Abstract
Apoptosis, or programmed cell death, is essential in development and homeostasis in multi‐cellular organisms. It is also an important component of the cellular response to injury. Many cells undergo apoptosis in response to viral infection, with a consequent reduction in the release of progeny virus. Viruses have therefore evolved multiple distinct mechanisms for modulating host cell apoptosis. Viruses may interfere with either the highly conserved ‘effector’ mechanisms of programmed cell death or regulatory mechanisms specific to mammalian cells. In addition to conferring a selective advantage to the virus, the capacity to prevent apoptosis has an essential role in the transformation of the host cell by oncogenic viruses. This article provides a focussed review of apoptosis and illustrates how the study of viruses has informed our understanding of this process. Selected mechanisms by which viral gene products interfere with cell death are discussed in detail and used to illustrate the general principles of the interactions between viruses and apoptosis.
Keywords: apoptosis, cancer, caspases, death receptors, mitochondria, viruses
Introduction
Apoptosis, or programmed cell death, is a naturally occurring process that has an essential role in development and the maintenance of homeostasis in multicellular organisms. Cells undergoing apoptosis show highly characteristic morphological changes, including shrinkage, blebbing of the plasma membrane, chromatin condensation and DNA fragmentation (Kerr et al. 1972). These changes are distinct from those seen in cell death caused by necrosis, although it is now clear that the pathways that lead to necrosis and apoptosis are not entirely separate. The central processes of apoptosis are remarkably conserved, and cell death genes from the nematode C. elegans are functional in mammalian cells. In addition, however, mammals have evolved complex regulatory mechanisms for the modulation of apoptosis. In health, apoptotic cell death is tightly linked to cell multiplication and leads to the regulated turnover of cells crucial to the preservation and function of adult organisms. Defects in cell death pathways, or uncoupling of cell death and proliferation, can lead to developmental abnormalities and are a major part of the pathogenesis of a wide range of degenerative and neoplastic diseases.
Apoptosis is mediated by the sequential activation of caspases, a family of cysteine proteases with specificity for aspartate residues in their target substrate. Caspases are constitutively present in most cells as inactive single chain proenzymes and are activated by specific proteolytic cleavage and assembly into tetramers (Green 1998). Caspases have the capacity to activate each other and co-operate in an intracellular ‘cascade’ leading to the activation of downstream ‘effector’ caspases-3, –6 and –7. Engagement of effector caspases is a key event that commits the cell to the cleavage of essential substrates and results in the morphological changes of apoptosis and the degradation of chromosomal DNA (Enari et al. 1998). At least two kinds of pathways converge on the activation of caspase-3. These pathways were previously thought to be independent, but are now known to interact in a cell specific manner (Liet al. 1998). The first is regulated by mitochondria, which are now recognized to play a central and rate-limiting role in cell death (Green & Reed 1998; Kroemer & Reed 2000). The second pathway begins with the activation of caspases-8 and –10 by members of the death receptor family. Activated caspase-8 then acts directly on caspase-3 to initiate apoptosis (Ashkenazi & Dixit 1998).
In addition to a necessary role in homeostasis and development, apoptosis is an essential component of the cell response to injury (Thompson 1995). In particular, many cells will undergo programmed cell death following viral infection and this may abort the production and release of progeny virus. It is therefore not surprising that viruses have evolved distinct mechanisms for the modulation of host cell apoptosis. Viral gene products may interfere with either the conserved ‘effector’ mechanisms of apoptosis or the more highly evolved regulatory mechanisms present in mammalian cells. These mechanisms must confer a selective evolutionary survival advantage to either the virus or to the host cell and act in two ways: (i) the prevention of the premature death of the host cell during lytic infection, leading to enhanced release of progeny virions and (ii) the promotion, in concert with other viral genes, of the transformation and maintenance of the host cell during latent infections. It is axiomatic that viruses that drive the host cell to proliferate must also prevent apoptosis induced by aberrant growth. There is also some evidence that viruses can induce apoptosis and use the cell remnants as a vehicle for viral transmission and avoidance of the immune system. This review will consider the principle mechanisms of apoptosis and their potential for modulation by viral gene products. The aim is not to produce an exhaustive catalogue of the interaction of viruses and apoptotic pathways, but to provide a general background that will enhance understanding of the following series of articles on viruses and cancer. This review will therefore focus on the mechanisms used by oncogenic viruses to subvert apoptotic pathways.
Apoptosis and mitochondria
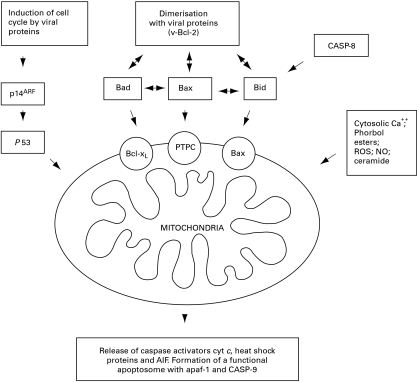
It has recently become evident that mitochondria play a pivotal role in both the life and death of the cell (Green & Reed 1998; Kroemer & Reed 2000). In particular, the selective permeabilization of mitochondrial membranes (mitochondrial membrane permeabilization, MMP) is a key event in the initiation of cell death by both apoptosis and necrosis. Mitochondria contain two discrete compartments known as the matrix and the intermembrane space. The matrix is surrounded by the inner membrane (IM) and the intermembrane space is enclosed by the outer membrane (OM). The IM contains components necessary for electron transport, together with an ATP synthase and adenine nucleotide translocator (ANT) which act to export ATP. Under normal physiological conditions, the IM is almost impermeable and can therefore maintain the electrochemical gradient essential to ATP production. In contrast, the OM contains an abundant voltage dependent anion channel (VDAC) and is permeable to solutes of up to 5 kDa in size. It is now recognized that a wide variety of apoptotic signals lead, either directly or indirectly, to alterations in the permeability of mitochondrial membranes. These signals can be modulated by local regulatory factors, such as members of the Bcl-2 protein family (Brenner & Kroemer 2000; Kroemer & Reed 2000) (Figure 1). The net effect of MMP is the selective release of soluble intermembrane proteins into the cytosol. The most important of these factors are cytochrome c, apoptosis inducing factor (AIF) and the mitochondrial caspases (Susin et al. 1999). In the cytosol, cytochrome c recruits apoptosis protease-activating factor 1 (apaf-1) and procaspase 9 to form a multicomponent holoenzyme, known as the ‘apoptosome’. In the presence of dATP, the apoptosome activates caspase-3 and initiates the apoptotic cascade (Li et al. 1997; Rodriguez & Lazebnik 1999). MMP is therefore a critical and irreversible event in many apoptotic pathways and it is important to understand the mechanisms that regulate MPP, the requirements for the subsequent formation of a functional apoptosome and the potential for these processes to be subverted by viruses.
