Abstract
Stings by Thalassophryne nattereri are responsible for envenomation of fishermen in north-eastern Brazil. Its venom induces prominent local tissue damage, characterized by pain, oedema and necrosis. The pathogenesis of acute muscle damage induced by T. nattereri venom was studied in mice. Intramuscular injection induced myonecrosis within the first hours. Some muscle cells presented a hypercontracted morphology, but most necrotic fibres were not hypercontracted, being instead characterized by a disorganization of myofibrils, with Z line loss, mitochondrial swelling and sarcolemmal disruption. In addition, thrombosis was observed histologically in venules and veins, together with vascular congestion and stasis, evidenced by intravital microscopy. Venom induced a rapid increment in serum creatine kinase (CK) levels, concomitant with a reduction in gastrocnemius muscle CK activity, whereas no increments in muscle lactic acid were detected. A rapid cytolytic effect was induced by the venom on C2C12 murine myoblasts in culture. The inflammatory reaction in affected muscle was characterized by oedema and scarce cellular infiltrate of polymorphonuclear leucocytes and macrophages, with a consequent delay in the removal of necrotic material. Skeletal muscle regeneration was partially impaired, as evidenced by the presence of regenerating fibres of variable size and by the increase of fibrotic tissue in endomysium and perimysium. It is suggested that T. nattereri venom affects muscle fibres by a direct cytotoxic effect, and that the vascular alterations described preclude a successful regenerative process.
Keywords: Thalassophryne nattereri, fish venom, myonecrosis, regeneration, thrombosis
Necrosis of skeletal muscle occurs in a variety of diseases such as muscular dystrophy (Mokri & Engel 1975; Hoffman et al. 1987), infections by Clostridum perfringens (Stevens et al. 1997), ischemia (Karpati et al. 1974) and envenomations associated with snakebites (Azevedo-Marques et al. 1985; Harris & Cullen 1990) and bee stings (França et al. 1994), among others. Snake venoms and toxins have become useful tools to develop experimental models of muscle degeneration and regeneration (Ownby 1990; Harris 1992; Gutiérrez & Lomonte 1997), including local myonecrosis as well as systemic myotoxicity associated with myoglobinuria (Gopalakrishnakone et al. 1997). It is, however, necessary to find new experimental models of acute muscle damage that may help to elucidate the mechanisms underlying basic degenerative and regenerative processes in mammalian skeletal muscle. It is important to develop models in which muscle cell injury occurs concomitantly with thrombosis, since these two pathological findings are present in a number of clinical examples of muscle damage. Stings caused by the fish Thalassophryne nattereri (niquim) are common on the shores of north-eastern Brazil, affecting mainly fishermen. These small fish are found buried under the mud in shallow waters. When people step on or grab them a spine protrudes from the fish injecting a toxic secretion produced by glands located at the base of the spine (Lopes-Ferreira et al. 1998). T. nattereri venom induces excruciating local pain, oedema and necrosis, observed both clinically (Froes 1933; Auto 1992) and experimentally (Lopes-Ferreria et al. 1998). Preliminary experimental observations in mice indicate that T. nattereri venom induces acute myonecrosis with histological features distinct from those characterizing myonecrosis caused by myotoxins isolated from snake venoms (Lopes-Ferreira et al. 2000). This study was carried out in order to describe the morphological and biochemical aspects of muscle damage and regeneration after experimental injections of T. nattereri venom, in order to investigate the pathogenesis of local tissue damage in this envenomation.
Materials and methods
Venom
Venom was obtained from specimens of T. nattereri collected on the shores of Maceio, State of Alagoas, Brazil. Venom was collected from the openings at the tip of the spines by applying pressure at their bases. It was lyophilized and kept at −20 °C until use. The batch of venom used was a pool obtained from 30 specimens.
Plasma creatine kinase activity and histological and ultrastructural studies
Groups of four Swiss-Webster mice (18–20 g body weight) were injected intramuscularly in the right gastrocnemius muscle with 100 µg venom (dissolved in 100 µL phosphate-buffered saline solution (PBS), pH 7.2). Control mice received 100 µL PBS under otherwise identical conditions. At various time intervals (1 h, 3 h, 6 h and 24 h) a blood sample was collected from the tail into heparinized capillary tubes. The creatine kinase (CK, EC 2.7.3.2) activity in plasma was determined using the kit no. 520 from Sigma Chemical Co. (St Louis, Missouri, USA). CK activity was expressed in Units/mL, one unit resulting in the phosphorylation of one nanomole of creatine per min at 25 °C. In some experiments, CK activity was determined using the Sigma kit no. 47-UV. Immediately after blood sampling, mice were sacrificed, the right gastrocnemius muscle was dissected out, and tissue samples were obtained from different areas. Besides the time intervals described above, tissue samples for histological studies were also collected at 30 min, 7, 14 and 28 days after envenomation. Samples were fixed for 2 h in Karnovsky's fixative (2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 m phosphate buffer, pH 7.2). Postfixation was performed for 1 h with 1% osmium tetroxide in 0.1 m phosphate buffer, pH 7.2. Samples were then dehydrated in ethanol and embedded in Spurr resin (Moreira et al. 1992). Thick (1 µm) sections were stained with toluidine blue for histological studies. Thin sections were stained with uranyl acetate and lead citrate, and observed on a H-7000 Hitachi electron microscope at the Electron Microscopy Unit, University of Costa Rica.
Quantification of wet weight and CK activity in muscle
Groups of five mice were injected with 100 µg venom, as described. After 3 h or one, seven and 28 days, mice were sacrificed and both gastrocnemius muscles were dissected out, weighed and immediately homogenized in 5 mL of PBS containing 0.1% Triton X-100 and centrifuged at 5000 X g. Supernatants were diluted 1:30 with PBS and the CK activity determined as described above. Muscle CK content was expressed as a percentage, taking as 100% the values of the left, nonenvenomated gastrocnemius.
