Abstract
Our aim was to compare the expression of EGFR and proliferative cell nuclear antigen (PCNA) in different histological and endoscopic diagnostic groups, in cases of Helicobacter pylori infection, in vivo. Paraffin embedded human gastric biopsy samples (86) were analysed by EGFR and PCNA immunohistochemistry and classified both on the basis of histology and endoscopic findings. In normal epithelia (NE), a positive correlation was found between PCNA and EGFR and in H. pylori-negative gastritis with and without intestinal metaplasia (P < 0.01). On the other hand, a negative correlation was detected between the two immunohistochemical findings in H. pylori-associated gastritis with intestinal metaplasia (HPGIM) and in the atrophic gastritis (AG) group. In HPGIM the percentage of EGFR-positive cells was significantly lower (32.4 ± 30.4) when compared to either the NE (50.3 ± 23.7) or H. pylori-negative gastritis with intestinal metaplasia (HNGIM) (48.3 ± 23.7). In AG, EGFR was significantly lower when compared to the NE (P < 0.05). Based on the endoscopic findings, a significant decrease of EGFR expression was found in gastric ulcer cases as compared to NE, gastritis or erosion cases (P < 0.01). PCNA showed no significant alterations between the NE and gastritis, AG groups. The presence of H. pylori has an inverse effect on PCNA and EGFR expression in HPGIM.
Keywords: endoscopic diagnosis, epidermal growth factor receptor, Helicobacter pylori infection, histological diagnosis, intestinal metaplasia, proliferative cell nuclear antigen
Introduction
Helicobacter pylori (H. pylori) infection is one of the most important agents in the development of chronic gastritis and plays a central role in the aetiology of peptic ulcer disease in humans. Persistent H. pylori infection results in delayed ulcer healing and ulcer recurrence. However, the mechanisms of the ulcerogenic action of this bacterium and the interference of H. pylori with ulcer healing are yet unknown.
Numerous studies have shown that H. pylori infection induces increased cell proliferation of epithelial cells (Konturek et al. 1997; Konturek et al. 1998; Smoot et al. 1999). However, it is not clear whether the increased cell proliferation is a direct effect of H. pylori or is a reflex increase in proliferation as a response to increased cell damage, indirectly caused by H. pylori (Granelli et al. 1997; Coyle et al. 1999; Wang et al. 2000).
The major defence mechanisms of gastric mucosa, continuous cell renewal and proliferation, are responsible for maintaining mucosal integrity, repair of injury and ulcer healing. The growth factors are the most important agents promoting all these processes (Fujiwara et al. 1997). The seemingly important growth factors are epidermal growth factor (EGF) and transforming growth factor alpha (TGFα). Both EGF and TGFα interact with a common receptor, called epidermal growth factor receptor (EGFR). The human EGFR has been cloned, and is a 170-kDa protein that consists of a cell surface ligand-binding domain, a single hydrophobic trans-membrane domain and a cytoplasmic tyrosine kinase domain. EGFR is localized to the basolateral cell membrane of the enterocytes (Hirono et al. 1995; Playford et al. 1996; Abe et al. 1997). The TGFα produced may be able to gain access to the receptor in normal gastric mucosa, but EGF, which is only present in the lumen of the bowel, may not. EGF is a potent mitogenic peptide, which plays a crucial role in promoting gastric epithelial cell migration and proliferation. The increased local production of EGF also leads to overexpression of EGFR (Konturek et al. 1998; Tarnawski et al. 1992; Playford et al. 1996; Abe et al. 1997; Fujiwara et al. 1997).