Figure 1.
The selective permeabilization of mitochondrial membranes (mitochondrial membrane permeabilization, MMP), is a key event in the induction of apoptosis. Members of the Bcl-2 family play an important role in regulating this process and may either be resident in the membrane as part of the permeability transition pore complex (PTPC) or translocate to the mitochondria in response to specific signals. The figure illustrates selected events that promote MMP, many of which can be modulated by viral proteins.
The regulation of mitochondrial membrane permeabilization
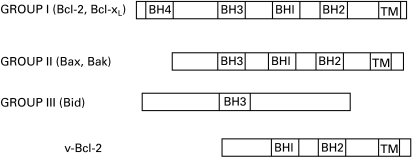
Mitochondria undergo MMP in response to a wide variety of apoptotic signal transduction pathways (Brenner & Kroemer 2000; Kroemer & Reed 2000). Members of the Bcl-2 family are extremely important in regulating this process, both as factors that can themselves induce MMP and as components of a multiprotein permeability transition pore complex that controls the integrity of the mitochondrial membranes. Bcl-2 family proteins can act either to promote cell survival or precipitate apoptosis. All members of the family possess at least one of four motifs, known as Bcl-2 homology domains (BH1 to BH4) (Adams & Corey 1998) (Figure 2). Bcl-2 itself is antiapoptotic and contains all four domains. Other antiapoptotic members of the family, such as Bcl-xL, contain at least the BH1 and BH2 domain. Pro-apoptotic members of the family belong to one of two groups. Members of the first group, such as Bax and Bak contain BH domains 1–3 and are closely homologous to Bcl-2. In contrast, a second group of pro-apoptotic proteins contain a short BH3 domain essential for their function, but are otherwise unrelated to Bcl-2. Bcl-2 family proteins can form homodimers and modify the function of other members by formation of heterodimers. Dimerization occurs by insertion of the BH3 domain of one ligand into an elongated hydrophobic cleft formed by the BH1, BH2 and BH3 domains of the second protein (Muchmore et al. 1996).
Figure 2.
The structure of Bcl-2 family members. Group I proteins (Bcl-2, Bcl-xL) contain four conserved Bcl-2 homology (BH) domains (BH1–4) and a C-terminal hydrophobic transmembrane sequence (TM). These proteins are all antiapoptotic. In contrast, Group II proteins lack the BH4 domains and promote apoptosis. Group III proteins contain only a BH3 domain and most members of this group are otherwise unrelated to members of the Bcl-2 family. Adenovirus 19K protein is an example of a group III protein. In contrast, the v-Bcl-2 proteins found in Epstein-Barr virus and HHV-8 appear to be divergent homologues of Bcl-2 and do not contain a BH3 domain.
Bcl-2 and Bcl-xL are resident proteins of the OM of the mitochondria and participate in formation of the permeability transition pore complex (Crompton 1999). The structure of Bcl-xL is similar to that of the membrane insertion domain of certain bacterial toxins, suggesting a mechanism by which these proteins may contribute directly to channel formation in mitochondrial membranes (Adams & Corey 1998). In contrast to the resident status of Bcl-2 and Bcl-xL, many pro-apoptotic members of the Bcl-2 family translocate to the mitochondrial membrane in response to apoptotic signals, where they induce MMP either directly or by heterodimerization with Bcl-2 or Bcl-xL (Desagher et al. 1999; Li et al. 1998; Marzo et al. 1998; Narita et al. 1998). Bcl-2 family members have been shown to cause MMP by interaction with both the ANT in the IM (Crompton 1999) and the VDAC in the OM (Shimizu et al. 1999). Interestingly, a growing number of apoptotic messengers, including transcription factors (Li et al. 2000) and p53 (Marchenko et al. 2000), have now been shown to share the capacity to translocate to the mitochondrial membrane, although the mechanisms by which this occurs are not clear.
Bcl-2 and Bcl-xL have been previously thought to work by either inhibiting the function of apaf-1 within the apoptosome, or by preventing MMP and the subsequent release of cytochrome c. The idea that Bcl-2 directly binds and inhibits apaf-1 is particularly attractive, as it is based on the studies of C. elegans that have informed so much of our understanding of apoptosis. Recent evidence, however, suggests that neither Bcl-2 nor Bcl-xL make direct contact with apaf-1 in vivo and, indeed, have distinct intracellular locations. (Hausmann et al. 2000). These proteins are more likely to act by stabilizing the mitochondrial membrane (Morishi et al. 1999; Newmeyer et al. 2000), thereby preventing the release of cytochrome c and formation of the apoptosome.
