Abstract
In man, chloramphenicol (CAP), induces two major haemotoxic effects. First, a reversible, dose-related reticulocytopenia and anaemia developing during treatment. Second, a non-dose-related aplastic anaemia (AA), developing weeks after treatment, is often irreversible and fatal. In previous studies, we developed a mouse model of the reversible reticulocytopenia/anaemia using CAP succinate (CAPS); attempts to induce AA in the mouse with CAPS were unsuccessful; in the rat, CAPS induced only minimal haemotoxicity. We therefore wished to investigate haematological changes caused by CAPS in a third rodent, particularly in relation to the induction of significant ‘late stage’ bone marrow depression (AA). Female guinea pigs were gavaged with CAPS in three experiments. In a dose ranging study, CAPS (at 2500 and 3500 mg/kg) was administered daily for 9 days, and blood examined at 1 day post dosing. CAPS induced increased erythrocyte values (an apparent haemoconcentration effect), and reduced reticulocytes and femoral marrow nucleated cell counts (FNCC). In a second experiment, CAPS was given at 333, 666 and 1000 mg/kg (13 days); haematological changes were compared with results from the initial study, with evidence of dose-related effects. In a final experiment, CAPS was administered (825 mg/kg, 16 days) and blood studied at 1, 12, 28 and 63 days post dosing. At day 1, erythrocyte values were decreased (NS), and reticulocytes and FNCC were reduced; the marrow was hypocellular with erythroid depletion. At 12 and 28 days, values returned towards the normal range. At 63 days, parameters were normal. Thus, CAPS (825 mg/kg for 16 days) induced changes comparable to the reversible bone marrow depression seen in man; but there was no evidence of ‘late stage’ (i.e. at 63 days) marrow depression, as would be seen in a developing or overt marrow aplasia (AA). The guinea pig (like the mouse) is a model for the early events, but is not a good model for CAP-induced AA in man.
Keywords: blood, bone marrow, chloramphenicol guinea pig, haemotoxicity
Introduction
Chloramphenicol (CAP) is a broad-spectrum antibiotic, first isolated from Streptomyces venezuelae in 1947. The antibiotic was originally used clinically in 1948 in the treatment of typhus fever (Volini et al. 1950). CAP then became widely employed in the treatment of serious infections such as typhoid fever and salmonellosis, and in severe infections due to Haemophilus influenzae, particularly meningitis, and other life-threatening infections of the respiratory tract and central nervous system. CAP is bactericidal, highly active, relatively cheap, has good pharmacokinetic properties, and can be given by the oral, intravenous, and intramuscular routes. For systemic use, the dose is generally 50 mg/kg/day, in divided doses, although higher dose levels have frequently been used; treatment may be continued for 10–15 days. CAP is currently used extensively in the developing world (Trevett et al. 1992; Kumar & Verma 1993; Kushwaha et al. 1994), but in the UK and USA the antibiotic is now mainly employed in the topical treatment of ear and eye infections (Dollery 1999; Parfitt 1999).
CAP, however, is haemotoxic in man, inducing three effects (FAO/WHO 1988; IARC 1990; Young & Alter 1994). First, a frequently occurring reticulocytopenia and mild anaemia, sometimes seen in association with leucopenia (granulocytopenia) and thrombocytopenia (Yunis & Bloomberg 1964). This form of bone marrow depression develops during treatment, is dose-related, and is rapidly reversible (in two to three weeks) on withdrawal of therapy (Krakoff et al. 1955; Scott et al. 1965; Best 1967). The bone marrow shows normal or reduced cellularity with decreased numbers of erythroid cells, an increased myeloid : erythroid (M : E ratio), and vacuolation of precursor cells (Rubin et al. 1958; Chaplin 1986). The second major haemotoxicity is aplastic anaemia (AA) (Rich et al. 1950; Welch et al. 1954; Wallerstein et al. 1969). This change is relatively rare, but is seen in peripheral blood as a severe pancytopenia. The effect is not related to the dose of the drug (Yunis & Bloomberg 1964; Yunis 1978; FAO/WHO 1988; Yunis 1988), develops weeks after therapy, and is often irreversible and fatal; the marrow is acellular or hypocellular (Yunis 1978). In CAP-induced AA it is considered that a genetic element is involved, giving rise to an individual metabolic predisposition towards the toxicity of the drug (FAO/ WHO 1988; Fraunfelder et al. 1993; Kumana et al. 1993; Festing et al. 2001). A third (unusual) haemotoxicity of CAP in man is seen in some individuals surviving AA; in these cases there is an increased incidence of leukaemia; it is therefore considered that there is some evidence for the carcinogenicity of CAP in man, and that AA is related to the development of leukaemia (Cohen & Creger 1967; FAO/WHO 1988; IARC 1990; Parfitt 1999).
We have studied the haemotoxicity of CAP succinate (CAPS) in the CD-1 mouse and demonstrated similarities in the murine response to the dose-dependent, reversible reticulocytopenia and anaemia seen in man (Turton et al. 1999). Studies in the Wistar Hanover rat demonstrated that in this species, CAP only induced mild haemotoxicity: after the administration of high dose levels (3600 and 4000 mg/kg for 19 days); haemoglobin (Hb) values were reduced but reticulocytes were unaffected (Turton et al. 1999). As CAP-induced AA in man is considered to involve a genetic element, CAPS haemotoxicity was investigated in different inbred mouse strains; clear differences between strains were demonstrated in the dose-dependent, reversible form of anaemia (Festing et al. 2001).
There are no reports in the literature of CAP inducing AA in laboratory rodents, and only a single report of the induction by CAP of AA experimentally in the calf (Krishna et al. 1981). Therefore, an investigation was carried out to assess the ability of CAPS, and the related antibiotic thiamphenicol (TAP), to induce AA in the BALB/c mouse (Turton et al. 2000); results showed that both agents caused the reversible form of anaemia, but AA was not induced.
Having demonstrated that CAPS is only mildly haemotoxic in the rat, and having been unsuccessful in attempts to induce AA in the BALB/c mouse, we wished to examine the haemotoxicity of the antibiotic in a third rodent species. We have therefore carried out a study to define the haemotoxicity of repeat dose, gavage administered, CAPS in the female Dunkin Hartley guinea pig; a preliminary report has been published (Havard et al. 1999). Of particular interest was whether any response would show similarities to the reversible reticulocytopenia/anaemia seen in man, and whether a ‘late stage’ marrow depression/aplasia (AA) would be induced in this species, making the guinea pig a suitable animal model for CAP-induced AA in man. No investigations on the haemotoxicity of CAP/CAPS in the guinea pig have previously been reported.
Materials and methods
Animals
Female, weanling, Dunkin Hartley guinea pigs (B & K Universal Ltd, Grimston, Aldborough, East Yorkshire HU11 4QE, UK) where caged in groups of 2–4 in conventional solid-bottomed rat cages with raised lids. Animals were bedded on wood shavings, with diet (Rabbit and Guinea Pig Maintenance Diet, B & K Universal Ltd) and mains drinking water ad libitum; additional hay, green vegetables and carrots were fed twice each week. A temperature of 19–22 °C was maintained, with a relative humidity of 45–65%, and a 12 : 12 h light : dark cycle (lights on at 07.00 hours). Animals were acclimatized for 7 days or more before the start of the experiments, and were observed at least twice daily for signs of ill health. Body weights were determined daily. In one experiment, water consumptions were carried out daily, using conventional water bottles. All animal procedures were conducted under local Ethical Committee guidelines and approval for Home Office Project and Personal Licences, and followed the UK Home Office (1989) ‘Code of Practice for the Housing and Care of Animals used in Scientific Procedures’.
Administration of drug
Solutions of chloramphenicol succinate (CAPS; Sigma Chemical Co Ltd, Poole, Dorset, UK) in distilled water were administered by gavage in dose volumes of 0.5–1.5 mL; control animals were given distilled water.
Haematological measurements
Guinea pigs were killed by ip injection of pentobarbitone sodium (Sagatal, Rhône Mérieux Ltd, Harlow, Essex, UK) and blood removed from the abdominal aorta. Blood (0.5 mL) was anticoagulated with 1.5 mg/mL dipotassium EDTA (Teklab, Sacriston, Durham, UK). The femur was removed, the proximal epiphysis cut off, and the femur contents flushed into 5–15 mL PBS to prepare a marrow cell suspension; a marrow smear was prepared from the contents of the left tibia. The sternum was removed and placed in 10.5% phosphate buffered formalin fixative (14 days) and decalcified (3 days) in Kristenson's solution. Sections (3–4 µm) were prepared and stained with haematoxylin and eosin (H&E) for histological examination.
