Abstract
Lectins are proteins or glycoproteins of nonimmune origin, which bind specifically to carbohydrate structures. They are widespread in the human diet, and many are resistant to digestion. High doses of lectins have been shown to stimulate intestinal and pancreatic growth. The aim of the present study was to investigate the long-term actions of low doses of lectins on the rat intestine and pancreas. A long-term carcinogenesis study was performed using low levels (40 µg/rat/day) of peanut (PNA) or mushroom lectin (ABA) which bind to O-linked (mucin-type) oligosaccharides in the gut. While this was primarily designed as a colon carcinogenesis study, the pancreas was also investigated. No significant changes in colon carcinogenesis were seen, however, the colons were slightly heavier in the lectin treated groups. The weight of the pancreas was significantly greater (by 18 and 23%) in both lectin treated groups (P < 0.03/0.001). The weights of the acini and septal tissue were also increased by 39–46% in PNA and ABA fed animals, respectively (P < 0.002); there was no significant change in the endocrine pancreas.
In conclusion, long-term feeding of low doses of lectin can influence pancreatic growth, and this trophic action may have potential adverse implications for the development of pancreatic cancer in humans.
Keywords: pancreas, gastrointestinal tract, cell division, lectin, peanut agglutinin, mushroom agglutinin
Introduction
The normal human diet contains many lectins (proteins or glycoproteins of non-immune origin which have specificity for carbohydrae structures) (Pusztai 1993, Nachbar & Oppenheim 1980). Although there is considerable literature about the effects on the intestine of toxic lectins such as red kidney bean (PHA) (Pusztai et al. 1982) and wheat germ agglutinin (WGA) (Brady et al. 1978), less is known about the effects of noncytotoxic lectins. Several factors suggest that such ingested lectins could have a major effect on the proliferation and metabolism of the human intestine. For example: (i) many lectins, particularly those of plant origin, are tightly globular and thus highly protease-resistant and are able to survive passage through the mammalian intestine without digestion (Barondes et al. 1994) (ii) the intestinal mucosal glycocalyx has numerous and diverse potential binding sites for lectins (Weaver & Bailey 1987) (iii) most lectins have been shown to have significant biological effects on cells to which they bind (Kaplowitz 1985).
One such group of lectins bind to galactose β1–3 N-acetylgalactosamine α– (Galβ1–3GalNAc(α -), the Thomsen Friedenreich (TF) blood group antigen that is the Type I core structure on O-linked (mucin-type) oligosaccharides. This structure can be expressed not only by secreted mucins (Melchior & Gerace 1995) but also by epithelial cell surface glycoconjugates (Campbell et al. 1995).
All the TF-binding lectins identified in plants have so far been found to be noncytotoxic and include constituents of a variety of food sources that include peanuts and mushrooms. Peanut lectin has proliferative effects on colon cell lines (Ryder et al. 1992), and on human colonic mucosal biopsies in culture (Ryder et al. 1994). The addition of 100 g peanuts per day for one week to a normal diet caused a 40% increase in rectal mucosal mitotic index in humans (patients with irritable bowel syndrome but normal colonic histology) who express the lectin receptor in their rectal mucosa (Ryder et al. 1998). Another group has demonstrated a proliferative effect of ingested peanut lectin on mouse small intestine (Henney et al. 1990) and we have also shown marked effects of PNA on both the small and large intestine of rats (Jordinson et al. 1999).
Conversely, we have found that the TF-binding lectin from the edible mushroom (Agaricus bisporus, ABA) inhibits proliferation in a wide range of epithelial cells without apparent cytotoxicity (Yu et al. 1993) and also inhibits invasion of HCT116 colon cancer cells through collagen gels (Yu et al. 1999). ABA differs in its specificity from peanut lectin, binding also to sialyl-Galβ1–3GalNAcα– (sialyl TF).