Expression of EGFR or proliferative cell nuclear antigen (PCNA) has been demonstrated in the gastric tissue of H. pylori-infected samples using immunohistochemical methods (Konturek et al. 1997; Tarnawski et al. 1992; Iida et al. 1995; Abe et al. 1997; Luan et al. 1997; Konturek et al. 1998). We have found in our previous work that there was a strong association between histological classification of gastritis and proliferating fraction of cells determined by TV image cytometry and immunohistochemistry (Szaleczky et al. 2000). However, there has been no investigation of the relationship between the expression of EGFR and the PCNA labelling index (LI) in H. pylori-positive gastric mucosa with or without intestinal metaplasia, and correlations to endoscopic findings were also not defined. Our aim was to examine whether the presence of H. pylori and intestinal metaplasia in the stomach affects the expression of EGFR and proliferation ratio of gastric mucosal cells from different human paraffin-embedded tissue samples.
Materials and methods
Eighty-six patients underwent routine upper gastrointestinal endoscopy in the Endoscopy Laboratory of the Second Department of Medicine, Semmelweis University (45 male, 41 female, of average age 58.5 years, with a range of 33–87 years). All subjects gave informed consent, and the project was reviewed and accepted by the ethical committee of the Semmelweis University, Budapest, Hungary. During endoscopy, gastric samples were taken for routine histology. Modified Giemsa staining and a rapid urease test were used to detect H. pylori. All cases were classified according to the endoscopic findings: normal epithelial (n = 9), endoscopic gastritis (n = 49), erosion (n = 13) and ulceration (n = 15). Samples were divided into five groups according to the routine histology: normal epithelial (n = 9), chronic gastritis (n = 28), chronic gastritis with intestinal metaplasia (n = 10), H. pylori-associated gastritis (n = 29), and H. pylori-associated gastritis with intestinal metaplasia (n = 10). These specimens contained foci of significant atrophy (n = 7), including intestinal metaplasia (n = 20), and had different degrees of inflammation (n = 50). The specimens were classified in accordance with the Sydney classification. Factors in this classification included the activity of gastritis, the severity of gastritis, atrophy and intestinal metaplasia.
Samples were tested for the presence of EGFR and proliferating cell nuclear antigen (PCNA) by immunohistochemical methods.
Immunohistochemistry
For immunohistochemistry all biopsy specimens were fixed in buffered formalin and embedded in paraffin. Sections 4 µm thick were cut and mounted on glass slides.
After deparaffinization in xylene and rehydration through graded ethanol, sections were submerged in methanol containing 3% hydrogen peroxide for 30 min to block endogenous peroxidase activity. Then tissue sections were washed in phosphate-buffer saline (PBS) and were placed in citrate buffer pH 6.0 twice for 7 min (Antigen Retrieval Citrate, Biogene, San Ramon, CA, USA). Then sections were exposed to non-immune serum obtained from animals of the same species from which secondary antibodies were prepared. Primary antibodies were reacted with tissue sections at optimal dilution overnight at 37 °C. Optimal dilutions for primary antibodies against EGFR (Kirkegaard & Perry Laboratories, Inc., USA) and PCNA (Monoclonal Mouse Anti-PCNA Clone PC10, DAKO, UK) were 1 : 100 and 1 : 80, respectively. Reaction with primary antibodies was followed by incubation with biotinylated secondary antibodies for 60 min at room temperature and with peroxidase-conjugated streptavidin for 30 min at room temperature. Each step was followed by two 7-min. washes in PBS. Following final washing, sections were visualized by aminoethylcarbazole (AEC) (LSAB2 Kit, DAKO, UK) for 15 min. Immunostained sections were counterstained with haematoxylin.
Known EGFR-positive and PCNA-positive tissue sections were used as positive controls, and negative control sections were processed immunohistochemically after having replaced the primary antibody by PBS. None of these control sections exhibited immunoreactivity. The result of EGFR staining was evaluated in 500 neighbouring cells in three different regions of gastric mucosa (the surface, the neck region and the basal region of glandular structures) in accordance with the criteria described previously (Tarnawski et al. 1992; Abe et al. 1997; Konturek et al. 1997; Konturek et al. 1998). The EGFR expression was defined as the percentage of the EGFR-positive cells over the total cells counted. In the case of PCNA staining, the nuclei stained brown completely, and they were uniformly considered as positive. The average number of nuclei recorded per specimen was 1000. The PCNA labelling index (LI) was defined as the percentage of the PCNA-positive nuclei over the total nuclei counted (Konturek et al. 1998; Iida et al. 1995).
