Abstract
Late structural changes such as interstitial fibrosis in the renal cortex and tubular atrophy have been detected after severe acute tubular necrosis (ATN). The aim of this study was to investigate the expression of fibronectin, α-smooth muscle actin and macrophages during the evolution of the ATN induced by glycerol and their relationship with the late structural changes observed in the kidneys of these animals. Forty-nine male Wistar rats were injected with a 50% glycerol solution, 8 mL/kg (4 mL/kg applied i.m. to each hind leg) and 14 with 0.15 m NaCl solution. Before glycerol injection on day 1, water was removed for 17 h. Blood and urine samples were collected 1 day after the injection to quantify sodium and creatinine. The animals were killed 5, 30 and 60 days after the injections and the kidneys removed for histological and immunohistochemical studies. The results of the histological and immunohistochemical studies were scored according to the extent of lesion or staining in the cortical tubulointerstitium, respectively. The percentage of tubulointerstitial lesions was determined by morphometry. Glycerol-injected rats presented a transitory increase in plasma creatinine levels and in fractional sodium excretion. The immunohistochemical studies showed increased fibronectin, α-smooth muscle actin (α-SM-actin), TGF-β and ED-1 (macrophages) staining in the renal cortex from rats killed 5, 30 and 60 days after glycerol injection (P < 0.05) compared to control. The animals killed on day 30 and 60 also presented chronic lesions (fibrosis, tubular dilatation and atrophy) in the renal cortex, despite the recovery of renal function. Macrophages, TGF-β and myofibroblasts may have contributed to the development of renal fibrosis in these rats.
Keywords: Glycerol-induced ATN, fibrosis, myofibroblasts, macrophages, fibronectin
Introduction
Hypertonic glycerol injection is one of the most frequently used models of experimental acute renal failure (ARF). The pathogenesis of ARF induced by glycerol may involve, among other causes, decreased renal blood flow, reactive oxygen metabolites and rhabdomyoglobin release from muscle damage (Abulezz et al. 1991; Zager et al. 1995; Shimizu et al. 1998). Renal function and structure may not fully return to normal level after acute renal ischemic or nephrotoxic injury (Cronin & Henrich 2000; Fox 1967; Finn 1980; Pagtalunan et al. 1999, 2000). Late structural changes such as interstitial fibrosis in the renal cortex and tubular atrophy were found after severe acute ischemia (Fox 1967; Pagtalunan et al. 1999). Tubulointerstitial disease, proteinuria and glomerular lesions were observed after acute renal injury ischemia to a solitary kidney (Pagtalunan et al. 1999, 2000). However, a long-term study with the evaluation of TGF-β, α-SM-actin, fibronectin and ED1 (macrophages/monocytes) expression and their relationship with renal function and structure in rats with ATN induced by glycerol has not been performed. The increase in renal production of transforming growth factor-β (TGF-β), endothelin and angiotensin II (AII) was observed in acute tubular necrosis (ATN) induced by different agents (Goes et al. 1995; Basile et al. 1996; Shimizu et al. 1998; Forbes et al. 1999; Pagtalunan et al. 2000). These polypeptides can provoke proliferation and modify the phenotypes of renal and extrarenal cells (Desmouliere et al. 1993; Eddy 1996; Forbes et al. 1999; Xu et al. 2001). After activation, these cells transform into myofibroblasts, increase the production of extracellular matrix components and start to express α- SM-actin, a protein that normally is expressed in renal cortex only by vascular smooth muscle cells. An interstitial infiltrate of macrophages is present in all kidneys with progressive renal disease (Klahr et al. 1988; Cameron 1992; Nikolic-Patterson et al. 1994; Kliem et al. 1996). It has been shown that cultured macrophages can synthesize collagen I and fibronectin and can also produce and release several fibrogenic cytokines including TGF-β (Eddy 1996; Vaage & Lindblad 1990; Kliem et al. 1996). The aim of this study was to investigate the expression of α-SM-actin, ED1 (monocyte/macrophage), fibronectin and TGF-β in the kidney during the evolution of ATN induced by glycerol and their relationship with long-term histological changes and renal function of these animals.
