Abstract
Failure of total joint replacement (TJR) is a major problem and it is estimated that 15–20% of TJR will fail within 5–10 years after implantation. Most TJR is attributed to aseptic loosening of the implants in association with resorption of related bone due to the release of bone-associated cytokines. IL-15 is a cytokine that activates T cells and natural killer (NK) cells. IL-15 protein is ubiquitous and is expressed in many tissues and cell types. Using immunohistochemical techniques, we demonstrated the expression of IL-15 and its receptors IL-15Rα and IL-2Rβ in the interface tissues obtained from revision surgery. Both IL-15 protein and IL-15Rα were observed in macrophages, multinucleated giant cells and endothelial cells around blood vessels. Both the SDS-PAGE and western blot revealed multiple bands and after stages of glycosylation, this resulted in a band at 13 KDa which corresponds to the IL-15 protein. Again RT-PCR results demonstrated a band of 420 bp corresponding to the IL-15 protein. In addition, using U937 cells, the expression of both IL-15 protein and IL-15Rα were considerably up-regulated when challenged with retrieved metal particles. Our results illustrated the IL-15 to be an intact protein and that it is stored in the cytoplasm. A dye exclusion cell viability test displayed an increase in toxicity with an increase in the amount of metal particles added. There was a discrepancy between abundant IL-15 mRNA, intracellularly detectable IL-15 protein and apparently inefficient secretion. This suggests that IL-15 protein production is predominantly regulated post-transcriptionally and this is indicated by its strict regulation, especially at cell trafficking. Finally, unlike IL-2, IL-15 plays a certain role in bone resorption that leads to failed joint prostheses. It is apparent that this cytokine is an important T cell mediated immune response which needs further research.
Keywords: interleukin 15, cytokine, implant protheses, inflammatory response, metal particles
Introduction
Aseptic loosening of the prosthetic components, often in association with resorption of bone (osteolysis) around the implants, is by far the most common cause of failure of TJR (Revell et al. 1997). The treatment of patients with a failed total hip replacement has become an increasing problem; furthermore, almost 7500 revisions are now performed annually in the UK (Hubble & Smith 1996). A revision operation is often difficult in patients with a significant loss of bone stock. The inflammatory response produced by wear particles is a major cause of bone loss occurring adjacent to implants. Cobalt chrome and titanium alloy along with stainless steel are the major metallic materials used in orthopaedic prosthetic surgery. Metals are not biologically inert, so therefore, suffer various forms of degradation such as pitting, fretting, and crevice corrosion. The wear debris products have been found to induce adverse biological effects such as cytotoxicity (Kelly et al. 1998), hypersensivity (Faleiro et al. 1996) and immunogenicity (Wang et al. 1997). The components tend to migrate as the loosening progresses and these changes are often accompanied by significant osteolysis. The release of bone-associated cytokines has been related to the development of osteolysis in patients with prostheses (Revell & Jellie 1998).
Interleukin 15 (IL-15) is a cytokine that is produced by monocytes. It is an activator of T lymphocytes and a stimulator of natural killer cell proliferation and activation. Furthermore, IL-15 induces cytokine production by monocytes and polymorphonuclear cells and stimulates the antimicrobial activity of phagocytes. IL-15 is a 14–15 KDa member of the α–helix bundle families of cytokines and shares several functional activities with IL-2 in different cell systems. Although IL-15 does not show sequence homology with IL-2, it uses components of the IL-2 receptor for binding and signal transduction. Revell & Jellie (1998) showed that the IL-2 cytokine, an activator and differentiator of T lymphocytes, was not expressed in any of the interface tissues examined. The role IL-15 protein plays in the development of osteoclasts has been recently reported by Ogata et al. (1999). Unlike IL-2, IL-15 was shown to stimulate the differentiation of osteoclast progenitors into preosteoclasts. Our objectives were to demonstrate the role of IL-15 and its receptors in failed joint prostheses.
Materials
Most of the antibodies (anti-CD68, anti-CD3, anti-IL-15, anti, IL-2, anti-IL2Rβ and anti-IL2Rγ) and recombinant human IL protein were purchased from R & D Systems Europe Ltd (Oxfordshire, UK). Anti-IL-15R polyclonal antibody was bought from Santa Cruz Biotechnology, Inc. (Wiltshire, UK). Sodium Chloride (NaCl), glycerol, Tris-HCl, dithiothreitol (DTT), ethylenediaminetetraacetic acid (EDTA), phenylmethylsulphonyl fluoride (PMSF), aprotinin, N-glycopeptidase F, sodium phosphate, lauryl sulphate (SDS), RPMI 1640, Hanks balanced salt solution, phorbol 12-myristate 13-acetate (PMA), lipopolysaccharide (LPS) and bovine serum albumin (BSA) were all obtained from Sigma-Aldrich (Poole, UK).
