Abstract
Pulmonary complications and graft-vs.-host disease (GVHD) remain severe threats to survival after bone marrow transplantation (BMT). Idiopathic pneumonia syndrome (IPS) accounts for nearly 50% of all the cases of interstitial pneumonitis after BMT. IPS is characterized by an early inflammatory phase followed by chronic inflammation and fibrosis of lung tissue; however, the immunopathogenesis of this disease is not yet clearly understood. This biphasic syndrome has been reported to be associated with pre‐transplant radiation conditioning in some studies while others have suggested that GVHD or autoimmune phenomena may be responsible for its development. The early post-BMT phase is characterized by the presence of inflammatory cytokines whose net effect is to promote lymphocyte influx into lungs with minimal fibrosis, that leads to an acute form of graft-vs.-host reaction-mediated pulmonary tissue damage. Gradual changes over time in leucocyte influx and activation lead to dysregulated wound repair mechanisms resulting from the shift in the balance of cytokines that promote fibrosis. Using data from new animal models of IPS and information from studies of human IPS, we hypothesize that cytokine-modulated immunological mechanisms which occur during the acute and chronic phases after bone marrow transplantation lead to the development of the progressive, inflammatory, and fibrotic lung disease typical of idiopathic pneumonia syndrome.
Idiopathic pneumonia syndrome following bone marrow transplantation
In the past few decades, bone marrow transplantation (BMT) has become an integral part of the therapy of several haematologic and nonhaematologic disorders. Despite the increasing clinical success rate of BMT, lung disease has remained a significant cause of morbidity and mortality among patients (Thomas et al. 1977; Breuer et al. 1993; Quabeck 1994; Soubani et al. 1996). Pulmonary disease is also a major cause of death after BMT in humans, although generally the lung has not been considered a critical target organ in animal models of graft-vs.-host disease (GVHD). While the occurrence of infectious complications of the lung after BMT is high among humans transplanted with bone marrow, these do not generally occur in experimental animals maintained in pathogen-free environments. Yet chronic lung interstitial pneumonitis and fibrosis can still occur in these controlled experimental circumstances. Clinically, the development of pulmonary inflammation and fibrosis after bone marrow transplantation in the absence of identifiable infectious agents has been termed idiopathic pneumonia syndrome (IPS). This syndrome accounts for as many as 50% of the total cases of pneumonia after BMT (Meyers et al. 1982; Cordonnier et al. 1986; Weiner et al. 1989; Ettinger & Trulock 1991). IPS in humans is a severely debilitating disease with an average survival time from onset of clinical symptoms to death ranging between 4 and 6 years. Once established, the mortality rate of IPS can be as high as 70% (Quabeck 1994) and there are currently no promising therapeutic options available for treatment.
IPS is characterized pathologically by the presence of interstitial and alveolar pneumonitis, and interstitial fibrosis, in the absence of an identifiable infectious agent. Interstitial pneumonitis and fibrosis lead to alveolar congestion and decreased lung compliance, which is clinically manifested as dyspnea, tachypnea and hypoxemia. The cellular and molecular mechanisms that give rise to IPS remain an enigma.
Chronic lung inflammation and fibrosis
The lung is constantly exposed to the external environment with the attendant airborne allergens, pathogens, toxic chemicals, and natural and synthetic dusts. The natural defence against such offensive environmental agents is primarily clearance by resident alveolar macrophages. However, ineffective clearance and degradation by alveolar macrophages and/or tissue damage by the insulting agent is commonly followed by an inflammatory response by leucocytes into the lungs (referred to as interstitial pneumonitis), which aids in effective clearing of insulting environmental agents from the lungs. Pulmonary inflammation may also represent the initial component of a progressive disorder such as fibrotic lung disease. Lung ‘fibrosis’ is a general term referring to inflammatory disorders of the lower respiratory tract associated with periluminal, intra-alveolar, and interstitial collagen deposition (Crystal et al. 1984; Crystal et al. 1991). Collagen deposition in the pulmonary interstitium and intra-alveolar spaces is a process of normal wound healing; but while this normal process is localized in space and confined in time, fibrotic lung diseases may involve the entire organ and are often chronic, progressive processes (Crouch 1990; Wolff & Crystal 1997). The severity of fibrosis has been found to correlate with the degree of inflammatory cellularity in fibrotic lungs (Fulmer et al. 1979; Cherniack et al. 1991). Current concepts on the pathogenesis of pulmonary fibrosis propose an initial stage involving an influx of inflammatory cells into the interstitium, which together with activated resident cells, are thought to release a variety of cytokines and other polypeptide mediators that stimulate fibroblast proliferation and collagen secretion. Genetic factors have also been proposed to influence the development of fibrosis, although there is little evidence for the presence of a ‘fibrosis gene’ (Marshall et al. 1997). A more likely genetic influence on fibrosis is the genetic control of cytokine synthesis and/or the response of cells to these cytokines in lung tissue. A number of cytokines secreted by inflammatory cells and resident lung cells have been implicated in the pathogenesis of pulmonary fibrosis. Pro-inflammatory cytokines such as interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), and IL-6 (Agelli & Wahl 1986; Martinet et al. 1996; White & Das 1997), chemokines such as IL-8, macrophage inflammatory protein-1α (MIP-1α), and macrophage chemotactic protein-1 (MCP-1) (Berman et al. 1990; Driscoll 1994; Martinet et al. 1996), and cell surface adhesion molecules (Dupuis & Mcdonald 1997; Southcott et al. 1998), are produced by cells in the lungs during inflammatory processes. Cytokines such as IL-2, interferon-γ (IFN-γ), and IL-12, that direct type-1 helper T cell (Th1) responses, and IL-4, IL-10, and IL-13, that promote type-2 helper T cell (Th2) responses, have also been identified in the lungs during acute and chronic inflammation (Agelli & Wahl 1986; Marshall et al. 1996; Martinet et al. 1996). Collagen gene expression by fibroblasts is known to be regulated by various cytokines, such as IL-1β, TNF-α, transforming growth factor-β (TGF-β), and IFN-γ (Elias et al. 1990a; Grande et al. 1997; Coker & Laurent 1998). Importantly, TNF-α (Piguet et al. 1993; Grande et al. 1997), TGF-β (Khalil et al. 1996; Grande et al. 1997), IL-6 (Shahar et al. 1996), and IL-1β (Kline et al. 1993; Zhang et al. 1993) have all been demonstrated in the fibrotic lungs of patients (Cantin et al. 1988). Additionally, chemokines such as MIP-1 (Standiford et al. 1993b), MCP-1 (Standiford et al. 1993a; Smith et al. 1995), and IL-8 (Ogushi et al. 1997) and cell adhesion molecules such as endothelial leucocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) (Nakao et al. 1995), have also been hypothesized to play key roles in the recruitment of leucocytes to fibrotic lungs and promotion of the pro-fibrogenic environment therein. Furthermore, reduced levels of antifibrogenic factors such as prostaglandin E2 (Wilborn et al. 1995) and IFN-γ (Narayanan et al. 1992) have also been found in fibrotic lungs, suggesting that the interplay between pro- and antifibrotic factors in the lung may be critical for maintaining homeostatic levels of collagen in lung tissue.
Association of IPS with graft-versus-host disease
The onset of IPS after BMT often coincides with the occurrence of graft-vs.-host disease (GVHD). Although a massive amount of information on GVHD mechanisms has accumulated over the years through animal experimentation, few studies have considered the lung as a critical target organ of GVHD. The major target organs are typically considered to be skin, spleen, gastrointestinal tract, and liver; however, the designation of IPS as a complication of BMT has increased awareness of the need to understand the mechanisms by which the lung becomes a target of injury in those patients who develop IPS and to elucidate whether IPS is a unique disease or yet another target site of GVHD. The graft-vs.-host reaction is complex, involving recognition of host histocompatibility antigens by donor T cells, followed by cellular activation and cytokine secretion at both the afferent and efferent phases of the reaction (Ferrara 1993; Ferrara et al. 1996). The incidence of GVHD is dependent not only upon the extent of donor-host histoincompatibility, but also on sex mismatch, recipient age, insufficient T cell-depletion of bone marrow, pre-transplant myeloablative conditioning, post-transplant immunosuppressive therapy, and status of the underlying disease (Chao 1992). It has been shown that mature T cells contaminating the marrow graft are responsible for the initiation of the two forms of GVHD: acute and chronic (Cantor 1972; Korngold & Sprent 1978; Vanbekkum 1980; Ferrara & Deeg 1991). Purging the marrow of T lymphocytes prior to transplant, an approach used by some BMT centres, has been found to correlate with decreased risk and severity of both forms of GVHD (Kernan et al. 1986; Mitsuyasu et al. 1986; Ash et al. 1990); however, the transfusion of T cell-depleted bone marrow causes other complications, such as poor engraftment and a higher risk of cancer relapse.
