Abstract
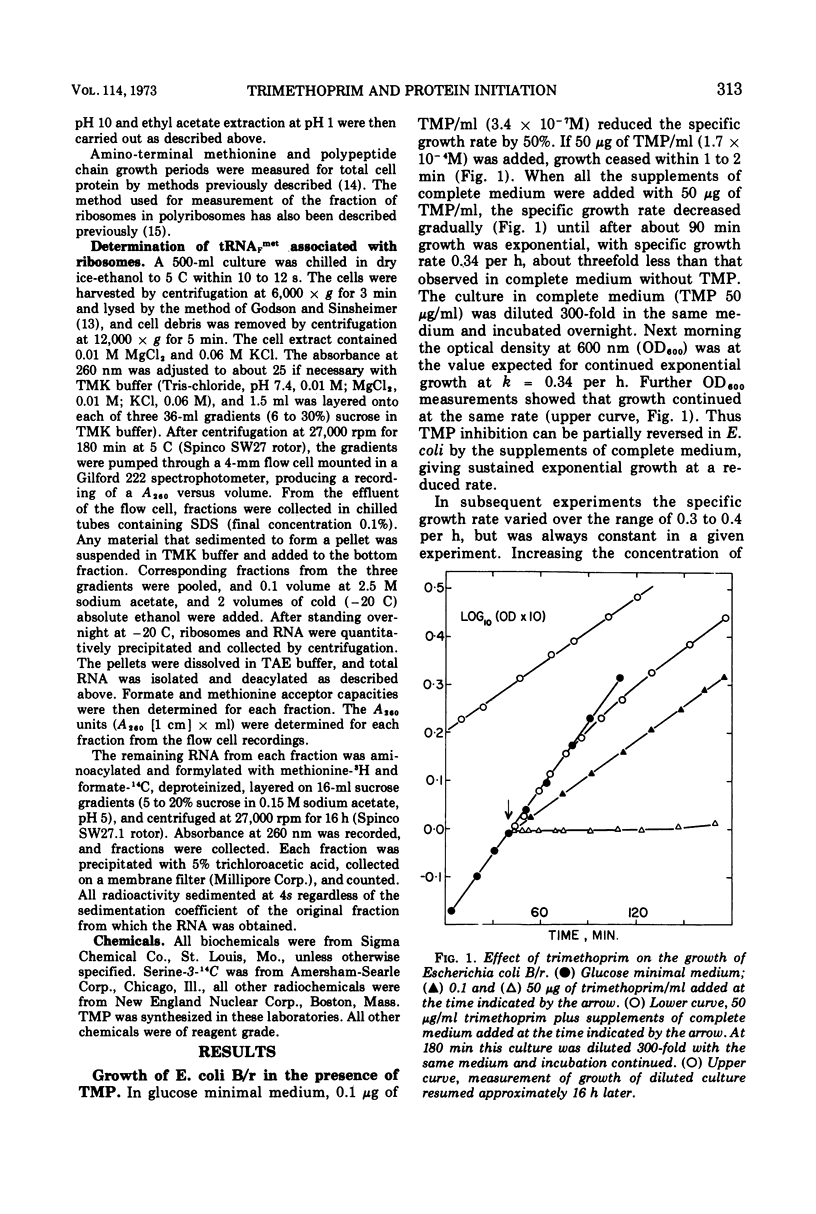
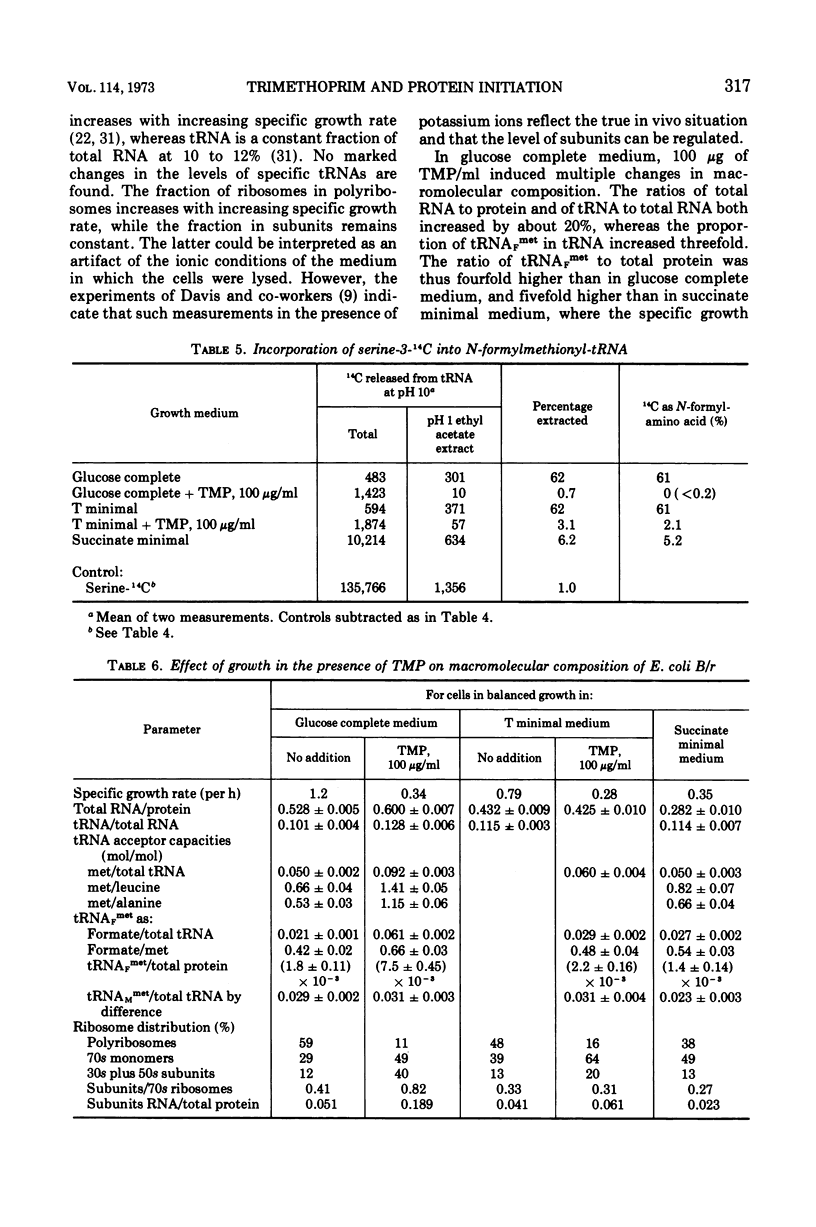
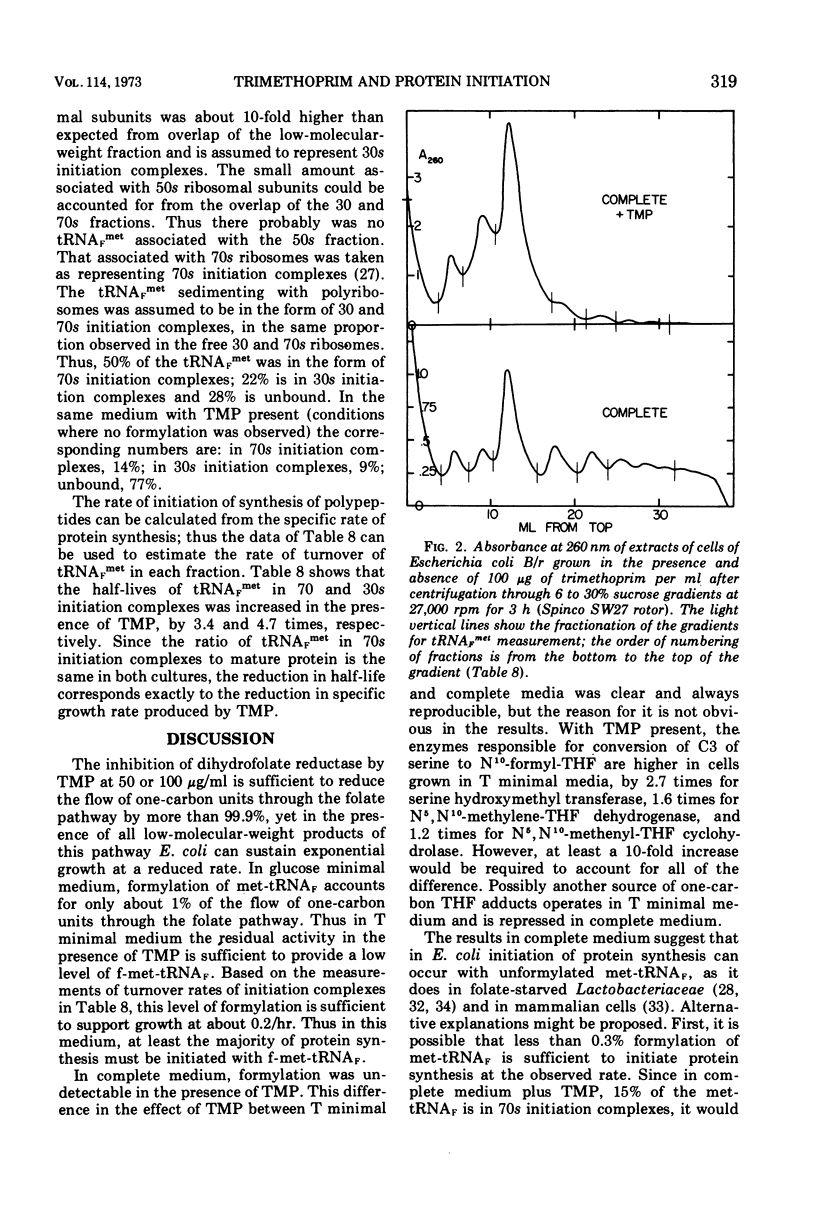
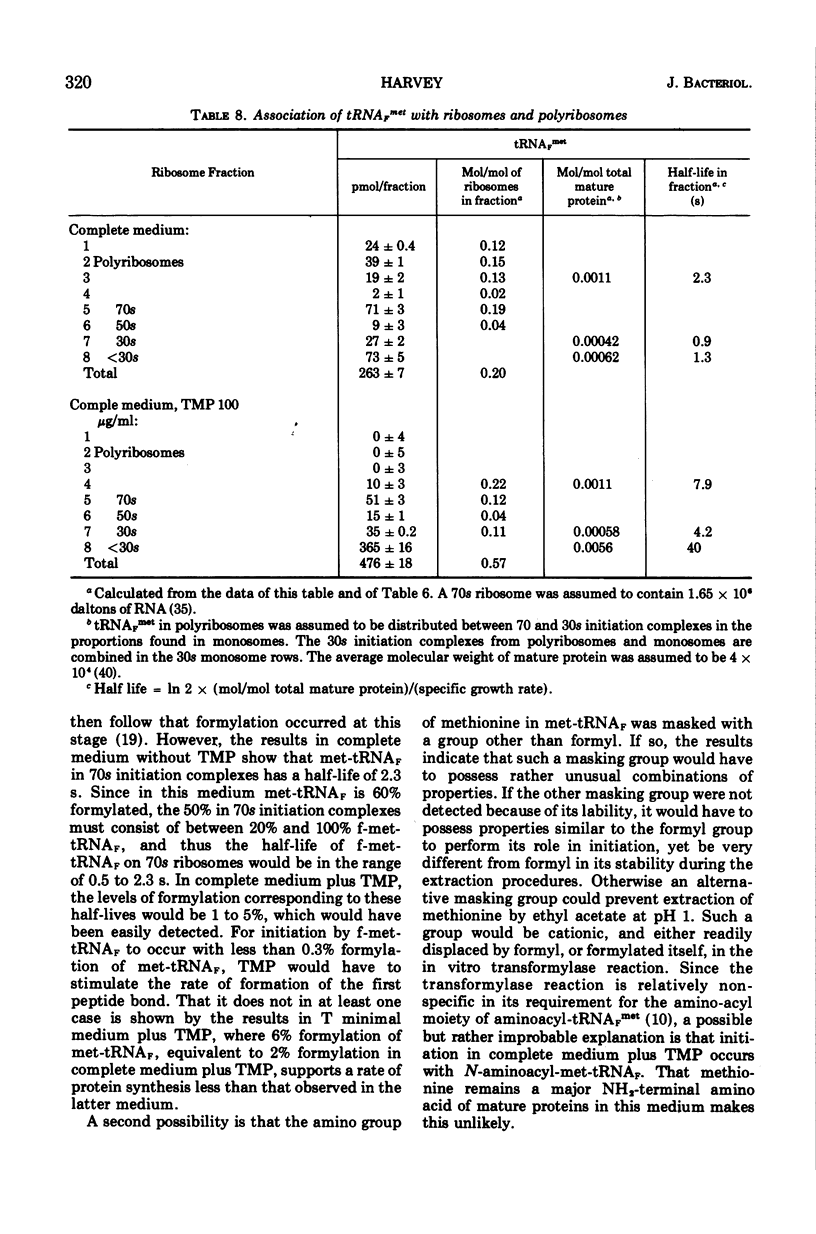
Escherichia coli grew exponentially at a reduced rate in the presence of 50 or 100 μg of trimethoprim/ml if the low-molecular-weight products of folate metabolism or their precursors (thymidine, purines, methionine, glycine, and pantothenate) were supplied in the medium. Folate metabolism was inhibited 99.9% by these concentrations of trimethoprim, but a low level of formylation of methionyl transfer ribonucleic acid (met-tRNAF) could be detected. However, in a medium containing all major amino acids, nucleosides, and vitamins, formylation of met-tRNAF was undetectable in the presence of trimethoprim. No other amino-masked amino acids were detected, and methionine remained a major amino-terminal amino acid of mature proteins. met-tRNAF was rapidly labeled with exogenous methionine and was associated with 30s ribosomal subunits and 70s ribosomes. It was concluded that initiation of protein synthesis can occur with unformylated met-tRNAF in E. coli. Changes in macromolecular composition were associated with the lack of formylation, in particular a fourfold increase in both met-tRNAF and ribosomal subunits. These changes would tend to compensate for the low specific rate of initiation with unformylated met-tRNAF.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Marcker K. A. Polypeptidyl-sigma-ribonucleic acid and amino-acyl-sigma-ribonucleic acid binding sites on ribosomes. Nature. 