Requirements for a functional apoptosome
Procaspases and related proteins exist as monomers with low intrinsic enzymatic activity. Oligomerization of the caspase, often mediated by a caspase activation and recruitment domain (CARD) (Green 1998), enhances activity sufficiently to permit self-cleavage and release of the fully active enzyme. Consistent with this theme, there is an absolute requirement for cytochrome c mediated, dATP-dependent oligomerization of apaf-1 for procaspase-9 activation (Jiang & Wang 2000; Saleh et al. 1999). Oligomerization is mediated by a CARD-like domain in apaf-1 known as the WD-40 repeat region (Hu et al. 1998). Heat shock proteins are also released following MMP, and may regulate the activity of the apoptosome. Heat shock protein 70 inhibits apoptosis by preventing the recruitment of procaspase-9 to apaf-1 (Beere et al. 2000) and heat shock protein 90 prevents oligomerization of apaf-1 (Pandey et al. 2000). Further, the presence of cytochrome c and dATP are essential for apoptotsis. In cells significantly depleted of either of these substances, the apoptosome does not form and MMP leads to cell death by necrosis (Kroemer & Reed 2000).
Viruses and mitochondria
Interaction with the Bcl-2 family
The first evidence that the modulation of apoptosis determines the outcome of viral infection came from the study of adenoviruses. Infection with strains of adenovirus that lacked a functional EIB 19 kDa gene was observed to produce an entirely different phenotype than infection with wild-type viruses (Ezoe et al. 1981). This phenotype was characterized by a dramatically enhanced cytopathic effect, together with degradation of host cell chromosomal DNA and viral DNA typical of apoptosis. The inability of EIB 19K loss-of-function mutants to prevent apoptosis resulted in premature host cell death and reduction in the yield of progeny virus. Further, the capacity of the adenovirus E1A oncogene to transform the host cell was also found to be dependent on the coexpression of the E1B 19K protein (Chiou et al. 1994). These functions of the E1B 19K protein could be complemented by over-expression of Bcl-2, and comparison of the amino acid sequence of the viral protein and Bcl-2 identified a region of limited sequence homology (the BH domain) which proved to be essential for function (Chiou et al. 1994). These observations established both the need for adenoviruses to delay apoptosis of the host cell in order to propagate efficiently and the capacity of the virus to ‘hijack’ host cell apoptotic machinery.
The adenovirus E1B 19K protein has been consistently shown to interact with other members of the Bcl-2 family. Indeed, E1B 19K was used as ‘bait’ in yeast two-hybrid screens to identify human Bax (Han et al. 1996) and Bak (Farrow et al. 1995). The interaction of E1B 19K with these proteins is functional and E1B 19K has been shown to block Bax induced apoptosis (Han et al. 1998). E1B 19K also interacts with members of the BH3 protein family, including BNIP3 (Yasuda et al. 1998) and Bnip3L (Imazu et al. 1999). Adenovirus E1B 19K protein therefore inhibits apoptosis by forming heterodimers with a variety of pro-apoptotic proteins of the Bcl-2 family, such as Bax, Bak, BNIP3 and Bnip3L. The net effect of the Bcl-2 protein family depends on the relative concentration of constituent pro- and antiapoptotic members (Adams & Corey 1998) and high levels of expression of EIB 19K during acute adenovirus infection therefore favour stabilization of the mitochondrial membrane and prevent MMP. It is interesting that EIB 19K has also been shown to inhibit death by necrosis in certain cell types (Subramanian et al. 1995), a function we now know to be consistent with its potential to stabilize mitochondrial membranes.
Other families of viruses in addition to adenovirus contain homologues of the Bcl-2 protein family. The most important of these is the herpesvirus family. Herpesviruses can be subdivided into alpha-, beta and gamma herpesviruses on the basis of differences in biological properties in vitro and in vivo (Honess 1984). Gammaherpesviruses can be further divided into gamma-1 and gamma-2 viruses on the basis of the mean (A + T) content of their genomes. Gammaherpesviruses are the only herpesviruses which have been unambiguously associated with cancer in their natural host and it is therefore interesting that members of this subfamily encode homologues of Bcl-2.
Epstein-Barr virus (EBV) is a gamma-1 herpesvirus that lytically infects epithelial cells and establishes latent infection in B cells. The transformation of B cells by EBV is the result of a co-ordinated programme of viral gene expression that both promotes entry into cell cycle and inhibits host cell apoptosis (Gregory et al. 1991). Prevention of apoptosis depends in part on the upregulation of host cell Bcl-2 by the multifunctional viral protein LMP-1 (Henderson et al. 1991). LMP-1 also upregulates cellular antiapoptotic proteins A20 (Fries et al. 1996) and bfl-1 (D'Souza et al. 2000). In addition, EBV encodes a homologue of Bcl-2, designated BHRF-1 (Henderson et al. 1993). BHRF1 confers cell specific protection against apoptosis induced by a variety of agents, including infection with E1B 19K defective adenovirus (Foghsgaard & Jaattela 1997; Henderson et al. 1993; Tarodi et al. 1994). The patterns of expression of cellular and viral Bcl-2 during EBV infection are, however, entirely different. Induction of the cellular gene by LMP-1 occurs during latent infection of immortalized B cells. In contrast, BHRF1 is expressed at high level during productive infections of epithelial cells and B cells (Rickinson & Kieff 1996). BHRF1 therefore does not contribute to cellular transformation by EBV, but acts to promote release of virus during lytic infection. BHRF1 is highly conserved at both the sequence and functional level among different isolates of EBV (Khanim et al. 1997), further supporting an important functional role for this protein. It is notable that BHRF1 is expressed in nasopharyngeal carcinoma (Liu et al. 2000a) and may have a distinct role in this important epithelial tumour. EBV has recently been found to encode a second homologue of Bcl-2 that can interact with both Bax and Bak to inhibit apoptosis (Marshall et al. 1999). The gamma-2 herpesviruses human herpesvirus 8 (HHV-8) and herpesvirus saimiri (HVS) also encode homologues of Bcl-2. The HHV-8 homologue has been shown to block apoptosis efficiently in mammalian cells (Cheng et al. 1997; Sarid et al. 1997) and has been reported, using different techniques, either to heterodimerize with Bcl-2 (Sarid et al. 1997) or not to heterodimerize with Bcl-2, Bax or Bak (Cheng et al. 1997). The HHV-8 ‘v-Bcl-2’ is expressed predominantly during lytic infection (Sarid et al. 1998) and is therefore also likely to act to increase the yield of progeny virus during productive infections. It is interesting that all these herpesvirus homologues of Bcl-2 contain relatively well conserved BH1 and BH2 domains, but show poor conservation of the BH3 domain (Figure 2). These observations imply that, in contrast to its essential role in pro-apoptotic proteins, BH3 may not be necessary for antiapoptotic functions.