Acute GVHD by definition occurs within the first 100 days following BMT and is observed in 30–80% of allogeneic transplant patients (Ferrara & Deeg 1991; Chao 1992). The target organs for acute GVHD are usually considered to be the skin, liver, gastrointestinal tract, and the immune system, leading to profound immunosuppression. In the acute GVHD response, alloactivated donor T cells primarily produce Th1-type cytokines, IL-2 and IFN-γ. These and other cytokines can, in turn, activate macrophages to secrete pro-inflammatory cytokines, including IL-1β and TNF-α (Ferrara 1993; Ferrara et al. 1996). This sequence of cytokine production which is associated with acute GVHD (Jadus & Wepsic 1992; Ferrara 1993; Ferrara et al. 1996) has been termed the ‘cytokine storm’ (Antin & Ferrara 1992). By inducing elevated expression of cell adhesion molecules in target organs, inflammatory cytokines produced during the acute GVHD process may facilitate binding of inflammatory cells in target tissues thereby increasing the possibility of recipient cell damage (Norton & Sloane 1991; Ferrara et al. 1996). The second form of GVHD, chronic GVHD, affects approximately 30–60% of patients and is defined as occurring beyond 100 days after grafting (Chao 1992). Clinical manifestations of chronic GVHD include scleroderma, liver dysfunction, rare gastrointestinal involvement, and the development of abnormalities in immune responsiveness such as hypergammaglobulinaemia and autoantibody production. Pulmonary involvement in chronic GVHD is quite common (Soubani et al. 1996). Infiltration and activation of CD4+ T cells have been associated with chronic GVHD in animal studies (Parkman 1986). While the role of Th1-type cytokines has been elucidated in acute GVHD, the network of pathogenic cytokines that cause and maintain chronic GVHD in BMT patients has not been established. However, studies in animal models have implicated a role for B cell-activating Th2-type cytokines such as IL-4 (Doutrelepont et al. 1991; Ushiyama et al. 1995), IL-5 (Dobashi et al. 1987), IL-10 (Dewit et al. 1993), and a selective loss of Th1-type cytokine production, such as that of IFN-γ, during chronic GVHD (Allen et al. 1993; Rus et al. 1995).
Idiopathic pneumonia syndrome temporally overlaps the two forms of graft-vs.-host disease (GVHD) (Soubani et al. 1996). Work from several groups (Stein-Streilein et al. 1981; Piguet et al. 1989b; Breuer et al. 1993) including our own (unpublished observations) has delineated the importance of graft-vs.-host reactivity. Both lung disease and GVHD fail to develop in mice reconstituted with T cell-depleted allogeneic BM alone, demonstrating a common requirement for mature alloreactive T cells in the development of GVHD and pneumonitis (Shankar et al. 1998). Analyses of cytokine mRNA expression in lung tissue of GVHD mice have consistently shown that steady-state levels of IL-1β, TNF-α, IFN-γ, and IL-12 are significantly elevated at 3 weeks post-BMT (acute, nonfibrotic phase). Mice transplanted with allogeneic bone marrow in the absence of alloreactive T cells also displayed elevated expression of these cytokines with the exception of IFN-γ, indicating that expression of cytokines may have been induced by pre-transplant conditioning and/or the transplant procedure and is unrelated to the presence of alloreactive T cells in the graft. In contrast, at 12 weeks after transplantation (chronic, fibrotic phase) only TNF-α and IL-12 levels remained elevated in GVHD mice, suggesting prolonged macrophage activation. Moreover, expression of TNF-α and IL-12 in the chronic phase was dependent on the presence of alloreactive T cells in the recipient. Lung cytokine mRNA expression during the disease could not be classified strictly as a Th1-or Th2-type (Shankar et al. 1998) and since in vitro stimulated lung lymphocytes could secrete both Th1 and Th2 cytokines (unpublished observations) we believe that IPS may not involve cytokine changes characteristic of either ‘classical’ acute or chronic GVHD in the lungs.
Pre-transplant radiation conditioning and IPS
Total body irradiation has played an important role in the development of bone marrow transplantation therapy. While current human BMT protocols offer life-saving treatment for lethal malignancies and immunodeficiency diseases, a significant proportion of BMT recipients die from treatment-related side-effects such as GVHD and IPS. The development of IPS has been found to be associated with pre-transplant conditioning. A number of studies have demonstrated that the lungs are quite susceptible to tissue damage by irradiation (Travis 1980; Down & Steel 1983; Hill 1985). Lung shielding during radiation conditioning (Wilms et al. 1982; Lawton et al. 1989; Labar et al. 1992; Giacchino et al. 1993) and fractionated total body irradiation (Miale et al. 1987; Giacchino et al. 1993; Cosset et al. 1994; Penny et al. 1994) have been shown to reduce (albeit not eliminate) the incidence of IPS in patients, suggesting that radiation-induced lung damage may participate in the initiation of this syndrome.
The severity of GVHD has been related to the extent of pre-transplant radiation conditioning (Deeg et al. 1991; Xun et al. 1994; Hill et al. 1997; Holler et al. 1997; Shankar et al. 1999). Acute GVHD has been found to be closely associated with radiation conditioning in both humans and animals (Deeg et al. 1991; Biggs et al. 1992) while its role in chronic GVHD is unresolved. Inflammatory reactions induced by radiation injury very likely play a role in the induction of immune responses (Milburn et al. 1990; Holler et al. 1997) and may play an important role in localized GVHD. For example, irradiated skin was found more permissive to GVHD lesion formation than unirradiated skin in the setting of systemic GVHD (Desbarats et al. 1994). Indeed, modulation of pre-transplant radiation conditioning paradigms has been shown to ameliorate post-BMT mortality and injury to target organs (Cosset et al. 1994; Penny et al. 1994; Vriesendorp et al. 1994). Studies utilizing animal models have shown that pre-transplant radiation conditioning when combined with allogeneic BMT significantly enhances GVHD-associated mortality (Lehnert et al. 1986; Truitt & Atasoylu 1991). Whether pre-transplant radiation exposure also sensitizes BMT recipients to develop IPS is not clear. Radiation dose fractionation is a suggested option at several BMT centres since it has been demonstrated to reduce the incidence of restricted ventilation and impaired gas exchange (Tait et al. 1991). The occurrence of IPS among patients treated by BMT was correlated in one study with the absolute dose of radiation to the lung (Keane et al. 1981).
Cytokines induced by pre-transplant radiation conditioning and GVHD
Is IPS a result of synergy between pre-transplant radiation-induced lung damage and the graft-vs.-host reaction? This question has not yet been conclusively answered. We have demonstrated in a murine model that BMT with alloreactive T cells and radiation conditioning act synergistically to induce GVHD and IPS under conditions when neither could do so individually; GVHD and IPS could also be induced in the absence of radiation, but only if a tenfold higher dose of alloreactive T cells were transplanted (Shankar et al. 1999). This observation suggests that radiation may lower the threshold for development of IPS and GVHD via increased expression of cytokines, alloantigens and possibly costimulatory molecules. The high incidence of IPS in BMT patients with GVHD compared to those without ongoing GVHD, suggests that the lung must also be considered as a critical target tissue for the graft-vs.-host reaction and that lung disease may be due to the additive or synergistic effects of both radiation injury and the graft-vs.-host reaction. Cytokines have been implicated in mediating GVHD, and both CD4+ and CD8+ T cells are sources of these factors (Jadus & Wepsic 1992). However, it must be noted that many of the T cell cytokines implicated in GVHD can also be secreted by a variety of non-T cells.