Methods
Tissue samples
Between 1994 and 1999, specimens of tissue from the bone implant interface were retrieved from 40 patients' hip joints, in which a metal femoral stem component and a polyethylene acetabular cup were present. The primary diagnosis before the first procedure of TJR was osteoarthritis. The ages of the patients varied from 65 to 79 and the duration of the implantation ranged from 2 to 10 years. The reason for revision was aseptic loosening of femoral and/or acetabular components with a thick interface membrane in all cases, often with a granulomatous inflitrate. All were negative on microbiological culture for organisms.
Immunohistochemistry
Cryostat sections (5 μm thickness) of interface membranes (n = 40) were cut and placed on poly-l-lysine coated slides. The sections were stained using antibody markers for human IL-15, IL-15Rα, IL-2Rβ, IL-2Rγ, CD68 and CD3 in an alkaline phosphatase-streptavidin method.
Western blot
Frozen sections were homogenized using Potter-Elvehjem tissue grinders (Jencons). Tissues were lysed for 10 min at 100 °C in 200μl of buffer containing NaCl (150 mm), glycerol (5%), Tris-HCl, pH 7.5 (25 mm), DTT (2 mm), EDTA (1 mm) PMSF (1 (M) and aprotinin (10 (μg/mL). Cellular debris was removed by centrifugation (14 000 g) and the proteins in the supernatants were separated on 10% SDS-PAGE under reducing conditions by using a semi-dry transblot apparatus (Bio-Rad Laboratories Ltd, Hertfordshire, UK) and transferred onto a nitrocellulose membrane (Amersham International Plc, London, UK). Membranes were developed with IL-15, IL-2 and IL-15Rα antibodies and detected using ECL Western blotting reagents (Amersham). The same amount of protein was loaded into each well.
Enzymatic treatments
Protein samples (50 μL) were treated overnight with N-glycopeptidase F (0.1 mU) at 37 °C in 10 mm sodium phosphate pH 7.1, containing 150 mm NaCl and 10 mm EDTA. The samples were treated with 5% SDS and 5% β– mercaptoethanol at 95 °C and protein separation and characterization by SDS-PAGE was performed as described above.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was purified by an RNAse purification system as specified by the manufacturer's instructions (Bioline Ltd, London, UK). cDNAs were prepared from 2 μg of total cellular RNA by reverse transcription at 70 °C for 15 min in a 25 μL reaction. cDNA was synthesized in the presence of Tth DNA Polymerase (4.0 U), using 2.5 μmol/L random primer and the reaction condition described by manufacturer (Bioline Ltd, London, UK). A 3 μL aliquot of the cDNA obtained was amplified in a 40 μL reaction containing 50 mmol/L MgCl2, 10 × NH4 buffer consisting of: [160 mm (NH4)2SO4, 670 mm Tris-HCl (pH 8.8 at 25 °C), 0.1% tween-20], 25 μmol/L of each primer and 2.5 U of Taq polymerase.
Reaction conditions were 35 s melting at 94 °C, 35 s annealing at 60 °C and 60 s extension at 72 °C for 35 cycles in a Techne PHC-3 Thermal cycler. The following oligonucleotides were used; IL-15 (5′ and 3′GAATC AATTGCAATCAAGAAGTTG) βActin (5′GAGCGGGAA ATGGTGCGTGACATT and 3′GAAGGTAGTTTCGTGG ATGCC). A sample (10 μL) of each PCR reaction was electrophoresed through a 2% agarose gel and visualized with ethidium bromide. All RT-PCR assays included several negative controls.
Cell culture
The human monocyte cell line U937 (8710802 ECAC) was cultured in a culture medium RPMI 1640 containing 10% heat inactivated fetal calf serum, 1% streptomycin and 5% l-glutamine. Following 8 days in culture, the cells were washed three times in Hanks balanced salt solution and were divided into 6 well plates (Costar, Cambridge, MA) at a density of 5 × 105 cells/well in RPMI 1640, containing 1% fetal bovine serum, 1% streptomycin and 1% l-glutamine and incubated with stimulatory agents at 37 °C in 5% CO2 in air for 48 h.
Particle preparation
Particles were extracted from interface tissues obtained during revision surgery of total hip replacements according to the method of Yamac (1999), sterilized in 70% ethanol overnight, subsequently washed three times with Hanks, then resuspended in Hanks and sonicated prior to addition to the cells.
Electron microscope
Samples of U937 cells treated with stainless steel particles were fixed for 24 h in 1% paraformaldehyde/0.5% glutaraldehyde in phosphate buffer saline (PBS) (pH 7.4) at 4 °C. They were rinsed in PBS, post fixed in 1% osmium tetroxide, rinsed in distilled water and then dehydrated in graded ethanols and embedded in LEMIX Epoxy resin. Ultrathin sections were cut and stained with uranyl acetate and lead citrate and examined in a Philips CM120 transmission electron microscope.
Stimulation of cells
The cells were stimulated by adding 1 ng/mL of PMA, 5 μg/mL of LPS, 5 ng/mL of stainless steel and 10 ng/mL of TiAlV. In some cases a combination of PMA and metal particles was added to examine the enhanced phagocytic ability after activation by PMA.