Quantification of lactic acid in muscle
Groups of four mice (18–20 g) were injected intramuscularly in the right gastrocnemius with 100 µg venom, dissolved in 100 µL PBS. Control mice received 100 µL PBS alone. At various time intervals (1 h, 3 h and 6 h) mice were sacrificed and their injected muscles excised and homogenized in 2 mL of distilled water. Then, 4 mL of 10% trichloroacetic acid was added and, after mixing, tubes were incubated 5 min at 4 °C. Immediately afterwards, they were centrifuged at 2000 × g for 10 min and the supernatants were collected and assayed for lactic acid using the Sigma kit 826-UV.
Intravital microscopy
Male mice (18–22 g) were anaesthetized with an intraperitoneal injection of sodium pentobarbital (2 mg/100 g body weight), placed on a water-heated bed (at 37 °C), and the cremaster muscle was exposed (Lomonte et al. 1994). Solutions containing various amounts of venom, in 20 µL PBS, were applied onto the exposed muscle and immediately covered with MylarR to prevent dehydration. Control experiments were performed by applying 20 µL PBS under otherwise identical conditions. Muscle preparations were observed in a light microscope (BX50, Olympus, Japan), equipped with a colour video camera (DXC-151/151P, Sony, Japan), a VCR (SLV-X510, Sony), and a colour video monitor (PVN-2030, Sony). Five minutes of observation were recorded before application of the venom to analyse the dynamics in control tissue. Four experiments were carried out and tissue reactions were observed for up to 40 min.
Cell culture studies
Cytotoxicity was determined on C2C12 murine myoblasts in culture, as previously described (Lomonte et al. 1999). Briefly, venom was diluted in assay medium (Dulbecco's modified Eagle's Medium, DMEM, supplemented with 1% fetal calf serum) and added to cell cultures growing in 96-well plates in a total volume of 150 µL per well. Controls for 0 and 100% cytotoxicity were prepared using assay medium and assay medium with 0.1% Triton X-100, respectively. After 3 h incubation, an aliquot of 100 µL supernatant was collected and lactic dehydrogenase (LDH) activity released from cells to the medium was determined using a colourimetric end-point assay (Sigma kit no. 500).
Results
Pathological changes induced by T. nattereri venom
Macroscopic observations
Mice injected with PBS did not present locomotion problems nor any type of distress. In contrast, animals receiving venom had difficulties in mobilizing their right hind leg within the first 2 min after injection and presented evident swelling as early as 10 min after envenomation. Swelling persisted for up to 24 h and subsided afterwards. By 7 and 28 days mice mobilized their hindlegs normally, although the apparent muscle mass in the right gastrocnemius was reduced. Gross haemorrhage was not observed at any time interval.
Histological and ultrastructural changes
Mice injected with PBS showed normal histological and ultrastructural features in the gastrocnemius muscle. Tissue from animals receiving T. nattereri venom showed pathological alterations early after injection. By 30 min there were few muscle cells having discrete lesions at the periphery of the fibres, resembling ‘delta lesions’ previously described in other muscle pathologies (Mokri & Engel 1975; Gutiérrez et al. 1984a). Areas of myofilament hypercontraction were observed in these fibres (Figure 1a). By 1, 3 and 6 h large areas of muscle tissue were affected. Although some affected muscle cells showed the typical hypercontracted morphology described after injection of snake venom myotoxins (Gutiérrez & Lomonte 1997), the majority of affected fibres presented a predominantly different morphological pattern, characterized by the scarcity of hypercontraction and by a disorganization of myofibrillar material (Figure 1b). At the ultrastructural level, these cells presented alterations in all organelles. The plasma membrane was interrupted or absent in large portions, whereas the basal lamina remained at the periphery of the fibres (Figure 2a). Mitochondria were swollen and some of them had disrupted membranes (Figure 2a). The myofibrils were disorganized, lacking their typical longitudinal orientation in the cellular space and the Z line was absent, with the consequent disorganization of sarcomeres (Figure 2b). Few hypercontrated myofibrils were observed in these necrotic cells.
Figure 1.
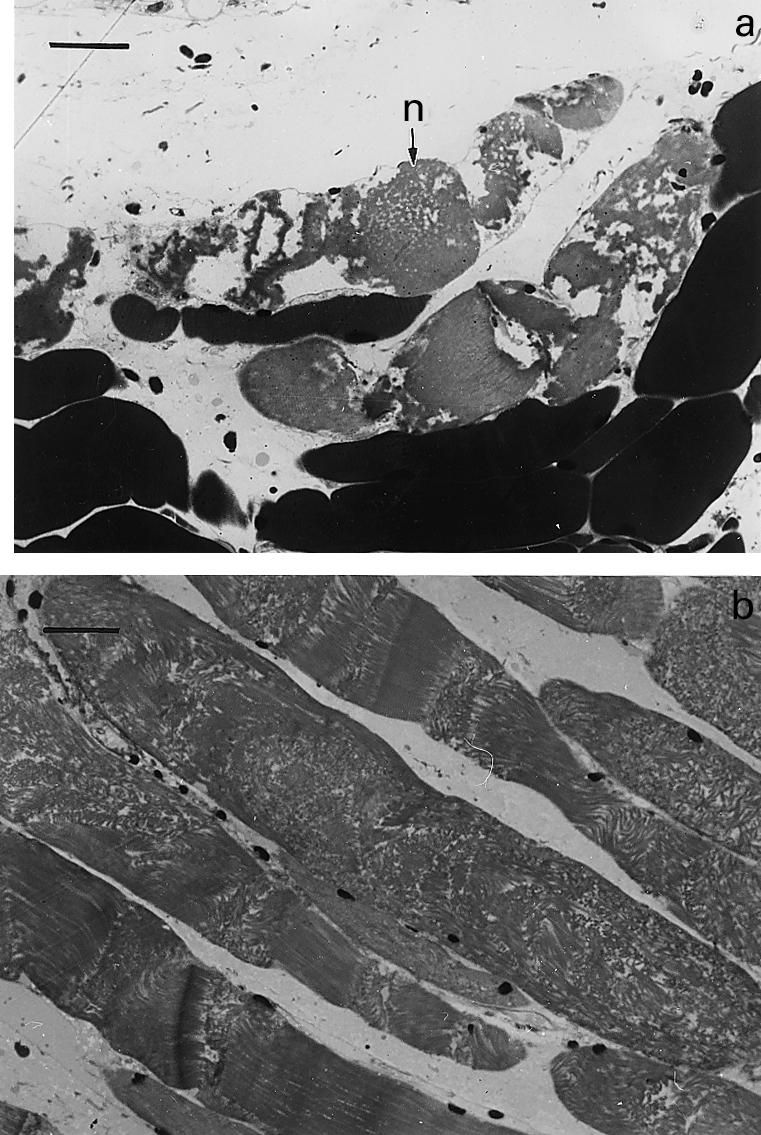
Light micrographs of sections of gastrocnemius muscle taken 3 h after intramuscular injection of T. nattereri venom. (a) Necrotic muscle fibres (n) showing hypercontraction and clumping of myofilaments. Bar represents 25 µm (b) Group of necrotic fibres in which there is disorganization of myofibrillar structure with very few areas of hypercontraction. Bar represents 25 µm.