Radiation exposure is known to cause local and systemic cytokine production in the early period after irradiation and can persist for several weeks or months after radiation exposure (Tartakovsky et al. 1993; Morgan & Breit 1995; Rubin et al. 1995). The importance of radiation-induced cytokines in the development of BMT-related GVHD was addressed in a study by Xun et al. (Xun et al. 1994). These investigators found that BMT after a critical ‘window period’ of 4–7 days following irradiation alleviated acute GVHD. Total body irradiation of severe combined immunodeficiency (SCID) mice rapidly induced synthesis of IL-1β, IL-6 and TNF-α. These pro-inflammatory cytokines were postulated to prime host tissues for the optimal expression of target antigens or, alternatively, prime donor T-cells for maximal allo-activation.
There are several direct effects of radiation on lung tissue which could promote IPS. Ionizing radiation exposure to the lungs has been demonstrated to induce synthesis of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, fibroblast growth factors such as TGF-β, and vascular adhesion molecules such as ICAM-1, ELAM-1, and selectins (Hallahan et al. 1989; Krivenko et al. 1992; Johnston et al. 1996; Thornton et al. 1996; Hallahan & Virudachalam 1997b). Moreover, direct irradiation of several cell types in vitro can increase expression of histocompatibility molecules (Hauser et al. 1993) and adhesion molecules such as E-selectin, ICAM-1, and VCAM-1 (Gaugler et al. 1997; Hareyama et al. 1998; Heckmann et al. 1998), which could promote better extravasation into lung tissues and enhance allorecognition by T cells. Finally, gamma irradiation can enhance the ability of stimulated monocytes to produce hydrogen peroxide (Gallin & Green 1987) and nitric oxide (Ibuki & Goto 1997), which may suggest an increased capacity for causing tissue damage via peroxidation of proteins, lipids, or nucleic acids, by peroxides and peroxynitrites.
Thoracic irradiation of mice has been shown to induce cytokine expression (Rubin et al. 1995; Johnston et al. 1996). We have discovered that pro-inflammatory cytokines are expressed in the lungs of mice that received either total body irradiation or thoracic irradiation (unpublished observations). The expression of TGF-α in the lungs following radiation has been shown to correlate with development of radiation pneumonitis and pulmonary fibrosis in both humans and rodents (Anscher et al. 1994; Finkelstein et al. 1994). Recent studies in transgenic animals have shown that constitutive expression of TNF-α by lung epithelial cells leads to progressive mononuclear cell infiltration and lung fibrosis (Miyazaki et al. 1995; Thrall et al. 1997; Sime et al. 1998). Thus, local production of cytokines in the lungs clearly can affect the development of lung inflammation and fibrosis. However, the contribution of circulating cytokines produced by cells outside of the lung after total body irradiation cannot be excluded and could exacerbate direct radiation effects in the lung.
A recent study on pneumonitis following allogeneic BMT demonstrated that alveolar macrophages from BMT patients were abnormal with respect to an increase in the number of alveolar monocytes and a decrease in expression of HLA class II (Milburn et al. 1993). However, whether alveolar or interstitial macrophages also display altered production of cytokines has not been addressed in humans with IPS. In contrast, several animal studies have given hints about the immunopathogenesis of IPS after BMT. Piguet et al. demonstrated using a murine GVHD model that a progressive lung disorder displaying signs of diffuse alveolar haemorrhage, alveolitis, and mononuclear interstitial pneumonitis, developed in recipients that received allogeneic donor T cells and thereby had ongoing GVHD. The lungs of these mice exhibited elevated levels of mRNA for TNF-α (Piguet et al. 1989b), a finding consistent with studies in our laboratory (Shankar et al. 1998). In the murine model of bleomycin-induced lung fibrosis TNF-α levels have been found to be elevated (Piguet et al. 1989a). It has also been reported that blocking radiation-induced TNF-α reduced the incidence of GVHD (Holler et al. 1995) inhibited the development of lung inflammation (Ulich et al. 1994).
A hypothetical mechanism for the induction of IPS after BMT: roles of radiation conditioning and graft-versus-host reactivity
The response of alloreactive T cells against class I and II MHC molecules or minor histocompatibility antigens is an attractive hypothesis for initiating and sustaining IPS. Although it is unclear which cells mediate GVHD and IPS, or what the exact specificity of the reactive cell is, it is reasonable to hypothesize at this juncture that chronic activation of macrophages and T cells, and subsequent cytokine release in the interstitial space, is responsible for tissue remodelling after injury – a consequence that results in fibrosis in the lungs. Given the importance of both CD4+ and CD8+ T cells in GVHD (Jadus & Wepsic 1992; Kelemen et al. 1993) and the well-described importance of T cells in a variety of chronic lung injury models, it is highly conceivable that T cells contribute to the onset and progression of IPS, directly through effector cell functions and indirectly through cytokine release and cytokine-mediated effects on other cells. The importance of progressive development of CD4+ T cell inflammation in the development of fibrosis may be two-fold. First, these T cells can amplify the disruption of the normal tissue architecture by directly contributing to local injury via release of cytokines. Second, inflammatory T cells may promote the accumulation of fibroblasts in the local milieu and thus contribute to the repair process and the development of fibrosis. It is equally likely that interstitial and alveolar macrophages participate in development of IPS through release of cytokines such as IL-1β, TNF-α and IL-12. Over the last decade, considerable evidence has emerged to suggest that cytokines constitute important stimuli for collagen deposition in pulmonary fibrosis (Agelli & Wahl 1986; Elias et al. 1990b; Rubin et al. 1995; Martinet et al. 1996; Coker & Laurent 1998). Resident lung cells of the host and donor-derived alloreactive T cells may release cytokines after BMT that can modulate fibroblast proliferation and collagen secretion.
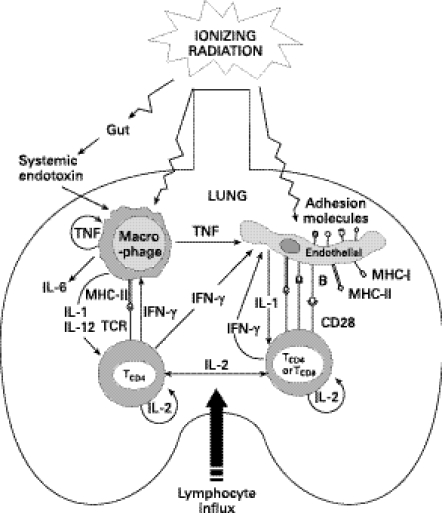
Given the association of lung irradiation with IPS one can envision how lung irradiation could lead to tissue damage or could enhance the damage caused by another ongoing process, such as GVHD (Figure 1). Radiation injury can induce pro-inflammatory cytokine secretion such as IL-1β from macrophages or endothelial cells. Macrophage-derived IL-12, a potent inducer of Th1 responses mediated by CD4+ T cells, can also be induced by irradiation. Interferon-γ secreted by activated T cells in turn can activate macrophages. Cytokines such as IFN-γ and TNF-α can directly or indirectly upregulate endothelial adhesion molecules (Hanseleit et al. 1995; Shen et al. 1997; Mohamadzadeh et al. 1998) thereby promoting lymphocyte homing to the lungs (Morgan & Breit 1995; Panes et al. 1995; Hallahan & Virudachalam 1997a). Lung irradiation may also directly induce or upregulate the expression of histocompatibility (MHC class I and II) antigens on lung epithelial and endothelial cells. In addition, several cytokines are also known to promote the expression of costimulatory molecules such as B-7 (CD80/CD86) and CD40 on the surface of endothelial cells (Behrends et al. 1994; Briscoe et al. 1997) which, along with MHC class II expression, can provide these cells with a potent capacity to activate CD4+ T cells. Finally, extraneous factors such as increased circulating endotoxin due to radiation-induced gastrointestinal tract damage (Hill et al. 1997), may activate resident macrophages (Nestel et al. 1992) and further induce pro-inflammatory cytokines in the lung. Endotoxin (bacterial lipopolysaccharide, LPS) has been shown to superinduce radiation-induced adhesion molecule expression in vivo (Eissner et al. 1996) and the presence of LPS in bronchoalveolar lavage fluid has been reported to correlate with the extent of lung injury (Cooke et al. 1996). In the presence of alloreactive T cells, these radiation-induced events could initiate a lung-specific immune reaction or nonspecific tissue injury leading to the inflammatory and fibrotic lesions characteristic of idiopathic pneumonia syndrome. Indeed, modulation of pre-transplant radiation conditioning has been suggested as a means to control post-BMT mortality that is associated with noninfectious lung complications (Cosset et al. 1994; Penny et al. 1994; Vriesendorp et al. 1994). While high-dose thoracic irradiation alone (i.e. in the absence of BMT) can cause pneumonitis, low doses up to 10–11 Gy have been shown to be relatively noninflammatory in murine models (Travis 1980; Travis et al. 1980). However, studies utilizing animal models have also shown that radiation treatment in this range, when combined with allogeneic BMT, significantly enhanced GVHD-associated mortality (Lehnert et al. 1986; Truitt & Atasoylu 1991). We have demonstrated that a TBI dose as low as 6 Gy in conjunction with allogeneic BMT in mice was able to enhance the development of GVHD as well as IPS (Shankar et al. 1999). Thus, even low-level pre-transplant irradiation can enhance graft-vs.-host reactions.