Materials and methods
Animals and experimental protocols
Forty-nine male Wistar rats were injected with a 50% glycerol solution, 4 mL/kg applied i.m. to each hind leg and 14 with 0.15 m NaCl solution. Before glycerol injection on day 1, water was removed for 17 h. Twenty-four glycerol-injected animals died after injection. Blood and urine samples were collected from the 25 surviving animals 1, 5, 30 and 60 days after the glycerol injection to quantify sodium and creatinine. The animals were killed 5, 30 and 60 days after the injections, the organs were perfused with PBS solution (0.15 m NaCl and 0.01 m sodium phosphate buffer, pH 7.4) and the kidneys removed for histological and immunohistochemical studies.
Renal function studies
Plasma creatinine was measured by the Jaffé method and plasma and urine sodium (Na+) and potassium (K+) by flame photometry (Micronal, model 262, São Paulo, Brazil), and urine osmolality was determined by freezing point depression (Fiske OS Osmometer, Norwood, MS). Glomerular filtration rate (GFR) was measured by inulin clearance in 8 control animals and in 8 rats killed on day 5, 8 on day 30 and 8 on day 60 after glycerol injection. The animals were anaesthetized with an i.p. injection of 50 mg/kg sodium thionembutal. After tracheostomy, the femoral artery and vein were cannulated to collect blood samples and to inject fluids, and the ureters were cannulated to collect urine. The animals received a priming inulin dose of 12 mg/100 g followed by a maintenance dose of 30 mg/100 g/ h of inulin. After stabilization for about 60 min, urine was collected for a period of 60 min and blood was sampled at 30 and 60 min. Plasma and urine inulin was measured by the method of Füehr et al. (1955).
Blood pressure
The femural artery were cannulated in five animals in order to measure the mean arterial pressure. Blood pressure was measured in these animals before and 1, 4, 24, 48, 96 and 120 h after glycerol injection through a pressure probe connected to a polygraph.
Light microscopy
The kidneys from 6 control animals and 25 rats killed 5, 30 and 60 days after glycerol injection were fixed in 4% paraformaldehyde, postfixed in Bouin's solution for 4–6 h, and processed for paraffin embedding. Four-μm histological sections were stained with Masson's trichrome and examined under the light microscope. The percentage of glomeruli with glomerulosclerosis was determined by scoring at least 100 glomeruli in a section of each kidney. Tubulointerstitial injury was defined as tubular necrosis, inflammatory cell infiltrate, tubular dilatation and/or atrophy, or interstitial fibrosis. Injury was graded according to Shih et al. (1988) on a scale of 0–4 (0 = normal; 0.5 = small focal areas; 1 = involvement of less than 10% of the cortex; 2 = involvement of 10–25% of the cortex; 3 = involvement of 25–75% of the cortex; 4 = extensive damage involving more than 75% of the cortex). The percentage of histological alterations characteristics of chronic nephropathy such as interstitial fibrosis, tubular atrophy and dilatation and cellular infiltrate was also evaluated by morphometric studies in 8 animals killed 30 and in 9 animals killed 60 days after glycerol injection and in 6 control rats. The morphometric studies were performed with a light camera connected to an image analyser (Kontron Electronic System KS 300, Eching, Germany). Twenty grid fields from the renal cortex measuring 0.245 mm2 in a section of each kidney were evaluated. Encircled damaged areas were traced manually on a video screen, and determined by computerized morphometry (Baroni et al. 2000).
Antibodies
Primary antibodies included: a) a purified IgG fraction of polyclonal rabbit antirat fibronectin (Chemicon, International Inc., Temecula, CA, USA); (b) a murine monoclonal antibody to an NH2-terminal synthetic form of α-smooth muscle actin (α-SM-actin) (Dako, Glostrup, Denmark), (c) a mouse antirat ED1 antibody to a cytoplasmic antigen present in macrophages and monocytes (Serotec, Oxford, UK), and (d) a purified fraction of polyclonal rabbit antibody against TGF-β1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) that shows no cross-reactivity with TGF-β2 or TGF-β3.