Figure 2.
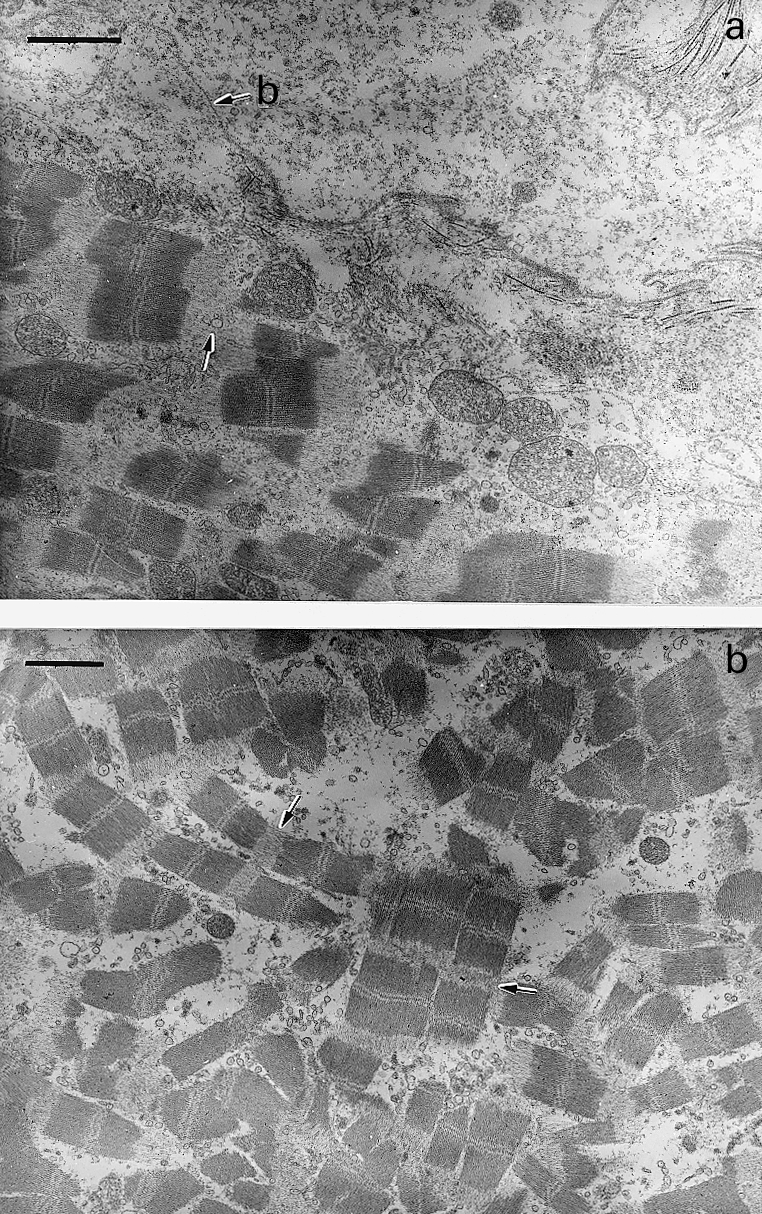
Electron micrographs of sections of gastrocnemius muscle taken 6 h after intramuscular injection of T. nattereri venom. (a) Portion of a necrotic muscle fibre showing Z line loss (arrows) and swollen mitochondria. Plasma membrane has been lost and only the basal lamina (b) remains at the periphery of the fibre. Bar represents 2 µm (b) Portion of a necrotic muscle fibre showing disorganization of myofibrils associated with Z line loss (arrow). Notice the lack of hypercontraction of myofilaments. Bar represents 2 µm.
There was no haemorrhage in muscle tissue at any time, as no erythrocytes were present in the interstitial space. Abundant thrombi were observed in venules and small veins (Figure 3) as early as 1 h after injection. Additionaly, some vessels showed congestion. The inflammatory reaction following myonecrosis was characterized by a scarce infiltrate of polymorphonuclear leucocytes and macrophages. This scarcity of inflammatory cellular influx was particularly evident at 24 h, where areas of abundant necrotic fibres had very few phagocytic cells and, as a consequence, necrotic material had not been removed by phagocytosis. Thrombosis in veins and venules was still observed at this time period. Seven and 14 days after venom injection there were histological features of regeneration, although this process was partially impaired. Regenerative muscle fibres had variations in their diameter and presented centrally located nuclei (Figure 4a). Some fibres were of small diameter and there were split regenerating fibres. Areas of fibrosis were present, together with increments in fibrotic tissue interspersed with regenerating muscle cells (Figure 4b). A qualitatively similar histological pattern was present 28 days after envenomation, in which regenerated muscle fibres were intermixed with areas of fibrosis, although at this time interval the size and abundance of regenerating fibres had increased when compared to samples collected at 7 days.
Figure 3.