The evaluation of staining intensity (i.e. no. of positive cells) for EGFR and PCNA was performed by two investigators independently, without knowledge of the histology and the result of the other investigator. There was less than 5% variance between the results of the two counts.
Statistical analysis
One-way anova (Kruskal–Wallis), a paired t-test and correlation analysis (Pearson) using css statistica (Statsoft, USA) software were used for the evaluation of the results. The null hypothesis was rejected if its probability was less than 0.05. For further evaluation a post hoc least-squares-deviation (LSD) test was performed.
Results
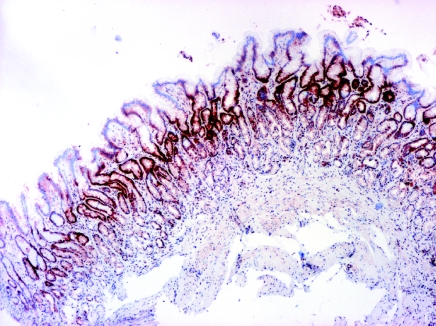
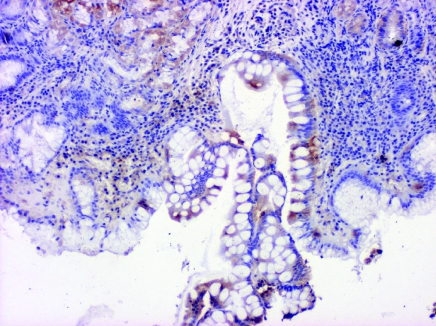
In normal gastric mucosa a strong positive EGFR immunoreactivity was expressed predominantly in some mucous neck cells of the proliferative zone. Positive EGFR immunoreactivity was observed at the basolateral membrane, cytoplasm and in the supranuclear area of mucosal cells. Positive EGFR immunoreactivity was also expressed in some parietal cells (in cytoplasm and plasma membrane), but it was generally weaker compared with neck cells. Positive PCNA immunoreactivity was also demonstrated in the proliferative zone, however, the parietal cells did not express PCNA. Poorly or aberrantly differentiated cells lining dilated glands of the ulcer scar showed PCNA immunoreactivity (Figures 1 and 2).
Figure 1.
PCNA labelling in ulcer scar, PCNA immunoreactivity in ulcer margin, magnification ×4
Figure 2.
PCNA labelling in H. pylori-positive gastritis without intestinal metaplasia, moderate inflammation, strong PCNA immunoreactivity in the neck region of glands, magnification ×4.
In normal epithelia a positive correlation was found between PCNA and EGFR. However, negative correlations were detected in H. pylori-positive gastritis with and without intestinal metaplasia by the Pearson test. Also a significant negative correlation was obtained in the atrophic gastritis groups between PCNA and EGFR expression (P < 0.05) (Table 1).
Table 1.
EGFR and PCNA in atrophic gastritis.
| PCNA | EGFR | Correlation | |
|---|---|---|---|
| Normal epithelium, N = 9 | 47.9±11.26 | 50.3±23.7** | 0.3 |
| Atrophic gastritis, N = 7 | 59.8±16.1 | 27.7±21.7** | −0.84† |
Significant difference
P < 0.01
Significant correlation
P < 0.05.