1966 Jul 23;211(5047):380–384. doi: 10.1038/211380a0. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. N-formyl-methionyl-sigma-ribonucleic acid and chain initiation in protein biosynthesis. Polypeptide synthesis directed by a bacteriophage ribonucleic acid in a cell-free system. Nature. 1966 Jul 23;211(5047):378–380. doi: 10.1038/211378a0. [DOI] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. The role of N-formyl-methionyl-sRNA in protein biosynthesis. J Mol Biol. 1966 Jun;17(2):394–406. doi: 10.1016/s0022-2836(66)80150-x. [DOI] [PubMed] [Google Scholar]
- Clark F. C., Marcker K. A. Coding response of N-fromyl-methionyl-sRNA to UUG. Nature. 1965 Sep 4;207(5001):1038–1039. doi: 10.1038/2071038b0. [DOI] [PubMed] [Google Scholar]
- Cohn W. E. The Separation of Purine and Pyrimidine Bases and of Nucleotides by Ion Exchange. Science. 1949 Apr 15;109(2833):377–378. doi: 10.1126/science.109.2833.377. [DOI] [PubMed] [Google Scholar]
- Davis B. D. Role of subunits in the ribosome cycle. Nature. 1971 May 21;231(5299):153–157. doi: 10.1038/231153a0. [DOI] [PubMed] [Google Scholar]
- Eisenstadt J., Lengyel P. Formylmethionyl-tRNA dependence of amino acid incorporation in extracts of trimethoprim-treated Escherichia coli. Science. 1966 Oct 28;154(3748):524–527. [PubMed] [Google Scholar]
- Friedman H., Lu P., Rich A. Temperature control of initiation of protein synthesis in Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):105–121. doi: 10.1016/0022-2836(71)90209-9. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Use of Brij lysis as a general method to prepare polyribosomes from Escherichia coli. Biochim Biophys Acta. 1967 Dec 19;149(2):489–495. doi: 10.1016/0005-2787(67)90176-1. [DOI] [PubMed] [Google Scholar]