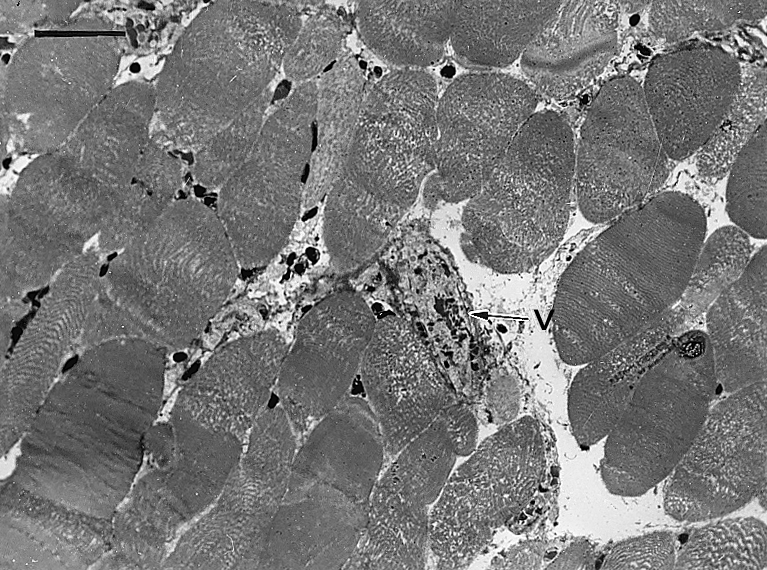
Light micrograph of a section of gastrocnemius muscle 3 h after injection of T. nattereri venom. There is widespread myonecrosis, with fibres showing a morphology characterized by disorganization of myofibrillar material with very few hypercontracted regions. A thrombosed venule is observed (v). Bar represents 50 µm.
Figure 4.
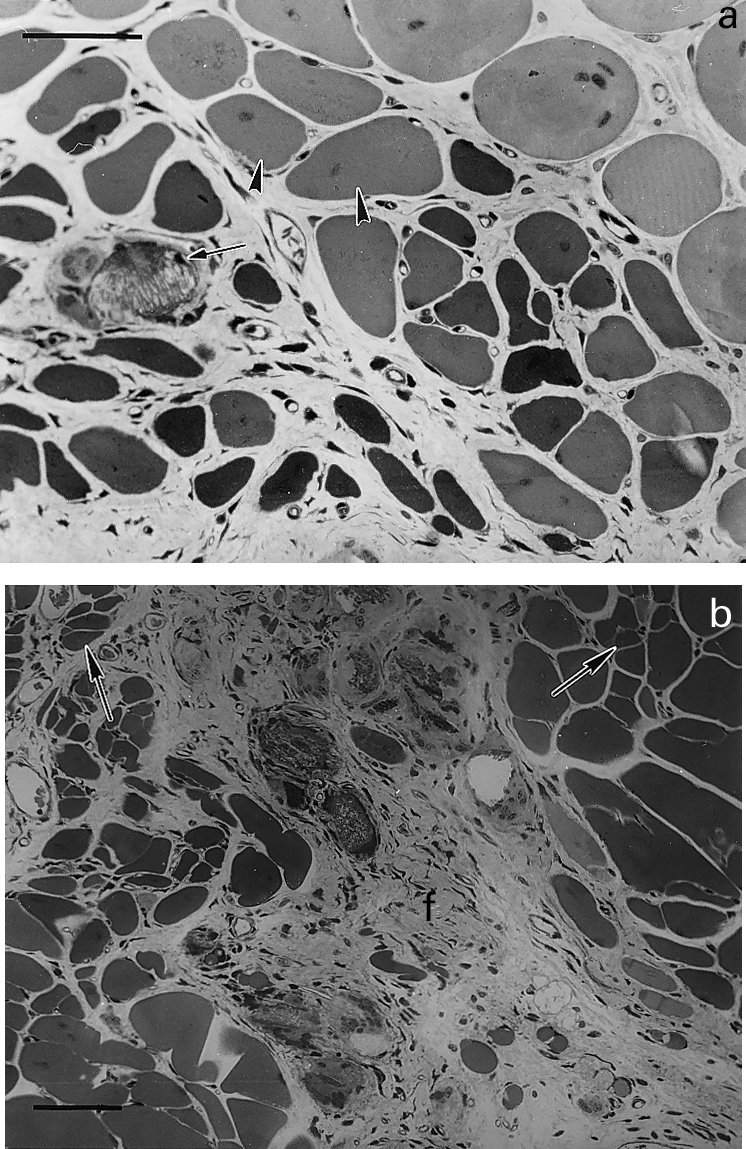
Light micrographs of sections of gastrocnemius muscle taken at different times after intramuscular injection of T. nattereri venom. (a) 14 days; abundant regenerating fibres of varying size, some of them showing centrally located nuclei (arrow heads). Notice a calcified necrotic cell with a small regenerating fibre in its vicinity. Bar represents 50 µm (b) 28 days; portion of tissue in which regenerating fibres of varying size are observed, some of them showing fibre splitting (arrows). Prominent fibrosis (f) is observed in an area of poor muscle regeneration. Bar represents 50 µm.