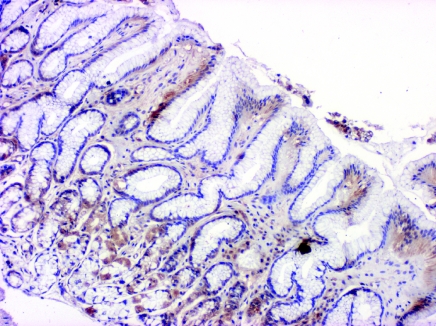
In H. pylori-positive gastritis with intestinal metaplasia the percentage of EGFR-positive cells was significantly decreased (32.4 ± 30.4) as compared to the normal epithelium (50.3 ± 23.7) and H. pylori-negative gastritis with intestinal metaplasia (48.3 ± 23.7) (Table 2, Figures 3 and 4). A similar, however, not significant difference was detected in chronic gastritis without intestinal metaplasia. The number of PCNA-positive cells was the same in the H. pylori-associated and chronic gastritis group. However, in the intestinal metaplasia group the H. pylori-associated cases had higher PCNA positivity compared to the H. pylori-negative ones.
Table 2.
PCNA and EGFR in H. pylori positive and negative gastritis with and without intestinal metaplasia
| PCNA | EGFR | Correlation | |
|---|---|---|---|
| Normal epithelium, N = 9 | 47.9±11.25 | 50.3±23.7** | 0.30 |
| H. pylori positive gastritis without | 52.9±16.5 | 45.3±25.1 | −0.08 |
| intestinal metaplasia, N = 29 | |||
| H. pylori negative gastritis without | 51.7±17.9 | 47.33±21.18 | 0.2 |
| intestinal metaplasia, N = 28 | |||
| H. pylori positive gastritis with | 52.02±18.9 | 32.4±30.4** | −0.89† |
| intestinal metaplasia, N = 10 | |||
| H. pylori negative gastritis with | 45.8±11.1 | 48.3±23.7* | 0.13 |
| intestinal metaplasia, N = 10 | |||
Significant difference
P <0.05.
Significant differences
P <0.01.
Significant correlation
P <0.05.
Figure 3.
EGFR labelling in H. pylori-positive gastritis with intestinal metaplasia, mild chronic inflammation in the interstice, antral mucosa, magnification × 10.
Figure 4.
EGFR labelling in H. pylori-negative gastritis without intestinal metaplasia, mild chronic inflammation, EGFR immunoreactivity in the neck region of glands, antral mucosa, magnification × 10.
Based on the endoscopic findings, the number of EGFR-positive epithelial cells was significantly (P < 0.01; Kruskal–Wallis test) lower in gastric ulcers (36.2 ± 24.4), compared to normal epithelium (57.1 ± 21.4), gastritis (46.8 ± 26.4) and erosion (54.3 ± 29.9) (Table 3). The number of PCNA, which are proliferating cells, was the highest in the erosion group (60.3 ± 21.5); however, the difference to the normal group (46.6 ± 19.4) was not significant.
Table 3.
Correlation of PCNA and EGFR with endoscopic findings.
| PCNA | EGFR | Correlation | |
|---|---|---|---|
| Normal epithelium, N = 9 | 46.6±19.4 | 51.7±21.4* | 0.29 |
| Endoscopic gastritis, N = 49 | 53.1±15.7 | 46.8±26.4** | −0.1 |
| Erosion, N = 13 | 60.3±21.5 | 54.3±29.9* | −0.63 |
| Ulceration, N = 15 | 49.1±17.3 | 36.2±24.4** | −0.13 |
Significant difference
P < 0.05.
Significant difference
P < 0.01.
Discussion
Persistent H. pylori infection results in delayed ulcer healing and ulcer recurrence, but the role of this bacterium within this process has not been completely elucidated. In vitro exposure of gastric mucosal cells to H. pylori leads to increased production of growth factors (Chang et al. 1993; Smoot et al. 1999). Induction of these growth factors is not sufficient to counteract the growth inhibitory effect exerted by H. pylori (Romano et al. 1998). Inhibition of cell growth does seem to be due to inhibition of EGFR activation by H. pylori. Helicobacter pylori may act by altering the EGFR signal transduction pathway, inhibiting the association of EGF to its receptor and phosphorylation of the EGFR, both of which are essential in the gastric mucosa cell proliferation and repair process. Intensive researches indicated that a H pylori-derived, yet unidentified low molecular (<12 kDa) mass component, secreted by H. pylori, interferes with the binding of EGF to its receptor, reduces cell migration and inhibits proliferative response of human gastric mucosal cells (Ricci et al. 1996; Fujiwara et al. 1997; Romano et al. 1998; Russo et al. 1998).
