Abstract
The Epstein-Barr virus (EBV) is a herpes virus which establishes a life-long persistent infection in over 90% of the human adult population world-wide. Based on its association with a variety of lymphoid and epithelial malignancies, EBV has been classified as a group 1 carcinogen by the International Agency for Research on Cancer. In this article we discuss the evidence supporting an aetiological role for EBV in the pathogenesis of human tumours. The biology of EBV infection will be described with special emphasis on viral transforming gene products. A brief survey of EBV-associated tumours is followed by a discussion of specific problems. Evidence is presented which suggests that failures of the EBV-specific immunity may play a role in the pathogenesis of EBV-associated tumours also in patients without clinically manifest immunodeficiencies. Finally, the timing of EBV infection in the pathogenesis of virus-associated malignancies is discussed. There is good evidence that EBV infection precedes expansion of the malignant cell populations in some virus-associated tumours. However, this is clearly not always the case and for some of these tumours there are indications that clonal genetic alterations may occur prior to EBV infection. Thus, whilst there is good evidence to suggest that EBV is a human carcinogen, its precise role(s) in the development of virus-associated human tumours requires clarification.
Keywords: Epstein-Barr virus, infectious mononucleosis, malignant lymphomas, Burkitt lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma
Introduction
In the last 10–15 years, the Epstein-Barr virus (EBV) has been detected in the tumour cells of a range of diverse neoplasms. In addition to Burkitt's lymphoma (BL) and nasopharyngeal carcinoma (NPC), the list of EBV-associated tumours now includes Hodgkin's disease (HD), post-transplant lymphoproliferative disorders (PTLD), T-cell non-Hodgkin lymphomas (NHL), gastric carcinomas, possibly breast and hepatocellular carcinomas, and smooth muscle cell-derived tumours in immunodeficient individuals. This has greatly stimulated research into EBV-associated tumours, attracting scientists from different backgrounds into the field. However, the expansion in the number of known EBV-associated tumours has now reached a point where it appears that the virus can be found anywhere if one only looks hard enough. In addition, the EBV association of human tumours shows a range of different patterns. For most of these tumours, the association with EBV is variable depending on factors such as geographical origin of the patient (e.g. BL) or histological subtype (e.g. HD). In some tumours, the virus is present only in a proportion of neoplastic cells (e.g. T-cell lymphomas), raising questions regarding the timing of EBV infection in the pathogenesis of these tumours. Moreover there are at least three different patterns of viral gene expression in EBV-associated tumours. Thus, it is now important to define the role(s) of EBV in the pathogenesis of virus-associated tumours. A preliminary attempt has been made by the International Agency for Research on Cancer (IARC) which, in 1997, classified EBV as a group 1 carcinogen based on its association with BL, HD, PTLD, sinonasal angiocentric T-cell NHL, and NPC (IARC 1997). The purpose of the present article is not to present a comprehensive review of EBV-associated tumours. Rather, we discuss issues relating to the role of EBV in the pathogenesis of human tumours emphasizing the biology of EBV infection, the contribution of viral transforming genes, the role of the immune system and the timing of EBV infection.
Biology of EBV infection
EBV genome
EBV is a member of the lymphocryptovirus genus of the γ herpesvirus family. The structure of EBV is that of other herpesviruses. In the infectious virion, the viral genome is present as a linear, double-stranded, ca.172 kbp DNA molecule. In infected cells, the viral genome persists mainly as an extrachomosomal episome. Episome formation is mediated by a set of 0.5 kbp terminal repetitive (TR) sequences located at either end of the linear molecule and results in a TR region with a variable number of repeats. It is believed that individual infection events lead to episomes which differ in their number of TRs. Thus, analysis of the TR region by Southern blot hybridization can provide evidence as to the clonality of the viral genomes and by implication of the cell population harbouring the virus (Raab-Traub & Flynn 1986).
The viral genome contains potentially over 100 open reading frames (ORFs). The nomenclature of these ORFs is based on a BamHI restriction map of the viral genome (Baer et al. 1984). For example, BZLF1 is the first leftward ORF in the BamHI Z fragment (BamHI Z Leftward open reading Frame 1) (IARC 1997).