Blood was analysed with a Bayer H*1 haematology analyser with guinea pig-specific software (Bayer Diagnostics UK Ltd, Newbury, Berks., UK), as described previously (Turton et al. 1999). Reticulocyte analysis was performed with a Sysmex R-1000 (Sysmex UK Ltd, Milton Keynes, Bucks., UK) with voltage gain adjusted optimally for the guinea pig. Three equal divisions of the total number of reticulocytes gave the percentage low (LFR), mid (MFR) and high fluorescence reticulocytes (HFR). For femoral marrow cell suspensions in PBS, the total nucleated cell count (femoral nucleated cell count; FNCC) was obtained from the basophil channel of the H*1. Femoral marrow smears were stained with May-Grünwald-Giemsa and differential counts performed by eye on 200 cells to give the myeloid : erythroid (M : E) ratio.
Statistical analysis
Treated and control (vehicle-treated) groups were compared using Student's t-tests for paired and unpaired samples using Microsoft Excel (Microsoft Corporation).
Experimental design
Experiment 1: Dose ranging study
Guinea pigs (mean body weight 269 g; n = 3 or 5) were randomised into 3 groups and gavage dosed daily with water, or CAPS at 2500 and 3500 mg/hg for 9 days. Animals were killed at 1 day after the final CAPS dose and haematological parameters and femoral marrow nucleated cell counts assessed.
Experiment 2: Dose–response study
Guinea pigs (mean body weight 236 g; n = 3 or 5) were randomised into four groups and gavage daily for 13 days with water, or CAPS at 333, 666 and 1000 mg/kg. Animals were killed at 1 day after the final CAPS dose and haematological parameters and femoral marrow nucleated cell counts assessed.
Experiment 3: Reversibility study
Guinea pigs (mean body weight 301 g; n = 3 or 5), were randomised into two groups and gavaged daily with water or CAPS at 825 mg/kg for 16 days. Daily water consumptions were carried out. Animals were sacrificed at 1, 12, 28 and 63 days after the final dose, and blood parameters and marrow nucleated cell counts studied, and marrow smears (differential counts) assessed.
Results
Experiment 1: Dose ranging study
Body weight changes, clinical signs, autopsy observations
Groups of guinea pigs (n = 3 or 5) were dosed at 0, 2500 and 3500 mg/kg CAPS daily for 9 days and killed at 1 day after the final dose. During the dosing period, control animals increased from a mean body weight of 264.0 g to 365.4 g, an increase of 38.4%. At 2500 mg/kg CAPS, there was an overall reduction in mean body weight over the dosing period (10.2%), and at 3500 mg/kg the reduction was 10.6%. Some clinical evidence of CAPS toxicity was seen at both CAPS dose levels; there was some loss of condition of the fur (becoming rough and staring from day 3/4 of dosing), mild body alopecia (from day 4), some staining of the fur in the urinogenital region (from day 4/5), and soft faeces (from day 5/6). At autopsy, gross examination of the major internal organs showed no abnormalities in CAPS-treated animals.
Haematological findings
Results are presented in Table 1. Values for RBC, HCT and Hb were increased in guinea pigs treated with CAPS at both 2500 and 3500 mg/kg. CAPS caused decreased values for MCV and MCH. Leucocytes were unaffected by CAPS treatment, apart from reduced eosinophil counts. Platelet counts were decreased (NS). Total reticulocyte counts, and low, mid and high fluorescence reticulocyte counts in general showed significant decreases. The FNCC was also significantly reduced in CAPS-treated animals. There was some evidence that the changes in RBC, Hb, MCV, MCH and FNCC were dose-related.
Table 1.
Haematological results† from female guinea pigs dosed with CAPS daily for 9 days at 2500 and 3500 mg/kg and sampled at 1 day after the final dose
| Dose level of CAPS (mg/kg) | |||
|---|---|---|---|
| Control | 2500 | 3500 | |
| RBC | 5.00 (0.37) | 6.31 (0.19)*** | 6.53 (0.33)*** |
| HCT | 0.431 (0.048) | 0.524 (0.018)** | 0.521 (0.013)*** |
| Hb | 13.5 (1.4) | 15.8 (0.3)** | 16.0 (0.4)*** |
| MCV | 86.0 (3.6) | 83.1 (1.4) | 79.9 (2.7)** |
| MCH | 26.8 (1.1) | 25.0 (0.4)* | 24.5 (1.1)** |
| MCHC | 31.2 (0.6) | 30.1 (0.6) | 30.7 (0.5) |
| Retic | 171.1 (89.8) | 20.5 (13.2)* | 44.4 (17.8)** |
| LFR | 101.6 (41.6) | 19.4 (12.6)* | 42.2 (17.7)* |
| MFR | 38.2 (25.0) | 0.9 (0.7)* | 1.7 (0.9)** |
| HFR | 31.3 (25.0) | 0.2 (0.1) | 0.5 (0.2)* |
| Plt | 805 (197) | 507 (45) | 582 (193) |
| WBC | 1.3 (0.8) | 1.2 (0.5) | 1.5 (0.8) |
| Neut | 0.39 (0.13) | 0.69 (0.52) | 0.79 (0.44) |
| Lymph | 0.81 (0.63) | 0.43 (0.12) | 0.54 (0.31) |
| Mono | 0.01 (0.00) | 0.01 (0.00) | 0.04 (0.04) |
| Eo | 0.03 (0.02) | 0.00 (0.00)* | 0.00(0.01)** |
| Baso | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.01) |
| LUC | 0.07 (0.05) | 0.04 (0.02) | 0.07 (0.04) |
| FNCC | 12.20 (2.88) | 5.24 (3.65)* | 3.99 (2.31)*** |
Values are means, SD in parentheses.
Asterisks denote significant differences from control animals
P <0.05
P <0.01
P <0.001.
n = 5 (control and 3500 mg/kg CAPS), n = 3 (2500 mg/kg CAPS).
Abbreviations and units: RBC, red blood cells, ×1012/L; HCT, haematocrit, L/L; Hb, haemoglobin, g/dL; MCV, mean cell volume, fl: MCH, mean cell haemoglobin, pg; MCHC, mean cell haemoglobin concentration, g/dL; Retic, absolute reticulocyte count, ×109/L; LFR, MFR, HFR, low, mid and high fluorescence reticulocytes, ×109/L; Plt, platelets, ×109/L; WBC, white blood cells, ×109/L; Neut, neutrophils, ×109/L; Lymph, lymphocytes, ×109/L; Mono, monocytes, ×109/L; Eo, eosinophils, ×109/L; Baso, basophils, ×109/L; LUC, large unstained cells, ×109/L; FNCC, femoral marrow nucleated cell count, ×107.
The increase in erythrocyte parameters (RBC, HCT, Hb) in this experiment was unexpected, suggesting a possible haemoconcentration effect in association with reduced diet and water intake. A second experiment was therefore designed to examine if the increase in RBC, HCT and Hb could be prevented by the administration of lower dose levels of CAPS, and whether the myelotoxicity of CAPS at these lower levels was dose-related.
Experiment 2: Dose–response study
Body weight changes, clinical signs, autopsy observations
CAPS at 0, 333, 666 and 1000 mg/kg was administered to guinea pigs (n = 3–5) daily for 13 days. Blood and marrow samples were taken at 1 day after the final CAPS dose. During the 13 day dosing period, control guinea pigs increased in mean body weight from 189.6 g to 307.6 g (a 62.2% increase). In animals treated with CAPS, body weight increases over the dosing period were less than the controls, being 29.9% (at 333 mg/kg), 16.9% (666 mg/kg) and 12.6% (1000 mg/kg). At all CAPS dose levels there were no clinical signs of toxicity, except slight staining in the urinogenital area in two of three animals treated at 1000 mg/kg. At the autopsy, gross examination of the internal organs of all animals showed no CAPS-induced changes.
Haematological findings
As seen in the first experiment, RBC, HCT and Hb were, in general, significantly increased above control values (Table 2), and the effects on HCT and Hb were dose-related. Similarly, there was a trend for decreased values of MCV and MCH at all CAPS dose levels. The WBC, individual leucocyte and platelet counts, showed no clear effects of CAPS administration, at any dose level, except for a dose-related trend for a reduction in eosinophil counts. Counts for total reticulocytes, and low, mid and high fluorescence reticulocytes, were significantly decreased at all CAPS dose levels. The FNCC was also reduced by all dose levels of CAPS, but at 333 mg/kg the effect was not statistically significant. The effects on reticulocytes and FNCC showed some evidence of being dose-related.
Table 2.