Changes in plasma and muscle CK activity
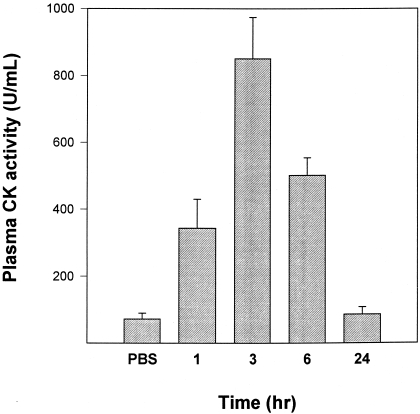
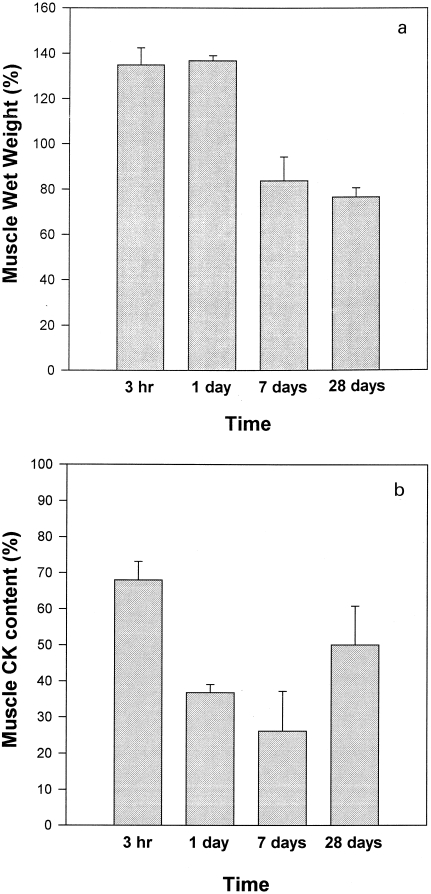
A rapid and prominent increment in plasma CK activity was observed after injection of T. nattereri venom (Figure 5). Activity peaked at 3 h and decreased afterwards. By 24 h plasma CK activity was similar in mice injected with venom and in those injected with PBS (Figure 5). CK activity in gastrocnemius muscle homogenates decreased drastically after injection, as by 3 h it corresponded to 68 ± 5% of activity of contralateral, noninjected gastrocnemius (Figure 6b). Contralateral muscle was not affected in this experimental model as its CK activity did not differ from that in control, noninjected mice (results not shown). Activity decreased to 45 ± 10% by 24 h, at which time muscle wet weight had increased to 137 ± 12% with respect to contralateral gastrocnemius (Figure 6a). Wet weight decreased during the first week, reaching 84 ± 11% of contralateral muscle by the 7th day, whereas CK activity of muscle homogenates was further reduced at this time period to 26 ± 11% of the activity of contralateral gastrocnemius. By 28 days, muscle mass in envenomated muscle remained significantly lower than in control muscle (76 ± 4%), and CK activity of injected gastrocnemius showed an increment to a value corresponding to 50 ± 10% of CK in contralateral muscle.
Figure 5.
Changes in plasma CK activity of mice injected intramuscularly in the gastrocnemius with 100 µg T. nattereri venom dissolved in 100 µL PBS. Control animals received PBS alone. Mice were bled at different time intervals and plasma CK quantified. In the case of mice injected with PBS, CK activity was determined 3 h after injection. Results are presented as mean ± SD (n = 4).
Figure 6.
(a) Changes in wet weight of mouse gastrocnemius muscle after intramuscular injection of 100 µg T. nattereri venom dissolved in 100 µL PBS. At various time intervals mice were sacrificed and both injected and contralateral muscles were excised and weighed. Results are expressed as a percentage, taking as 100% the weight of contralateral, noninjected muscle. (b) CK content of mouse gastrocnemius muscle at different time intervals after intramuscular injection of 100 µg T. nattereri venom dissolved in 100 µL PBS. Both injected and contralateral gastrocnemius muscles were homogenized and, after centrifugation, CK activity of the supernatant was quantified. CK activity in envenomated muscle is expressed as a percentage, taking as 100% the CK activity of contralateral, noninjected gastrocnemius. Results are presented as mean ± SD (n = 5).
Changes in muscle lactic acid content
Lactic acid concentration in muscle of mice injected with PBS was 27 ± 5 µmol/g tissue and levels of this metabolite in venom-injected muscle were significantly reduced (P < 0.05) to a value of 18 ± 4 µmol/g tissue 1 h after envenomation. Lactic acid levels in muscles obtained 3 and 6 h after venom injection were 20 ± 3 and 21 ± 4 µmol/g tissue, respectively (P > 0.05 when compared to control muscle).
Intravital microscopy observations
Within 3–4 min of venom application (20 µg/20 µL) onto cremaster muscle there were muscle contractions followed by alterations in some muscle fibres. Few fibres showed hypercontraction, evidenced by retraction of myofibrillar material, with formation of dense clumps of myofibrillar masses. Such changes were often observed at various points in the same muscle fibre. Concomitantly with muscle alterations, there were prominent changes in the microvasculature, starting about 2 min after venom application. These were characterized by congestion and the appearance of abundant thrombi of varying size in veins and venules (Figure 7), which often became emboli. Blood flow in venules decreased throughout this process, resulting in stasis and halting in some vessels. Flow did not resume during the 40 min observation period. There was no evidence of haemorrhage in the cremaster muscle.
Figure 7.

Light micrograph of mouse cremaster muscle examined in intravital microscopy 7 min after topical application of 20 µg of T. nattereri venom, dissolved in 20 µL of PBS. A prominent thrombus is observed in a venule (arrow), whereas blood flow was halted in a nearby smaller venule (arrow head). Notice the presence of myofibrillar hypercontraction in an affected muscle cell (M). Bar represents 100 µm.
Cytotoxic activity on myoblasts
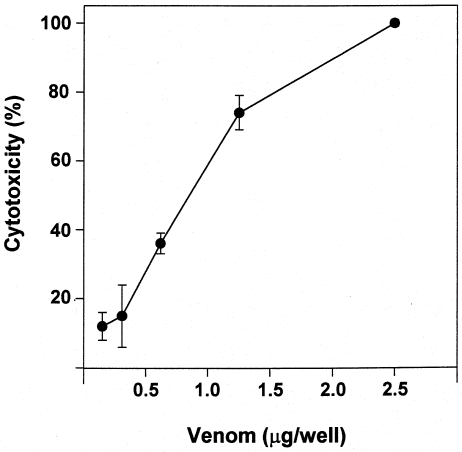
Venom induced a prominent cytotoxic effect when incubated with C2C12 myoblasts in culture. A dose-dependent release of LDH was observed (Figure 8), and this effect occurred even at venom concentrations as low as 5 µg/mL.
Figure 8.
Cytotoxic activity of T. nattereri venom on C2C12 myoblasts in culture. Various concentrations of venom were added to myoblasts and incubated for 3 h at 37 °C. Cytotoxicity was estimated by the release of lactic dehydrogenase to the supernatant. Cytotoxic effect was expressed as a percentage release, taking as 100% the lactic dehydrogenase activity of supernatants of wells in which cells were incubated with assay medium containing 0.1% Triton X-100. Results are presented as mean ± SD (n = 3).