EBV target cells in vitro
The identification of EBV specific sequences in human tumours of lymphoid or epithelial origin suggests that the virus has the ability to infect cells belonging to very different lineages. However, the efficiency of infection in vitro varies dramatically according to the cell type used. EBV readily infects primary resting B-cells from all lymphoid compartments in vitro (Rickinson & Kieff 1996). Virus entry includes adhesion to the target cell and entry itself, both phenomena mediated by viral glycoproteins contained in the viral envelope (Rickinson & Kieff 1996). Infection of B lymphocytes has been shown to be mediated by binding of the BLLF1 viral glycoprotein (gp350/220) to the CD21 antigen which also serves as the lymphocyte receptor for the C3d molecule (C3d receptor, CR2) (Fingeroth et al. 1984; Nemerow et al. 1985; Tanner et al. 1987). The BLLF1 glycoprotein is the most abundant glycoprotein in the viral envelope and the major antigen responsible for stimulating the production of neutralizing antibodies in vivo (Thorley-Lawson & Geilinger 1980). The BLLF1 glycoprotein not only mediates EBV adsorption to CD21 but binding also induces capping of the receptor and endocytosis of the virus into B lymphocytes. The viral protein BXLF2 (gp85, gH) is critical for the second step of viral infection, i.e. fusion of the virus with B cells, as has been shown by using a viral recombinant with the neomycin cassette inserted in the BXLF2 open reading frame (Shimizu et al. 1996).
Despite isolated reports of successful infection of T lymphocytes, dendritic cells or primary epithelial cells, the efficiency of infection of these cells with EBV has generally been very low, and the experiments difficult to reproduce (Sixbey et al. 1983; Watry et al. 1991; Lindhout et al. 1994). Mature T-lymphocytes from blood or lymphoid tissues appear to be refractory to conventional in vitro infection, but a subpopulation of foetal thymocytes expressing the CD21 antigen can be infected and the cells kept in culture for some weeks in the presence of IL2 or thymic presenting cells (Watry et al. 1991). These infected thymocytes are reported to express some EBV-encoded antigens but do not show long-term growth (Kelleher et al. 1996). In contrast, the T cell leukaemia cell line HSB-2 and the HTLV1 infected cell line MT-2 are readily infected by laboratory strains of EBV (Hedrick et al. 1992; Koizumi et al. 1992). Interestingly, HSB-2 is CD21 negative, whereas the receptor for EBV entry in MT-2 seems to be an isoform of CD21 which differs from that seen on B-cells.
The binding of EBV to epithelial cells was also thought to result mainly from the interaction of gp350/220 with CD21, and indeed most instances of experimentally induced viral entry into non-B cells has occurred either through ectopic expression of CD21 on these cells or, it has been argued, through very low CD21 levels naturally expressed in the target cell surface (Birkenbach et al. 1992; Li et al. 1992). This mechanism has also been proposed to mediate EBV infection in the 293 epithelial cell line. However, we have recently shown that a viral EBV mutant lacking gp350/220 still infects the 293 epithelial cell line, as well as B-cells, albeit with a reduced efficiency (Janz et al. 2000). These observations therefore point towards the existence of additional viral receptors whose primary function may be the specific infection of non B-cells. In line with this assertion, Wang and Hutt-Fletcher have shown that the gp42 glycoprotein encoded by the viral BZLF2 gene is critical for the infection of B cells but not of epithelial cells (Wang & Hutt-Fletcher 1998). In contrast, the BDLF3 glycoprotein (gp150) was not essential for infection of B cells, and BDLF3 knockout EBV mutants demonstrated an enhanced ability to infect epithelial cells (Borza & Hutt-Fletcher 1998). However, it should be pointed out that the epithelial cells used by this group are stably transfected with the CD21 gene, and therefore represent a rather artificial system.
In summary, the situation is reminiscent of that which has been described for Herpes simplex virus I (HSV1) in which numerous glycoproteins were initially thought to be dispensable for viral infection. In fact some of these glycoproteins were later proved to be critical for infection of primary cells, underlining the need for an appropriate cellular system (Spear et al. 2000).
Cocultivation of EBV infected cells with several epithelial cell lines has been shown to be more efficient than infection of the same cells with free viral particles, indicating the potential importance of direct cell-to-cell spread of the infection either via mature or possibly via immature virus particles (Imai et al. 1998). This alternative way of infection proved to be remarkably efficient with epithelial cell lines derived from adenocarcinomas of various origins. Whether EBV can be transmitted from one cell type to the other in vivo remains to be determined.