Haematological results† from female guinea pigs dosed with CAPS daily for 13 days at 333, 666 and 1000 mg/kg and sampled at 1 day after the final dose
| Dose level of CAPS (mg/kg) | ||||
|---|---|---|---|---|
| Control | 333 | 666 | 1000 | |
| RBC | 4.18 (0.11) | 4.78 (0.40)* | 4.74 (0.26)** | 4.77 (0.23)** |
| HCT | 0.358 (0.008) | 0.376 (0.026) | 0.389 (0.017)** | 0.394 (0.024)* |
| Hb | 10.8 (0.2) | 11.6 (0.8) | 12.1 (0.6)** | 12.2 (0.8)** |
| MCV | 85.6 (1.9) | 78.9 (2.1)*** | 82.1 (1.9)* | 82.2 (1.7)* |
| MCH | 25.8 (0.3) | 24.4 (0.7)** | 25.4 (0.2) | 25.6 (0.7) |
| MCHC | 30.2 (0.4) | 30.9 (0.3)* | 31.0 (0.5)* | 31.1 (0.2)** |
| Retic | 190.6 (22.1) | 89.7 (21.5)*** | 87.3 (76.5)* | 36.9 (5.4)*** |
| LFR | 106.0 (14.3) | 62.6 (11.0)** | 55.2 (41.6)* | 27.2 (4.2)*** |
| MFR | 43.4 (4.0) | 16.1 (5.3)*** | 18.3 (18.7)* | 6.5 (2.1)*** |
| HFR | 41.2 (5.0) | 11.0 (5.6)*** | 14.0 (16.8)** | 3.2 (0.5)*** |
| Plt | 669 (104) | 725 (112) | 490 (238) | 622 (151) |
| WBC | 1.2 (0.4) | 2.7 (1.1)* | 1.5 (1.1) | 1.3 (0.4) |
| Neut | 0.48 (0.17) | 1.08 (0.44)* | 0.70 (0.49) | 0.69 (0.26) |
| Lymph | 0.68 (0.29) | 1.44 (0.59)* | 0.74 (0.53) | 0.60 (0.32) |
| Mono | 0.02 (0.01) | 0.04 (0.01)* | 0.04 (0.03) | 0.20 (0.00) |
| Eo | 0.03 (0.02) | 0.02 (0.01) | 0.02 (0.02) | 0.00 (0.01)* |
| Baso | 0.01(0.01) | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.00) |
| LUC | 0.03 (0.02) | 0.08 (0.10) | 0.03 (0.03) | 0.02 (0.02) |
| FNCC | 15.02 (1.74) | 9.41 (6.0) | 10.03 (2.79)* | 4.37 (2.10)*** |
Values are means, SD in parentheses.
n = 4 (333 and 666 mg/kg CAPS), n = 5 (control), n = 3 (1000 mg/kg CAPS).
All other information as Table 1.
Experiment 3: Reversibility study
Body weight changes, clinical signs, water consumption and autopsy observations
Groups of guinea pigs (n = 3 or 5) were dosed at 0 (vehicle control) and 825 mg/kg CAPS, daily for 16 days; blood and marrow samples were examined at 1, 12, 28 and 63 days after the final CAPS dose. During the period of study, control guinea pigs increased from a mean body weight of 293.1 g (day 1 of dosing), to 458.2 g (day 16 of dosing; a 56.3% increase), and to 872.7 g (day 63 post dosing; a 197.7% increase); comparable figures for CAPS-treated animals were 321.1 g (day 1 of dosing), 388.3 g (day 16 of dosing; a 21.0% increase), and 835.8 g (day 63 post dosing; a 160.3% increase). During the dosing period, control guinea pigs were normal in appearance; in animals treated with CAPS, there was a slight loss of condition of the fur in a few animals, but otherwise there was no evidence of CAPS-induced toxicity.
Measurements of water consumption were carried out daily during the 16-day period of CAPS administration. In control animals, consumption, expressed as relative consumption (i.e. mL of water consumed/kg of body weight/day), remained relatively constant over the 16-day period. In guinea pigs dosed with CAPS, consumption on days 1 and 2 was significantly increased, but fell to a low level (days 3–11), and then rose to normal levels (days 12–16). Mean consumption figures were: control animals; 163.2 mL/kg/day on days 1 and 2, 169.9 mL/kg (days 3–11), and 156.5 mL/kg (days 12–16); for CAPS-treated animals the comparable figures were 196.4 (P < 0.05), 102.7 (P < 0.001) and 155.7 (NS) mL/kg, over the same periods, respectively. Overall, over the whole 16-day period, the average relative consumptions were 163.7 mL/kg/day (control) and 130.2 mL/kg/day (CAPS-treated; P < 0.001). At autopsy, CAPS-dosed guinea pigs showed no gross changes of the major internal organs.
Haematological findings
Haematology results are given in Table 3. At day 1 post dosing, in guinea pigs treated with CAPS, there were slight decreases (NS) in RBC, HCT, and Hb; MCV and MCH were also slightly reduced (NS). WBC, neutrophils, lymphocytes, monocytes and platelets were unaffected; eosinophils were reduced (P < 0.05). Total reticulocyte count was significantly reduced, as were low, mid and high fluorescence reticulocytes. The FNCC was significantly decreased.
Table 3.
Haematological data† from female guinea pigs dosed with CAPS daily for 16 days at 825 mg/kg and sampled at 1, 12, 28 and 63 days after the final dose
| Day of sampling | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 12 | Day 28 | Day 63 | |||||
| Control | CAPS | Control | CAPS | Control | CAPS | Control | CAPS | |
| RBC | 4.89 (0.53) | 4.58 (0.56) | 4.74 (0.54) | 4.35 (0.28) | 4.75 (0.10) | 4.66 (0.26) | 5.07 (0.30) | 5.21 (0.14) |
| HCT | 0.423 (0.030) | 0.394 (0.053) | 0.410 (0.041) | 0.371 (0.022) | 0.412 (0.009) | 0.402 (0.014) | 0.435 (0.02) | 0.440 (0.012) |
| Hb | 13.0 (0.9) | 12.1 (1.6) | 13.0 (1.2) | 11.6 (0.6) | 13.1 (0.2) | 12.3 (0.4)* | 14.1 (0.7) | 14.3 (0.4) |
| MCV | 86.9 (3.1) | 86.0 (1.8) | 86.5 (1.3) | 85.4 (1.9) | 86.6 (0.9) | 86.4 (3.3) | 85.8 (0.8) | 84.9 (1.3) |
| MCH | 26.6 (1.0) | 26.3 (0.7) | 27.5 (0.6) | 26.6 (0.5) | 27.6 (0.4) | 26.5 (1.0) | 27.7 (0.2) | 27.5 (0.2) |
| MCHC | 30.6 (0.1) | 30.6 (0.4) | 31.8 (0.2) | 31.2 (0.4) | 31.8 (0.3) | 30.7 (0.9) | 32.3 (0.1) | 32.4 (0.5) |
| Retic | 131.2 (26.4) | 16.0 (10.9)*** | 124.9 (28.4) | 206.4 (41.6)* | 71.4 (18.1) | 125.6 (62.1) | 54.0 (29.9) | 51.1 (23.2) |
| LFR | 77.6 (17.3) | 10.9 (5.7)*** | 79.8 (19.6) | 121.1 (20.9)* | 43.3 (12.2) | 73.9 (34.0) | 30.8 (9.8) | 34.3 (14.8) |
| MFR | 34.7 (10.2) | 3.1 (2.9)** | 26.8 (9.1) | 45.5 (8.9)* | 16.7 (4.3) | 27.3 (15.0) | 11.7 (8.8) | 9.4 (5.1) |
| HFR | 23.8 (5.6) | 2.1 (2.4)*** | 18.3 (12.3) | 39.8 (13.5) | 11.5 (2.3) | 24.3 (13.6) | 11.6 (11.7) | 7.4 (4.4) |
| Plt | 761 (34) | 673 (176) | 695 (9) | 731 (147) | 685 (50) | 724 (79) | 672 (150) | 460 (73)* |
| WBC | 1.6 (0.3) | 1.2 (0.8) | 2.3 (0.5) | 2.2 (0.7) | 2.4 (1.1) | 1.8 (0.6) | 2.7 (1.7) | 1.4 (0.6) |
| Neut | 0.66 (0.10) | 0.56 (0.34) | 0.74 (0.17) | 0.81 (0.28) | 0.77 (0.34) | 0.65 (0.23) | 0.58 (0.35) | 0.33 (0.12) |
| Lymph | 0.73 (0.41) | 0.60 (0.37) | 1.31 (0.42) | 1.17 (0.45) | 1.42 (0.77) | 1.07 (0.80) | 1.71 (1.08) | 0.86 (0.37) |
| Mono | 0.04 (0.02) | 0.03 (0.03) | 0.02 (0.01) | 0.04 (0.03) | 0.02 (0.02) | 0.07 (0.08) | 0.03 (0.03) | 0.03 (0.04) |
| Eo | 0.08 (0.01) | 0.02 (0.03)* | 0.07 (0.03) | 0.07 (0.07) | 0.12 (0.09) | 0.11 (0.06) | 0.12 (0.09) | 0.09 (0.12) |
| Baso | 0.01 (0.01) | 0.00 (0.01) | 0.02 (0.01) | 0.01 (0.01) | 0.00 (0.01) | 0.03 (0.04) | 0.02 (0.01) | 0.01 (0.01)* |
| LUC | 0.06 (0.02) | 0.02 (0.02)* | 0.12 (0.02) | 0.09 (0.06) | 0.08 (0.06) | 0.08 (0.08) | 0.20 (0.16) | 0.04 (0.02) |
| FNCC | 22.37 (3.80) | 6.25 (2.32)*** | 18.92 (4.22) | 14.41 (4.10) | 14.79 (2.90) | 18.04 (9.31) | 16.82 (2.39) | 13.01 (3.12) |
Values are means, SD in parentheses.
n = 3 (controls), n = 5 (CAPS) at all time points.