Immunohistochemical studies
Six control animals, 8 animals killed 5 days after glycerol injection, 8 animals killed 30 days after injection and 9 rats killed 60 days after injection were used for this study. The rats were subjected to aortic perfusion with PBS (0.15 m NaCl and 0.01 phosphate buffer, pH 7.4) until the kidneys were blanched. The kidneys were then perfused with 4% paraformaldehyde, fixed in paraformaldehyde 4% for 2 h, postfixed in Bouin's solution for an additional 4–6 h, rinsed with 70% ethanol to eliminate picric acid, dehydrated through a graded alcohol series, embedded in paraffin, sectioned into 3-μm slices, deparaffinized, and subjected to immunohistochemical staining (Kliem et al. 1996; Baroni et al. 2000).
The sections were incubated overnight at 4 °C with 1/500 antifibronectin and 1/30 anti-TGF-β polyclonal antibodies produced in rabbits or 1/1000 anti α-SM-actin (overnight) and 1/2500 anti-ED-1 (1 h) monoclonals antibodies. The reaction product was detected with an avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA, USA). The colour reaction was developed with 3, 3-diamino-benzidine (Sigma Chemical Company, St. Louis, MO, USA), and the material was counterstained with methylgreen, dehydrated and mounted. Nonspecific protein binding was blocked by incubation with 20% goat serum in PBS for 20 min. Negative controls consisted of replacement of primary antibody with equivalent concentrations of normal rabbit IgG or mouse IgG for polyclonal and monoclonal antibodies, respectively.
For evaluation of immunoperoxidase staining for fibronectin, TGF-β and α-SM-actin each tubulointerstitial grid field was graded semiquantitatively, and the mean score per kidney was calculated (Kliem et al. 1996). Each score reflected mainly changes in the extent, rather than the intensity, of staining and depended on the percentage of grid field showing positive staining: 0, absent or less than 5%; I, 5–25%; II, 25–50%; III, 50–75%, and IV, >75%. To obtain mean numbers of infiltrating macrophages/monocyte cells in the renal cortical tubulointerstitium, 30 grid fields measuring 0.245 mm2 each were evaluated, and mean counts per kidney were calculated.
Statistical analysis
Data were submitted to analysis of variance with multiple comparisons by the Tukey test, with the level of significance set at P < 0.05.
Results
Renal Function
All glycerol-treated rats presented a transitory increase in plasma creatinine levels and in fractional excretion of sodium and potassium (Table 1) that peaked at day 5 after glycerol injection (P < 0.01) and returned to normal levels at day 30. The GFR measured on day 30 after glycerol injection was 0.62 ± 0.05 mL/min/100 g body weight and did not differ from the control group (0.85 ± 0.09 mL/min/100 g). We also observed a transitory decrease in urine osmolality after glycerol injection compared to control (Table 1).
Table 1.
Parameters of renal function of control rats and glycerol (G)-injected rats
| Groups | C (N=8) | G-5days (N=8) | G-30days (N=8) | G-60days (N=8) |
|---|---|---|---|---|
| FENa% | 0.33±0.04 | 9.22±1.09** | 0.44±0.05 | 0.43±0.038 |
| Fek% | 30.26±2.48 | 214.10±36.44** | 46.05±2.84 | 32.08±4.73 |
| Uosm | 2160±215 | 752±77** | 1817±133 | 1951±259 |
| Pcreat | 0.61±0.07 | 1.84±0.17** | 0.71±0.05 | 0.63±0.05 |
| V | 11.09±0.77 | 18.86±4.09 | 12.71±1.15 | 12.88±3.37 |
| GFR | 0.85±0.09 | 0.22±0.04** | 0.62±0.05 | 0.62±0.09 |
Data are expressed as mean±SEM. FE, fractional excrection, GFR, glomerular filtration rate (ml/min/100g), Pcreat., plasma creatinine (mg/dL), Uosm, urine osmolality (mOsm/kg H2O), V, urinary volume (μm/min.).
P<0.001 vs. control
Blood pressure
A transitory reduction in mean arterial pressure was observed 24 and 48 h after glycerol injection, returned to normal level 3 days after injection. However, two of these animals died 4 days after the glycerol injection. The mean arterial pressure was 109.0 ± 3.3 mmHg before glycerol injection and 95.8 ± 1.3 mmHg and 96.0 ± 2.5 mmHg 24 and 48 h after, respectively.