Cell staining
Adherent cells were recovered by scraping, centrifugation and resuspension in RPMI. Cytospin preparations of the cells were fixed with cold methanol/acetone (1 : 1) for 5 min, washed three times and blocked with 1% BSA. The cells were then incubated with anti-huIL-15 mAb, anti-huIL15Rα, anti-huIL-2Rβ and anti-huIL2Rγ. Bound primary mAb was detected using a biotinylated horse antimouse IgG and avidin-Texas Red complex, followed by counterstaining with DAPI.
Elisa
Supernatants from the culture were collected at time zero, 24, 48 and 72 h, then stored at − 70 °C. They were thawed and concentrated (5x) using Cetricon (Millipore Ltd, Hertfordshire, UK), before an ELISA for huIL-15 was performed according to the manufacturers’ instructions (Cytimmune Sciences, Inc). Standards for the ELISA were prepared by serial dilution of rhIL-15 in RPMI 1640 containing 1% foetal bovine serum.
Statistical analysis
Data were expressed as the mean ± SD for three or four replicates. The difference among the means of groups was compared by using student's t-test. P < 0.05 was considered a significant difference between the two groups (untreated and treated).
Results
Interface tissue
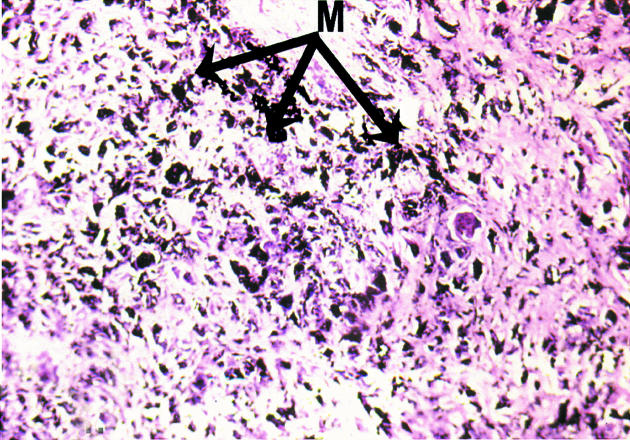
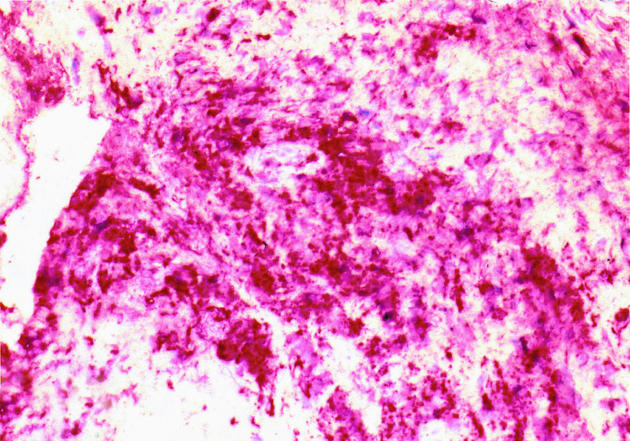
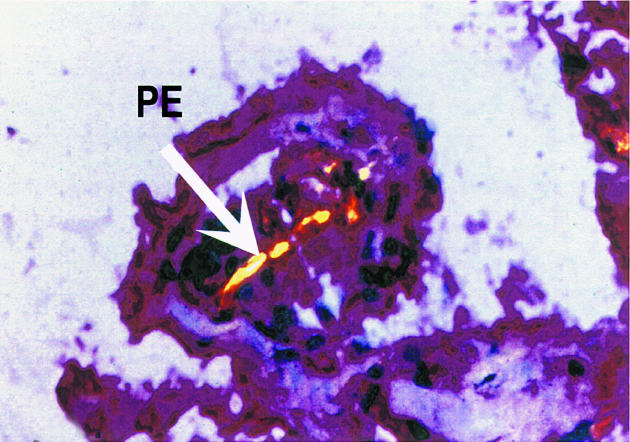
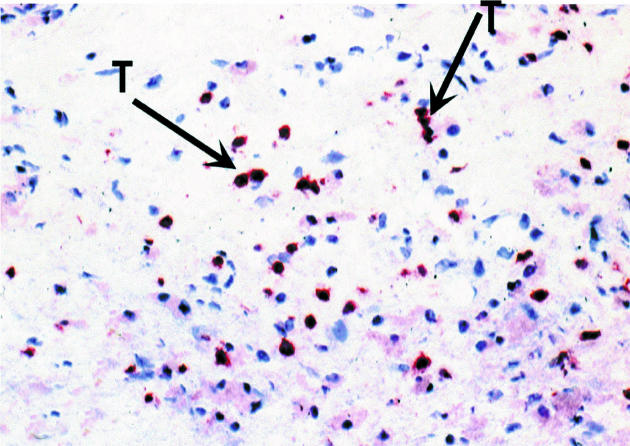
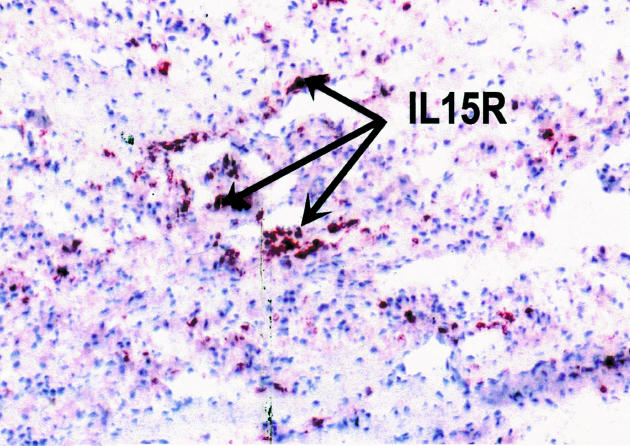
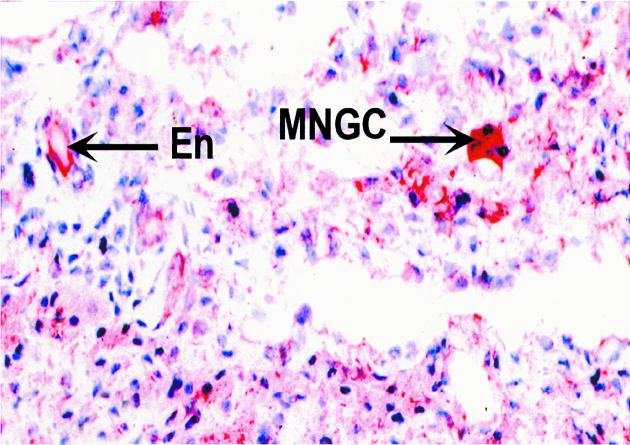
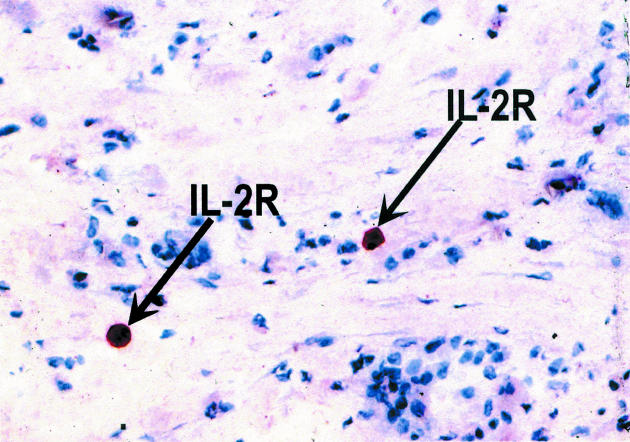
Histology examination of retrieved interface tissues from revision surgeries (Figure 1) shows heavy deposits of black metal wear debris produced from orthopaedic implants. Infiltrate of foreign body granuloma with heavy macrophage and MNGC are present. Immunohistochemical studies demonstrated the expression of IL-15 in most