All other information as Table 1.
At day 12 post dosing, in CAPS-treated animals, values for RBC, HCT and Hb were still slightly lower than the controls, and MCV and MCH were also slightly reduced (all changes NS). The total reticulocyte count was slightly increased above the control level (P < 0.05), as were low (P < 0.05), mid (P < 0.05), and high (NS) fluorescence reticulocytes. The reduced FNCC evident at day 1 appeared to be returning towards normal, but the count was still slightly depressed (NS).
In CAPS-treated guinea pigs at day 28 post dosing, the RBC, HCT, Hb, MCV and MCH remained slightly depressed (all NS, except Hb P < 0.05). The total, and low, mid and high fluorescence reticulocyte counts were also slightly raised above the control values (all NS). At day 63, in CAPS-dosed guinea pigs, all peripheral blood parameters, and the FNCC, were normal; there was no evidence of myelosupression.
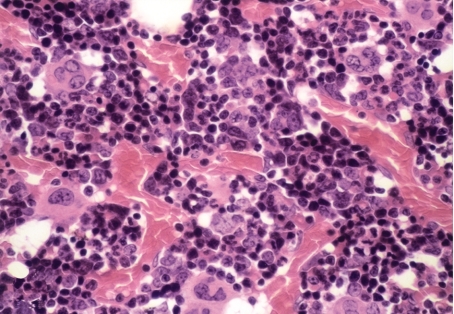
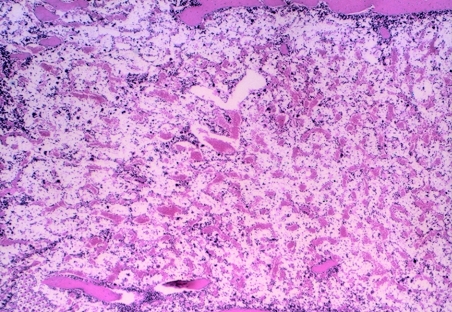
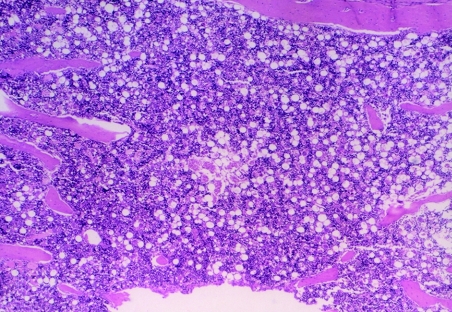
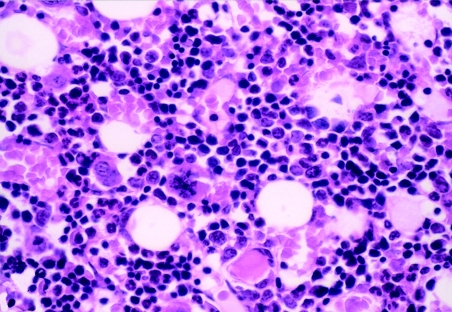
An examination of sternal marrow sections illustrated that at day 1 post dosing in CAPS-treated guinea pigs, the marrow was hypoplastic (Figures 1, 2 and 3), with erythroid depletion and an uneven distribution of cells (Figure 4). At day 12 post dosing, the marrow showed normal cellularity (Figure 5), but with some evidence of erythroid hyperplasia (Figure 6). At days 28 and 63, erythroid hyperplasia was not evident, and the marrow appeared normal. It was noted that the number of fat spaces in the sternal marrow sections showed considerable variation between individual animals in both control and CAPS-treated guinea pigs, at each time point.
Figure 1.
Sternal marrow from a control (CAPS vehicle) treated guinea pig at day 1 post dosing; the marrow is fully cellular with only a small number of fat spaces. H&E; original magnification (OM) × 40.
Figure 2.
Sternal marrow from a control guinea pig at 1 day post dosing; at this higher magnification granulocytic and erythroid elements, and megakaryocytes, are all clearly represented. H&E; OM × 400.
Figure 3.
Sternal marrow from a guinea pig treated with CAPS at 825 mg/kg for 16 days, and sampled at day 1 post dosing; the marrow is markedly hypoplastic. H&E; OM × 40.
Figure 4.
Sternal marrow from a CAPS-treated (825 mg/kg/16 days) guinea pig at day 1 post dosing; the marrow is hypocellular and erythroid elements are depleted. The residual haemopoiesis is patchy in nature but the megakaryocytes have been relatively spared. H&E; OM × 400.
Figure 5.
A CAPS-treated guinea pig (825 mg/kg/16 days) at 12 days post dosing; the cellularity of the sternal marrow has returned to normal. The fatty appearance of this section is comparable to control animals at this time point. H&E; OM × 40.
Figure 6.
A CAPS-treated guinea pig (825 mg/kg/16 days) at day 12 post dosing; whilst the granulocytic and erythroid series are both present in the sternal marrow, there is a slight increase in the number of early erythroid cells. H&E; OM × 400.
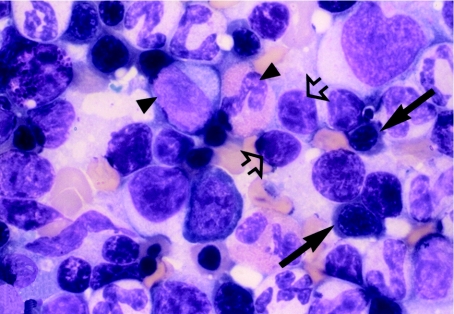
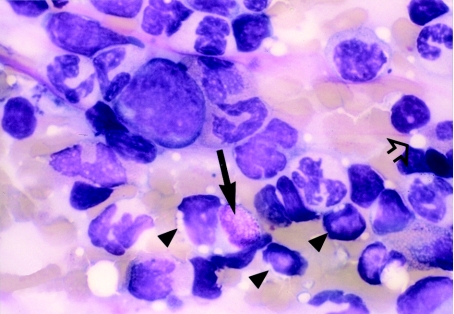
Differential counts were carried out on femoral marrow smears to determine the proportion of cells (as percentages) in the myeloid, erythroid and lymphoid lineages; using the femoral marrow cell count (FNCC) from the opposing limb (Table 3), the absolute number of cells making up each lineage was calculated (Table 4). Examination of marrow smears from CAPS-treated guinea pigs at day 1 post dosing showed a hypocellular picture, with erythroid suppression and fatty replacement; cytoplasmic vacuolation of precursor cells was evident, particularly in myeloid and lymphoid cells (Figures 7 and 8). Vacuolation of promyelocyctes was frequently seen. Cellular counts demonstrated that erythroid cells in CAPS-dosed animals were reduced to 0.57% of the control figure, and the number of myeloid and lymphoid cells were also significantly decreased (Table 4; Figure 9); the M : E ratio was 0.86 : 1 in the control animals and 79.61 : 1 in the CAPS-treated guinea pigs.
Table 4.
Counts (×107) of myeloid, erythroid and lymphoid cells, and the myeloid : erythroid (M : E) ratio in femoral marrow smears of control and CAPS-treated guinea pigs; animals were treated with CAPS at 825 mg/kg, or vehicle solution daily for 16 days, and sampled at 1, 12, 28 and 63 days after the final dose†
| Day of sampling | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 12 | Day 28 | Day 63 | |||||
| Control | CAPS | Control | CAPS | Control | CAPS | Control | CAPS | |
| Myeloid | 6.73 (1.95) | 3.35 (1.03)* | 6.42 (2.47) | 3.61 (1.16) | 4.99 (1.07) | 6.40 (2.88) | 7.05 (1.16) | 5.66 (1.19) |
| Erythroid | 7.72 (0.94) | 0.04 (0.02)** | 6.37 (2.16) | 6.64 (1.96) | 5.06 (1.51) | 5.82 (3.45) | 5.44 (1.27) | 3.90 (1.29) |
| Lymphoid | 7.13 (1.53) | 1.91 (0.80)** | 5.52 (0.42) | 3.69 (1.20)* | 4.46 (1.02) | 5.54 (3.20) | 4.09 (0.33) | 3.60 (1.19) |
| Other | 0.79 (0.23) | 0.95 (0.57) | 0.61 (0.23) | 0.48 (0.38) | 0.31 (0.17) | 0.28 (0.16) | 0.23 (0.14) | 0.24 (0.19) |
| M:E ratio | 0.86 (0.19) | 79.61 (26.50)** | 1.01 (0.13) | 0.57 (0.24)** | 1.06 (0.48) | 1.22 (0.46) | 1.35 (0.43) | 1.53 (0.45) |
Figure 7.