EBV-mediated immortalization
Resting normal B-lymphocytes infected with EBV continuously grow under standard culture conditions in vitro (Rickinson & Kieff 1996). This unique property has provided a very useful tool to dissect the immortalizing properties of the virus. The lymphoblastoid cell lines (LCL), also called immortalized B-cell lines, represent an excellent model for the post-transplant lymphoproliferative disorders observed in patients with immune deficiencies. However, in many other cases of EBV-associated tumours, the viral proteins identified in vitro as central for B-cell immortalization are not expressed, showing that the virus can contribute to cell growth in different ways (Rickinson & Kieff 1996). Cell lines derived from highly malignant Burkitt's lymphomas tumour cells frequently carry the viral genome, and their study has provided important information about the physiology of EBV infection (Rickinson & Kieff 1996).
In EBV infected B-cells, viral multiplication, i.e. lytic replication, is rather unusual. Instead, the viral DNA is replicated by the cellular machinery and is transmitted to daughter cells after cell division, ensuring that the viral genome is faithfully maintained in the infected cell population (Rickinson & Kieff 1996). Only a few of almost 100 genes identified in the EBV genome are actively transcribed in immortalized B-cells, the so-called latent genes. The intracellular localization of these genes has led to the inclusion of their expression products into the EBNA (Epstein-Barr nuclear antigen 1, 2, -LP, 3A, 3B and 3C) and LMP (latent membrane protein 1, 2A and 2B) protein families. Furthermore, two small nonpolyadenylated nuclear RNAs, EBER1 and EBER2, are abundantly expressed in latent infection (Howe & Steitz 1986). The study of mutant laboratory strains, followed by the construction of purpose made viral recombinants, has led to the conclusion that the expression of the majority of latent genes, if not of all of them, is required for efficient B-cell immortalization (Delecluse & Hammerschmidt, 2000). However, none of these gene products is able to immortalize B-lymphocytes on its own, even if one of them, LMP1, has been shown to transform rodent cells in vitro (Wang et al. 1985; Baichwal & Sugden 1988). This has led to the concept of latency programs, characterized by distinct patterns of latent gene expression (Table 1). EBV immortalized LCLs express all latent genes (latency type III, Table 1), but in other EBV infected cells the observed pattern of expression can be more restricted, e.g. BL (latency I, Table 1) or HD (latency II, Table 1). In latency III, the EBNAs are transcribed as a long primary transcript originating from one of two promoters located in the BamHI C or W fragments of the viral genome (Cp or Wp). In latencies I and II, EBNA1 alone is transcribed from a different promoter in the BamHI F fragment (Fp) (Schaefer et al. 1995; Speck & Strominger 1989).
Table 1.
Patterns of EBV latency
| Latency | EBERs* | EBNA1* | EBNA2* | EBNA3 A,B,C | EBNA-LP | LMP1* | LMP2 A*B |
|---|---|---|---|---|---|---|---|
| I e.g. BL | + | + | – | – | – | – | – |
| II e.g. HD | + | + | – | – | – | + | + |
| III e.g. PTLD | + | + | + | + | + | + | + |
viral gene products detectable in formalin-fixed, paraffin-embedded tissue sections by in situ hybridization (EBERs) or by immunohistochemistry (all others).
Viral infection has been shown to be tightly linked to neoplastic transformation in several animal and human tumours. In many cases, viral products directly interfere with the mechanisms that positively or negatively control cell growth. Similarly, EBV infection permanently induces the physiological molecular pathways that lead to B-cell activation, ultimately leading to cell division. The use of conditional systems, in which the expression of one latent gene can be switched on or off, has shown that continuous B-cell proliferation is strictly dependant on viral gene expression. Thus B-cell immortalization is a reversible phenomenon. This is in contrast with common tumour cell lines characterized by the accumulation of irreversible genetic abnormalities. Immortalization, rather than transformation, therefore more accurately describes the immediate consequences of EBV infection in B cells.