In addition to their effects on the intestine, we have recently reported that some lectins, especially peanut lectin, can increase plasma cholecystokinin (CCK) levels and also increase the weight of the pancreas (Jordinson et al. 1999). Such effects may also occur by blood borne lectins, as peanut lectin can be detected in the blood of humans minutes after eating peanuts (Wang et al. 1998), and systemic infusion of peanut lectin into rats can stimulate colonic cell division (Jordinson et al. 2000).
There are significant geographical differences in the incidence of pancreatic cancer (Warshaw & Fernandez-del Castillo 1992). In the USA, and in the UK, incidence has increased sharply since the 1930s but seems to have plateaued since the 1970s. In Japan, there has been a more recent increase, from 1.8 per 100000 in 1960 to 5.2 in 1985 (Hirayama 1989). The reasons for these marked geographical differences are unclear but the marked increase in incidence since the 1940s in Westernised countries suggests that environmental factors are very important. Dietary risk factors seem to be similar to colon cancer with some evidence to support a high intake of meat or fat as risk factors with fruit and vegetables as protective factors (Farrow & Davis 1990) (Norell et al. 1986) (Howe & Burch 1996). The evidence for these dietary factors is however, generally considered to be weaker than that for those in colon cancer (Warshaw & Fernandez-del Castillo 1992). Little is known about the possible roles for plant lectins in colon and pancreatic cancer but their ability to alter cell proliferation warrants their investigation.
The present paper reports our findings of pancreatic growth following lectin feeding. The data was generated as part of a study primarily designed to investigate intestinal proliferation and carcinogenesis. The rats were treated with the colon carcinogen dimethylhydrazine, but the results of the intestine were inconclusive; however, the multifactorial design of the study enabled us to investigate the actions on the pancreas with effective statistical power.
Materials and methods
Seventy-two male Wistar rats, aged between 6 and 8 weeks old and of mean weight (213.0 ± 3.6 g) were used. They were placed into one of the six following groups; control group, DMH group, peanut lectin group, mushroom lectin group, DMH plus peanut lectin group and DMH plus mushroom lectin. After two weeks on the diets the rats were then given 16 weekly sc injections of either DMH (1,2-dimethylhydrazine, Sigma Poole, Dorset, UK) at a dosage of 20 mg/kg body weight (n = 36), or the same volume of vehicle (n = 36; 1 mL /kg body weight) (Park etal. 1997). Peanut and mushroom lectin were purchased from EY Laboratories, Leicestershire, UK.
The diet was based on a standard rat chow (prepared by SDS, Witham, Essex, UK) to which was added 2 mg/kg PNA or ABA, the diet was then pelleted and irradiated.
Rats had free access to food and water and were killed by C02 and cervical dislocation, 6 weeks after the last injection of DMH and 24 weeks after the first injection of the DMH.
All animals were maintained in a temperature-controlled room with a 12 : 12 h light-dark cycle. Food and water were available ad libitum and the animals were weighed weekly. All animal experiments were approved by the Cancer Research UK Animals Ethical Committee and by the Home Office Animal procedures (1986) act.
Whole pancreata were dissected out, weighed and formalin-fixed. The first six pancreases from each group were used for quantitative stereological examination (Howard et al. 1997). By employing stereology, significant changes of about 1/10th the magnitude of those detectable by qualitative methods can be achieved (Howard et al. 1998).
Pancreata were uniformly randomly sampled (Gundersen & Jensen 1987) and ‘vertical’ blocks taken (Baddeley et al. 1986), embedded in Historesin (TAAB Laboratories Equipment Ltd, Aldermaston, Berkshire, UK) and sectioned at both 5 µm and 30 µm. Absolute volume and volume fraction of islet tissue, acinar tissue and septal tissue within the pancreas were estimated by point counting and the Cavalieri method (Howard et al. (1998).
Statistical analyses
All results are presented as the group mean ± standard error of the mean (SEM). Two way analysis of variance (anova) was performed to test any effect of lectin or DMH and any interactions between these. If there was no interaction the test was re-run without the interaction term. Analysis was performed using Minitab Statistical Software, Release 10.5 Xtra (Minitab Ltd, Coventry, UK).