Light microscopy studies
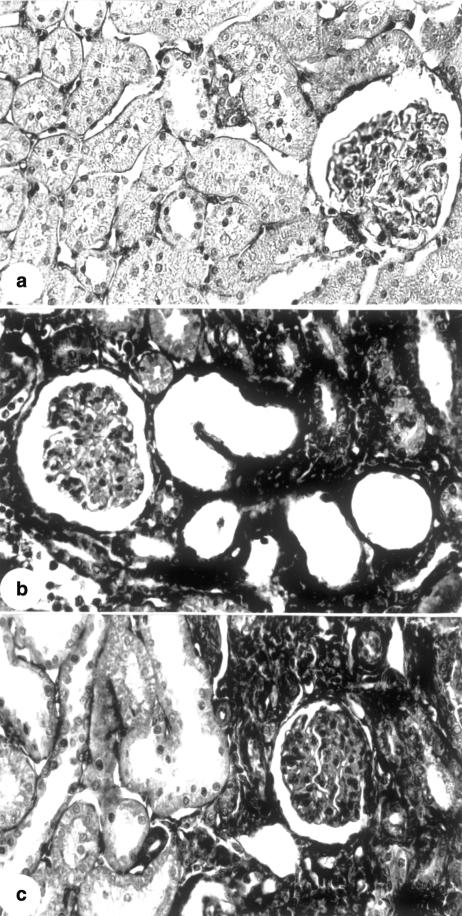
Light microscopy studies showed the following morphological features characteristic of ATN in the renal cortex of rats on day 5 after glycerol injection: tubular cell necrosis, focal areas of denuded basement membrane, intraluminal casts, swelling and flattening of proximal tubular cells with brush border loss, diffuse interstitial oedema, and interstitial inflammatory cell infiltrates (Fig. 1c). Glomerular morphology remained unchanged. However, in the animals killed on day 30 and 60 after glycerol injection we found histological alterations characteristic of chronic nephropathy such as interstitial fibrosis, tubular atrophy and dilatation and inflammatory cell infiltrates (Figs 1b, d). The animals killed 30 and 60 days after glycerol treatment also showed 6.20% ± 0.97 and 7.01%± 0.92, respectively, of the glomeruli with focal or global sclerosis, whereas the control rats presented 1.41%±0.40 of glomeruli with focal or global sclerosis.
Figure 1.
Masson trichrome-stained histological sections from renal cortex (a, b, c and d) of a control rat (a) and of rats killed 5 (c) and 30 days (b and d) after glycerol injection. Note the presence of tubular dilatation, swelling and flattening of proximal tubular cells with brush border loss, diffuse interstitial oedema and interstitial inflammatory cellular infiltrates in (c) and of fibrosis, interstitial inflammatory cellular infiltrate and tubular dilatation and atrophy in (b) and (d) X120, (a) and (b); X280, (c) and (d).
Immunohistochemical studies
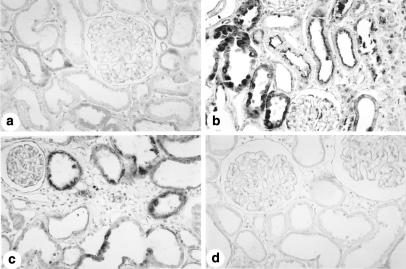
The immunohistochemical studies showed increased fibronectin and ED-1 (monocyte/macrophage) staining in the tubulointerstitium from the renal cortex of rats killed 5, 30 and 60 days after glycerol injection compared to control (P < 0.05), with a diffuse distribution on day 5 and a focal distribution and primarily located in the damaged areas on days 30 and 60. We also observed an increase in α-SM-actin staining in the animals killed 5 and 30 days after glycerol injection. The increased fibronectin, α-SM-actin and ED-1 reaction peaked on day 5 (Table 2, Figs 2 and 3). The control rats presented α-SM-actin staining confined to arterial smooth muscle cells and a small number of macrophages in the renal cortex (Table 1, Fig. 3a and 3b). Higher immunohistochemical staining for TGF-β in interstitial and tubular cells was also observed in the glycerol-injected animals that peaked at day 5 (Table 2, Fig. 4b, c). The immunostaining for this peptide presented a diffuse distribution in the renal cortical tubulointerstitium on day 5 and a focal distribution localized in the damaged areas on days 30 and 60. The control animals presented reaction for TGF-β of mild intensity in the cytoplasm of some tubule cells (Fig. 4a).
