editors. Vascular Morphogenesis in the Female Reproductive System. Boston: Birkhauser; 2001. pp. 245–270. [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Niswender GD, Nett TM. The corpus luteum and its control. In: Knobil E, et al., editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 489–525. [Google Scholar]
- North RA, Ferrier C, Long D, Townend K, Kincaid-Smith P. Uterine artery Doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth retardation. Obstet. Gynecol. 1994;83:378–386. [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc. Natl. Acad. Sci. USA. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plendl J. Angiogenesis and vascular regression in the ovary. Anat. Histol. Embryol. 2000;29:257–266. doi: 10.1046/j.1439-0264.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- Ravindranath N, Little-Ihrig L, Phillips HS, Ferrara N, Zeleznik AJ. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinology. 1992;131:254–260. doi: 10.1210/endo.131.1.1612003. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Dai Y, Li J, et al. Characterization and expression of vascular endothelial growth factor (VEGF) in the ovine corpus luteum. J. Reprod. Fertil. 1996;108:157–165. doi: 10.1530/jrf.0.1080157. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Doraiswamy V, Bortnem BJ, et al. Evidence for a role of capillary pericytes in vascular growth of the developing ovine corpus luteum. Biol. Reprod. 2001;65:879–889. doi: 10.1095/biolreprod65.3.879. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Grazul AT, Kirsch JD, Reynolds LP. Angiogenic activity of bovine corpus luteum at several stages of luteal development. J. Reprod. Fertil. 1988;82:627–634. doi: 10.1530/jrf.0.0820627. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Kirsch JD, Grazul AT. In vitro production of angiotropic factor by bovine corpus luteum partial characterization of activities that are chemotactic and mitogenic for endothelial cells. In: Mahesh VB, Dhindsa DS, Anderson E, Kalra SP, editors. Regulation of Ovarian and Testicular Function. Adv. Exp. Medical Biol. Vol. 219. New York: Plenum Press; 1987. pp. 683–688. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Kirsch JD, Reynolds LP. Production of mitogenic factors by cell types of estrogen-active and estrogen-inactive preovulatory bovine follicles. J. Anim. Sci. 1991;69:237–245. doi: 10.2527/1991.691237x. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Reynolds LP. Angiogenesis in the ovary. Rev. Reprod. 1996;1:182–192. doi: 10.1530/ror.0.0010182. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Rone JD, Goodman AL. Evidence for a non-steroidal angiotropic factor from the primate corpus luteum: Stimulation of endothelial cell migration in vitro. Proc. Soc. Exp. Biol. Medical. 1985;179:136–140. doi: 10.3181/00379727-179-1-rc3. [DOI] [PubMed] [Google Scholar]
- Reynolds LP. Utero–ovarian interactions during early pregnancy: Role of conceptus-induced vasodilation. J. Anim. Sci. 1986;62(Suppl. 2):47–61. doi: 10.1093/ansci/62.2.47. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the corpus luteum. Endocrine. 2000;12:1–9. doi: 10.1385/ENDO:12:1:1. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Killilea SD, Grazul-Bilska AT, Redmer DA. Mitogenic factors of corpora lutea. Progr. Growth Factor Res. 1994;5:159–175. doi: 10.1016/0955-2235(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Killilea SD, Redmer DA. Angiogenesis in the female reproductive system. FASEB J. 1992;6:886–892. [PubMed] [Google Scholar]
- Reynolds LP, Kirsch JD, Kraft KC, Redmer DA. Time-course of the uterine response to estradiol-17â in ovariectomized ewes: Expression of angiogenic factors. Biol. Reprod. 1998a;59:613–620. doi: 10.1095/biolreprod59.3.613. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Kirsch JD, Kraft KC, Knutson DL, McClaflin WJ, Redmer DA. Time-course of the uterine response to estradiol-17â in ovariectomized ewes: Uterine growth and microvascular development. Biol. Reprod. 1998b;59:606–612. doi: 10.1095/biolreprod59.3.606. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Millaway DS, Kirsch JD, Infeld JE, Redmer DA. Growth and in vitro metabolism of placental tissues of cows from Day 100 to Day 250 of gestation. J. Reprod. Fertil. 1990;89:213–222. doi: 10.1530/jrf.0.0890213. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Growth and microvascular development of the uterus during early pregnancy in ewes. Biol. Reprod. 1992;47:698–708. doi: 10.1095/biolreprod47.5.698. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J. Anim. Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Expression of the angiogenic factors, basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), in the ovary. J. Anim. Sci. 1998;76:1671–1681. doi: 10.2527/1998.7661671x. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Growth and development of the corpus luteum. J. Reprod. Fertil. 1999;54(Supplement):179–189. [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol. Reprod. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- Rider V, Piva M. Bazer FW. The Endocrinology of Pregnancy. Totowa, NJ: Humana Press; 1998. Role of growth factors of uterine and fetal-placental origin during pregnancy; pp. 83–124. [Google Scholar]