interfaces studied (Figure 2), reactivity ranging from strong to moderate. IL-15 was expressed within the synovium-like layer and in the underlying connective tissue. CD68 staining revealed the cells expressing IL-15 to be macrophages; there was no apparent staining with all control samples. The protein was also expressed by endothelial cells and multinucleate giant cells (Figure 3). Other cells in the infiltrates included CD3 positive T lymphocytes (Figure 4). Ten out of the 40 cases were examined for the expression of IL-15Rα, IL-2R and IL-2Rγ. IL-15Rα (Figure 5) was expressed in six samples by macrophages, multinucleate giant cells and endothelial cells (Figure 6), with weak expression in four further interfaces. IL-2Rβ (Figure 7) was apparent in 6 cases and there was no sign of IL-2Rγ expression in any of the cases. The IL-2Rβ was expressed only by T lymphocytes.
Figure 1.
In routine preparation of histological sections, heavy deposits of black metal wear debris are demonstrated in interface tissue obtained from failed joint prosthesis, × 200. M = metal wear debris.
Figure 2.
Immunohistochemical staining of IL-15 protein in interface tissue obtained from failed joint prosthesis. The binding of specific antibody to IL-15 was detected by alkaline phosphate-streptavidin method, × 200.
Figure 3.
Immunohistochemical staining of IL-15 protein expressed by multinucleated giant cell containing a large piece of polyethylene debris (arrow), viewed by compensated polarization microscopy. The binding of specific antibody to IL-15 was detected by an alkaline phosphate-streptavidin method, × 250.
Figure 4.
Immunohistochemical staining of T lymphocytes in interface tissue. The binding of specific antibody to CD3 (arrows) was detected by an alkaline phosphate-streptavidin method, × 200.