Table 2.
Score and percentage (morphometric studies, MS) of tubulointerstitial lesions (TIL) and scores for α- SM-Actin, fibronectin, and TGF-β staining in cortical tubulointerstitium and number of cells ED1 positive per grid field of renal cortex measuring 0.245 mm2 for control rats (C) and for rats killed 5 (G-5 days), 30 (G-30 days) and 60 days (G-60 days) after glycerol (G) injection
| Groups | C (N=6) | G-5days (N=8) | G-30days (N=8) | G-60days (N=9) |
|---|---|---|---|---|
| TIL, score | 0.10±0.10 | 3.75±0.16*** | 1.94±0.24***### | 2.00±0.24***### |
| %TIL, MS | 0.63±0.28 | 13.82±2.74* | 17.44±2.70** | |
| α-SM-actin | 0.025±0.01 | 1.48±0.14*** | 0.33±0.07*### | 0.27±0.05### |
| Fibronectin | 0.38±0.04 | 1.31±0.03*** | 0.97±0.13**# | 1.11±0.08*** |
| ED1+cells | 5.05±1.20 | 44.76±4.67*** | 19.00±3.45*### | 18.42±2.08*### |
| TGF-β | 0.66±0.07 | 1.52±0.04*** | 1.12±0.03***### | 1.14±0.05***### |
Data are expressed as mean±SEM
P < 0.05 vs. control
P < 0.01 vs. control
P < 0.001 vs. control
P < 0.05 vs. G-5days
P < 0.01 vs. G-5days
P < 0.001 vs. G-5days.
Figure 2.
Immunolocalization of fibronectin in the renal cortex from a control rat (a) and from rats killed 5 (b) and 30 days (c) after glycerol injection. Observe that the immunoreaction for fibronectin is more intense in glycerol-injected rats (b and c) than in the control animal (a). X280.
Figure 3.
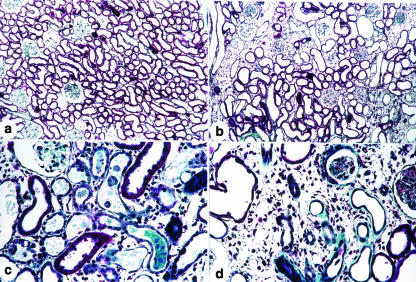
Immunolocalization of α-SM-actin (a, c and e) and ED1 (b, d and f) in the renal cortex from a control rat (a and b) and from rats killed 5 (c and d) and 30 days (e and f) after glycerol injection. Note that the staining for α-SM-actin is more intense in c and e than in a and the increase of ED1 + cells (monocytes/macrophages) is more marked in d and f compared to b. X280.
Figure 4.
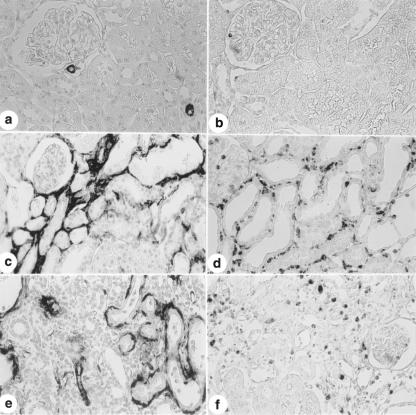
Immunolocalization of TGF-β in the renal cortex from a control rat (a) and from rats killed 5 (b) and 30 days (c) after glycerol injection using a polyclonal anti-TGF-β antibody. Note that the staning for TGF-β in tubular and interstitial cells is more intense in b and c than in a. d, Immunolocalization of TGF-β in the renal cortex from rats killed 30 days after glycerol injection using a normal rabbit IgG; the staining was abolished by this treatment. X280.