Numerous observations suggest that the vacA virulence factor of H. pylori directly inhibits gastric epithelial cell proliferation by altering the growth factor-regulated homeostasis of the gastric epithelium (Chang et al. 1993; Pai et al. 1998; Romano et al. 1998; Tarnawski & Jones 1998). Moreover vacA may enter into cells by endocytosis mediated by the EGFR; thus EGFR may play a significant role in the development of vacuolation caused by vacA (Seto et al. 1998). Endocytosis may have inactivated EGFR and made it undetectable. However, this mechanism has not yet been completely validated (Iida et al. 1995; Tokunaga et al. 1995).
In our human data the expression of EGFR is decreased in H. pylori-positive intestinal metaplasia cases, as compared to the normal epithelia. These results are consistent with previous reports showing reduced rather than increased release of EGFR into the gastric juice (Calabro et al. 1990; Polk et al. 1992). However, an EGFR overexpression was found by Konturek et al. (1997) and Coyle et al. (1999) in H. pylori infection. Our results have demonstrated that H. pylori infection significantly reduced the EGFR expression and phosphorylation in intestinal metaplasia; in addition, no significant changes were detected in gastritis. In previous reports, increased expression of EGFR was described in the intestinal metaplastic areas compared with normal mucosa but H. pylori-positive samples were not examined (Luan et al. 1997; Slesak et al. 1998). However, Granelli et al.'s (1997) preliminary data suggest that EGFR overexpression seemed rather infrequent in the metaplastic gastric mucosa of normal patients.
A very specific situation was observed in atrophic gastritis. The PCNA LI was increased, however, the expression of EGFR was the lowest of all. Similar significant negative correlation was found between PCNA and EGFR expression in H. pylori-positive gastritis with and without intestinal metaplasia. A close positive correlation was observed between the intensities of EGFR and PCNA by Konturek et al. (1997) and Luan et al. (1997) in H. pylori-positive cases of gastritis.
Several splendid studies have suggested that H. pylori infection of the gastric mucosa is associated with an increase in gastric epithelial cell proliferation (Brenes et al. 1993; Cahill et al. 1995; Konturek et al. 1998; Rokkas et al. 1999; Xia & Talley 2001).
Our PCNA data are consistent with some previous reports (Ricci et al. 1996; Romano et al. 1998; Smoot et al. 1999). We have not found any significant increase in PCNA LI, neither in H. pylori-negative, nor H. pylori-positive gastritis. The lower PCNA labelling index observed in gastritis with intestinal metaplasia compared to gastritis without intestinal metaplasia, corresponds with our previous results (Szaleczky et al. 2000).
The comparison of endoscopic findings with the immunohistochemical data showed that erosion and ulceration have a different PCNA and EGFR expression pattern. In ulceration both the PCNA and EGFR were lower. These results are consistent with immunohistochemical studies by Abe et al. (1997). The discrepancy in EGFR expression from previous studies may be due to differences in the antibody employed, tissue preparation or stage of the gastric ulcers examined. In erosion these parameters were elevated as compared to the normal epithelium.
Our data suggest that increased cell proliferation is not a specific response of the host to cell damage in gastritis, since the number of PCNA-positive cells is equal both in H. pylori-positive and negative gastritis. However, EGFR is a better immunohistochemical marker for the detection of altered gastric epithelial cell function.