- Rogers PAW, Gargett CE. The vascular system in the endometrium: Introduction and overview. In: Augustin HG, Iruela-Arispe ML, Rogers PAW, Smith SK, editors. Vascular Morphogenesis in the Female Reproductive System. Boston: Birkhauser; 2001. pp. 209–222. [Google Scholar]
- Rogers PAW, Lederman R, Kooy J, Taylor NH, Healy DL. Endometrial vascular smooth muscle oestrogen and progesterone receptor distribution in women with and without menorrhagia. Hum. Reprod. 1996;11:2003–2008. doi: 10.1093/oxfordjournals.humrep.a019533. [DOI] [PubMed] [Google Scholar]
- Schiffenbauer YS, Abramovitch R, Meir G, et al. Loss of ovarian function promotes angiogenesis in human ovarian carcinoma. Proc. Natl. Acad. Sci. USA. 1997;94:13203–13208. doi: 10.1073/pnas.94.24.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood island formation and vasculogenesis in flk-1 deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Short RV. Species differences in reproductive mechanisms. In: Austin CR, Short RV, editors. Reproduction in Mammals Book 4. 2. Cambridge, UK: Cambridge University Press; 1984. pp. 24–61. [Google Scholar]
- Short RE, Bellows RA, Staigmiller RB, Berardinelli JG, Custer EE. Physiological mechanisms controlling anestrus and infertility in postpartum beef cattle. J. Anim. Sci. 1990;68:799–816. doi: 10.2527/1990.683799x. [DOI] [PubMed] [Google Scholar]
- Silvester LM, Luck MR. Distribution of extracellular matrix components in the developing ruminant corpus luteum: a wound repair hypothesis for luteinization. J. Reprod. Fertil. 1999;116:187–198. doi: 10.1530/jrf.0.1160187. [DOI] [PubMed] [Google Scholar]
- Slack JMW, Darlington BG, Heath JK, Godsave SF. Mesoderm induction in early xenopus embryos by heparin-binding growth factors. Nature. 1987;326:197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Stirling DT, Waterman MF, Simpson ER. Expression of mRNA encoding basic fibroblast growth factor (bFGF) in bovine corpora lutea and cultured cells. J. Reprod. Fertil. 1991;91:1–8. doi: 10.1530/jrf.0.0910001. [DOI] [PubMed] [Google Scholar]
- Taraska T, Reynolds LP, Redmer DA. In vitro secretion of angiogenic activity by ovine follicles. In: Hirshfield AN, editor. Growth Factors and the Ovary. New York: Plenum Press; 1989. pp. 267–272. [Google Scholar]
- Toutges MJ, Grazul-Bilska AT, Kirsch JD, Reynolds LP, Redmer DA. Effects of luteinizing hormone (LH) and oxygen (O2) levels on vascular endothelial growth factor (VEGF) mRNA expression by ovine luteal cells in culture. Biol. Reprod. 1999;60(Suppl. 1):280. [Google Scholar]
- Trudinger BJ, Giles WB, Cook CM. Uteroplacental blood flow velocity-time waveforms in normal and complicated pregnancy. Br. J. Obstet. Gynecol. 1985;92:39–45. doi: 10.1111/j.1471-0528.1985.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Eriksson H, Sahlin L. Estrogen receptors alpha and beta in the female reproductive tract of the rat during the estrous cycle. Biol. Reprod. 2000;63:1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- WinSPIRS SilverPlatter International N.V. 2001. http://www.silverplatter.com/
- Wulff C, Wilson H, Rudge JS, Wiegand SJ, Lunn SF, Fraser HM. Luteal angiogenesis: Prevention and intervention by treatment with vascular endothelial growth factor trapA40. J. Clin. Endocrinol. Metab. 2001;86:3377–3386. doi: 10.1210/jcem.86.7.7662. [DOI] [PubMed] [Google Scholar]
- Yamagishi S-I, Yonekura H, Yamamoto Y, et al. Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Laboratory Invest. 1999;79:501–509. [PubMed] [Google Scholar]
- Zheng J, Bird IM, Melsaether AN, Magness RR. Activation of the mitogen-activated protein kinase cascade is necessary but not sufficient for basic fibroblast growth factor- and epidermal growth factor-stimulated expression of endothelial nitric oxide synthase in ovine fetoplacental artery endothelial cells. Endocrinology. 1999;140:1399–1407. doi: 10.1210/endo.140.3.6542. [DOI] [PubMed] [Google Scholar]
- Zimmermann RC, Hartman T, Bohlen P, Sauer MV, Kitajewski J. Preovulatory treatment of mice with anti-VEGF receptor 2 antibody inhibits angiogenesis in corpora lutea. Microvasc. Res. 2001;62:15–25. doi: 10.1006/mvre.2001.2312. [DOI] [PubMed] [Google Scholar]