Figure 5.
Immunohistochemical staining of IL-15Rα in interface tissue. The binding of specific antibody to IL-15Rα (arrows) was detected by an alkaline phosphate-streptavidin method, × 200.
Figure 6.
Immunohistochemical staining of IL-15Rα of endothelial cell (arrow) and giant cell (arrow). The binding of specific antibody to IL-15Rα was detected by an alkaline phosphate-streptavidin method, × 250.
Figure 7.
Immunohistochemical staining of IL-2Rβ in interface tissue. The binding of specific antibody to IL-2Rβ (arrows) in lymphocytes was detected by an alkaline phosphate-streptavidin method, × 250.
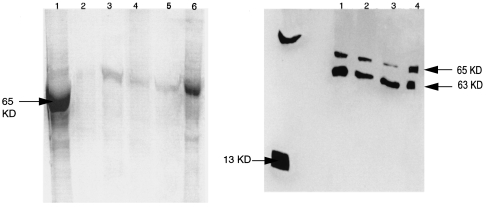
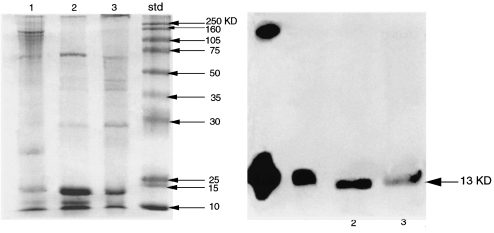
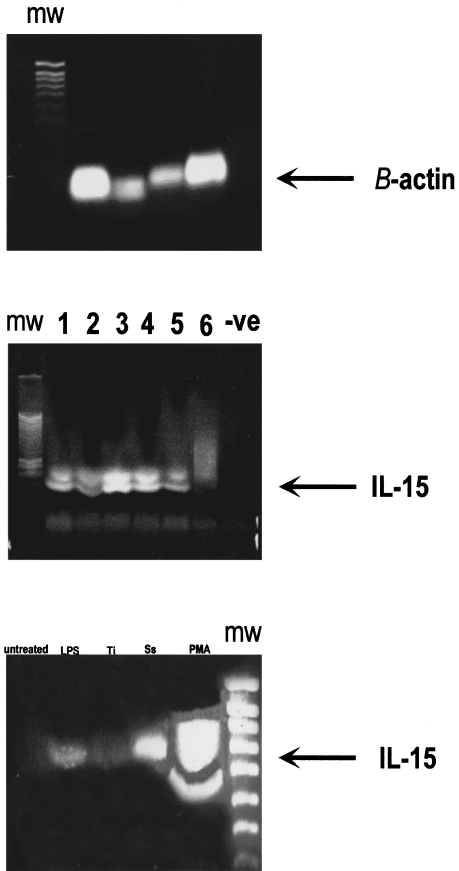
SDS-PAGE and western blot were performed on the same tissues and these demonstrated a band with a MW of 65 KDa (Figure 8a,b). Enzymatic fragmentation by glycosidation gave bands with MW of 35 and 13 KDa. (Figure 9a,b), respectively. The latter coincides with the known MW of recombinant huIL-15. The IL-2 protein was not detected by the western blot confirming the results observed in the immunostaining of the interface tissues. To support these observations, RT-PCR amplification was carried out on mRNAs prepared from several interface tissues. The interface tissues were the same as those used for the biochemical analysis and the results revealed two bands at 420 bp and 400 bp (Figure 10a).
Figure 8.
SDS-PAGE results of precursor IL-15 protein (a) and western blotting of IL-15 protein extracted from interface tissues (b). The arrows indicate the position of the extracted IL-15 protein and the rhIL-15 protein used as positive control. Randomly chosen cases from patient's interface tissues obtained from revision surgery are specified by numbers.
Figure 9.
SDS-PAGE results of IL-15 protein after subjecting to enzymatic glycosylation (a) and western blotting of IL-15 protein extracted from interface tissues (b). The arrows indicate the position of the extracted IL-15 protein and the rhIL-15 protein used as the positive control. Randomly chosen cases from patient's interface tissues obtained from revision surgery are specified by numbers.
Figure 10.
RT-PCR analysis of mRNA from β–Actin (10a). RT-PCR analysis of mRNA extracted from interface tissue (10b), cases 1–6 are randomly chosen interface tissues obtained from revision surgery. RT-PCR analysis of mRNA extracted from U937 cell lysates (10c) of untreated and treated with LPS = lipopolysaccharide, Ti = titanium, Ss = stainless steel, PMA = phorbol 12-myristate 13-acetate.