Discussion
The results of this study show that glycerol-injected rats presented histological alterations in the renal cortex characteristic of ATN on day 5 after treatment and histological features of chronic nephropathy such as interstitial fibrosis, tubular atrophy or dilatation on days 30 and 60. All glycerol-injected rats showed a transitory increase in plasma creatinine levels and in fractional excretion of sodium and potassium which returned to normal levels on day 30. The GFR of these animals measured on day 30 and 60 after glycerol injection did not differ from control despite the morphological alterations. The percentage of glomeruli with sclerosis was 6.20 and 7.01, respectively, and probably these glycerol-injected animals had enough normal glomeruli and tubulointerstitium areas to maintain renal function. However, disturbances in renal function can persist in some patients with transplanted kidneys or with prior renal disease (Brophy et al. 1980; Tilney & Guttmann 1997). Residual areas of interstitial fibrosis in gentamicin-treated animals were observed by Cronin & Henrich (2000). Fox (1967) described late structural changes such as interstitial fibrosis and round cell infiltration following acute renal artery clamping in mice. Finn (1980) showed that there was a reduction in the number of functioning nephrons at 4 weeks after acute ischemic injury in rats. Pagtalunan et al. (1999) found tubulointerstitial disease, proteinuria and glomerular lesions at 20 weeks after acute ischemic injury to a solitary kidney. Some abnormalities of renal function with long-term implications were also observed in patients with acute tubular necrosis after renal transplantation (Brophy et al. 1980; Tilney & Guttmann 1997).
The immunohistochemical studies showed increased immunoreaction for α-SM-actin and fibronectin and a larger number of ED1-positive cells (macrophages/monocytes) in the tubulointerstitial area from the renal cortex of glycerol-injected animals which persisted until day 30 and 60. Marked increases in macrophage and myofibroblast content and collagen accumulation in renal cortex were also found by Forbes et al. (1999, 2000) in a model of ischemic acute renal failure. This increase was observed only in the first week postischemia. The only marker of renal injury remaining 64 days postischemia detected by these authors was the accumulation of collagen III in the inner cortical region. An interstitial infiltrate of macrophages is present in all kidneys with progressive renal disease (Klahr et al. 1988; Cameron 1992; Nikolic-Patterson et al. 1994; Kliem et al. 1996). It has been shown that cultured macrophages can synthesize collagen I and fibronectin (Eddy 1996; Vaage & Lindblad 1990) and can also produce and release several fibrogenic cytokines including TGF-β (Eddy 1996; Kliem et al. 1996).
Myofibroblasts and macrophages can be involved in the fibrosis observed in glycerol-injected rats by the release of fibrogenic peptides such as TGF-β, interleukin-1, endothelin and AII and, in addition, these cells can produce collagen and other ECM components such as several types of collagens and fibronectin. Fibronectin usually appears first, forming a scaffold for the deposition of other proteins and acting as a fibroblast chemoattractant (Eddy 1996). It was observed that α-SM-actin overexpression in the course of several renal diseases is associated with fibrosis (Eddy 1996; El Nahas et al. 1996; Strutz et al. 1996; Geleilete et al. 2001). Fibroblasts derived from fibrotic human kidneys synthesize more total collagen than fibroblasts derived from normal kidneys (Rodemann & Muller 1991). We found an increased staining for α-SM-actin and ED1 (monocytes/macrophages) with a diffuse distribution on day 5 after glycerol injection, and on day 30 and 60 we observed a focal distribution for these proteins in the tubulointerstitium from the renal cortex of these animals primarily located in the damaged areas.
The glycerol-injected animals also presented increase immunostaining for TGF-β in renal cortex located primarily in the cytoplasm of tubule cells and in interstitial cells. Higher TGF-β expression was found in experimental models of acute renal failure provoked by ischemic injury (Goes et al. 1995; Basile et al. 1996). This polypeptide can transform fibroblasts into myofibroblasts (Desmouliere et al. 1993; Eddy 1996). TGF-β has been considered to be one of the major fibrogenic cytokines. This polypeptide enhances the synthesis of matrix components and blocks matrix degradation, thus promoting ECM deposition (Coimbra et al. 1991; Sharma & Ziyadeh 1994; Eddy 1996).
Angiotensin II may also be involved in the development of renal fibrosis in these glycerol-treated rats. AII is a potent inducer of TGF-β and PDGF and some of its effects are mediated by the expression of these factors (Border & Noble 1998; Sharma & Ziyadeh 1994). Pagtalunan et al. (2000) found that reduction of angiotensin activity by treatment with enalapril prevents late secondary glomerular injury and reduces the proteinuria provoked by acute renal ischemia. However, this treatment did not modify the interstitial fibrosis observed during the recovery from ischemic injury.