Romanowsky stained femoral marrow smear from a control (CAPS vehicle) treated guinea pig at day 1 post dosing; representative cells from the granulocytic (arrowhead), erythroid (arrow) and lymphoid (open arrow) lineages are clearly evident. OM × 1000.
Figure 8.
Femoral marrow smear (Romanowsky stained) from a guinea pig treated with CAPS at 825 mg/kg for 16 days, at day 1 post dosing; erythroid hypoplasia is apparent. Erythroid precursors are absent, and vacuolation is present in three lymphocytes (arrowhead), one of which contains a Kurloff body (arrow). Fat spaces are also evident (open arrow). OM × 1000.
Figure 9.
Bone marrow smear (Romanowsky stained) from a CAPS-treated guinea pig (825 mg/kg/16 days) at day 1 post dosing; there is erythroid hypoplasia and only granulocytic and lymphoid cells are seen. A myelocyte shows cytoplasmic vacuolation (arrow). OM × 1000.
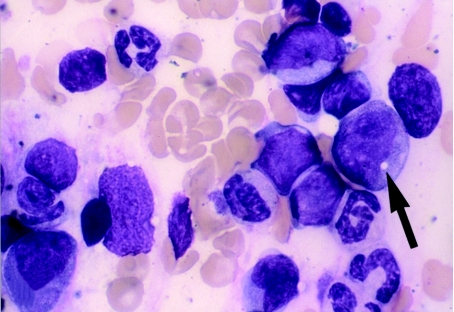
Examination of marrow smears at day 12 showed evidence of an ‘erythroid rebound’ in CAPS-treated animals (Figure 10); cell counts demonstrated that the number of erythroid cells was approximately similar in CAPS-dosed and control animals (Table 4). However, the counts of myeloid and lymphoid cells remained slightly decreased (NS, and P < 0.05, respectively), and the M : E ratio was also reduced (P < 0.01). The FNCC was slightly decreased at this time (NS; Table 3). Examination of femoral marrow smears, and counts of cells, at days 28 and 63 post dosing showed a normal picture. There was no evidence of a ‘late stage’ myelosupression.
Figure 10.
Romanowsky stained femoral marrow smear from a guinea pig treated with CAPS (825 mg/kg/16 days), at day 12 post dosing; there is an erythroid rebound. The M : E ratio is reduced and erythroid activity is evident as clusters of normoblasts (arrow). OM × 500.
Discussion
Sutherland & Festing (1987) discussed several aspects of the use of the guinea pig in biomedical research, pointing out that the species has been employed experimentally since the early nineteenth century (Mason 1940), that the now commonly used Dunkin Hartley stock was first established in 1926 (Dunkin et al. 1930), and that the species is the fifth most frequently used experimentally in the UK. The guinea pig is a species widely used in biochemistry, physiology, and pharmacology investigations, and has also found particular application in immunology studies (delayed hypersensitivity, anaphylactic shock, and genetic control of the immune response), infectious disease (Mycobacterium spp. and tuberculosis), otology, and vitamin C metabolism. In toxicology, and other disciplines, the guinea pig is not often employed, but is sometimes selected as a contrasting species for possible use after the mouse, rat and rabbit (Sutherland & Festing 1987). In the present toxicological investigations the guinea pig was selected as a third rodent species, as we had been unable to demonstrate CAPS-induced late-stage marrow aplasia (AA) in the mouse or rat (Turton et al. 1999, 2000).
In general, blood parameters of control guinea pigs in the present study were comparable with other reports of normal values (Loeb et al. 1978; Moore 2000). However, the guinea pig has several unusual haematological characteristics. The erythrocyte is the largest of the common laboratory species (7.2–7.8 µm diameter) and the M : E ratio is reported as high (1.45 : 1.0 or 1.6 : 1.0). A unique feature is the presence in 5–8% of cells of the lymphocytic series of a single, large, PAS-positive, cytoplasmic inclusion body, the Kurloff body (see Figure 8). These cells, Kurloff cells, are considered to be natural killer cells, and the Kurloff body itself is a lysosomal organelle (Landemore et al. 1994; Pouliot et al. 1996; Taouji et al. 1996). Kurloff bodies are not found in monocytes.
Literature searches have provided few references on CAP/CAPS toxicity in guinea pigs. Gruhzit et al. (1949) reported early toxicity studies with chloromycetin ( = CAP) in the mouse, rat, dog, rabbit and guinea pig. With CAP incorporated in the diet, guinea pigs showed an aversion to eating, with resulting decreases in body weight and cachexia, with six of eight animals dying. There was a ‘haemoglobin concentration’ in two surviving animals, and the maximum tolerated dose was given as 250 mg/kg/day, and the LD50 as 538 mg/kg.
In our earlier investigations on CAPS haemotoxicity, the antibiotic was administered in repeat dose studies at levels up to 3000 mg/kg in the mouse (Festing et al. 2001), and up to 4000 mg/kg in the rat (Turton et al. 1999). Thus, in the present dose ranging study (Experiment 1), CAPS was given to the guinea pigs at 2500 and 3500 mg/kg for 9 days. The changes induced were a trend for a dose-related increase in RBC, HCT and Hb, and reduced MCV and MCH; leucocytes were unaffected, except for a decrease in eosinophils; reticulocytes and FNCC were also decreased. The increases in RBC, HCT and Hb were unexpected, although comparable changes had been seen in previous studies with CAPS in mice, where the effect was considered to relate to a haemoconcentration phenomenon in association with reduced water and diet intake (Holt et al. 1998). Experiment 2 involved CAPS administration at lower dose levels (333, 666 and 1000 mg/kg) for 13 days. The results showed that RBC, HCT and Hb were increased at all dose levels; MCV and MCH, eosinophils, reticulocytes and FNCC were lowered. There were indications that the effects on HCT and Hb, eosinophils, reticulocytes and FNCC were related to CAPS dose levels.
In Experiment 3, CAPS was dosed for 16 days at 825 mg/kg, and animals sampled at day 1–63 post dosing. At day 1 post dosing, RBC, HCT and Hb were slightly reduced (NS), as were MCV and MCH (NS); eosinophils, reticulocytes and FNCC were lowered. Parameters were returning towards normal at days 12 and 28, and at day 63 post dosing all parameters were normal. There was no evidence of ‘late stage’ blood changes. Sternal marrow at day 1 showed a hypoplastic picture with significant erythroid depletion; at days 28 and 63, the marrow picture was normal. Marrow smears at day 1 confirmed erythroid suppression, with precursor cell vacuolation and an increased M : E ratio; there was a return towards normal at day 12, with normal values at day 63; no myelodepression was evident at day 63.
The increases in erythrocyte values in the first two experiments are considered to be related to haemoconcentration, and a reduced diet and water consumption. A reduction in water consumption in Experiment 3 was confirmed between days 3–11 of dosing, with an increase to normal levels from days 12–16. Similar haemoconcentration effects with CAPS have been seen in earlier studies in the mouse (Holt et al. 1998), and in rats treated with the related antibiotic, thiamphenicol (TAP) (Turton et al. 2002). Related changes in the peripheral blood of rodents given limited water and diet have been described by other workers; Maejima & Nagase (1991), and Zaporowska & Wasilewski (1991) reported increased erythrocyte values in starved rats with reduced intakes of water, and Boyne & Arthur (1990) and Levin et al. (1993) described similar results in rats with lowered diet consumptions.
At day 1 post dosing in Experiment 3, in CAPS-treated animals, the total absolute reticulocyte count was reduced to 12.2% of the control figure. At day 12, the count was increased above normal by 65.3%, and on day 28 by 75.9%; levels had returned to normal on day 63. This reticulocyte response at day 12 and 28 is a ‘rebound reticulocytosis’, and has been observed previously in mice dosed with TAP (Turton et al. 2002), and is often associated with a previous reduction in erythrocyte values. The mechanism may be related to an increase in erythropoietin production by the kidney to stimulate erythropoiesis, and the release of reticulocytes from the bone marrow.
Cytoplasmic and nuclear vacuolation has been reported in the marrow cells of CAP-treated patients (Rosenbach et al. 1960; Saidi et al. 1961; Scott et al. 1965), and has become one of the criteria indicative of CAPS-induced toxicity. Vacuolation frequently involves the erythroblast (pro-erythroblast) but granulocytes, megakaryocytes and plasma cells may also be affected. The formation of vacuoles in the myeloid and erythroid marrow cells of the CAP-treated rat was reported by Schrober et al. (1972). In Experiment 3, cytoplasmic vacuolation was seen in the myeloid and lymphoid marrow precursor cells of CAPS-treated guinea pigs, but particularly in promyelocytes. We have previously reported vacuolation in the marrow cells of TAP-treated mice and rats, but not in CAPS-treated animals (Turton et al. 2000, 2002).
In Experiment 1, significant reductions in eosinophil counts were evident at both CAPS dose levels; at 1000 mg/kg in Experiment 2, and at day 1 post dosing in Experiment 3. Other leucocytes did not show any significant or consistent pattern of change. The basis of this effect on the eosinophil count is unclear; a search of the literature has not indicated any previous comparable findings with CAP, CAPS or TAP.