U937 cells
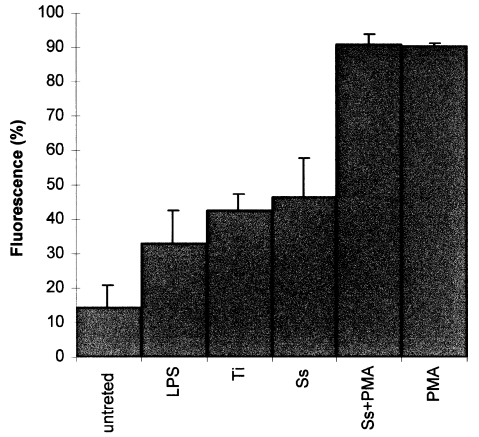
Few of the untreated cells were found to express IL-15 (14%) (Figure 11), while PMA dramatically up-regulated the IL-15 expression by a 5.3 fold increase to 90.3% (P < 0.05) compared to the untreated cells, and induced them to phagocytose the metal particles. The addition of LPS stimulated U937 cells up-regulated the expression of IL-15 by 1.3 fold (32.9%) (P < 0.05). Metal particles induced the expression of IL-15, stainless steel increased the reactivity by 2.2 fold (46.5%) (P < 0.05) and titanium increased it by 1.9 fold (42%) (P < 0.05). Addition of a mixture of PMA and metal particles to the cells produced the greatest reactivity of 91% (P < 0.05). The staining was stronger around the cell membrane, however some staining was evident within the cytoplasm. mRNA for IL-15 was abundantly expressed in the U937 cells challenged with LPS, PMA and retrieved metal particles (Figure 10c) and LPS and PMA are known stimulant of U937 cells.
Figure 11.
The expression of IL-15 by U937 cells showing both untreated and treated with stimulatory agents. Data were calculated as the mean ± SD for four replicates and expressed as a percentage of the controls. P < 0.05. LPS = lipopolysaccharide, Ti = titanium, Ss = stainless steel, PMA = phorbol 12-myristate 13-acetate.
The cells were challenged with retrieved metal particles of stainless steel and titanium alloy for a period of 24 h and 48 h. The trypan blue viability test showed the stainless steel particles to be most toxic (Table 1), the survival of the cells was found to be time and dosage dependent, 20 ng/mL of metal particles at 48 h was more lethal compared to 5 ng/mL (results not shown). There was correlation between cell death and the amount particles added. After 24 h the stainless steel increased cell death by 20% (P < 0.05), while titanium increased it by 8% (P < 0.05). A larger percentage of the cells were dead (34%, P < 0.05) when challenged with stainless steel and with titanium the cell death was increased by 20% (P < 0.05) after 48 h (Table 1).
Table 1.
The cell viability test results, data were calculated as the mean ± SD for three replicates and expressed as a percentage of the controls. P<0.05.
| Treatment | 24h mean | SD | 48h mean | SD |
|---|---|---|---|---|
| Untreated | 96.2 | ± 1.76 | 87.0 | ± 2.86 |
| LPS | 97.5 | ± 1.64 | 80.9 | ± 5.71 |
| Ti | 88.4 | ± 3.51 | 77.2 | ± 8.45 |
| Ss | 77.1 | ± 2.72 | 63.4 | ± 17.30 |
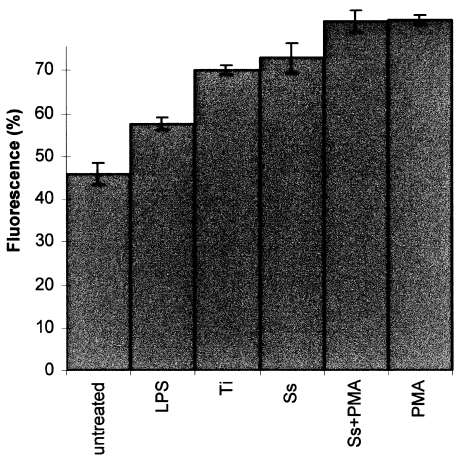
The immunostaining for IL-15Rα produced similar results to those for IL-15 in all the groups. However the percentage of untreated cells expressing the IL-15Rα was much higher (47%) than those untreated cells expressing IL-15 protein (14%) (Figure 11, Figure 12). PMA stimulated the IL-15Rα expression in the U937 cells by 78.5% (P < 0.05) relative to the untreated cells (Figure 12). LPS up-regulated the expression of IL-15Rα by 25% (P < 0.05) and stainless steel induced the reactivity by 58.7% (P < 0.05) and titanium increased it by 52.4% (P < 0.05). Addition of a mixture of PMA and metal particles to the cells increased the reaction by 77.4%. Similarly, the staining was stronger around the cell membrane, around the nucleus and within the cytoplasm. No evidence of IL-2Rβ or IL-2Rγ was found in any group. The ELISA on the supernatants from the cultured cells revealed an insignificant level of IL-15 to be present in all samples.
Figure 12.
The expression of IL-15Rα by U937 cells showing both untreated and treated with stimulatory agents. Data were calculated as the mean ± SD for four replicates and expressed as a percentage of the controls. P < 0.05. LPS = lipopolysaccharide, Ti = titanium, Ss = stainless steel, PMA = phorbol 12-myristate 13-acetate.