P53
In addition to interacting directly with mediators of MMP, viral gene products modulate the activity of a number of regulatory networks that can direct the cell towards apoptosis. The most important and relevant of these networks centres on p53. p53 is an essential tumour-suppressor gene that induces cell cycle arrest or apoptosis in response to DNA damage, aberrant growth signals and an array of other cytotoxic insults (Vogelstein et al. 2000). p53 is negatively regulated by the oncoprotein Mdm2, which inactivates p53-dependent transactivation and shuttles between the nucleus and cytoplasm, where it targets p53 for ubiquitin-mediated proteolysis (Piette et al. 1997). The function of Mdm-2 is in turn inhibited by the tumour-suppressor protein p14ARF, which prevents Mdm-2 mediated shuttling and consequent degradation of p53 (Tao & Levine 1999). Small DNA tumour viruses, such as adenovirus, simian virus 40 and high risk human papillomaviruses, drive infected cells to deregulated growth by releasing the E2F family of transcription factors from an inactive complex with the tumour-suppressor protein pRB (reviewed by Swanton and Jones in this series). In addition to activating genes necessary for progression through cell cycle, E2F activates p53 by upregulating the expression of p14ARF (Bates et al. 1998; Palermo et al. 1998). The induction of p53 mediated apoptosis is therefore a direct consequence of activation of the cell cycle by E2F, and provides a vital defence against aberrant growth. The small DNA tumour viruses have therefore each evolved distinct mechanisms to counter the effects of p53. Adenovirus E1B-55K protein, like Mdm2, is a highly active shuttle protein (Kratzer et al. 2000) and both E1B-55K and the adenovirus E4 ORF6 gene product bind directly to p53 and block p53-mediated transcriptional activation (Dobner et al. 1996; Martin & Berk 1998). The SV40 large T antigen sequesters p53 and prevents binding to its cognate recognition element in cellular DNA (Yanai & Obinata 1994). In contrast, the human papillomavirus E6 protein promotes the degradation of p53 by forming a complex with E6-AP, a ubiquitin protein ligase (Scheffner et al. 1990), although recent evidence suggests alternative mechanisms of interference with the function of intact p53 (Thomas et al. 1999). It is interesting that the LNA-1 nuclear antigen of the large oncogenic herpesvirus, HHV-8, has recently been shown to target pRB/E2F and to interact with p53 (Radkov et al. 2000).
Apoptosis and death receptors
Death receptors are a family of type I transmembrane proteins which belong to the tumour necrosis factor (TNF) receptor gene superfamily (Ashkenazi & Dixit 1998; Krammer 2000). Death receptors are each characterized by a cysteine-rich extracellular domain and a homologous cytoplasmic sequence termed the ‘death domain’. In addition to the TNF receptors themselves, there are a large and growing family of death receptors, including death receptor 3 (DR3, Apo3, WSL-1, TRAMP), DR4 (TRAIL-R1) and DR5 (Apo2, TRAIL-R2) (Ashkenazi & Dixit 1998; Ju et al. 1999; Krammer 2000). The most widely studied death receptor, however, is CD95 (Fas). CD95 is a ubiquitously expressed glycoprotein of approximately 45 kDa in size. The interaction of CD95 and its natural ligand, CD95L, results in the immediate recruitment of adapter molecule FADD and procaspase-8 (FLICE) to form a death-inducing signalling complex (DISC) (Figure 3). The interaction of FADD and CD95 is dependent on the death domain in each protein. FADD, however, also contains a second domain known as the ‘death effector domain’ (DED) essential for the interaction of FADD with procaspase-8 and the formation of DISC (Ashkenazi & Dixit 1998; Ju et al.1999; Krammer 2000). It has until recently been assumed that trimerization of the CD95 receptor following binding of CD95L was essential for the formation of DISC, but recent reports suggest that receptor oligomers form in the absence of ligand-binding (Siegel et al. 2000).
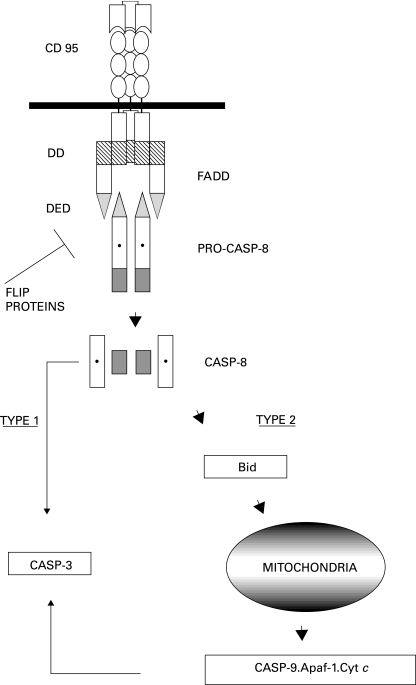
Figure 3.
Signal transduction in the CD95 pathway. Activated, trimerized CD95 receptor forms a death inducing signalling complex (DISC) with adapter molecules FADD and procaspase-8. FADD binds to CD95 via a domain known as the death domain (DD) present in both proteins. Procaspase-8 is recruited by homologous interaction with a second domain in FADD, the death effector domain (DED). The formation of DISC leads to the release of activated caspase-8 in the form of a heterotetramer. Thereafter, caspase-8 either acts directly on caspase-3 (CASP-3) in type I cells or activates Bid to induce MMP and formation of a functional apoptosome in type II cells. Viral and cellular FLIP proteins inhibit the formation of DISC.