There was some evidence (NS) that in Experiment 1, values for MCV and MCH were reduced in CAPS-treated guinea pigs at all dose levels. This was also evident in Experiment 2. This change was also seen in mice treated with CAPS (Turton et al. 2000). The mechanisms causing these decreases are unclear, however, Manyan & Yunis (1970),Manyan et al. (1972), and Rosenberg & Marcus 1974) in studies in the dog and rabbit, demonstrated that CAP inhibited marrow mitochondrial ferrocheletase, and aminolaevulinic acid synthetase activity, causing a block on mitochondrial haem and haemoglobin synthesis, with a resulting depression of erythropoiesis. The reduction in MCV and MCH in the present studies therefore may be related to a CAPS-induced adverse effect on haemoglobin synthesis.
As mentioned earlier, in man, CAP induces two major haematological toxicities, a reversible bone marrow depression, and AA. The reversible depression is mainly characterized by mild anaemia and reticulocytopenia, and the change is dose-related, develops during treatment and rapidly reverses on drug withdrawal. Marrow shows normal or reduced cellularity, an increased M : E ratio, decreased erythroid cells and vacuolated precursors. CAP-induced AA in man is characterized by pancytopenia that develops weeks after treatment and is not dose-related; the marrow is acellular or hypocellular, and the changes are often irreversible. Therefore, in the present guinea pig studies, CAPS-induced haematological changes show many effects directly comparable to the reversible marrow depression seen in man. At 28/63 days post dosing there was no evidence of an overt or developing marrow aplasia (AA), with an associated peripheral pancytopenia and acellular/hypocellular marrow.
The reasons for the failure to induce of AA in the guinea pig are unclear, but this failing is in line with attempts by other workers to induce AA with CAP, and with other drugs and chemicals, in laboratory rodents. If the aetiology of AA in man is considered against this background, the human disease may be associated with several rare congenital or inherited conditions (e.g. Fanconi's anaemia, dyskeratosis congenita, Schwachman –Diamond syndrome, etc.) (Alter & Young 1998; Freedman 2000). However, in the majority of AA cases the disease is acquired. Although a number of causative agents are associated with the development of AA, in about 70% of patients, no causal link can be identified (idiopathic AA) (Young & Maciejewski 1997). Nevertheless, in the remaining 30% of cases there are three principal causes: infectious agents, radiation, and drugs and chemicals (Alter et al. 1978; Young & Maciejewski 2000). First, the majority of infectious agents linked to AA are viral (i.e. antecedent viral illness), e.g. hepatitis virus (non-A, non-B, non-C), Epstein–Barr virus, human immunodeficiency virus, etc. (Kurtzman & Young 1989). Second, myelosupression may be a predictable response to radiation, especially gamma-irradiation, and in man, doses exceeding 1.5–2.0 Gy induce bone marrow hypoplasia (Knospe 1988; Young & Maciejewski 2000). Chronic low dose irradiation is associated with AA, and the incidence of the condition may be increased in patients given radiotherapy, and in radiologists. Third, AA has been linked to a variety of chemicals (Young & Alter 1994), for example, benzene and insecticides (e.g. lindane, chlordane, DDT), and also to a wide range of drugs (e.g. chloramphenicol, anti-neoplastic agents, phenylbutazone and many other non-steroidal anti-inflammatory agents, gold, penicillamine, anticonvulsants, sulphonamides, etc.) (Young & Alter 1994).
Against this background of the aetiology of the human disease, there have been various attempts to produce experimental models of AA in small laboratory animals using infectious agents, radiation, drugs and chemicals, and antibodies (Alter et al. 1978; Haak 1980; Vincent 1984). Of these, probably the most useful model has proved to be that of Morley & Blake (1974) who developed a mouse model of chronic hypoplastic marrow failure (CHMF) (i.e. AA) in the busulphan-treated mouse. However, there are no literature reports of CAP being used as the sole agent in attempts to induce AA in laboratory rodents before our own studies (Turton et al. 2000). Indeed, there has been a view (Chaplin 1986) that rodents are not susceptible to CAP-induced haemotoxicity. Nevertheless, Morley et al. (1976) evaluated the capacity of CAPS to induce CHMF in his busulphan mouse model of AA (Morley & Blake 1974). In this study, mice were treated with busulphan and CAPS was then given in the drinking water at 5 mg/mL and caused a progressive fall in nucleated marrow cells, marrow CFU-S and CFU-GM. However, using the experimental design of Morley et al. (1976), we were unable to demonstrate that CAPS (4 mg/mL in the drinking water) induced AA, or indeed caused any haemotoxic effects (Andrews et al. 1998; Andrews 2000). It would appear that other workers have also examined the myelotoxicity of CAP in the busulphan–CAP mouse model of Morley et al. (1976), but their results, similarly, do not closely parallel those of Morley (Pazdernik & Corbett 1980; Robin et al. 1981).
In our studies with the busulphan–CAP mouse model, water consumption figures showed that over a 13-month study period, mice consumed about 176–370 mg CAPS/kg/day. We subsequently demonstrated that at these dose levels, CAPS is not haemotoxic, and that dose levels of 1400–1700 mg/kg in the CD-1 mouse are required to induce significant myelotoxicity (Holt et al. 1998; Turton et al. 1999). More recently, it was demonstrated (Turton et al. 2000) that CAPS administered as a sole agent at 2000 mg/kg day for 17 days to the BALB/c mouse, failed to induce AA. Also, in the Wistar Han rat, levels of 3600 and 4000 mg/kg/day for 19 days, only induced very mild haemotoxicity (Turton et al. 1999). Against the background of these dose levels in rodents, the therapeutic dose of CAP in man is generally given as 50 mg/kg/day, in divided doses, although higher dose levels (up to 100 mg/kg) have frequently been used; the time period of treatment is often 10–15 days. It is this range of dose levels which, in man, induce both the reversible form of haemotoxicity, and AA.
The mechanisms of toxicity underlying the reversible haemotoxicity of CAP are thought to relate to the ability of the drug to inhibit mitochondrial protein (haem) synthesis (Yunis et al. 1970; Manyan et al. 1972). However, the basal mechanism of CAP-induced AA in man has not been determined. Nevertheless, there is some evidence of an association with a genetic predisposition, in that CAP inhibits DNA synthesis at lower concentrations in the marrow cells of patients (and in marrow from the relatives of patients), with CAP-associated AA, than in the marrow of normal individuals (Yunis 1973). Also, there are case reports of CAP-associated AA in identical twins (Best 1967; Nagao & Mauer 1969).
Yunis & Bloomberg (1964) speculated that the genetic predisposition to CAPS susceptibility had a metabolic–biochemical basis. Four possible mechanisms were proposed: (a), inability to excrete the drug normally, leading to high blood levels of drug; (b), the selective concentration of the drug in the bone marrow; (c), abnormal drug metabolism with the production of toxic metabolites; (d), an inborn error of metabolism (e.g. an enzyme deficiency) causing susceptibility to the parent drug or its metabolites. Yunis and colleagues (Murray et al. 1982; Yunis 1988) postulated that CAP-induced AA resulted from the interaction of highly reactive metabolic reduction products of the antibiotic with bone marrow stem cells. In this regard, nitroso-chloramphenicol (NO-CAP) which contains a nitroso group in place of the p-NO2 moiety of CAP, was suspected as being the potential mediator of drug-induced AA, and was shown to induce significant DNA damage (Murray et al. 1982).
It was suggested by Andrews (2000), that susceptibility to the various forms of CAP haemotoxicity in different animal species, and animal strains, may relate to inherent variations in absorption, distribution, metabolism and excretion of the drug. This variation in susceptibility to CAP-induced haemotoxicity has been demonstrated in different mouse strains in vitro, where the sensitivity of marrow erythroid cells to CAP was shown to be genetically determined (Miller et al. 1978). Similarly, Festing et al. (2001) reported significant differences in the haematological response of several strains of mice to CAPS, and with the present investigations in the guinea pig our own studies now show differing responses in the mouse, rat and guinea pig; this variation in response in different species also applies to the related antibiotic thiamphenicol (Turton et al. 2002). In the mouse, attempts to induce AA with CAPS were unsuccessful (Turton et al. 2000), and this lack of success now extends to the guinea pig. Literature searches have revealed no reports of CAP-induced AA in small laboratory animals, nor in the cat or dog (where CAP has been widely used therapeutically). The only report of CAP-induced AA in a non-human species appears to be in the calf, where there is a single report (Krishna et al. 1981).
In conclusion therefore it is suggested that the inability to induce AA with CAPS in the guinea pig, and previously in the mouse (Turton et al. 2000), may relate to aspects of the metabolism of the drug in these species, and possibly the non-formation of nitroso-chloramphenicol at requisite toxic levels in the bone marrow. The metabolic pathways of CAP in the mouse and other laboratory species are unknown (Holt et al. 1993, 1995). Further work in this area is required to shed light on these problems and to identify the mechanistic basis of CAP-induced AA in man.