Figure 1.
Pre-transplant irradiation can induce cytokines that lead to the expression of MHC, costimulatory and/or cell adhesion molecules on pulmonary tissue. Cytokine networks may induce an environment that promotes homing and activation of allogeneic T cells in the lungs.
In our experience with a murine model of IPS, the kinetics of lung disease differ markedly from the development of GVHD (Shankar et al. 1998; Shankar et al. 1999). While GVHD pathology is evident in liver, gut, and skin, early after BMT (‘acute phase’, up to 3 weeks post-BMT), lung pathology develops progressively beginning around 7–9 weeks post-BMT and remains unresolved beyond 25 weeks (‘chronic phase’). This late onset IPS is similar to the onset of post-BMT lung disease in many patients (Soubani et al. 1996). Histological analysis of lung tissue from GVHD mice in the acute phase shows minor immunopathologic changes compared to control mice. In addition, there is a complete lack of collagen deposition at the acute stage. In contrast, lungs of GVHD mice in the chronic phase display histopathological hallmarks of interstitial pneumonitis, such as prominent periluminal mononuclear cell infiltration, areas of alveolar congestion, and significant periluminal and diffuse interstitial fibrosis. Flow cytometric analyses of lung interstitial cells of GVHD mice revealed an increase of CD8+ T cells during the acute phase, which decreased to normal levels at the later chronic phase. Conversely, the percentage of CD4+ T cells, which is normal in the acute phase, increased progressively during the chronic phase. Analyses of cytokine mRNA expression in lung tissue in several experiments have shown that the steady-state levels of IL-1β, TNF-α, IFN-γ, and IL-12 are significantly elevated in the acute phase in lungs of mice with GVHD compared to untreated controls. In contrast, in the chronic phase after BMT only TNF-α and IL-12 levels remained elevated in GVHD mice, suggesting prolonged macrophage activation.
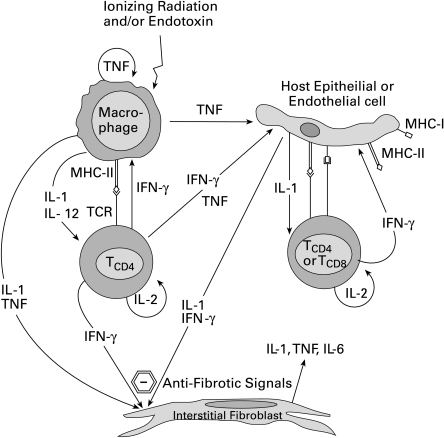
As mentioned earlier, pulmonary complications during acute GVHD are minimal in humans and are most commonly diagnosed during chronic GVHD (Soubani et al. 1996). A recently published review article on lung fibrosis by Coker and Laurent (Coker & Laurent 1998) concluded that fibrosis resulted from an imbalance in the normal cytokine regulatory pathways within the lung. Thus, based on our own observations and those of other laboratories, we propose that the acute phase may be characterized by the prevalence of an antifibrotic cytokine milieu that progressively changes into a chronic, pro-fibrotic environment. As shown in Figure 2, the first event in the initiation of an alloreactive response in the lungs after BMT may be mediated by donor-derived mature CD8+ and/or CD4+ T cells that recognize recipient-specific alloantigens in the context of MHC class I or class II, respectively. Secretion of IFN-γ as a result of T cell activation may act directly on macrophages that subsequently secrete IL-1β, TNF-α and IL-12. Radiation exposure can have direct effects such as induction of cytokine secretion and cell surface molecule expression on various host cell types including macrophages, lymphocytes, endothelial cells, epithelial cells, and fibroblasts (Goldstein & Lewis 1973; Eissner et al. 1996; Thornton et al. 1996; Heckmann et al. 1998). Activated endothelial cells, for instance, possess enhanced capability for augmentation of T cell-derived IFN-γ, via costimulatory molecules (Briscoe et al. 1997). IFN-γ can in turn induce adhesion molecule and costimulatory molecule expression by endothelial and epithelial cells (Westphal et al. 1993). Endothelial cell expression of adhesion molecules, induced by pro-inflammatory cytokines (Ray et al. 1997; Shen et al. 1997; Mohamadzadeh et al. 1998) and ionizing radiation (Gaugler et al. 1997; Heckmann et al. 1998), may be key to the influx of alloreactive cells into the lungs (Hanseleit et al. 1995). Importantly, the key antifibrogenic stimuli in the acute phase may be provided by IFN-γ in combination with IL-1β and TNF-α. While IFN-γ is a potent inhibitor of fibroblast growth and function (collagen synthesis), IL-1β and TNF-α are each known to be pro-fibrogenic. Numerous in vitro studies have demonstrated that IL-1β, IL-6, TGF-β, or TNF-α individually are pro-fibrogenic, promoting fibroblast proliferation and collagen production (Matsushima et al. 1985; Elias et al. 1987a; Elias 1988; Elias & Lentz 1990; Elias & Reynolds 1990; Elias et al. 1990a; Elias et al. 1990b), while IL-1β and TNF-α in combination with IFN-γ deliver inhibitory, and thereby antifibrogenic, signals to fibroblasts (Elias et al. 1987b; Elias et al. 1988a; Elias et al. 1988b; Elias et al. 1990b; Narayanan et al. 1992). The pro-fibrogenic role of TNF-α is well documented since expression of a TNF-α transgene in lungs induces spontaneous lung inflammation and fibrosis (Miyazaki et al. 1995; Sime et al. 1998), and blocking of TNF-α in lungs inhibits lung inflammation and fibrosis (Ulich et al. 1994). The secretion of chemokines and pro-inflammatory cytokines may be sufficient to attract the influx of T cells into the lungs. However, the presence of high levels of IL-1β, TNF-α, and IFN-γ may preclude the development of fibrosis in the lungs in the acute phase after BMT.
Figure 2.
The initial inflammatory phase in the lungs after bone marrow transplantation is an antifibrotic phase. Homing and activation of alloreactive T cells to the lungs results in a cytokine milieu that promotes cellular influx into lungs while maintaining a nonfibrotic microenvironment.
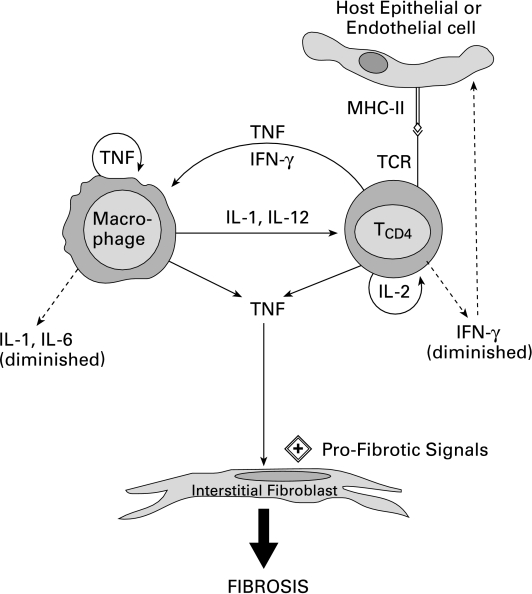
As shown in Figure 3, the chronic phase after BMT is characterized mainly by the presence of CD4+ T cells and elevated levels of TNF-α. Other pro-inflammatory cytokines return to normal constitutive levels at this time (Shankar et al. 1998). These basal levels of IL-1β and IFN-γ may be sufficient for their homeostatic roles in the immune response, such as T cell and macrophage stimulation. However, the reduced expression of IFN-γ and the continued presence of TNF-α, in the absence of elevated IL-1β, may shift the balance from an antifibrogenic environment to a profibrogenic milieu in lung tissue. We suggest that one mechanism by which fibrosis occurs after BMT may be due to elevated levels of TNF-α, which promotes fibrosis via stimulation of collagen expression by fibroblasts in an environment containing reduced levels of IL-1β and IFN-γ.