The consequences of DISC formation are dependent on the cell type (Figure 3). In cells designated type I, DISC generates large amounts of activated caspase-8, which acts directly on caspase-3 to initiate the ‘executioner’ phase of apoptosis (Scaffidi et al. 1998). Inhibition of the apoptogenic functions of mitochondria, either by blocking the release of cytochrome c and AIF or by the heterologous expression of antiapoptotic members of the Bcl-2 family, does not prevent apoptosis in type I cells. In contrast, in type II cells very little DISC is formed and the caspase cascade is amplified by the mitochondria. Caspase-8 cleaves BID, a pro-apoptotic member of the Bcl-2 family. Truncated BID translocates to the mitochondrial membrane, where it induces MMP and leads to the formation of a functional apoptosome (Li et al. 1998). In type II cells therefore, apoptotic signals transduced by CD95 are dependent on MMP and can be inhibited by over expression of antiapoptotic members of the Bcl-2 family, such as Bcl-2 and Bcl-xL (Scaffidi et al. 1999).
The CD95/CD95L system is of particular importance in the immune system. It is essential for normal T-cell and B-cell development and is central to the regulated control of immune responses. CD95/CD95L mediates the deletion of activated T cells in the periphery and therefore plays a key role in regulating the clonal expansion of T cells in response to antigen. Signals transduced by CD95 are also responsible for the elimination of T cells that receive only a single signal via the T cell receptor. An important function of the second signal delivered by costimulatory molecules is the prevention of both type I and II CD95 mediated apoptosis by the upregulation of Bcl-xL and FLICE-inhibitory proteins, specific inhibitors of DISC formation (see below) (Krammer 2000). CD95 also plays a key role in both the positive and negative selection of B cells during development and is essential for the maintenance of immunological tolerance (Wang & Watanabe 1999). The dual function of CD95 in B cells is regulated by signals transduced by the B cell antigen receptor and the CD40 receptor (Tsubata et al. 1993; Rothstein et al. 1995; Rathmell et al. 1996). Further, the CD95/CD95L system is an important mechanism for the elimination of virally infected and malignant cells by CD95L positive cytotoxic T lymphocytes (Ju et al. 1999; Krammer 2000). The expression of functional CD95 on premalignant or malignant B cells therefore provides a potential mechanism of tumour suppression and evasion of CD95 mediated apoptosis is likely to be a key step in lymphomagenesis. In support of this concept, low grade non-Hodgkin's lymphomas (NHLs) express CD95, whereas aggressive NHLs of B cell lineage are usually CD95 negative (Nguyen et al. 1996; Gregory et al. 1987). Furthermore, many of those NHLs that express CD95 have evolved complex mechanisms for evasion of CD95 mediated apoptosis (Plumas et al. 1998; Medema et al. 1999; Tepper & Seldin 1999; Gutierrez et al. 1999). Overall, it is unsurprising that viruses infecting cells of the immune system, particularly those viruses that lead to cellular transformation, have evolved specific mechanisms to evade apoptotic signals transduced by CD95.
Viruses and death receptors
In 1997, three independent groups identified a novel family of viral proteins with the potential to interfere with death receptor signalling pathways (Bertin et al. 1997; Hu et al. 1997; Thome et al. 1997). These proteins each contained two domains homologous to the DED domains of FADD and procaspase-8 and were called viral FLICE inhibitory proteins or v-FLIPS. v-FLIPS were found in the gamma-herpesviruses HVS (encoded by open reading frame 71, ORF71), HHV-8 (ORF 71), equine herpesvirus 2 (EHV2) (E8), bovine herpesvirus 4 (BHV4) (BORFE2) and the poxvirus molluscum contagiosum (MCV) (ORF 159 L and 160 L). The E8 protein of EHV2, HVS ORF71, MCV 159 L and BORFE2 have all been shown to confer protection from CD95-mediated apoptosis and to bind to either FADD or procaspase-8 (Bertin et al. 1997; Hu et al. 1997; Thome et al. 1997; Wang et al. (1997). In addition, in at least some experiments, v-FLIPs protected against signals transduced by death receptors TNFR1, TRAMP, TRAIL-R1 and TRAIL-R2. The discovery of viral FLIP proteins led to the identification of a family of cellular homologues, termed cellular FLIPs (c-FLIPs) which modulate signals transduced by CD95 and other death receptors (Wallach 1997).
It is striking that all of the viruses found to encode v-FLIPs have the capacity to transform cells in vitro and are associated with tumours in susceptible hosts. HVS causes lymphoma and leukaemia in susceptible primates and induces stable growth transformation in human T-cells in vitro (Meinl et al. 1995). HHV-8 is associated with Kaposi's sarcoma (Chang et al. 1994), multicentric Castleman's disease (Soulier et al. 1995) and a rare B-cell lymphoma known as body-cavity based lymphoma (BCBL) (Cesarman et al. 1995). It is also striking that all the herpesvirus that encode v-FLIPs contain homologues of Bcl-2. The coacquisition of genes with the capacity to inhibit both type I and type II CD95 mediated apoptosis strongly suggests that v-FLIPs might facilitate cellular transformation. There is, however, no clear evidence that this is the case. HVS ORF71 has been shown to provide an antiapoptotic function, but is not expressed in T-cells growth transformed by HVS and is not essential for viral replication or host cell transformation and proliferation (Glycofrydes et al. 2000; Thome et al. 1997). In contrast HHV-8 ORF71 is expressed in at least some cell lines derived from BCBL (Talbot et al. 1999) and has recently been shown to confer protection against CD95-mediated apoptosis and to promote tumour progression in a murine model of B cell lymphoma (Djerbi et al. 1999). Intriguingly, however, BCBL lines persistently infected with HHV-8 do not express CD95 or any other known death receptor (BJ Thomson & AP Haynes, unpublished). It is highly likely that, in common with c-FLIPs, the expression and function of v-FLIPs will vary in a cell type, cell cycle and differentiation dependent manner.