Discussion
As abnormal bone resorption is the main cause of aseptic loosening, determining the destructive mechanisms operating in bone adjacent to implants may help to solve this problem. In our study we showed that IL-15 protein is present and expressed in a large quantity of macrophages within the interface tissues obtained during the revision of failed THR with aseptic loosening. IL-15 has been indicated previously to be a unique cytokine that is expressed by a variety of tissues and cell types, including monocyte/macrophages, kidney epithelial cells, keratinocytes, fibroblasts, nerve cells, placenta, skeletal muscle and heart tissues (Giri et al. 1994; Grabstein et al. 1994; Quinn et al. 1995; Doherty et al. 1996; Tagaya et al. 1996).
The biochemical analysis revealed the existence of an IL-15 protein in the interface tissues which was intact and N-glycosylated. IL-15 has been reported to be N-glycosylated and to form oligomers (Balknill 1996). The SDS-PAGE results demonstrated three different molecular weights in interface macrophages upon digestion, suggesting the protein to be a trimer. The function of the glycosylation is obscure, but may affect the half-life of cytokines in vivo and their distribution. Although IL-15 is mainly produced by macrophages it is also produced by MNGC and endothelial cells of the blood vessels. The IL-15 produced by the endothelial cells may attract T cells and sustain their growth in the interface membrane. In order to be certain that the wear debris produced from the implants are the cause of the release of IL-15 by macrophages, experiments were performed using the U937 cell line activated by various known stimulant agents such as LPS and PMA and retrieved metal wear particles. Both the mRNA and IL-15 protein were found expressed by the cells, and the staining for the protein was strong around the cell membrane and the cytoplasm. This is not the first time that metal particles have been reported to induce production of inflammatory cytokines by monocytes/macrophages in vitro. There are reports by Wang et al. (1997), Shanbhag et al. (1995) and Haynes et al. (1993), however, none of these showed IL-15.
Despite the widespread distribution of IL-15 mRNA, it has been difficult to demonstrate IL-15 in the supernatants of many cells that express its message. The ELISA assay performed on the supernatants collected at intervals in the present study showed a discrepancy between the detectable IL-15 protein (even at 10-fold concentration) and its mRNA and protein in tissues as visualized by Western blot and RT-PCR. This strange behaviour observed for IL-15 coincides with work undertaken on the regulation of IL-15 secretion at transcriptional and translational levels (Tagaya et al. 1997; Bamford et al. 1998; Musso et al. 1999). Most of these studies stressed that unlike IL-2, the synthesis and secretion of IL-15 is controlled at the level of mRNA translation (e.g. initiation, elongation and termination). This is not unique to IL-15 but has also been reported for other important proteins including insulin (Welsh et al. 1986). There are 10 upstream AUG peptide sequences present which have been confirmed to inhibit translation. When these upstream AUGs were deleted experimentally in some cell lines the secretion of IL-15 protein was increased 4- to 5-fold relative to untreated cells.
Our study has also shown IL-15 protein to be stored in the cytoplasm. This strengthens the findings of Onu and colleagues (Onu et al. 1997) who found that fully synthesized IL-15 enters the secretory pathway through the cytoplasmic pool in a regulated manner. This is neither dependent on IL-15 SP or the 10 upstream AUGs that burden the efficient translation of IL-15 protein (Kozak 1989). Two IL-15 isoforms have been identified that differ in the length of the signal peptide IL-15 associated with the short signal peptide (SS-IL-15). This is not secreted but rather stored intracellularly, which explains the staining observed in this study in the cytoplasm. The alternative isoform, characterized by the longer signal peptide (LPS-IL-15) is located in the endoplasmic reticulum and is supposed to follow a pathway ending in its secretion (Kozak et al. 1989). Factors that control the secretion of IL-15 are still a mystery. However, one can deduce that having a ready IL-15 protein within the cytoplasm provides the cell with a mechanism by which it may respond quickly and effectively to intracellular infections or other stimuli, such as foreign indigestible inorganic particles, as in the present study. Once again this justifies the importance of IL-15 being the main innate defence mechanism of the cell.
Retrieved metal debris was found to enhance the production of the IL-15 cytokine and its receptor IL15Rα by U937 cells without prior stimulation of the cells. This could be due the formation of soluble metal ions during incubation with cell medium. Metal ions have been reported to enter cells and bind kinases or nuclear factors involving an alteration of signal transduction and cytokine gene expression (Daffada et al. 1994). Cell death was only observed with an addition of a mixture of metal debris and PMA and this was related to the phagocytosis of metals by cells. Similarly Shanbhag et al. (1995) demonstrated that Ti and TiAlV particles caused more cell death than did PE particles and Haynes et al. (1993) found that Co-Cr alloy produced early cell death. It has been demonstrated in a previous paper that MNGCs resemble osteoclasts and express osteoclast surface markers, especially when activated, and are able to resorb bone (Kadoya et al. 1994). Furthermore, U937 cells form MNGCs in culture with metal particles (Bainbridge et al. 2000). These findings are intriguing and we envisage further work to examine the role of metal ions, their oxidation state in vitro and in vivo and how this relates to the clinical manifestation of changes related to joint prostheses.