Figure 3.
The late phase of IPS is characterized by a chronic fibrotic phase in the lungs. The balance of cytokines in the pulmonary microenvironment shifts to higher expression of tumour necrosis factor (TNF) and reduced expression of IFN-γ which promotes collagen secretion by interstitial fibroblasts, thereby leading to lung fibrosis.
Hence, the outcome of pulmonary fibrosis following BMT is likely the result of an abnormal balance between cytokine signals that modulate the activity of fibroblasts in the lung. What regulates the progressive resolution of elevated levels of IFN-γ and IL-1β, yet maintains sustained elevated levels of TNF-α, needs to be categorically elucidated. It is hoped that the recently described animal models of IPS may be the necessary tools to discern the roles of these cytokines in the immunopathogenesis of this BMT-associated lung disease.
Acknowledgments
Studies conducted by the authors were supported by grant number HL-53246 from the National Institutes of Health, USA. GS was supported by a Research Challenge Trust Fellowship from the University of Kentucky, USA.
References
- Agelli M, Wahl SM. Cytokines and fibrosis. Clin. Exp Rheumatol. 1986;4:379–388. [PubMed] [Google Scholar]
- Allen RD, Staley TA, Sidman CL. Differential cytokine expression in acute and chronic murine graft-versus-host disease. European J. Immunology. 1993;23:333–337. doi: 10.1002/eji.1830230205. [DOI] [PubMed] [Google Scholar]
- Anscher MS, Murase T, Prescott DM, et al. Changes in plasma TGFβ levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 1994;30:671–676. doi: 10.1016/0360-3016(92)90954-g. [DOI] [PubMed] [Google Scholar]
- Antin JH, Ferrara JLM. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- Ash RC, Casper JT, Chitambar CR, et al. Successful allogeneic transplantation of T-cell-depleted bone marrow from closely HLA-matched unrelated donors. N. Engl. J. Med. 1990;322:485–494. doi: 10.1056/NEJM199002223220801. [DOI] [PubMed] [Google Scholar]
- Behrends U, Peter RU, Hintermeier-Knabe R, et al. Ionizing radiation induces human intercellular adhesion molecule-1 in vitro. J. Invest Dermatology. 1994;103:726–730. doi: 10.1111/1523-1747.ep12398607. [DOI] [PubMed] [Google Scholar]
- Berman JS, Beer DJ, Theodore AC, et al. Lymphocyte recruitment to the lung. Am. Rev. Respir. Dis. 1990;142:238–257. doi: 10.1164/ajrccm/142.1.238. [DOI] [PubMed] [Google Scholar]
- Biggs JC, Szer J, Crilley P, et al. Treatment of chronic myeloid leukemia with allogeneic bone marrow transplantation after preparation with BuCy2. Blood. 1992;80:1352–1357. [PubMed] [Google Scholar]
- Breuer R, Lossos IS, Berkman N, et al. Pulmonary complications of bone marrow transplantation. Respir. Med. 1993;87:571–579. doi: 10.1016/s0954-6111(05)80259-8. [DOI] [PubMed] [Google Scholar]
- Briscoe DM, Henault LE, Geehan C, et al. Human endothelial cell costimulation of T cell IFN-gamma production. J. Immunol. 1997;159:3247–3256. [PubMed] [Google Scholar]
- Cantin AM, Boileau R, Begin R. Increased procollagen III aminoterminal peptide-related antigens and fibroblast growth signals in the lungs of patients with idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1988;137:572–578. doi: 10.1164/ajrccm/137.3.572. [DOI] [PubMed] [Google Scholar]
- Cantor H. The effects of anti-theta antiserum upon graft-versus-host activity of spleen and lymph node cells. Cell. Immunol. 1972;3:461–469. doi: 10.1016/0008-8749(72)90251-1. [DOI] [PubMed] [Google Scholar]
- Chao NJ. Graft versus host disease following allogeneic bone marrow transplantation. Curr. Opin. Immunol. 1992;4:571–576. doi: 10.1016/0952-7915(92)90028-d. [DOI] [PubMed] [Google Scholar]
- Cherniack RM, Crystal RG, Kalica AR. NHLBI Workshop summary. Current concepts in idiopathic pulmonary fibrosis: a road map for the future. Am. Rev. Respir. Dis. 1991;143:680–683. doi: 10.1164/ajrccm/143.3.680. [DOI] [PubMed] [Google Scholar]
- Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998;11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- Cordonnier C, Bernaudin FJ, Bierling P, et al. Pulmonary complications occurring after allogeneic bone marrow transplantation: a study of 130 consecutive transplanted patients. Cancer. 1986;58:1047–1054. doi: 10.1002/1097-0142(19860901)58:5<1047::aid-cncr2820580512>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Cosset J-M, Socie G, Dubray B, et al. Single dose versus fractionated total body irradiation before bone marrow transplantation: radiobiological and clinical considerations. Int. J. Radiat. Oncol. Biol. Phys. 1994;30:477–492. doi: 10.1016/0360-3016(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Crouch E. Pathobiology of pulmonary fibrosis. Am. J. Physiol. 1990;259:L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- Crystal RG, Bitterman PB, Renard SI, et al. Interstitial lung disease of unknown cause: disorders characterized by chronic inflammation of the lower respiratory tract. New England J. Med. 1984;310:235–244. doi: 10.1056/NEJM198401263100406. [DOI] [PubMed] [Google Scholar]
- Crystal RG, Ferrans VJ, Basset F. Biologic basis of pulmonary fibrosis. In: Crystal RG, West JB, editors. The Lung: Scientific Foundations. New York: Raven Press; 1991. pp. 2031–2046. [Google Scholar]
- Deeg HJ, Spitzer TR, Cottler-Fox M, et al. Conditioning-related toxicity and acute graft-versus-host disease in patients given methotrexate/cyclosporine prophylaxis. Bone Marrow Transplant. 1991;7:193–198. [PubMed] [Google Scholar]
- Desbarats J, Seemayer TA, Lapp WS. Irradiation of the skin and systemic graft-versus-host disease synergise to produce cutaneous lesions. Am. J. Pathol. 1994;144:883–888. [PMC free article] [PubMed] [Google Scholar]
- Dewit D, Mechelen MV, Zanin C, et al. Preferential activation of Th2 cells in chronic graft-versus-host reaction. J. Immunol. 1993;150:361–366. [PubMed] [Google Scholar]
- Dobashi K, Ono S, Murakami S, et al. Polyclonal B cell activation by a B cell differentiation factor, B151‐TRF2. III. B151‐TRF2 as a B cell differentiation factor closely associated with auto immune disease. J. Immunol. 1987;138:780–787. [PubMed] [Google Scholar]
- Doutrelepont JM, Moser M, Leo O, et al. Hyper IgE in stimulatory graft-versus-host disease: role of interleukin-4. Clin. Exp. Immunol. 1991;83:133–136. doi: 10.1111/j.1365-2249.1991.tb05602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Down JD, Steel GG. The expression of early and late damage after thoracic irradiation: a comparison between CBA and C57BL mice. Radiat. Res. 1983;96:603–610. [PubMed] [Google Scholar]
- Driscoll KE. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- Dupuis M, McDonald DM. Dendritic-cell regulation of lung immunity [comment] Am. J. Respir. Cell Mol. Biol. 1997;17:284–286. doi: 10.1165/ajrcmb.17.3.f136. [DOI] [PubMed] [Google Scholar]
- Eissner G, Lindner H, Behrends U, et al. Influence of bacterial endotoxin on radiation-induced activation of human endothelial cells in vitro and in vivo. Transplantation. 1996;62:819–827. doi: 10.1097/00007890-199609270-00020. [DOI] [PubMed] [Google Scholar]
- Elias JA. Tumor necrosis factor interacts with interleukin-1 and interferons to inhibit fibroblast proliferation via fibroblast prostaglandin-dependent and -independent mechanisms. Am. Rev. Respir. Dis. 1988;138:652–658. doi: 10.1164/ajrccm/138.3.652. [DOI] [PubMed] [Google Scholar]