CD95 mediated signals can be subverted by viruses in other ways. Adenoviruses produce a receptor internalization and degradation complex, encoded by the E3 region, that mediates removal of CD95 from the cell surface by internalization and degradation within lysozymes (Tollefson et al. 1998). In addition, many nontransforming viruses have evolved distinct mechanisms for the modulation of signals transduced by the CD95 receptor. The most important of these viruses, of course, is HIV. There is a complex heirarchy of interactions between HIV gene products and components of both the type I and type II CD95 apoptotic pathways in infected and bystander cells (Kaplan & Sieg 1998; Krammer 2000). These mechanisms are central to the pathogenesis of infection with HIV, but are not directly relevant to a series on viruses and cancer and will therefore not be reviewed in this article.
The final common pathway
The activation of executioner caspases-3, ‐6 and ‐7, whether by the direct action of caspase-8 or the formation of a functional apoptosome following MPP, is the final common pathway in programmed cell death. There is, however, evidence that even this ‘final’ commitment to cell death need not be irreversible. Baculovirus p35 protein and the cowpox CrmA gene product have been shown to directly inhibit executioner phase caspases. Another family of proteins first described in baculovirus, known as the inhibitors-of-apoptosis-proteins (IAP), are potent and specific inhibitors of caspase-3 and can reverse apoptosis following MMP, at least temporarily (Crooke et al. 1993; Clem & Miller 1994). IAP proteins have subsequently been identified in mammalian cells (Duckett et al. 1996; Liston et al. 1996). The importance of the regulatory role played by IAP proteins is further supported by the recent discovery of a specific inhibitor of IAP in mammalian cells, known as Smac/Diablo (Verhagen et al. 2000; Du et al. 2000). This protein is normally resident in the mitochondria and is released into the cytosol following MPP, where it binds to IAP proteins in a structure that has already been defined by crystallography (Wu et al. 2000; Liu et al. 2000).
Conclusions
This article has sought to review the principal mechanisms by which cells undergo apoptosis and to provide examples of the many ways in which viral gene products can interact with this process. It is clear that modulation of the death programme is an important component of the pathogenesis of viral infection and that prevention of apoptosis is an absolute requirement for the sustained growth-transformation of the host cell by oncogenic viruses. It is also clear that the study of mechanisms by which viruses interact with apoptotic pathways has provided invaluable insight into the function of cellular genes. Specific examples of how viruses subvert the regulation of cell cycle regulation and apoptosis to cause some of the most important human cancers will be reviewed in this series of articles.
References
- Adams JM, Corey S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Bates S, Phillips AC, Clark PA, et al. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, et al. Heat shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the apaf-1 apoptosome. Nat. Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Bertin J, Armstrong RC, Ottilie S, et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner C, Kroemer G. Apoptosis. Mitochondria – the death signal integrators. Science. 2000;289:1150–1151. doi: 10.1126/science.289.5482.1150. 10.1126/science.289.5482.1150. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based-lymphomas. N. Eng. J. Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Cheng EH-Y, Nicholas J, Bellows DS, et al. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerise with Bax or Bak. Proc. Natl. Acad. Sci. USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SK, Tseng CC, Rao L, White E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J. Virol. 1994;68:6553–6566. doi: 10.1128/jvi.68.10.6553-6566.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RJ, Miller LK. Control of programmed cell death by the baculovirus genes p35 and iap. Mol. Cell. Biol. 1994;14:52121–52122. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999;341:233–249. 10.1042/0264-6021:3410233. [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptotis. J. Cell. Biol. 1999;274:31734–31739. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerbi M, Screpanti V, Catrina AI, et al. The inhibitor of death receptor signalling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 1999;190:1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4 orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- D'Souza B, Rowe M, Walls D. The bfl-1 gene I transcriptionally upregulated by the Epstein-Barr virusLMP1-, and its expression promotes the survival of a Burkitt's lymphoma cell line. J. Virol. 2000;74:6652–6658. doi: 10.1128/jvi.74.14.6652-6658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome-c dependent caspase activation by eliminating IAP inhibition. Cell. 2000;101:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Duckett CS, Nava VE, Gedrich RW, et al. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Ezoe H, Fatt RBL, Mak S. Degradation of intracellular DNA in KB cells infected with cyt mutants of adenovirus-type-12. J. Virol. 1981;40:20–27. doi: 10.1128/jvi.40.1.20-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow SN, White JH, Martinou I, et al. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- Foghsgaard L, Jaattela M. The ability of BHRF1 to inhibit apoptosis is dependent on stimulus and cell type. J. Virol. 1997;71:7509–7517. doi: 10.1128/jvi.71.10.7509-7517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries KL, Miller WE, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis. J. Virol. 1996;70:8653–8659. doi: 10.1128/jvi.70.12.8653-8659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glycofrydes D, Niphuis H, Kuhn EM, et al. Herpesvirus saimiri v FLIP provides an anti-apoptotic function but is not essential for viral replication, transformation or proliferation. J. Virol. 2000;74:11919–11927. doi: 10.1128/jvi.74.24.11919-11927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1321. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gregory CD, Dive C, Henderson S, et al. Activation of Epstein-Barr virus latent genes protects human-B cells from death by apoptosis. Nature. 1991;349:612–614. doi: 10.1038/349612a0. [DOI] [PubMed] [Google Scholar]