In conclusion, taken together, these data show that the renal damage induced by glycerol can progress to fibrosis despite the recovery of renal function and suggest that TGF-β myofibroblasts and macrophages may contribute to this process.
Acknowledgments
The authors would like to thank Erika Dellaiogono and Rubens Fernando de Melo for expert technical assistance.
Funding
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil. RSC and TMC are recipients of fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico, DF, Brazil.
References
- Abulezz SR, Walker PD, Shah SV. Role of glutathione in an animal- model of myoglobinuric acute-renal-failure. Proc. Natl. Acad. Sci. USA. 1991;88:9833–9837. doi: 10.1073/pnas.88.21.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni EA, Costa RS, Da Silva CGA, Coimbra TM. Heparin treatment reduces glomerular injury in rats with adriamycin-induced nephropathy but does not modify tubulointerstitial damage or the renal production of transforming growth factor-beta. Nephron. 2000;84:248–257. doi: 10.1159/000045585. [DOI] [PubMed] [Google Scholar]
- Basile DP, Rovak JM, Martin DR, Hammerman MR. Increased transforming growth factor-(1 expression in regenerating rat tubules following ischemic injury. Am. J. Physiol. 1996;39:F500–F509. doi: 10.1152/ajprenal.1996.270.3.F500. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31(Suppl.):181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- Brophy D, Najarian JS, Kjellstrand CM. Acute tubule necrosis after renal transplantation. Transplantation. 1980;29:245–248. doi: 10.1097/00007890-198003000-00016. [DOI] [PubMed] [Google Scholar]
- Cameron J S. Tubular and interstitial factors in the progression of glomerulonephritis. Pediatr. Nephrol. 1992;6:292–303. doi: 10.1007/BF00878382. [DOI] [PubMed] [Google Scholar]
- Coimbra TM, Wiggins RC, Noh JW, Merritt S, Phan S. Transforming growth factor-(production in anti-glomerular basement membrane in the rabbit. Am. J. Pathol. 1991;138:223–234. [PMC free article] [PubMed] [Google Scholar]
- Cronin RE, Henrich WL. Toxic Nephropathies. In: Brenner B M, editor. The Kidney. Philadelphia: W.B. Saunders Company; 2000. pp. 1563–1596. [Google Scholar]
- Desmouliere A, Geinoz A, Gabiani F, Gabbiani G. Transforming growth factor-beta induces alpha-smooth-muscle actin expression in granulation-tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell. Biol. 122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy AA. Molecular insights into renal interstitial fibrosis. J. Am. Soc. Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- El Nahas AM, Muchaneta-Kubara EC, Zhang G-Z, Adam A, Goumenos D. Phenotypic modulation of renal cells during experimental and clinical scarring. Kidney Int. 1996;49(Suppl. 54):S23–S27. [PubMed] [Google Scholar]
- Finn WF. Enhanced recovery from post-ischemic acute renal failure-Micropuncture studies in the rat. Circ. Res. 1980;46:440–448. doi: 10.1161/01.res.46.3.440. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Hewitson TD, Becker GJ, Jones CL. Ischemic acute renal failure: Long-term histology of cell and matrix changes in the rat. Kidney International. 57:2375–2385. doi: 10.1046/j.1523-1755.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- Forbes JM, Leaker B, Hewitson TD, Becker GJ, Jones CL. Macrophage and myofibroblast accumulation in ischemic acute renal failure is attenuated by endothelin receptor antagonists. Kidney Int. 1999;55:198–208. doi: 10.1046/j.1523-1755.1999.00253.x. [DOI] [PubMed] [Google Scholar]
- Fox M. Progressive renal fibrosis following acute tubular necrosis. An experimental study. J. Urol. 1967;97:196–202. doi: 10.1016/S0022-5347(17)63012-X. [DOI] [PubMed] [Google Scholar]