- Harvey R. J. Regulation of ribosomal protein synthesis in Escherichia coli. J Bacteriol. 1970 Feb;101(2):574–583. doi: 10.1128/jb.101.2.574-583.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelmers A. D., Novelli G. D., Stulberg M. P. Separation of transfer ribonucleic acids by reverse phase chromatography. J Biol Chem. 1965 Oct;240(10):3979–3983. [PubMed] [Google Scholar]
- Kolakofsky D., Nakamoto T. The initiation of viral protein synthesis in e. Coli extracts. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1786–1793. doi: 10.1073/pnas.56.6.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Eggerston G., Eisenstadt J., Lengyel P. Ribosome formation from subunits: dependence on formylmethionyl-transfer RNA in extracts from E. coli. Nature. 1968 Oct 26;220(5165):368–371. doi: 10.1038/220368a0. [DOI] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis,I. Effect of formylation of methionyl-tRNA on codon recognition. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1579–1585. doi: 10.1073/pnas.56.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Friedman H., Rich A. Temperature effects in the formation of the initiation complex in protein synthesis: a note of caution. Biochim Biophys Acta. 1971 May 13;238(2):343–346. doi: 10.1016/0005-2787(71)90102-x. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- Marcker K. The formation of N-formyl-methionyl-sRNA. J Mol Biol. 1965 Nov;14(1):63–70. doi: 10.1016/s0022-2836(65)80230-3. [DOI] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V. PHAGE f2 RNA-DIRECTED BINDING OF FORMYLMETHIONYL-TRNA TO RIBOSOMES AND THE ROLE OF 30S RIBOSOMAL SUBUNITS IN INITIATION OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1967 Sep;58(3):946–953. doi: 10.1073/pnas.58.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J., Gordon B., Sarimo S. S. Protein initiation without folate in Streptococcus faecium. Biochim Biophys Acta. 1969 Apr 22;179(2):439–447. doi: 10.1016/0005-2787(69)90052-5. [DOI] [PubMed] [Google Scholar]
- Rosset R., Julien J., Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966 Jul;18(2):308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., D'Ari L., Rabinowitz J. C. Evidence against the folate-mediated formylation of formyl-accepting methionyl transfer ribonucleic acid in Streptococcus faecalis R. J Biol Chem. 1970 Oct 10;245(19):5115–5121. [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Soska J. Growth of Lactobacillus acidophilus in the absence of folic acid. J Bacteriol. 1966 May;91(5):1840–1847. doi: 10.1128/jb.91.5.1840-1847.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Bock R. M. Isolation and physical properties of the ribosomal ribonucleic acid of Escherichia coli. Biochemistry. 1965 Jul;4(7):1302–1311. doi: 10.1021/bi00883a014. [DOI] [PubMed] [Google Scholar]