- Gregory CD, Tursz T, Edwards CF, et al. Identification of a subset of normal B cells with a Burkitt's lymphoma (BL) -like phenotype. J. Immunol. 1987;139:313–318. [PubMed] [Google Scholar]
- Gutierrez MI, Cherney B, Hussain A, et al. Bax is frequently compromised in Burkitt's lymphomas with irreversible resistance to Fas-induced apoptosis. Cancer Res. 1999;59:696–703. [PubMed] [Google Scholar]
- Han J, Modha D, White E. Interaction of E1B 19K with Bax is required to block Bax-induced loss of mitochondrial membrane potential and apoptosis. Oncogene. 1998;17:2993–3005. doi: 10.1038/sj.onc.1202215. [DOI] [PubMed] [Google Scholar]
- Han J, Sabbatini P, Perez D, et al. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53 inducible and death-promoting Bax protein. Genes. Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- Hausmann G, O'Reilly LA, van Driel R, et al. Pro-apoptotic apoptosis protease-activating factor 1 (Apaf-1) has a cytoplasmic location distinct from Bcl-2 or Bcl-x (L) J. Cell. Biol. 2000;149:623–634. doi: 10.1083/jcb.149.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S, Huen D, Rowe M, et al. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B-cells from programmed cell death. Proc. Natl. Acad. Sci. USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S, Rowe M, Gregory C, et al. Induction of Bcl-2 expression by Epstein-Barr virus latent membrane protein-1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Honess RW. Herpes simplex and ‘the herpes complex’: diverse observations and a unifying hypothesis. The eighth Fleming lecture. J. Gen. Virol. 1984;65:2077–2107. doi: 10.1099/0022-1317-65-12-2077. [DOI] [PubMed] [Google Scholar]
- Hu S, Vincenz C, Buller M, Dixit VM. A novel family of death effector domain-containing molecules that inhibit both CD-95 and tumor necrosis factor receptor-1-induced apoptosis. J. Biol. Chem. 1997;272:9621–9624. doi: 10.1074/jbc.272.15.9621. 10.1074/jbc.272.15.9621. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ding L, Spencer DM, Nunez G. WD-40 repeat region regulates Apaf-1 self-association and procaspase-9 activation. J. Biol. Chem. 1998;273:33489–33494. doi: 10.1074/jbc.273.50.33489. 10.1074/jbc.273.50.33489. [DOI] [PubMed] [Google Scholar]
- Imazu T, Shimizu S, Tagami S, et al. Bcl-2/E1B 19 Kda-interacting protein 3-like protein (Bnip3L) interacts with bcl-2/Bcl-xL and induces apoptosis by altering mitochondrial membrane permeability. Oncogene. 1999;18:4523–4529. doi: 10.1038/sj.onc.1202722. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to apaf-1. J. Biol. Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- Ju ST, Matsui K, Ozdemirli M. Molecular and cellular mechanisms regulating T and B cell apoptosis through Fas/FasL interaction. Int. Rev. Immunol. 1999;18:485. doi: 10.3109/08830189909088495. [DOI] [PubMed] [Google Scholar]
- Kaplan D, Sieg S. Role of the Fas/Fas ligand apoptotic pathway in human immunodeficiency virus type 1 disease. J. Virol. 1998;72:6279–6282. doi: 10.1128/jvi.72.8.6279-6282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide ranging implication in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanim F, Dawson C, Meseda CA, et al. BHRF1, a viral homologue of the Bcl-2 oncogene, is conserved at both the sequence and functional level in different Epstein-Barrr virus isolates. J. Gen. Virol. 1997;78:2987–2999. doi: 10.1099/0022-1317-78-11-2987. [DOI] [PubMed] [Google Scholar]
- Krammer PH. CD95′s deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- Krazter F, Rosorius O, Heger P, et al. The adenovirus type 3 E1B-55K oncoprotein is a highly active shuttle protein and shuttling is independent of E4 orf6, p53 and MDM-2. Oncogene. 2000;19:850–857. doi: 10.1038/sj.onc.1203395. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nature Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Li H, Kolluri SK, Gu J, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of a nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu C-J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:4379–4489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Liston P, Roy N, Tamai K, et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- Liu MY, Shih YY, Li LY, et al. Expression of the Epstein-Barr virus BHRF1 gene, a homologue of Bcl-2, in nasopharyngeal carcinoma tissue. J. Med. Virol. 2000a;61:241–250. doi: 10.1002/(sici)1096-9071(200006)61:2<241::aid-jmv11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Liu Z, Sun C, Olejniczak ET, et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1008–1012. doi: 10.1038/35050006. 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Zaika A, Moll UM. Death signal-induced localisation of p53 to mitochondria. A potential role in apoptosis signalling. J. Biol. Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- Marshall WL, Yim C, Gustafson E, et al. Epstein-Barr virus encodes a novel homolog of the bcl-2 oncogene that inhibits apoptosis and associates with Bax and Bak. J. Virol. 1999;73:5181–5185. doi: 10.1128/jvi.73.6.5181-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ME, Berk AJ. Adenovirus E1B-55K represses p53 activation in vitro. J. Virol. 1998;72:3146–3154. doi: 10.1128/jvi.72.4.3146-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzani N, et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- Medema JP, de Jong J, van Hall T, Melief CJ, Offringa R. Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J. Exp. Med. 1999;190:1033–1038. doi: 10.1084/jem.190.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinl E, Hohlfield R, Wekerle H, Fleckenstein B. Immortalisation of human T cells by herpesvirus saimiri. Immunol. Today. 1995;16:55–58. doi: 10.1016/0167-5699(95)80087-5. [DOI] [PubMed] [Google Scholar]