Results
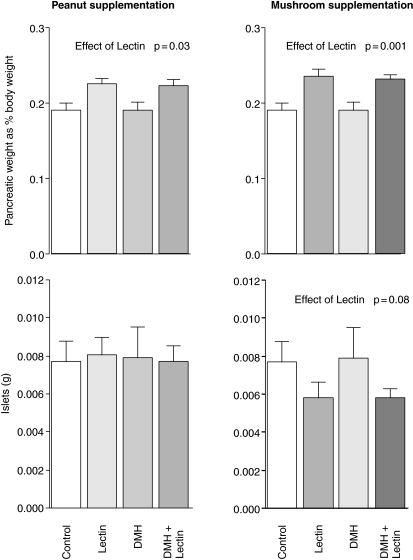
The rats increased their body weight by approximately 66% over the period of study, with no significant differences in weight gain between groups. The colons were slightly heavier in both the lectin treated groups, and this was statistically significant (P = 0.018) for the PNA group. Only one tumour per group was seen in the DMH plus PNA and DMH plus ABA treated groups, however, one rat fed PNA was put down early due to a large Wilm's like tumour in the peritoneal cavity. None of these differences was statistically significant. Peanut lectin was associated with an 18% increase in pancreatic weight (P = 0.03 Fig. 1), while mushroom lectin was associated with a 23% increase (P < 0.001). PNA had no effect on the volume of the islet cells, but ABA appeared to be associated with a 25% decrease in islet cell mass which approached statistical significance (P = 0.08). The pancreatic tissue was reviewed by a pathologist and was of normal histological appearance. There was no evidence of inflammation in any of the tissue examined.
Figure 1.
Effects of peanut and mushroom lectins on pancreatic weight (expressed as percentage of total body weight) and on the mass of pancreatic islet cells. Data was tested by two-way analysis of covariance and significant effects are presented in the figure.
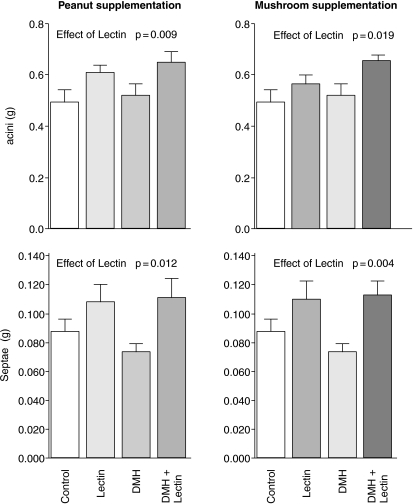
PNA was associated with a 24% increase in the mass of the acini (Fig. 2, P = 0.009) and of the septae (39%P = 0.012). ABA also increased the mass of the acini (by 21%P = 0.019) and had a larger effect of 46% on the mass of the septae (P = 0.004).
Figure 2.
Effects of peanut and mushroom lectins on the mass of pancreatic acini and septae. Data was tested by two-way analysis of covariance and significant effects are presented in the figure.
Discussion
The principal aim of the study was to investigate the actions of the two lectins on gastrointestinal carcinogenesis, unfortunately the results for this were inconclusive. Nevertheless, the finding of a tumour in the non-DMH treated PNA fed group is a source of concern and further studies on the actions of PNA in carcinogenesis models are in progress. While there was an indication of increased gastrointestinal tissue mass, especially with PNA, this was not significant, however, the two lectins both significantly increased the weight of the pancreas. These results were statistically significant despite the relatively low dose of lectin used.