- Elias JA, Freundlich B, Adams S, et al. Regulation of human lung fibroblast collagen production by recombinant interleukin-1, tumor necrosis factor, and interferon-gamma. Ann. N. Y. Acad. Sci. 1990a;580:233–244. doi: 10.1111/j.1749-6632.1990.tb17932.x. [DOI] [PubMed] [Google Scholar]
- Elias JA, Freundlich B, Kern JA, et al. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990b;97:1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- Elias JA, Gustilo K, Baeder W, et al. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J. Immunol. 1987a;138:3812–3816. [PubMed] [Google Scholar]
- Elias JA, Gustilo K, Freundlich B. Human alveolar macrophage and blood monocyte inhibition of fibroblast proliferation. Evidence for synergy between interleukin-1 and tumor necrosis factor. Am. Rev. Respir. Dis. 1988a;138:1595–1603. doi: 10.1164/ajrccm/138.6.1595. [DOI] [PubMed] [Google Scholar]
- Elias JA, Jimenez SA, Freundlich B. Recombinant gamma, alpha, and beta interferon regulation of human lung fibroblast proliferation. Am. Rev. Respir. Dis. 1987b;135:62–65. doi: 10.1164/arrd.1987.135.1.62. [DOI] [PubMed] [Google Scholar]
- Elias JA, Krol RC, Freundlich B, et al. Regulation of human lung fibroblast glycosaminoglycan production by recombinant interferons, tumor necrosis factor, and lymphotoxin. J. Clin. Invest. 1988b;81:325–333. doi: 10.1172/JCI113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JA, Lentz V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL- 6 production and stabilize IL-6 messenger RNA. J. Immunol. 1990;145:161–166. [PubMed] [Google Scholar]
- Elias JA, Reynolds MM. Interleukin-1 and tumor necrosis factor synergistically stimulate lung fibroblast interleukin-1 alpha production. Am. J. Respir. Cell Mol. Biol. 1990;3:13–20. doi: 10.1165/ajrcmb/3.1.13. [DOI] [PubMed] [Google Scholar]
- Ettinger NA, Trulock EP. Pulmonary considerations of organ transplantation, part 2. Am. Rev. Respir. Dis. 1991;144:213–223. doi: 10.1164/ajrccm/144.1.213. [DOI] [PubMed] [Google Scholar]
- Ferrara JLM. Cytokine dysregulation as a mechanism of graft versus host disease. Curr. Opin. Immunol. 1993;5:794–799. doi: 10.1016/0952-7915(93)90139-j. [DOI] [PubMed] [Google Scholar]
- Ferrara JLM, Cooke KR, Pan L, et al. The immunopathophysiology of acute graft-versus-host-disease. Stem Cells. 1996;14:473–489. doi: 10.1002/stem.140473. [DOI] [PubMed] [Google Scholar]
- Ferrara JLM, Deeg HJ. Graft-versus-host disease. NEnglJMed. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- Finkelstein JN, Johnston CJ, Baggs R, et al. Early alterations in extracellular matrix and TGF beta expression in mouse lung indicative of late radiation fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 1994;28:621–631. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Fulmer JD, Roberts WC, Von Gal ER, et al. Morphologic-physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis. J. Clin. Invest. 1979;63:665–676. doi: 10.1172/JCI109349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin EK, Green SW. Exposure to gamma-irradiation increases phorbol myristate acetate-induced H2O2 production in human macrophages. Blood. 1987;70:694–701. [PubMed] [Google Scholar]
- Gaugler MH, Squiban C, Vandermeeren A, et al. Late and persistent up-regulation of intercellular adheson molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. Int. J. Radiat. Biol. 1997;72:201–209. doi: 10.1080/095530097143428. 10.1080/095530097143428. [DOI] [PubMed] [Google Scholar]
- Giacchino M, Busca A, Miniero R, et al. Pulmonary complications after bone marrow transplantation. Minerva Pediatr. 1993;45:141–150. [PubMed] [Google Scholar]
- Goldstein E, Lewis JP. Patterns of pulmonary alveolar macrophage function following radiation injury. J. Lab Clin. Med. 1973;82:276–286. [PubMed] [Google Scholar]
- Grande JP, Melder DC, Zinsmeister AR. Modulation of collagen gene expression by cytokines: stimulatory effect of transforming growth factor-beta1, with divergent effects of epidermal growth factor and tumor necrosis factor-alpha on collagen type I and collagen type IV [see comments] J. Lab Clin. Med. 1997;130:476–486. doi: 10.1016/s0022-2143(97)90124-4. [DOI] [PubMed] [Google Scholar]
- Hallahan DE, Sprigs DR, Beckett MA, et al. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc. Natl. Acad. Sci. (USA) 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc. Natl. Acad. Sci. (USA) 1997a;94:6432–6437. doi: 10.1073/pnas.94.12.6432. 10.1073/pnas.94.12.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan DE, Virudachalam S. Ionizing radiation mediates expression of cell adhesion molecules in distinct histological patterns within the lung. Cancer. Res. 1997b;57:2096–2099. [PubMed] [Google Scholar]
- Hanseleit U, Steinbrink K, Sunderkotter C, et al. Expression of murine VCAM-1 in vitro and in different models of inflammation in vivo: correlation with immigration of monocytes. Exp. Dermatol. 1995;4:249–256. [PubMed] [Google Scholar]
- Hareyama M, Imai K, Oouchi A, et al. The effect of radiation on the expression of intercellular adhesion molecule-1 of human adenocarcinoma cells. Int. J. Radiat. Oncol. Biol. Phys. 1998;40:691–696. doi: 10.1016/s0360-3016(97)00860-2. 10.1016/s0360-3016(97)00860-2. [DOI] [PubMed] [Google Scholar]
- Hauser SH, Calorini L, Wazer DE, et al. Radiation-enhanced expression of major histocompatibility complex class I antigen H-2Db in B16 melanoma cells. Cancer. Res. 1993;53:1952–1955. [PubMed] [Google Scholar]
- Heckmann M, Douwes K, Peter R, et al. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp. Cell. Res. 1998;238:148–154. doi: 10.1006/excr.1997.3826. [DOI] [PubMed] [Google Scholar]
- Hill GR, Crawford JM, Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- Hill RP. Response of mouse lung to irradiation at different dose-rates. Int. J. Radiat. Oncol. Biol. Phys. 1985;9:1043–1047. doi: 10.1016/0360-3016(83)90395-4. [DOI] [PubMed] [Google Scholar]
- Holler E, Ertl B, Hintermeier-Knabe R, et al. Inflammatory reactions induced by pretransplant conditioning – an alternative target for modulation of acute GVHD and complications following allogeneic bone marrow transplantation. Leuk. Lymphoma. 1997;25:217–224. doi: 10.3109/10428199709114161. [DOI] [PubMed] [Google Scholar]
- Holler E, Kolb HJ, Mittermuller J, et al. Modulation of acute graft-versus-host-disease after allogeneic bone marrow transplantation by tumor necrosis factor alpha (TNF alpha) release in the course of pretransplant conditioning: role of conditioning regimens and prophylactic application of a monoclonal antibody neutralizing human TNF alpha (MAK 195F) Blood. 1995;86:890–899. [PubMed] [Google Scholar]
- Ibuki Y, Goto R. Enhancement of NO production from resident peritoneal macrophages by in vitro gamma-irradiation and its relationship to reactive oxygen intermediates. Free Radic. Biol. Med. 1997;22:1029–1035. doi: 10.1016/s0891-5849(96)00500-x. 10.1016/s0891-5849(96)00500-x. [DOI] [PubMed] [Google Scholar]
- Jadus MR, Wepsic HT. The role of cytokines in graft-versus-host reactions and disease. Bone Marrow Transplant. 1992;10:1–14. [PubMed] [Google Scholar]
- Johnston CJ, Piedboeuf B, Rubin P, et al. Early and persistent alteratons in the expression of interleukin-1 alpha, interleukin-1 beta, and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat. Res. 1996;145:762–767. [PubMed] [Google Scholar]