- Morishi K, Huang DC, Cory S, Adams JM. Bcl-2 family members do not inhibit apoptosis by binding the caspase activator apaf-1. Proc. Natl. Acad. Sci. USA. 1999;96:9683–9688. doi: 10.1073/pnas.96.17.9683. 10.1073/pnas.96.17.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, et al. X-ray and NMR studies of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Narita M, Shimizu S, Ito T, et al. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer DD, Bossy-Wetzel E, Kluck RM, et al. Bcl-xL does not inhibit the function of Apaf-1. Cell Death Differ. 2000;7:402–407. doi: 10.1038/sj.cdd.4400665. [DOI] [PubMed] [Google Scholar]
- Nguyen PL, Harris NL, Ritz J, Robertson MJ. Expression of CD95 antigen and Bcl-2 protein in non-Hodgkin's lymphomas and Hodgkin's disease. Am. J. Pathol. 1996;148:847–853. [PMC free article] [PubMed] [Google Scholar]
- Palermo I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998;395:125–126. doi: 10.1038/25870. 10.1038/25870. [DOI] [PubMed] [Google Scholar]
- Pandey P, Saleh A, Nakazawa A, et al. Negative regulation of cytochrome c-mediated oligomerization of apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J, Neel H, Marechal V. Mdm2: keeping p53 under control. Oncogene. 1997;15:1001–1010. doi: 10.1038/sj.onc.1201432. [DOI] [PubMed] [Google Scholar]
- Plumas J, Jacob M-C, Chaperot L, et al. Tumor B cells from non-Hodgkin's lymphoma are resistant to CD95 (Fas/Apo-1) -mediated apoptosis. Blood. 1998;8:2875–2885. [PubMed] [Google Scholar]
- Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nature Med. 2000;10:1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95) -ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Fields BN, Knipe & DM, Howley PM, editors. Fields Virology. 3. Philadelphia: Lippincot-Raven; 1996. pp. 2397–2446. [Google Scholar]
- Rodriguez J, Lazebnik Y. Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev. 1999;13:3179–3184. doi: 10.1101/gad.13.24.3179. 10.1101/gad.13.24.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein TL, Wang JK, Panka DJ, et al. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Acharya S, et al. Cytochrome c and dATP-mediated oligomerisation of Apaf-1 is a prerequisite for procaspase-9 activation. J. Biol. Chem. 1999;274:17941–17945. doi: 10.1074/jbc.274.25.17941. 10.1074/jbc.274.25.17941. [DOI] [PubMed] [Google Scholar]
- Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in body cavity-based lymphoma cell line (BC-1) J. Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. Kaposi's sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nature Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signalling pathways. EMBO J. 1998;17:1657–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Schmit I, Zha J, et al. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus type-16 and type-18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Siegel RM, Frederiksen JK, Zacharias DA, et al. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler F, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- Subramanian T, Tarodi B, Chinnadurai G. p53-independent apoptotic and necrotic cell deaths induced by adenovirus infection: suppression by E1B 19K and Bcl-2 proteins. Cell Growth Differ. 1995;6:131–137. [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzani N, et al. Mitochondrial release of caspases-2 and −9 during the apoptosis process. J. Exp. Med. 1999;189:381–394. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Levine AJ. P19 (ARF) stabilises p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl. Acad. Sci. USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot SJ, Weiss RA, Kellam P, Boshoff C. Transcriptional analysis of human herpesvirus-8 open reading frames 71, 72, 73, K14, 74 in a primary effusion lymphoma cell line. Virology. 1999;257:84–94. doi: 10.1006/viro.1999.9672. [DOI] [PubMed] [Google Scholar]
- Tarodi B, Subramanian T, Chinnadurai G. Epstein-Barr virus BHRF1 protein protects against cell death induced by DNA-damaging agents and heterologous viral infection. Virology. 1994;201:404–407. doi: 10.1006/viro.1994.1309. [DOI] [PubMed] [Google Scholar]
- Tepper CG, Seldin MF. Modulation of caspase-8 and FLICE-inhibitory protein expressions as a potential mechanism of Epstein-Barr virus tumorigenesis in Burkitt's lymphoma. Blood. 1999;94:1727–1737. [PubMed] [Google Scholar]
- Thomas M, Pim D, Banks L. The role of the E6–p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- Thome M, Schneider P, Hofmann K, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death recptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tollefson AE, Hermiston TW, Lichtenstein DL, et al. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature. 1998;392:726–730. doi: 10.1038/33712. 10.1038/33712. [DOI] [PubMed] [Google Scholar]
- Tsubata T, Wu J, Honjo T. B-cell apoptosis induced by antigen receptor cross-linking is blocked by a T cell signal through CD40. Nature. 1993;364:645. doi: 10.1038/364645a0. [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Ekert PG, Pakusch M, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and recognising IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Wallach D. Apoptosis. Placing death under control. Nature. 1997;388:123–126. doi: 10.1038/40516. 10.1038/40516. [DOI] [PubMed] [Google Scholar]
- Wang G-H, Bertin J, Wang Y, et al. Bovine herpesvirus 4 BORFE2 protein inhibits Fas- and tumor necrosis factor receptor 1-induced apoptosis and contains death effector domains shared with other gamma-2 herpesviruses. J. Virol. 1997;71:8928–8932. doi: 10.1128/jvi.71.11.8928-8932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Watanabe T. Expression and function of Fas during differentiation and activation of B cells. Int. Rev. Immunol. 1999;18:367–379. doi: 10.3109/08830189909088489. [DOI] [PubMed] [Google Scholar]
- Wu G, Chai J, Suber TL, et al. Structural basis for IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- Yanai N, Obinata M. Apoptosis is induced at nonpermissive temperature by a transient increase in p53 in cell-lines immortalised with temperature sensitive SV40 large T-antigen gene. Exp. Cell. Res. 1994;211:296–300. doi: 10.1006/excr.1994.1090. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Theodarakis P, Subramanian T, Chinnadurai G. Adenovirus E1B 19K/BCL-2 interacting protein BNIP3 contains a mitochondrial targeting sequence. J. Biol. Chem. 1998;273:12415–12421. doi: 10.1074/jbc.273.20.12415. 10.1074/jbc.273.20.12415. [DOI] [PubMed] [Google Scholar]