Acknowledgments
This work was supported by EPSRC funding to the IRC in Biomedical Materials.
References
- Bainbridge J, Dalby M, Curtis P, Knight M, Revell P. Cytoskeletal changes in macrophages and gaint cells following exposure to wear debris. 2000 Abstract submitted to the UK Society for Biometrials. [Google Scholar]
- Balknill F. Oxford: IRL Press; 1996. Cytokine: A practical approach. [Google Scholar]
- Bamford RN, Defilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide and coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J. Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- Daffada AAL, Murray EJ, Young SP. Control of activator protein 1 and nuclear factor Kappa β activity by international interleukin-6 and metals in HEPG2 cells. Biochim. Biophys. Acta. 1994;1222:234–240. doi: 10.1016/0167-4889(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- Faleiro C, Codinho I, Reus U, de Sousa M. Cobalt-chromium-molybdenum but not titanium-6 alumnium-4 vanadium alloy discs inhibit human T cell activation in vitro. Biometals. 1996;9:321–326. doi: 10.1007/BF00140600. [DOI] [PubMed] [Google Scholar]
- Giri JG, Ahdieh M, Eisenman J, et al. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2828. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein KH, Eisenman J, Shanebeck K. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Haynes DR, Rogers SD, Bapp SH, Pearcy MJ, Howei SW. The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J. Bone Joint Surg. 1993;75A:825–834. doi: 10.2106/00004623-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Hubble MJW, Smith EJ. Revision of failed total hip replacement. Review. Br. J. Hosp. Med. 1996;55:432–436. [PubMed] [Google Scholar]
- Kadoya Y, Al-Saffar N, Kobayashi A, Revell P. The expression of osteoclast markers on foreign body gaint cells. Bone Miner. 1994;27:85–96. doi: 10.1016/s0169-6009(08)80211-5. [DOI] [PubMed] [Google Scholar]
- Kelly CJ, Frishberg Y, Gold DP. An appraisal of T cell subsets and the potential for autoimmune injury. Kid. Intern. 1998;53:1574–1584. doi: 10.1046/j.1523-1755.1998.00917.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation, an update. J. Cell Biol. 1989;108:229. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso T, Calosso L, Zucca M, Millesino M, Ravarino D. Human monocyte constitutively express membrane-bound biologically active and interferon-γ upregulated interleukin-15. Blood. 1999;93:3531–3539. [PubMed] [Google Scholar]
- Ogata Y, Kukita T, Komine M, Miyahara S, Miyazaki A, Kohashi O. A novel role of IL-15 in the development of osteoclasts: inability to replace its activity with IL-2. J. Immunol. 1999;162:2754–2760. [PubMed] [Google Scholar]
- Onu A, Pohl T, Krause H, Bulfone-Paus S. Regulation of IL-15 secretion via the leader peptide of two Il-15 isoforms. J. Immunol. 1997;158:255–267. [PubMed] [Google Scholar]
- Quinn LS, Haugh KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136:3669. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- Revell PA, Al-Saffar N, Kobayashi A. Biological reaction to debris in relation to joint prostheses. Proc. Instn. Mech. Engrs. 1997;211:187–197. doi: 10.1243/0954411971534304. [DOI] [PubMed] [Google Scholar]
- Revell PA, Jellie SE. Interleukin 15 production by macrophages in the implant interface membrane of aseptically loosened joint replacements. J. Mater. S. Materials Med. 1998;9:727–730. doi: 10.1023/a:1008903018885. [DOI] [PubMed] [Google Scholar]
- Shanbhag AS, Jacobs JJ, Black J, Galente JO, Glant TT. Human monocyte responses to particulate biomaterials generated in vivo and in vitro. J. Orthopaed. 1995;13:792–801. doi: 10.1002/jor.1100130520. [DOI] [PubMed] [Google Scholar]
- Tagaya Y, Bamford RN, Defilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4:329–336. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- Tagaya Y, Kurys G, Thies TA, et al. Generation of secretable and non-secretable interleukin 15 isoforms through alternate usage of signal peptides. Proc. Natl. Acad. Sci. USA. 1997;94:14444–14454. doi: 10.1073/pnas.94.26.14444. 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Wicklund BH, Gustilo RB, Tsukayama DT. Prosthetic metals impair murine immune response and cytokine release in vivo and in vitro. J. Orthopaed. Res. 1997;15:688–699. doi: 10.1002/jor.1100150510. [DOI] [PubMed] [Google Scholar]
- Welsh M, Scherberg N, Gilmore R, Steiner DF. Translational control of insulin biosynthesis. Biochem. J. 1986;235:459–468. doi: 10.1042/bj2350459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamac T. University of London: 1999. PhD Thesis. [Google Scholar]