Acknowledgments
We wish to acknowledge the assistance of the technical staff at the School of Pharmacy for their care of the animals. JAT wishes to thank Glaxo SmithKline, Ware, for continued support, and Ms Patrizia Gargiulo and Ms Vicky Welsh for the preparation of the manuscript. CMA would like to thank the staff of the Clinical Pathology Unit, Glaxo SmithKline, for their assistance in the analysis of the blood and marrow samples.
References
- Alter BP, Potter NU, Li FP. Classification and aetiology of the aplastic anaemias. Clin. Haematol. 1978;7:431–465. [PubMed] [Google Scholar]
- Alter BP, Young NS. The bone marrow failure syndromes. In: Nathan DG, Orkin SH, editors. Haematology of Infancy and Childhood. Philadelphia: W.B. Saunders; 1998. pp. 111–222. [Google Scholar]
- Andrews CM. Studies on the haemotoxicty of busulphan and chloramphenicol in the B6C3F1 mouse. London: University of London; 2000. PhD Thesis. [Google Scholar]
- Andrews CM, Williams TC, Turton JA. Long-term haematological alterations in female B6C3F1 mice treated with bulsulphan. Comp. Haematol. Int. 1998;8:125–138. [Google Scholar]
- Best WR. Chloramphenicol-associated blood dyscrasias. J. Am. Med. Assoc. 1967;201:99–106. [Google Scholar]
- Boyne R, Arthur JR. Anaemia and changes in erythrocyte morphology associated with copper and selenium deficiencies and dietary restriction in rats. Res. Vet. Sci. 1990;49:151–156. [PubMed] [Google Scholar]
- Chaplin S. Bone marrow depression due to mianserin, phenylbutazone, oxyphenbutazone, and chloramphenicol—Part II. Adverse Drug React. Acute Poison. Rev. 1986;3:181–196. [PubMed] [Google Scholar]
- Cohen T, Creger WP. Acute myeloid leukemia following seven years of aplastic anemia induced by chloramphenicol. Am. J. Med. 1967;43:762–770. doi: 10.1016/0002-9343(67)90118-0. [DOI] [PubMed] [Google Scholar]
- Dollery C. Therapeutic Drugs. Edinburgh: Churchill Livingstone; 1999. pp. C168–C172. [Google Scholar]
- Dunkin GW, Hartley P, Lewis-Faning E, Russell WT. A comparative biometric study of albino and coloured guinea-pigs from the point of view of their suitability for experimental use. J. Hyg. (Camb). 1930;30:311–333. doi: 10.1017/s0022172400010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO. Toxicological Evaluation of Certain Veterinary Drug Residues in Food (WHO Food Additives Series 23) Geneva: World Health Organisation; 1988. Expert Committee on Food Additives. Chloramphenicol; pp. 1–71. [Google Scholar]
- Festing MFW, Diamanti P, Turton JA. Strain differences in haematological response to chloramphenicol succinate in mice: implications for toxicological research. Food Chem. Toxicol. 2001;39:375–383. doi: 10.1016/s0278-6915(00)00149-6. [DOI] [PubMed] [Google Scholar]
- Fraunfelder FT, Morgan RL, Yunis AA. Blood dyscrasias and topical ophthalmic chloramphenicol. Am. J. Ophthalmol. 1993;115:812–813. doi: 10.1016/s0002-9394(14)73653-0. [DOI] [PubMed] [Google Scholar]
- Freedman MH. Inherited forms of bone marrow failure. In: Hoffman R, et al., editors. Haematology: Basic Principles and Practice. New York: Churchill Livingstone; 2000. pp. 260–297. [Google Scholar]
- Gruhzit OM, Fisken RA, Reutner TF, Martino E. Chloramphenicol (chloromycetin), an antibiotic. Pharmacological and pathological studies in animals. J. Clin. Invest. 1949;28:943–952. doi: 10.1172/JCI102184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak HL. Experimental drug-induced aplastic anaemia. Clinics Haematol. 1980;9:621–639. [PubMed] [Google Scholar]
- Havard A, Andrews CM, Williams TC, Turton JA. Chloramphenicol induces reversible dose-related reticulocytopenia, not aplastic anaemia, in the guinea pig. Hum. Exp. Toxicol. 1999;18:764. [Google Scholar]
- Holt DE, Andrews CM, Payne JP, Williams TC, Turton JA. The myelotoxicity of chloramphenicol: in vitro and in vivo studies: II: in vivo myelotoxicity in the B6C3F1 mouse. Hum. Exp. Toxicol. 1998;17:8–17. doi: 10.1177/096032719801700102. [DOI] [PubMed] [Google Scholar]
- Holt D, Harvey D, Hurley R. Chloramphenicol toxicity. Adv. Drug React. Toxicol. Rev. 1993;12:83–95. [PubMed] [Google Scholar]
- Holt D, Hurley R, Harvey D. A reappraisal of chloramphenicol metabolism; detection and quantification of metabolites in the sera of children. J. Antimicrob. Chemother. 1995;35:115–127. doi: 10.1093/jac/35.1.115. [DOI] [PubMed] [Google Scholar]
- Home Office. Code of Practice for the Housing and Care of Animals Used in Scientific Procedures. London: Her Majesty's Stationary Office; 1989. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risk of Chemicals to HumansChloramphenicol. Vol. 50. Lyon: IARC; 1990. pp. 169–193. [PMC free article] [PubMed] [Google Scholar]
- Knospe WH. Long-term bone marrow damage after irradiation. In: Testa NG, Gale RP, editors. Hematopoiesis: Long-term Effects of Chemotherapy and Radiation. New York: Marcel Dekker; 1988. pp. 93–130. [Google Scholar]
- Krakoff IH, Karnofsky DA, Burchenal JH. Effects of large doses of chloramphenicol on human subjects. New Engl. J. Med. 1955;253:7–10. doi: 10.1056/NEJM195507072530102. [DOI] [PubMed] [Google Scholar]
- Krishna G, Aykac I, Siegel D. Recent studies on the mechanisms of chloramphenicol activation responsible for aplastic anaemia. In: Najean Y, Tognoni G, Yunis AA, editors. Safety Problems Related to Chloramphenicoland Thiamphenicol. New York: Raven Press; 1981. pp. 5–16. [Google Scholar]
- Kumana CR, Li KY, Kou M. Do chloramphenicol blood dyscrasias occur in Hong Kong? Adverse Drug React. Toxicol. Rev. 1993;12:97–106. [PubMed] [Google Scholar]
- Kumar P, Verma IC. Antibiotic therapy for bacterial meningitis in children in developing countries. Bull. World Health Org. 1993;71:183–188. [PMC free article] [PubMed] [Google Scholar]
- Kurtzman G, Young N. Viruses and bone marrow failure. Balliere's Clin. Haematol. 1989;2:51–67. doi: 10.1016/s0950-3536(89)80007-1. [DOI] [PubMed] [Google Scholar]
- Kushwaha KP, Verma RB, Singh YD, Rathi AK. Surveillance of drug induced diseases in children. Indian J. Pediatr. 1994;61:357–365. doi: 10.1007/BF02751889. [DOI] [PubMed] [Google Scholar]
- Landemore G, Quillec M, Letaief SE, Izard J. The proteoglycan skeleton of the Kurloff body as evidenced by cuprolinic blue staining. Histochem. J. 1994;26:571–581. doi: 10.1007/BF00158591. [DOI] [PubMed] [Google Scholar]
- Levin S, Semler D, Ruben Z. Effects of two weeks of feed restriction on some common toxicologic parameters in Sprague-Dawley rats. Toxicol. Pathol. 1993;21:1–14. doi: 10.1177/019262339302100101. [DOI] [PubMed] [Google Scholar]
- Loeb WF, Bannerman RM, Rininger BF, Johnston AJ. Haematologic disorders (Chapter II) In: Benirschke K, Garner FM, Jones TC, editors. Pathology of Laboratory Animals. New York: Springer Verlag; 1978. pp. 889–1050. [Google Scholar]
- Maejima K, Nagase S. Effect of starvation and refeeding on the circadian rhythms of haematological and clinico-biochemical values, and water intake of rats. Exp. Anim. Tokyo. 1991;40:389–393. doi: 10.1538/expanim1978.40.3_389. [DOI] [PubMed] [Google Scholar]
- Manyan DR, Arimura GK, Yunis AA. Chloramphenicol-induced erythroid suppression and bone marrow ferrochelatase activity in dogs. J. Laboratory Clin. Med. 1972;79:137–144. [PubMed] [Google Scholar]