- Keane TJ, Vandyk J, Rider WD. Idiopathic interstitial pneumonia following bone marrow transplantation: the relationship with total body irradiation. Int. J. Oncol. Biol. Phys. 1981;7:1365–1370. doi: 10.1016/0360-3016(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Kelemen E, Szebeni J, Petranyi GG. Graft-versus-host disease in bone marrow transplantation: experimental, laboratory, and clinical contributions of the last few years. Arch. Allergy Immunol. 1993;102:309–320. doi: 10.1159/000236577. [DOI] [PubMed] [Google Scholar]
- Kernan HNA, Collins NH, Juliano L, et al. Clonable T lymphocytes in T cell-depleted bone marrow transplants correlate with development of graft-versus-host disease. Blood. 1986;68:770–773. [PubMed] [Google Scholar]
- Khalil N, O'Connor RN, Flanders KC, et al. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am. J. Respir. Cell Mol. Biol. 1996;14:131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- Kline JN, Schwartz DA, Monick MM, et al. Relative release of interleukin-1 beta and interleukin-1 receptor antagonist by alveolar macrophages. A study in asbestos-induced lung disease, sarcoidosis, and idiopathic pulmonary fibrosis. Chest. 1993;104:47–53. doi: 10.1378/chest.104.1.47. [DOI] [PubMed] [Google Scholar]
- Korngold R, Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice: prevention by removing mature T-cells from marrow. J. Exp. Med. 1978;148:1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivenko SI, Dryk SI, Komarovskaya ME, et al. Ionizing radiation increases TNF/cachectin production by human peripheral blood mononuclear cells in vitro. Int. J. Hematol. 1992;55:127–130. [PubMed] [Google Scholar]
- Labar B, Bogdanic V, Nemet D, et al. Total body irradiation with or without lung shielding for allogeneic bone marrow transplantation. Bone Marrow Transplant. 1992;9:343–347. [PubMed] [Google Scholar]
- Lawton CA, Barber-Derus S, Murray KJ, et al. Technical modifications in hyperfractionated total body irradiation for T-lymphocyte deplete bone marrow transplant. Int. J. Radiat. Oncol. Biol. Phys. 1989;17:319–322. doi: 10.1016/0360-3016(89)90446-x. [DOI] [PubMed] [Google Scholar]
- Lehnert S, Rybka WB, Seemayer TA. Amplification of the graft-versus-host reaction by partial body irradiation. Transplantation. 1986;41:675–679. doi: 10.1097/00007890-198606000-00002. [DOI] [PubMed] [Google Scholar]
- Marshall BG, Wangoo A, Cook HT, et al. Increased inflammatory cytokines and new collagen formation in cutaneous tuberculosis and sarcoidosis [see comments] Thorax. 1996;51:1253–1261. doi: 10.1136/thx.51.12.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RP, McAnulty RJ, Laurent GJ. The pathogenesis of pulmonary fibrosis: is there a fibrosis gene? Int J. Biochem. Cell Biol. 1997;29:107–120. doi: 10.1016/s1357-2725(96)00141-0. 10.1016/s1357-2725(96)00141-0. [DOI] [PubMed] [Google Scholar]
- Martinet Y, Menard O, Vaillant P, et al. Cytokines in human lung fibrosis. Arch. Toxicol. 1996;18(Supplement):127–139. doi: 10.1007/978-3-642-61105-6_14. [DOI] [PubMed] [Google Scholar]
- Matsushima K, Bano M, Kidwell WR, et al. Interleukin 1 increases collagen type IV production by murine mammary epithelial cells. J. Immunol. 1985;134:904–909. [PubMed] [Google Scholar]
- Meyers JD, Flournoy N, Thomas ED. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years' experience. Rev. Infect. Dis. 1982;4:1119–1132. doi: 10.1093/clinids/4.6.1119. [DOI] [PubMed] [Google Scholar]
- Miale T, Mody N, Dick B, et al. Difficulty in establishing diagnosis from lung biopsies and bronchial washing analysis in children with leukemia following bone marrow transplantation. Cancer Detect Prev Supplement. 1987;1:165–172. [PubMed] [Google Scholar]
- Milburn HJ, Du Bois RM, Prentice HG, et al. Pneumonitis in bone marrow transplant recipients results from a local immune response. Clin. Exp. Immunol. 1990;81:232–237. doi: 10.1111/j.1365-2249.1990.tb03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn HJ, Prentice HG, Poulter LW. Abnormal alveolar macrophage populations in bone marrow transplant recipients with pneumonitis. Eur. Resp. J. 1993;6:1295–1300. [PubMed] [Google Scholar]
- Mitsuyasu RT, Champlin RE, Gale RP, et al. Treatment of donor bone marrow with monoclonal anti-T-cell antibody and complement for the prevention of graft-versus-host disease. A prospective, randomized, double-blind trial. Ann. Intern. Med. 1986;105:20–26. doi: 10.7326/0003-4819-105-1-20. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Araki K, Vesin C, et al. Expression of a tumor necrosis factor-α transgene in murine lung causes lymphocytic and fibrosing alveolitis. J. Clin. Invest. 1995;96:250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadzadeh M, Degrendele H, Arizpe H, et al. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J. Clin. Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int. J. Radiat. Oncol. Biol. Phys. 1995;31:361–369. doi: 10.1016/0360-3016(94)00477-3. 10.1016/0360-3016(94)00477-3. [DOI] [PubMed] [Google Scholar]
- Nakao A, Hasegawa Y, Tsuchiya Y, et al. Expression of cell adhesion molecules in the lungs of patients with idiopathic pulmonary fibrosis. Chest. 1995;108:233–239. doi: 10.1378/chest.108.1.233. [DOI] [PubMed] [Google Scholar]
- Narayanan AS, Whithey J, Souza A, et al. Effect of gamma-interferon on collagen synthesis by normal and fibrotic human lung fibroblasts. Chest. 1992;101:1326–1331. doi: 10.1378/chest.101.5.1326. [DOI] [PubMed] [Google Scholar]
- Nestel FP, Price KS, Seemayer TA, et al. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor α during graft-versus-host disease. J. Exp. Med. 1992;175:405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J, Sloane JP. ICAM-1 expression on epidermal keratinocytes in cutaneous graft-versus-host disease. Transplantation. 1991;51:1203–1206. doi: 10.1097/00007890-199106000-00011. [DOI] [PubMed] [Google Scholar]
- Ogushi F, Tani K, Maniwa K, et al. Interleukin-8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis or idiopathic pulmonary fibrosis. J. Med. Invest. 1997;44:53–58. [PubMed] [Google Scholar]
- Panes J, Anderson DC, Miyasaka M, et al. Role of leukocyte-endothelial cell adhesion in radiation-induced microvascular dysfunction in rats. Gastroenterology. 1995;108:1761–1769. doi: 10.1016/0016-5085(95)90138-8. [DOI] [PubMed] [Google Scholar]
- Parkman R. Clonal analysis of murine graft-versus-host disease. I. Phenotypic and functional analysis of T lymphocyte clones. J. Immunol. 1986;136:3543–3548. [PubMed] [Google Scholar]
- Penny DP, Siemann DW, Rubin P, et al. Morphological correlates of fractionated radiation of the mouse lung: early and late effects. Int. J. Radiat. Oncol. Biol. Phys. 1994;29:789–804. doi: 10.1016/0360-3016(94)90568-1. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, et al. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J. Exp. Med. 1989a;170:655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet PF, Grau GE, Collart MA, et al. Pneumopathies of the graft-versus-host reaction: alveolitis associated with an increased level of tumor necrosis factor mRNA and chronic interstitial pneumonitis. Lab. Invest. 1989b;61:37–45. [PubMed] [Google Scholar]
- Piguet PF, Ribaux C, Karpuz V, et al. Expression and localization of tumor necrosis factor-alpha and its mRNA in idiopathic pulmonary fibrosis. Am. J. Pathol. 1993;143:651–655. [PMC free article] [PubMed] [Google Scholar]
- Quabeck K. The lung as a critical organ in marrow transplantation. Bone Marrow Transplant. 1994;14:S19–S28. [PubMed] [Google Scholar]