Discussion
Venoms and toxins represent useful tools to investigate muscle degeneration and regeneration, since a synchronic lesion can be induced by these agents (Harris 1992). A variety of myotoxins directly affect the integrity of muscle plasma membrane, promoting a calcium influx which results in a variety of cellular derrangements (Harris & Cullen 1990; Trump & Berezesky 1995). Phospholipases A2, isolated from snake venoms, are well studied membrane-disrupting agents (Harris & Cullen 1990; Ownby 1990; Gopalakrishnakone et al. 1997; Gutiérrez & Lomonte 1997), however other toxins such as elapid cardiotoxins (Duchen et al. 1974; Ownby et al. 1993), bee venom melittin (Ownby et al. 1997) and tarantula venom (Ownby & Odell 1983) induce a similar degenerative pattern after an initial sarcolemmal disruption. In these cases microvasculature is not affected and muscle regeneration proceeds normally and successfully (Harris et al. 1975; Gutiérrez et al. 1984b, 1991).
Haemorrhagic metalloproteinases from snake venoms induce myonecrosis of relatively late onset, probably as a consequence of the ischemia that ensues in muscle tissue after microvessel disruption (Ownby et al. 1978; Gutiérrez et al1995). Whenever myonecrosis occurs concomitantly with local haemorrhage, as in the case of most crotaline snake venoms, regeneration is impaired due to insufficient blood supply during critical stages in the regenerative process (Gutiérrez et al. 1984b, 1995; Arce et al. 1991). The venom of T. nattereri constitutes a novel and valuable tool to study muscle pathology, since it induces direct damage to muscle fibres together with thrombosis and other alterations in the microvasculature, without inducing haemorrhage. Thus, despite its lack of haemorrhagic activity, this venom affects blood supply to muscle tissue by different mechanisms, thereby affecting muscle regeneration. To the best of our knowledge, this constitutes a novel model of myonecrosis coupled to thrombosis in muscle tissue, and may help to understand the effects of thrombosis in a concomitant acute muscle damage.
Various lines of evidence strongly suggest that T. nattereri venom directly affects the integrity of skeletal muscle plasma membrane as its basic mechanism of action in muscle cells: (a) venom is highly cytotoxic to myoblasts in culture in conditions where ischemia is precluded; (b) venom induces a prominent increase in plasma CK, and a concomitant decrease in muscle CK, in vivo rapidly after injection; (c) observations in intravital microscopy revealed early muscle cell lesions, even before thrombosis and stasis appear; and (d) conspicuous interruptions in the integrity of the plasma membrane of muscle cells were observed at the ultrastructural level. Thus, it is suggested that T. nattereri venom contains potent myotoxic components that need to be isolated and characterized.
Despite the observation that plasma membrane is affected at early stages in this model, conspicuous hypercontraction of myofilaments was observed in a reduced number of fibres. In contrast, the predominant morphological pattern of muscle cell damage was characterized by myofibrillar disorganization, with Z line loss, without the characteristic hypercontraction described in many models of muscle cell damage secondary to plasma membrane disruption (Gutiérrez et al. 1984a, 1991; Ownby & Odell 1983; Harris & Cullen 1990; Gopalakrishnakone et al. 1997). This apparent discrepancy might be associated with the effects that T. nattereri venom induces in blood vessels. Thrombosis and diminishment in blood flow would reduce the availability of interstitial calcium, thereby precluding a drastic increment in cytosolic calcium concentration required for hypercontraction. Such hypothesis is supported by experimental models of myocardial ischemia, in which there is sarcolemmal damage and small increments in cytosolic calcium, although hypercontraction does not develop until reperfusion occurs, with the concomitant supply of large quantities of calcium and the development of prominent hypercontraction, i.e. contraction band necrosis (Jennings & Reimer 1991; Reimer & Jennings 1992). In our model, the calcium influx that follows membrane damage is probably not enough to cause hypercontraction in the majority of the fibres, since thrombosis and stasis preclude the availability of large amounts of calcium in these areas. However, such small calcium increment would probably be enough to activate cytosolic, calcium-dependent proteinases and phospholipases A2, with the consequent cleavage of proteins associated with Z line (Ishiura et al. 1978; Sorimachi & Suzuki 1998) and membrane phospholipid degradation (Murakawi et al. 1998).
In the light of the observed thrombosis and stasis, it was of interest to determine if ischemia contributes to myonecrosis in this model. The lack of increment of lactic acid concentration in muscle argues against the hypothesis. This might be due to the fact that the majority of muscle fibres might have been affected by the direct action of venom's myotoxic components. Consequently, cells were already irreversibly damaged by the time blood flow was reduced and therefore there was no increment in the glycolytic pathway and no lactic acid accumulation in necrotic muscle cells.
Skeletal muscle regeneration is a complex process in which myogenic cells, known as satellite cells, are activated as a consequence of myonecrosis, becoming myoblasts which replicate under the influence of various growth factors (see review of Grounds 1991). Removal of necrotic material by phagocytic cells is required for regeneration to proceed. Myoblasts then fuse to form myotubes which later on become fully differentiated muscle fibres. For this process to succeed, several requirements must be met, among which removal of necrotic material by phagocytic cells, adequate blood supply, functional innervation and preservation of the basal lamina at the periphery of muscle fibres are critical (Allbrook 1981; Grounds 1991). T. nattereri venom does not seem to affect basal lamina, which remains morphologically intact throughout the degenerative process. However, thrombosis and stasis may affect regeneration in at least two critical steps. They preclude the development of an adequate cellular inflammatory response, evidenced by the low numbers of phagocytic cells during the first 24 h, and by the presence of necrotic material which had not been cleared out seven days after envenomation. In other models of myonecrosis, in which the microvasculature is not affected, phagocytosis of necrotic material is well advanced by 24 h and no remnants of necrotic muscle cells are observed after one week (Harris et al. 1975; Gutiérrez et al1991; Morini et al1998). Moreover, in mice injected with myotoxic phospholipases A2 from snake venoms, clusters of regenerating fibres are already observed a few days after injection (Gutiérrez et al. 1984b; Morini et al. 1998). Scarcity of macrophages may affect the regenerative process in at least another way, since these cells secrete a variety of growth factors that modulate the replication and differentiation of regenerating cells (Grounds 1991; Auger & Ross 1992).