The weight data was augmented by the use of stereological techniques, which are important tools for the study of the entire pancreas, providing an unbiased estimate of the separate volumes of each component of the pancreas, without the need to serially reconstruct the pancreas. There was a marked and highly significant increase in pancreatic weight and in the weight of the pancreatic acini and septae, in both lectin-fed groups, this was the more noteworthy considering the very low dose of lectin used. Con-A, PHA, WGA and PNA can all increase pancreatic weight when given in large doses (25 mg/rat/day) for short periods (Jordinson et al. 1999). Peanuts normally contain about 1 mg/g of PNA (Lotan et al. 1975), thus a 250-g rat eating 40 µg per day would take in 160 µg/kg, approximately equivalent to a 70-kg man eating 11 g peanuts per day. There are 100 µgrams of ABA per gram of mushroom (Sueyoshi et al. 1985), thus the dose is approximately equivalent to 110 g (one serving) of mushrooms per day.
These trophic actions may be direct or due to an indirect mechanism. The actions of PNA and ABA could be direct as some lectins can be absorbed and transported in the blood. Although lectins are well known to agglutinate red blood cells, PNA is only agglutinated if its sialic acid residues are removed to reveal the cryptic TF antigen. Other lectins may bind to the plasma proteins and thus not agglutinate the erythrocytes. A recent study has shown that peanut lectin is found in the blood of man soon after eating peanuts (Wang et al. 1998), and systemic infusion of peanut lectin into rats can stimulate cell division in the colon (Jordinson et al. 2000). It is not known how these lectins enter the blood but a possible route is via binding to the follicle-associated epithelium of the intestinal M cells (Clark et al. 1995), however, the speed of uptake (Wang et al. 1998) suggests that the stomach is also involved.
An alternative mechanism for the actions of these agents could be provided by the finding that several lectins (including PNA and ABA) can interact with the epidermal growth factor receptor, perhaps by disrupting the lateral mobility and aggregation of mitogen-receptor complexes (Kaplowitz 1985) (Zeng et al. 1995). However, Ryder (Ryder et al. 1992) found no competition between PNA and EGF; in fact there appeared to be a synergistic action of PNA and EGF when given to colonic explants.
The significance of these changes is as yet unknown, however experimental pancreatic cancer is consistently promoted by agents that induce pancreatic hyperplasia. Many of these, including raw soy flour, have been thought to induce pancreatic hyperplasia mediated via CCK release from the duodenum. Some of the soy effect can be attributed to soybean lectin (and other lectins) interacting with an as-yet-unidentified receptor on the duodenal mucosa, with resultant CCK release (Jordinson et al. 1996). Soy lectin is readily heat inactivated and raw soy does not form part of the human diet so this is unlikely to be an important promoter of human pancreatic cancer. It does however, raise the possibility that other dietary lectins, particularly those which are resistant to heat and digestion, might have similar effects on cholecystokinin release and hence pancreatic hyperplasia.
If these lectins also stimulate pancreatic growth in humans it would imply that consumption of peanuts (and possibly of other dietary heat- and digestion-resistant noncytotoxic galactose-binding dietary lectins) might promote intestinal and pancreatic cancer. Nonetheless, the very specific binding of these lectins may also provide a potential means of ameliorating such actions. Several dietary fibres, especially those from vegetables, are high in galactose residues, which raises the intriguing possibility that these fibres could bind and thus ‘inactivate’ the lectin. This would provide a alternative explanation for the protective actions of some fibres reported in epidemiological studies where the consumption of (galactose-containing) fruit and vegetable fibre is associated with decreased cancer risk. We intend to follow this hypothesis in the gut and pancreas in further studies using experimental models of pancreatic cancer.
In conclusion, we have shown that two lectins can alter pancreatic growth in vivo. This was achieved using low doses of the lectins, which could easily occur when eating a normal diet. This has worrying implications, as increased growth can be a promoter of carcinogenesis.
Acknowledgments
We thank the Biotechnology and Biological Sciences Research Council for funding Anthony FitzGerald & Ravinder Singh.