- Sundararajan T. A., Thach R. E. Role of the formylmethionine codon AUG in phasing translation of synthetic messenger RNA. J Mol Biol. 1966 Aug;19(1):74–90. doi: 10.1016/s0022-2836(66)80051-7. [DOI] [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Enzymes of clostridial purine fermentation. Methylenetetrahydrofolate dehydrogenase. J Biol Chem. 1967 Oct 10;242(19):4378–4385. [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Enzymes of the clostridial purine fermentation. Serine hydroxymethyltransferase. Arch Biochem Biophys. 1968 Feb;123(2):271–278. doi: 10.1016/0003-9861(68)90134-3. [DOI] [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Metabolism of formiminoglycine. Formiminotetrahydrofolate cyclodeaminase. J Biol Chem. 1967 Jan 10;242(1):24–31. [PubMed] [Google Scholar]
- WALLER J. P. THE NH2-TERMINAL RESIDUES OF THE PROTEINS FROM CELL-FREE EXTRACTS OF E. COLI. J Mol Biol. 1963 Nov;7:483–496. doi: 10.1016/s0022-2836(63)80096-0. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D. In vitro protein synthesis: chain initiation. Proc Natl Acad Sci U S A. 1966 Jan;55(1):155–161. doi: 10.1073/pnas.55.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegian C. D., Stent G. S., Martin E. M. Intracellular condition of Escherichia coli transfer RNA. Proc Natl Acad Sci U S A. 1966 Apr;55(4):839–846. doi: 10.1073/pnas.55.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir A., Leder P., Elson D. A ribosome-catalyzed reaction between N-formylmethionyl-trna and puromycin. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1794–1801. doi: 10.1073/pnas.56.6.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]