- Manyan DR, Yunis AA. The effect of chloramphenicol treatment on ferrochelatase activity in dogs. Biochem. Biophys. Res. Commun. 1970;41:926–931. doi: 10.1016/0006-291x(70)90172-5. [DOI] [PubMed] [Google Scholar]
- Mason JH. The date of the first use of guinea-pigs and mice in biological research. J. Afr. Vet. Med. Assoc. 1940;11:22–25. [Google Scholar]
- Miller AM, Arimura GK, Gross MA, Yunis AA. In vitro evidence for genetically determined variations in marrow erythroid sensitivity. Exp. Hematol. 1978;6:455–460. [PubMed] [Google Scholar]
- Moore DM. Haematology of the guinea pig. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm's Veterinary Haematology. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 1107–1119. [Google Scholar]
- Morley A, Blake J. An animal model of chronic hypoplastic marrow failure. Late marrow failure after busulphan. Blood. 1974;44:49–56. [PubMed] [Google Scholar]
- Morley A, Trainor K, Remes J. Residual marrow damage: possible explanation for idiosyncrasy to chloramphenicol. Br. J. Haematol. 1976;32:525–531. doi: 10.1111/j.1365-2141.1976.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Murray T, Downey KM, Yunis AA. Degradation of isolated deoxyribonucleic acid mediated by nitroso-chloramphenicol. Biochem. Pharmacol. 1982;31:2291–2296. doi: 10.1016/0006-2952(82)90117-4. [DOI] [PubMed] [Google Scholar]
- Nagao T, Mauer AM. Concordance for drug induced aplastic anaemia in identical twins. New Engl. J. Med. 1969;281:7–11. doi: 10.1056/NEJM196907032810102. [DOI] [PubMed] [Google Scholar]
- Parfitt K. Martindale: The Complete Drug Reference. 32. London: Pharmaceutical Press; 1999. pp. 182–184. [Google Scholar]
- Pazdernik TL, Corbett MD. Role of chloramphenicol reduction products in aplastic anaemia. Pharmacology. 1980;20:878–894. doi: 10.1159/000137348. [DOI] [PubMed] [Google Scholar]
- Pouliot N, Maghni K, Blanchette F, et al. Natural killer and lectin-dependent cytotoxic activities of Kurloff cells: target cell selectivity, conjugate formation, and Ca++ dependency. Inflammation. 1996;20:647–671. doi: 10.1007/BF01488802. [DOI] [PubMed] [Google Scholar]
- Rich ML, Ritterhoff RJ, Hoffmann RJ. A fatal case of aplastic anaemia following chloramphenicol (chloromycetin) therapy. Ann. Intern. Med. 1950;33:1459–1467. doi: 10.7326/0003-4819-33-6-1459. [DOI] [PubMed] [Google Scholar]
- Robin E, Berman M, Bhoopalam N, Cohen H, Fried W. Induction of lymphomas in mice by busulphan and chloramphenicol. Cancer Res. 1981;41:3478–3482. [PubMed] [Google Scholar]
- Rosenbach LM, Caviles AP, Mitus WJ. Chloramphenicol toxicity: reversible vacuolization of erythroid cells. New Engl. J. Med. 1960;263:274–278. doi: 10.1056/NEJM196010132631503. [DOI] [PubMed] [Google Scholar]
- Rosenberg A, Marcus O. Effect of chloramphenicol on reticulocyte aminolaevulinic acid synthetase in rabbits. Br. J. Haematol. 1974;26:79–83. doi: 10.1111/j.1365-2141.1974.tb00451.x. [DOI] [PubMed] [Google Scholar]
- Rubin D, Weisberger AS, Botti RE, Storaosli JP. Changes in iron metabolism in early chloramphenicol toxicity. J. Clin. Invest. 1958;37:1286–1292. doi: 10.1172/JCI103716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi P, Wallerstein RO, Aggeler PM. Effect of chloramphenicol or erythropoiesis. J. Lab. Clin. Med. 1961;57:247–256. [PubMed] [Google Scholar]
- Schrober R, Kosek JC, Wolf PL. Chloramphenicol-induced vacuoles. Their ultrastructure in bone marrow pronormoblasts and immature myeloid cells. Arch. Path. 1972;94:298–302. [PubMed] [Google Scholar]
- Scott JL, Finegold SM, Belkin GA, Lawrence JS. A controlled double blind study of the haematologic toxicity of chloramphenicol. New Engl. J. Med. 1965;272:1137–1142. doi: 10.1056/NEJM196506032722201. [DOI] [PubMed] [Google Scholar]
- Sutherland SD, Festing MFW. The guinea-pig. In: Poole TB, editor. The UFAW Handbook on the Care and Management of Laboratory Animals. 6. Harlow: Longman; 1987. pp. 393–410. [Google Scholar]
- Taouji S, Debout C, Izard J. Arylsulfatase B in Kurloff cells: increased activity of anionic isoforms in guinea pig acute lymphoblastic leukemia. Leukemia Res. 1996;20:259–264. doi: 10.1016/0145-2126(95)00152-2. [DOI] [PubMed] [Google Scholar]
- Trevett AJ, Naraqi S, Wembri J. Typhoid fever complicated by chloramphenicol toxicity, ataxia and psychosis. Papua New Guinea Med J. 1992;35:205–209. [PubMed] [Google Scholar]
- Turton JA, Andrews CM, Havard AC, et al. Haemotoxicity of thiamphenicol in the BALB/c mouse and Wistar Hanover rat. Food Chem. Toxicol. 2002;40:1849–1861. doi: 10.1016/s0278-6915(02)00178-3. [DOI] [PubMed] [Google Scholar]
- Turton JA, Havard AC, Robinson S, et al. An assessment of chloramphenicol and thiamphenicol in the induction of aplastic anaemia in the BALB/c mouse. Food Chem. Toxicol. 2000;38:925–938. doi: 10.1016/s0278-6915(00)00087-9. [DOI] [PubMed] [Google Scholar]
- Turton JA, Yallop D, Andrews M, Fagg R, York M, Williams TC. Haemotoxicity of chloramphenicol succinate in the CD-1 mouse and Wistar Hanover rat. Hum. Exp. Toxicol. 1999;18:566–576. doi: 10.1191/096032799678845098. [DOI] [PubMed] [Google Scholar]
- Vincent PC. In vitro evidence of drug action in aplastic anaemia. Blut. 1984;49:3–12. doi: 10.1007/BF00320378. [DOI] [PubMed] [Google Scholar]
- Volini IF, Greenspan L, Ehrlich L, Gunner JA, Felsenfeld O, Schwartz SO. Hemopoietic changes during administration of chloramphenicol (chloromycetin) J. Am. Med. Assoc. 1950;142:1333–1355. doi: 10.1001/jama.1950.02910350003002. [DOI] [PubMed] [Google Scholar]
- Wallerstein RO, Condit PK, Kasper CK, Brown JW, Morrison FR. Statewide study of chloramphenicol therapy and fatal aplastic anaemia. J. Am. Med. Assoc. 1969;208:2045–2050. [PubMed] [Google Scholar]
- Welch H, Lewis CN, Kerlan I. Blood dyscrasias. A nationwide survey. Antibiot. Chemother. 1954;4:607–623. [PubMed] [Google Scholar]
- Young NS, Alter BP. Aplastic Anemia: Acquired and Inherited. Philadelphia: W.B. Saunders; 1994. [Google Scholar]
- Young NS, Maciejewski JP. Aplastic anaemia. In: Hoffman R, et al., editors. Haematology: Basic Principles and Practice. New York: Churchill Livingston; 2000. pp. 297–331. [Google Scholar]
- Young NS Maciejewski. The pathophysiology of acquired aplastic anaemia. New Engl. J. Med. 1997;336:1365–1372. doi: 10.1056/NEJM199705083361906. [DOI] [PubMed] [Google Scholar]
- Yunis AA. Chloramphenicol-induced bone marrow suppression. Semin. Haematol. 1973;10:225–234. [PubMed] [Google Scholar]
- Yunis AA. Mechanisms underlying marrow toxicity from chloramphenicol and thiamphenicol. In: Silver R, Le Bue J, Gordon AS, editors. The Year in Haematology. New York: Plenum Press; 1978. pp. 143–170. [Google Scholar]
- Yunis AA. Chloramphenicol: relation of structure to activity and toxicity. Ann. Rev. Pharmacol. Toxicol. 1988;28:83–100. doi: 10.1146/annurev.pa.28.040188.000503. [DOI] [PubMed] [Google Scholar]
- Yunis AA, Bloomberg GR. Chloramphenicol toxicity, clinical features and pathogenesis. Prog. Haematol. 1964;4:138–159. [PubMed] [Google Scholar]
- Yunis AA, Smith US, Restreppo A. Reversible bone marrow suppression from chloramphenicol: A consequence of mitochondrial injury. Arch. Intern. Med. 1970;126:272–275. [PubMed] [Google Scholar]
- Zaporowska H, Wasilewski W. Significance of reduced food and water consumption with vanadium. Comp. Biochem. Physiol. 1991;99C:349–352. doi: 10.1016/0742-8413(91)90254-q. [DOI] [PubMed] [Google Scholar]