References
- Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J. Microscopy. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- Brady PG, Vannier AM, Banwell JG. Identification of the dietary lectin, wheat germ agglutinin, in human intestinal contents. Gastroenterology. 1978;75:236–239. [PubMed] [Google Scholar]
- Campbell BJ, Finnie IA, Hounsell EF, Rhodes JM. Direct demonstration of increased expression of Thomsen-Friedenreich (TF) antigen in colonic adenocarcinoma and ulcerative colitis mucin and its concealment in normal mucin. J. Clin. Invest. 1995;95:571–576. doi: 10.1172/JCI117700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MA, Jepson MA, Simmons NL, Hirst BH. Selective binding and transcytosis of Ulex europaeus 1 lectin by mouse Peyer's patch M-cells in vivo. Cell Tissue Res. 1995;282:455–461. doi: 10.1007/BF00318877. [DOI] [PubMed] [Google Scholar]
- Farrow DC, Davis S. Diet and the risk of pancreatic cancer in men. Am. J. Epidemiol. 1990;132:423–431. doi: 10.1093/oxfordjournals.aje.a115677. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microscopy. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Henney L, Ahmed EM, George DE, Kao KJ, Sitren HS. Tolerance to long-term feeding of isolated peanut lectin in the rat: evidence for a trophic effect on the small intestines. J. Nutritional Sci. Vitaminol. 1990;36:599–607. doi: 10.3177/jnsv.36.599. [DOI] [PubMed] [Google Scholar]
- Hirayama T. Epidemiology of pancreatic cancer in Japan. Jap J. Clin. Oncol. 1989;19:208–215. [PubMed] [Google Scholar]
- Howard CV. Quantitative microscopy in studies of intrauterine growth retardation [editorial] J. Pathol. 1997;183:129–130. doi: 10.1002/(SICI)1096-9896(199710)183:2<129::AID-PATH1172>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology, Quantitative 3-D Microscopy. Oxford: Bios Ltd; 1998. [Google Scholar]
- Howe GR, Burch JD. Nutrition and pancreatic cancer. Cancer Causes Control. 1996;7:69–82. doi: 10.1007/BF00115639. [DOI] [PubMed] [Google Scholar]
- Jordinson M, Deprez PH, Playford RJ, et al. Soybean lectin stimulates pancreatic exocrine secretion via CCK-A receptors in rats. Am. J. Physiol. 1996;270:G653–G659. doi: 10.1152/ajpgi.1996.270.4.G653. [DOI] [PubMed] [Google Scholar]
- Jordinson M, Fitzgerald AJ, Goodlad RA, et al. Systemic effects of peanut agglutinin following intravenous infusion in the rat. Alimentary Pharmacol. Therapeutics. 2000;14:835–840. doi: 10.1046/j.1365-2036.2000.00764.x. [DOI] [PubMed] [Google Scholar]
- Jordinson M, Goodlad RA, Brynes A, et al. Gastrointestinal responses to a panel of lectins in rats maintained on total parenteral nutrition. Am. J. Physiol. 1999;276:G1235–G1242. doi: 10.1152/ajpgi.1999.276.5.G1235. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB. Wheat germ agglutinin and concanavalin A inhibit the response of human fibroblasts to peptide growth factors by a post-receptor mechanism. J. Cellular Physiol. 1985;124:474–480. doi: 10.1002/jcp.1041240317. [DOI] [PubMed] [Google Scholar]
- Lotan R, Skutelsky E, Danon D, Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea) J. Biol. Chem. 1975;250:8518–8523. [PubMed] [Google Scholar]
- Melchior F, Gerace L. Mechanisms of nuclear protein import. Current Opinion Cell Biol. 1995;7:310–318. doi: 10.1016/0955-0674(95)80084-0. [DOI] [PubMed] [Google Scholar]