In addition, lack of blood supply impairs muscle regeneration (Allbrook 1981; Grounds 1991). In these circumstances, fibroblasts proliferate and fibrosis partially substitutes muscle tissue, as described in our experiments, resulting in a histological pattern where regenerating fibres of varying size are intermixed with a fibrotic matrix. It must be kept in mind, however, that regeneration was not fully halted in this model, since there were regenerating muscle fibres 7 and 28 days after envenomation, and since muscle CK contents increased significantly by 28 days. It is necessary to study the process of revascularization and its effect on the regenerative response in this model. It has been observed that regeneration is also impaired in skeletal muscle injected with Bothrops asper snake venom, which induces myonecrosis and haemorrhage (Gutiérrez et al. 1984b; Arce et al. 1991). In these cases, enzymatic degradation of basal lamina components resulting in the disruption of capillary blood vessels, with the consequent extravasation, is responsible for inadequate blood supply to muscle tissue and for the impairment in muscle regeneration. T. nattereri venom does not induce haemorrhage but, by causing thrombosis and stasis, it probably affects blood flow to the regenerating muscle.
In conclusion, T. nattereri venom induces a complex pattern of muscle damage characterized by direct myotoxic effect, together with vascular alterations characterized by blood stasis and thrombosis. The combination of these effects results in acute muscle damage followed by a partially impaired regenerative process. The isolation and characterization of venom components responsible for these effects is a relevant task.
Acknowledgments
The authors thank Rodrigo Mora and Rodrigo Chaves, Instituto Clodomiro Picado, as well as the staff of the Electron Microscopy Unit, Universidad de Costa Rica, for their collaboration. This study was supported by FAPESP (98/11312–1), CNPq (520636/96) and Vicerrectoría de Investigación (Universidad de Costa Rica).
References
- Allbrook D. Skeletal muscle regeneration. Muscle & Nerve. 1981;4:234–245. doi: 10.1002/mus.880040311. [DOI] [PubMed] [Google Scholar]
- Arce V, Brenes F, Gutiérrez JM. Degenerative and regenerative changes in murine skeletal muscle after injection of venom from the snake Bothrops asper: a histochemical and immunocytochemical study. Int. J. ExpPath. 1991;72:211–226. [PMC free article] [PubMed] [Google Scholar]
- Auger MJ, Ross JA. The biology of the macrophage. In: Lewis CE, MCGEE JOD, editors. The Macrophage. Oxford: IRL press; 1992. pp. 2–74. [Google Scholar]
- Auto HF. Acidentes por peixes peçonhentos Thalassophryne(Niquim), consideraçoes em torno de 32 casos. RevEscCiencias Médicas Alagoas. 1992;5:35–36. [Google Scholar]
- Azevedo-Marques MM, Cupo P, Coimbra TM, Hering SE, Rossi MA, Laure CJ. Myonecrosis, myoglobinuria and acute renal failure induced by South American rattlesnake(Crotalus durissus terrificus) envenomation in Brazil. Toxicon. 1985;23:631–636. doi: 10.1016/0041-0101(85)90367-8. [DOI] [PubMed] [Google Scholar]
- Duchen LW, Excell BJ, Patel R, Smith B. Changes in motor endplates resulting from muscle fibre necrosis and regeneration. A light and electron microscopic study of the effects of the depolarizing fraction (cardiotoxin) of Dendroaspis jamesoni venom. J. Neurol. Sci. 1974;21:391–417. doi: 10.1016/0022-510x(74)90041-0. [DOI] [PubMed] [Google Scholar]
- Franca FOS, Benvenuti LA, Fan HW, et al. Severe and fatal mass attacks by ‘killer’ bees (Africanized honey bees - Apis mellifera scutellata) in Brazil: clinicopathological studies with measurement of serum venom concentrations. QJMedicine. 1994;87:269–282. [PubMed] [Google Scholar]
- Froes HP. Sur un poisson toxiphore bresilien: Le niquim. Thalassophryne MaculosaRevSud-AmMedChir. 1933;3:871–878. [Google Scholar]
- Gopalakrishnakone P, Ponraj D, Thwin MM. Venom Phospholipase A2 EnzymesStructure, Function and Mechanism. Chichester: Wiley; 1997. Myotoxic phospholipases from snake venoms: general myoglobinuric and local myonecrotic toxins; pp. 287–320. [Google Scholar]
- Grounds M. Towards understanding skeletal muscle regeneration. Path. Res. Pract. 1991;187:1–22. doi: 10.1016/S0344-0338(11)81039-3. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Lomonte B. Structure, Function and Mechanism. Chichester: Wiley; 1997. Phospholipase A2 myotoxins from Bothrops snake venoms. In Venom Phospholipase A2Enzymes; pp. 321–352. [Google Scholar]
- Gutiérrez JM, Ownby CL, Odell GV. Pathogenesis of myonecrosis induced by crude venom and a myotoxin of Bothrops asper. Exp. Mol. Pathol. 1984a;40:367–379. doi: 10.1016/0014-4800(84)90054-6. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Ownby CL, Odell GV. Skeletal muscle regeneration after myonecrosis induced by crude venom and a myotoxin of the snake Bothrops asper (fer-de-lance) Toxicon. 1984b;22:719–731. doi: 10.1016/0041-0101(84)90155-7. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Núñez J, Díaz C, et al. Skeletal muscle degeneration and regeneration after injection of bothropstoxin II, a phospholipase A2 isolated from the venom of the snake. BothropsJararacussu. Exp. MolPathol. 1991;55:217–229. doi: 10.1016/0014-4800(91)90002-f. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Romero M, Núñez J, Chaves F, Borkow G, Ovadia M. Skeletal muscle necrosis and regeneration after injection of BaH1, a hemorrhagic metalloproteinase isolated from the venom of the snake Bothrops asper (terciopelo) Exp. Mol. Pathol. 1995;62:28–41. doi: 10.1006/exmp.1995.1004. [DOI] [PubMed] [Google Scholar]