References
- Abe S, Sasano H, Katoh K, et al. Immunohistochemical studies on EGF family growth factors in normal and ulcerated human gastric mucosa. Dig. Dis. Sci. 1997;42:1199–1209. doi: 10.1023/a:1018897922644. [DOI] [PubMed] [Google Scholar]
- Brenes F, Riuz B, Correa P, Hunter F, Rhamakrishnan T, Fontham E, Shi TY. Helicobacter pylori causes hyperproliferation of the gastric epithelium: pre- and posteradication indices of proliferating cell nuclear antigen. Am. J. Gastroenterol. 1993;88:1870–1875. [PubMed] [Google Scholar]
- Cahill RJ, Xia HHX, Kilgallen C, Beattie S, Hamilton H, O'Morain C. Effect of eradication of Helicobacter pylori infection on gastric epithelial cell proliferation. Dig. Dis. Sci. 1995;40:1627–1631. doi: 10.1007/BF02212681. [DOI] [PubMed] [Google Scholar]
- Calabro A, Orsini B, Brocchi A, Falchini M, Fedi P, Surrenti C. Gastric juice immunoreactive epidermal growth factor levels in patients with peptic ulcer disease. Am. J. Gastroenterol. 1990;85:404–407. [PubMed] [Google Scholar]
- Chang K, Fujiwara Y, Wyle F, Tarnawski A. Helicobacter pylori toxin inhibits growth and proliferation of cultured gastric cells-Kato III. J. Physiol. Pharmacol. 1993;44:17–22. [PubMed] [Google Scholar]
- Coyle WJ, Sedlack RE, Nemec R, et al. Eradication of Helicobacter pylori normalizes elevated mucosal levels of EGF and its receptor. Am. J. Gastroenterol. 1999;94:2885–2889. doi: 10.1111/j.1572-0241.1999.01432.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Wyle F, Arakawa T, et al. Helicobacter pylori culture supernatant inhibits binding and proliferative response of human gastric cells to EGF: implications for Helicobacter pylori interference with ulcer healing? Digestion. 1997;58:299–303. doi: 10.1159/000201458. [DOI] [PubMed] [Google Scholar]
- Granelli P, Fichera G, Zennaro F, et al. Expression of the EGFR gene in human intestinal metaplasia: a preliminary report. Scand. J. Gastroenterol. 1997;32:485–489. doi: 10.3109/00365529709025086. [DOI] [PubMed] [Google Scholar]
- Hirono Y, Tsugawa K, Fushida S, et al. Amplification of EGFR gene and its relationship to survival in human gastric cancer. Oncology. 1995;52:182–188. doi: 10.1159/000227455. [DOI] [PubMed] [Google Scholar]
- Iida A, Hirose K, Arai M, Yamaguchi A, Nakagawara G. Relationships among the expression of epidermal growth factor receptor, proliferating cell nuclear antigen labelling index, and lymph node metastasis in gastric cancer. Oncology. 1995;52:189–195. doi: 10.1159/000227456. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Bobrzynski A, Konturek SJ, et al. Epidermal Growth Factor and Transforming Growth Factor Alpha in duodenal ulcer and non-ulcer dyspepsia patients before and after Helicobacter pylori eradication. Scand. J. Gastroenterol. 1998;33:143–151. doi: 10.1080/00365529850166860. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Ernst H, Konturek SJ, et al. Mucosal expression and luminal release of epidermal and transforming growth factors in patients with duodenal ulcer before and after eradication of Helicobacter pylori. Gut. 1997;40:463–469. doi: 10.1136/gut.40.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan F, Wang M, You W. The correlation of TGF-alpha, EGFR in precancerous lesions and carcinoma of stomach with PCNA expression. Abstract. Chung. Hua. Ping. Li. Hsueh. Tsa. Chih. 1997;26:31–34. [PubMed] [Google Scholar]
- Pai R, Wyle FA, Cover TL, Itani RM, Domek MJ, Tarnawski AS. Helicobacter pylori culture supernatant interferes with EGF-activated signal transduction in human gastric KATO III. cells. Am. J. Pathol. 1998;152:1617–1624. [PMC free article] [PubMed] [Google Scholar]