- Nachbar MS, Oppenheim JD. Lectins in the United States diet: a survey of lectins in commonly consumed foods and a review of the literature. Am. J. Clin. Nutrition. 1980;33:2338–2345. doi: 10.1093/ajcn/33.11.2338. [DOI] [PubMed] [Google Scholar]
- Norell SE, Ahlbom A, Erwald R, et al. Diet and pancreatic cancer: a case-control study. Am. J. Epidemiol. 1986;124:894–902. doi: 10.1093/oxfordjournals.aje.a114479. [DOI] [PubMed] [Google Scholar]
- Park HS, Goodlad RA, Ahnen DJ, et al. Effects of epidermal growth factor and dimethylhydrazine on crypt size, cell proliferation, and crypt fission in the rat colon. Cell proliferation and crypt fission are controlled independently. Am. J. Pathol. 1997;151:843–852. [PMC free article] [PubMed] [Google Scholar]
- Pusztai A. Dietary lectins are metabolic signals for the gut and modulate immune and hormone functions. European J. Clin. Nutrition. 1993;47:691–699. [PubMed] [Google Scholar]
- Pusztai A, King TP, Clarke EM. Recent advances in the study of the nutritional toxicity of kidney bean (Phaseolus vulgaris) Lectins Rats'. Toxicon. 1982;20:195–197. doi: 10.1016/0041-0101(82)90192-1. [DOI] [PubMed] [Google Scholar]
- Ryder SD, Jacyna MR, Lev AJ, Rizzi PM, Rhodes JM. Peanut ingestion increases rectal proliferation in individuals with mucosal expression of peanut lectin receptor. Gastroenterology. 1998;114:44–49. doi: 10.1016/s0016-5085(98)70631-6. [DOI] [PubMed] [Google Scholar]
- Ryder SD, Parker N, Ecclestone D, Haqqani MT, Rhodes JM. Peanut lectin stimulates proliferation in colonic explants from patients with inflammatory bowel disease and colon polyps. Gastroenterology. 1994;106:117–124. doi: 10.1016/s0016-5085(94)94775-9. [DOI] [PubMed] [Google Scholar]
- Ryder SD, Smith JA, Rhodes JM. Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. J. Natl Cancer Inst. 1992;84:1410–1416. doi: 10.1093/jnci/84.18.1410. [DOI] [PubMed] [Google Scholar]
- Sueyoshi S, Tsuji T, Osawa T. Purification and characterization of four isolectins of mushroom (Agaricus bisporus) Biol. Chem Hoppe-Seyler. 1985;366:213–221. doi: 10.1515/bchm3.1985.366.1.213. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu G, Campbell BJ, Milton JD, Rhodes JM. Identification of intact peanut lectin in peripheral venous blood [letter] Lancet. 1998;352:1831–1832. doi: 10.1016/S0140-6736(05)79894-9. [DOI] [PubMed] [Google Scholar]
- Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl. J. Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Weaver LT, Bailey DS. Effect of the lectin concanavalin A on the neonatal guinea pig gastrointestinal mucosa in vivo. J. Pediatric Gastroenterol. Nutrition. 1987;6:445–453. doi: 10.1097/00005176-198705000-00023. [DOI] [PubMed] [Google Scholar]
- Yu LG, Fernig DG, Smith JA, Milton JD, Rhodes JM. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993;53:4627–4632. [PubMed] [Google Scholar]
- Yu LG, Fernig DG, White MR, et al. Edible mushroom (Agaricus bisporus) Lectin, Which Reversibly Inhibits Epithelial Cell Proliferation, Blocks Nuclear Localization Sequence-Dependent Nuclear Protein Import. J. Biol. Chem. 1999;274:4890–4899. doi: 10.1074/jbc.274.8.4890. [DOI] [PubMed] [Google Scholar]
- Zeng FY, Benguria A, Kafert S, Andre S, Gabius HJ, Villalobo A. Differential response of the epidermal growth factor receptor tyrosine kinase activity to several plant and mammalian lectins. Mol Cellular Biochem. 1995;142:117–124. doi: 10.1007/BF00928932. [DOI] [PubMed] [Google Scholar]