- Harris JB. Methods in Neurosciences. Vol. 8. San Diego: Academic Press; 1992. Natural toxins in study of degeneration and regeneration of skeletal muscle; pp. 298–310. Neurotoxins. [Google Scholar]
- Harris JB, Cullen MJ. Muscle necrosis caused by snake venoms and toxins. Electron MicroscRev. 1990;3:183–211. doi: 10.1016/0892-0354(90)90001-9. [DOI] [PubMed] [Google Scholar]
- Harris JB, Johnson MA, Karlsson E. Pathological responses of rat skeletal muscle to a single subcutaneous injection of a toxin isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin. Exp. PharmacPhysiol. 1975;2:383–404. [Google Scholar]
- Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Ishiura S, Murofushi H, Suzuki K, Imahori K. Studies of a calcium-activated neutral protease from chicken skeletal muscle. I. Purification and characterization. JBiochem. 1978;84:225–230. doi: 10.1093/oxfordjournals.jbchem.a132111. [DOI] [PubMed] [Google Scholar]
- Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu. Rev. Med. 1991;42:225–246. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- Karpati G, Carpenter S, Melmed C, Eisen AA. Experimental ischemic myopathy. J. Neurol. Sci. 1974;23:129–161. doi: 10.1016/0022-510x(74)90148-8. [DOI] [PubMed] [Google Scholar]
- Lomonte B, Lundgren J, Johansson B, Bagge U. The dynamics of local tissue damage induced by Bothrops asper snake venom and myotoxin II on the mouse cremaster muscle: an intravital and electron microscopic study. Toxicon. 1994;32:41–55. doi: 10.1016/0041-0101(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Lomonte B, Angulo Y, Rufini S, et al. Comparative study of the cytolytic activity of myotoxic phospholipases A2 on mouse endothelial (tEnd) and skeletal muscle (C2C12) cells in vitro. Toxicon. 1999;37:145–158. doi: 10.1016/s0041-0101(98)00171-8. [DOI] [PubMed] [Google Scholar]
- Lopes-Ferreira M, Barbaro KC, Cardoso DF, Moura da Silva AM, Mota I. Thalassophryne nattereri fish venom: biological and biochemical characterization and serum neutralization of its toxic activities. Toxicon. 1998;36:405–410. doi: 10.1016/s0041-0101(97)00115-3. [DOI] [PubMed] [Google Scholar]
- Lopes-Ferreira M, Rucavado A, Núñez J, et al. Local pathological changes induced by venom of the fish Talassophryne nattereri (niquim) Toxicon. 2000;38:523. [Google Scholar]
- Mokri B, Engel AG. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of muscle fiber. Neurology. 1975;25:1111–1120. doi: 10.1212/wnl.25.12.1111. [DOI] [PubMed] [Google Scholar]
- Moreira L, Gutiérrez JM, Borkow G, Ovadia M. Ultrastructural alterations in mouse capillary blood vessels after experimental injection of venom from the snake Bothrops asper (terciopelo) Exp. Mol. Pathol. 1992;57:124–133. doi: 10.1016/0014-4800(92)90004-u. [DOI] [PubMed] [Google Scholar]
- Morini CC, Pereira ECL, Ownby CL, Salvini TF. Injury and recovery of fast and slow skeletal muscle fibers affected by ACL myotoxin isolated from Agkistrodon contortrix laticinctus (broad-banded copperhead) venom. Toxicon. 1998;36:1007–1024. doi: 10.1016/s0041-0101(97)00112-8. [DOI] [PubMed] [Google Scholar]
- Murakawi M, Shimbara S, Kambe T, et al. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. J. Biol. Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- Ownby CL. Handbook of Toxinology. New York: Marcel Dekker; 1990. Locally acting agents: Myotoxins, hemorrhagic toxins and dermonecrotic factors; pp. 601–653. [Google Scholar]
- Ownby CL, Odell GV. Pathogenesis of skeletal muscle necrosis induced by tarantula venom. Exp. Mol. Pathol. 1983;38:283–296. doi: 10.1016/0014-4800(83)90069-2. [DOI] [PubMed] [Google Scholar]
- Ownby CL, Bjarnason JB, Tu AT. Hemorrhagic toxins from rattlesnake (Crotalus atrox) venom. Pathogenesis of hemorrhage induced by three purified toxins. Am. J. Pathol. 1978;93:201–218. [PMC free article] [PubMed] [Google Scholar]
- Ownby CL, Fletcher JE, Colberg TR. Cardiotoxin 1 from cobra (Naja naja atra) venom causes necrosis of skeletal muscle in vivo. Toxicon. 1993;31:697–709. doi: 10.1016/0041-0101(93)90376-t. [DOI] [PubMed] [Google Scholar]
- Ownby CL, Powell JR, Jiang MS, Fletcher JE. Melittin and phospholipase A2 from bee (Apis mellifera) venom cause necrosis of murine skeletal muscle in vivo. Toxicon. 1997;35:67–80. doi: 10.1016/s0041-0101(96)00078-5. [DOI] [PubMed] [Google Scholar]
- Reimer KA, Jennings RB. Heart and Cardiovascular System. New York: Raven Press; 1992. Myocardial ischemia, hypoxia, and infarction. in the; pp. 1875–1973. [Google Scholar]
- Sorimachi H, Suzuki K. Handbook of Proteolytic Enzymes. San Diego: Academic Press; 1998. M-calpain; pp. 649–654. [Google Scholar]
- Stevens DL, Tweten RK, Awad MM, Rood JI, Bryant AE. Clostridial gas gangrene: evidence that α and φ toxins differentially modulate the immune response and induce acute tissue necrosis. J. Infect. Dis. 1997;176:189–195. doi: 10.1086/514022. [DOI] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB J. 1995;9:219–228. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]