- Playford RJ, Boulton R, Ghatei MA, Bloom SR, Wright NA, Goodlad RA. Comparison of the effects of TGF-alpha and EGF on gastrointestinal proliferation and hormone release. Digestion. 1996;57:362–367. doi: 10.1159/000201358. [DOI] [PubMed] [Google Scholar]
- Polk WH, Dempsey PJ, Russell WE, et al. Increased production of transforming growth factor alpha following acute gastric injury. Gastroenterology. 1992;102:1467–1474. doi: 10.1016/0016-5085(92)91703-7. [DOI] [PubMed] [Google Scholar]
- Ricci V, Ciacci C, Zarrilli R, et al. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of vacA and cagA. Infect. Immun. 1996;64:2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokkas T, Ladas S, Liatsos C, et al. Relationship of Helicobacter pylori cagA status to gastric cell proliferation and apoptosis. Dig. Dis. Sci. 1999;44:487–493. doi: 10.1023/a:1026636803101. [DOI] [PubMed] [Google Scholar]
- Romano M, Ricci V, Di Popolo A, et al. Helicobacter pylori upregulates expression of epidermal growth factor-related peptides, but inhibits their proliferative effect in MKN 28 gastric mucosal cells. J. Clin. Invest. 1998;101:1604–1613. doi: 10.1172/JCI1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F, Messa C, Amati L, et al. The influence of Helicobacter pylori eradication on the gastric mucosal content of EGF, TGF-alpha and their common receptor. Scand. J. Gastroenterol. 1998;33:271–275. doi: 10.1080/00365529850170856. [DOI] [PubMed] [Google Scholar]
- Seto K, Hayashi-Kuwabara Y, Yoneta T, Suda H, Tamaki H. Vacuolation induced by cytotoxin from Helicobacter pylori is mediated by the EGF receptor in HeLa cells. FEBS Lett. 1998;431:347–350. doi: 10.1016/s0014-5793(98)00788-1. [DOI] [PubMed] [Google Scholar]
- Slesak B, Harlozinska A, Porebska I, et al. Expression of epidermal growth factor receptor family proteins (EGFR, c-erbB-2 and c-erbB-3) in gastric cancer and chronic gastritis. Anticancer Res. 1998;18:2727–2732. [PubMed] [Google Scholar]
- Smoot DT, Wynn Z, Elliott TB, et al. Effects of Helicobacter pylori on proliferation of gastric epithelial cells in vitro. Am. J. Gastroenterol. 1999;94:1508–1511. doi: 10.1111/j.1572-0241.1999.01134.x. [DOI] [PubMed] [Google Scholar]
- Szaleczky E, Pronai L, Molnar B, Berczi L, Feher J, Tulassay Z. Increased cell proliferation in chronic Helicobacter pylori positive gastritis and gastric carcinoma. Correlation between immunohistochemistry and Tv image cytometry. Anal. Cell. Pathol. 2000;20:131–139. doi: 10.1155/2000/830906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski AS, Jones MK. The role of EGF and its receptor in mucosal protection, adaptation to injury, and ulcer healing: involvement of EGF receptor signal transduction pathways. J. Clin. Gastroenterol. 1998;27(Suppl. 1):S12–S20. doi: 10.1097/00004836-199800001-00004. [DOI] [PubMed] [Google Scholar]
- Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102:695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Onda M, Okuda T, et al. Clinical significance of EGF, EGF receptor and c-erbB-2 in human gastric cancer. Cancer. 1995;75(Suppl. 6):1418–1425. doi: 10.1002/1097-0142(19950315)75:6+<1418::aid-cncr2820751505>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- Xia HHX, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am. J. Gastroenterol. 2001;96:16–26. doi: 10.1111/j.1572-0241.2001.03447.x. [DOI] [PubMed] [Google Scholar]