- Ray KP, Farrow S, Daly M, et al. Induction of the E-selectin promoter by interleukin 1 and tumour necrosis factor alpha, and inhibition by glucocorticoids. Biochem. J. 1997;328:707–715. doi: 10.1042/bj3280707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. 10.1016/0360-3016(95)00095-g. [DOI] [PubMed] [Google Scholar]
- Rus V, Svetic A, Nguyen P, et al. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. J. Immunol. 1995;155:2396–2406. [PubMed] [Google Scholar]
- Shahar I, Fireman E, Topilsky M, et al. Effect of IL-6 on alveolar fibroblast proliferation in interstitial lung diseases. Clin. Immunol. Immunopathol. 1996;79:244–251. doi: 10.1006/clin.1996.0075. [DOI] [PubMed] [Google Scholar]
- Shankar G, Bryson JS, Jennings CD, et al. Idiopathic pneumonia syndrome following allogeneic bone marrow transplantation in mice: the role of pre-transplant radiation conditioning. Am. J. Respir. Cell Mol. Biol. 1999;20:1116–1124. doi: 10.1165/ajrcmb.20.6.3455. [DOI] [PubMed] [Google Scholar]
- Shankar G, Bryson JS, Jennings CD, et al. Idiopathic pneumonia syndrome in mice after allogeneic bone marrow transplantation. Am. J. Respir. Cell Mol. Biol. 1998;18:235–242. doi: 10.1165/ajrcmb.18.2.2988. [DOI] [PubMed] [Google Scholar]
- Shen J, To‐To S, Schrieber L, et al. Early E-selectin, VCAM-1, ICAM-1, and late major histocompatibility complex antigen induction on human endothelial cells by flavivirus and comodulation of adhesion molecule expression by immune cytokines. J. Virol. 1997;71:9323–9332. doi: 10.1128/jvi.71.12.9323-9332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Marr RA, Gauldie D, et al. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am. J. Pathol. 1998;153:825–832. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Strieter RM, Zhang K, et al. A role for C-C chemokines in fibrotic lung disease. J. Leukoc. Biol. 1995;57:782–787. doi: 10.1002/jlb.57.5.782. [DOI] [PubMed] [Google Scholar]
- Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest. 1996;109:1066–1077. doi: 10.1378/chest.109.4.1066. [DOI] [PubMed] [Google Scholar]
- Southcott AM, Hemingway I, Lorimer S, et al. Adhesion molecule expression in the lung: a comparison between normal and diffuse interstitial lung disease. Eur Respir J. 1998;11:91–98. doi: 10.1183/09031936.98.11010091. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Rolfe MR, Kunkel SL, et al. Altered production and regulation of monocyte chemoattractant protein-1 from pulmonary fibroblasts isolated from patients with idiopathic pulmonary fibrosis. Chest. 1993a;103:121S. doi: 10.1378/chest.103.2_supplement.121s. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Rolfe MW, Kunkel SL, et al. Macrophage inflammatory protein-1 alpha expression in interstitial lung disease. J. Immunol. 1993b;151:2852–2863. [PubMed] [Google Scholar]
- Stein-Streilein J, Lipscomb MF, Hart DA, et al. Graft-versus-host reaction in the lung. Transplantation. 1981;32:38–44. doi: 10.1097/00007890-198107000-00008. [DOI] [PubMed] [Google Scholar]
- Tait RC, Burnett AK, Robertson AG, et al. Subclinical pulmonary function defects following autologous and allogeneic bone marrow transplantation: relationship to total body irradiation and graft-versus-host disease. Int. J. Radiat. Oncol. Biol. Phys. 1991;20:1219–1227. doi: 10.1016/0360-3016(91)90231-r. [DOI] [PubMed] [Google Scholar]
- Tartakovsky B, Goldstein O, Krautghamer R, et al. Low doses of radiation induce systemic production of cytokines: possible contribution to leukemogenesis. Int. J. Cancer. 1993;55:269–274. doi: 10.1002/ijc.2910550217. [DOI] [PubMed] [Google Scholar]
- Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511–533. [PubMed] [Google Scholar]
- Thornton SC, Walsh BJ, Bennett S, et al. Both in vitro and in vivo irradiation are associated with induction of macrophage-derived fibroblast growth factors. Clin. Exp. Immunol. 1996;103:67–73. doi: 10.1046/j.1365-2249.1996.898598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall RS, Vogel SN, Evans R, et al. Role of tumor necrosis factor-alpha in the spontaneous development of pulmonary fibrosis in viable motheaten mutant mice. Am. J. Pathol. 1997;151:1303–1310. [PMC free article] [PubMed] [Google Scholar]
- Travis EL. The sequence of histological changes in mouse lungs after single doses of X-rays. Int. J. Radiat. Oncol. Biol. Phys. 1980;6:345–347. doi: 10.1016/0360-3016(80)90145-5. [DOI] [PubMed] [Google Scholar]
- Travis EL, Down JD, Holmes SJ, et al. Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiat. Res. 1980;84:133–143. [PubMed] [Google Scholar]
- Truitt RL, Atasoylu AA. Impact of pretransplant conditioning and donor T cells on chimerism, graft-versus-host disease, graft-versus-leukemia reactivity, and tolerance after bone marrow transplantation. Blood. 1991;77:2515–2523. [PubMed] [Google Scholar]
- Ulich TR, Yi ES, Yin S, et al. Intratracheal administration of endotoxin and cytokines. VII. The soluble interleukin-1 receptor and the soluble tumor necrosis factor receptor II (p80) inhibit acute inflammation. Clin. Immunol. Immunopathol. 1994;72:137–140. doi: 10.1006/clin.1994.1117. [DOI] [PubMed] [Google Scholar]
- Ushiyama C, Hirano T, Miyajima H, et al. Anti-IL-4 antibody prevents graft-versus-host disease in mice after bone marrow transplantation. The IgE allotype is an important marker of graft-versus-host disease. J. Immunol. 1995;154:2687–2696. [PubMed] [Google Scholar]
- Vanbekkum DW. Immunologic basis of graft-versus-host disease. In: Gale RP, Fox CF, editors. Biology of Bone Marrow Transplantation. New York: Academic Press; 1980. pp. 175–194. [Google Scholar]
- Vriesendorp HM, Chu H, Ochran TG, et al. Radiobiology of total body radiation. Bone Marrow Transplant. 1994;14:S4–S8. [PubMed] [Google Scholar]
- Weiner RS, Horowitz MM, Gale RP, et al. Risk factors for interstitial pneumonia following bone marrow transplantation for severe aplastic anemia. Br. J. Hematol. 1989;71:535–543. doi: 10.1111/j.1365-2141.1989.tb06314.x. [DOI] [PubMed] [Google Scholar]
- Westphal JR, Willems HW, Tax WJ, et al. The proliferative response of human T cells to allogeneic IFN-gamma- treated endothelial cells is mediated via both CD2/LFA-3 and LFA-1/ICAM- 1 and -2 adhesion pathways. Transpl Immunol. 1993;1:183–191. doi: 10.1016/0966-3274(93)90045-a. [DOI] [PubMed] [Google Scholar]
- White CW, Das KC. Role of cytokines in acute lung injury. In: Crystal RG, editor. The Lung: Scientific Foundations. Philadelphia: Lippincott-Raven; 1997. pp. 2451–2464. [Google Scholar]
- Wilborn J, Crofford LJ, Burdick MD, et al. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J. Clin. Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms K, Link H, Meyer P, et al. Bone marrow transplantation in patients with leukemia. Klin Wochenschr. 1982;60:1279–1287. doi: 10.1007/BF01727484. [DOI] [PubMed] [Google Scholar]
- Wolff G, Crystal RG. Biology of pulmonary fibrosis. In: Crystal RG, West JB, et al., editors. The Lung: Scientific Foundations. Philadelphia: Lippincott-Raven; 1997. pp. 2509–2524. [Google Scholar]
- Xun CQ, Thompson JS, Jennings CD, et al. Effect of total body irradiation, Busulfan-Cyclophosphamide, or Cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- Zhang Y, Lee TC, Guillemin B, et al. Enhanced IL-1 beta and tumor necrosis factor-alpha release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J. Immunol. 1993;150:4188–4196. [PubMed] [Google Scholar]