- Füehr Y, Kaczmarszk Y, Kruttgen GD. Eine einfache colorimetrische Methode zur Inulin Bestimmung für Nieren Clearance-Untersuchungen bei Stoffwechselgesundend und Diabetikern. Klin. Wochenchr. 33:729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Geleilete TJM, Costa RS, Dantas M, Coimbra TM. α-Smooth muscle actin and proliferating cell nuclear antigen expression in focal segmental glomerulosclerosis: functional and structural parameters of renal disease progression. Braz. J. Med. Biol. Res. 2001;34:985–991. doi: 10.1590/s0100-879x2001000800003. [DOI] [PubMed] [Google Scholar]
- Goes N, Urmson J, Ramassar V, Halloran PF. Ischemic tubular necrosis induces an extensive local cytokine response. Evidence for induction of interferon-gamma, transforming growth factor-beta-1, granulocy-macrophage colony-stimulating factor, interleukin-2 and interleukin-10. Transplantation. 1995;59:565–572. [PubMed] [Google Scholar]
- Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N. Engl. J. Med. 1988;318:1657–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- Kliem V, Johnson RJ, Alpers CE, et al. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49:666–678. doi: 10.1038/ki.1996.95. [DOI] [PubMed] [Google Scholar]
- Nikolic-Patterson DJ, Lan HY, Hill PA, Atkins RC. Macrophages in renal injury. Kidney Int. 1994;45(Suppl.):S79–S82. [PubMed] [Google Scholar]
- Pagtalunan ME, Olson JL, Meyer TW. Contribution of angiotensin. II to late injury after acute ischemia. J. Am. Soc. Nephrol. 2000;11:1278–1286. doi: 10.1681/ASN.V1171278. [DOI] [PubMed] [Google Scholar]
- Pagtalunan ME, Olson JL, Tilney NL, Meyer TW. Late consequences of acute ischemic injury to a solitary kidney. J. Am. Soc. Nephrol. 1999;10:366–3783. doi: 10.1681/ASN.V102366. [DOI] [PubMed] [Google Scholar]
- Rodemann HP, Muller GA. Characterization of human renal fibroblasts in health and disease. II. In vitro growth, differentiation, and collagen synthesis of fibroblasts from kidneys with interstitial fibrosis. Am. J. Kidney. Dis. 1991;17:684–686. doi: 10.1016/s0272-6386(12)80352-0. [DOI] [PubMed] [Google Scholar]
- Sharma K, Ziyadeh FN. The emerging role of transforming growth factor β in kidney disease. Am. J. Physiol. 1994;266:F829–F842. doi: 10.1152/ajprenal.1994.266.6.F829. [DOI] [PubMed] [Google Scholar]
- Shih W, Hines WH, Neilson EG. Effects of cyclosporin A on the development of immune-mediated interstitial nephritis. Kidney Int. 1988;33:1113–1118. doi: 10.1038/ki.1988.119. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kuroda T, Ikeda M, Hata S, Fujimoto M. Potential contribution of endothelin to renal abnormalities in glycerol-induced acute renal failure in rats. J. Pharmacol. Exp. Ther. 1998;286:977–983. [PubMed] [Google Scholar]
- Strutz F, Muller GA, Neilson EG. Transdifferentition: a new angle on renal fibrosis. Exp. Nephrol. 1996;4:267–270. [PubMed] [Google Scholar]
- Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation. 1997;64:945–947. doi: 10.1097/00007890-199710150-00001. [DOI] [PubMed] [Google Scholar]
- Vaage J, Lindblad WJ. Production of collagen type I by mouse peritoneal macrophages. J. Leukocyte Biol. 1990;48:274–280. doi: 10.1002/jlb.48.3.274. [DOI] [PubMed] [Google Scholar]
- Xu SW, Denton CP, Dashwood MR, et al. Fibroblast matrix gene expression and connective tissue remodelingRole Endothelin-1. J. Invest. Dermatol. 2001;116:417–425. doi: 10.1046/j.1523-1747.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- Zager RA, Durkhart KM, Conrad DS, Gmur DJ. Iron, heme oxygenase, and glutathione: effects on myoglobinuric proximal tubular injury. Kidney Int. 1995;48:1624–1634. doi: 10.1038/ki.1995.457. [DOI] [PubMed] [Google Scholar]