EBNA1
This protein is invariably found to be expressed in all EBV infected cells, irrespective of their lineage or differentiation stage. This is not surprising as EBNA1 is crucial for persistence of the viral DNA within the infected cell, and recombinant viruses devoid of EBNA1 lose their ability to immortalize B-cells (Lee et al. 1999). EBNA1 binds to several viral DNA domains and in particular to the 20 tandem direct 30 bp repeats present in the oriP latent origin of replication as well as to human chromatin (Rawlins et al. 1985; Marechal et al. 1999). EBNA1 is therefore thought to tether the viral episomes to the chromatin via ori P, ensuring their transmission to the cell progeny. EBNA1 was classically thought to be required also for latent viral DNA replication. However, more recent work has cast some doubts on this notion. EBV-derived plasmids can be replicated in the absence of EBNA1 by the cellular machinery but are rapidly eliminated (Aiyar et al. 1998). Therefore, EBNA1 is not required for DNA synthesis itself, but allows maintenance of the newly synthetized plasmids. The central part of EBNA1 is characterized by Gly-Ala repeats that play a major role in inhibiting immune recognition. These repeats block processing of the protein by the proteasome and therefore inhibit killing of EBV infected cells by cytotoxic T-cells (Levitskaya et al. 1995; Levitskaya et al. 1997). More recently, it became clear that EBNA1 elicits a CD4-positive T-cell response, but the contribution of these cells to the anti-EBV response in vivo is still unknown (Münz et al. 2000). The binding of EBNA1 to ori P also leads to an activation of the Cp promoter (Reisman & Sugden 1986). This initiates the transcription of the EBNA common RNA from which the various EBNA proteins are translated. Transgenic mice expressing EBNA1 have been shown to develop tumours, but the actual contribution to immortalization of human cells is still a matter of debate (Wilson et al. 1996).
EBNA2, EBNA-LP
The P3HR1 EBV laboratory strain is unable to immortalize B-cells (Miller et al. 1974). This strain carries a genomic deletion encompassing the EBNA2 gene and the last two exons of the EBNA-LP gene. Introduction of the EBNA2 and LP genes from the B95.8 viral strain into the P3HR1 genome results in a virus with full immortalizing capacity, demonstrating that EBNA2 is absolutely required for immortalization (Cohen et al. 1989; Hammerschmidt & Sugden 1989). EBNA2 has transactivating properties, activating cellular (e.g. CD21, CD23, C-FGR, C-MYC) and viral promoters like the Cp promoter or the LMP1 promoter (Wang et al. 1987; Abbot et al. 1990; Fåhraeus et al. 1990; Knutson 1990; Wang et al. 1990; Wang et al. 1990; Kaiser et al. 1999). EBNA2 is an unusual transactivator as it does not bind directly to DNA but requires an ubiquitous cellular protein, RBP-Jκ, to exert its functions (Henkel et al. 1994). RBP-Jκ belongs to the Notch1 receptor signal transduction pathway and binds to specific DNA motifs (GTGGGAA) present in the promoter regions of its target genes (Goodbourn 1995). It then recruits EBNA2 to activate transcription via its acidic transactivating domain. In fact an activated version of the mouse Notch1 can induce the expression of EBNA2 targets which confirms that EBNA2 makes use of the physiological Notch pathway to activate its targets (Hofelmayr et al. 1999; Strobl et al. 2000). The PU.1 protein, another DNA binding protein has also been suggested to interact with EBNA2 to activate transcription in EBNA2 responsive promoters (Sjoblom et al. 1995). The construction of viral recombinants with deletion of some EBNA2 subdomains has led to a better understanding of the different functions of the protein. Up to now, at least four different subdomains have been defined as essential for immortalization (Cohen & Kieff 1991; Cohen et al. 1991; Cohen et al. 1992; Tong et al. 1994; Yalamanchili et al. 1994; Harada et al. 1998). Viruses harbouring a deletion of the EBNA-LP gene display a decreased ability to immortalize B-cells (Hammerschmidt & Sugden 1989; Mannick et al. 1991). EBNA-LP is currently seen as a coactivator, reinforcing the transactivating properties of EBNA2 (Nitsche et al. 1997).
EBNA3A, 3B, 3C
These constitute a related family of genes with limited sequence homology. All members of this family seem to act as transcriptional regulators, with repressing and activating properties. Transfection of an expression plasmid carrying EBNA3C silences the Cp promoter (Radkov et al. 1997), but upregulates the LMP1 promoter (Wang et al. 1990). EBNA3B transactivates the CD40 and the vimentin promoters (Silins & Sculley 1994). The EBNA3 proteins can bind to RBP-Jκ and therefore inhibit EBNA2 binding to RBP-Jκ (Robertson et al. 1995; Robertson et al. 1996). The interplay between EBNA2 and the EBNA3 family therefore builds a network that allows precise modulation of the EBV target genes. EBNA3C recruits the human histone deacetylase protein to its targets (Radkov et al. 1999). This can account for the silencing properties of the protein, as histone deacetylation represses transcription nonspecifically. Genetic analyses have shown that EBNA3C is absolutely required for B-cell immortalization, as an EBNA3C negative viral recombinant cannot immortalize B-cells (Tomkinson et al. 1993). The EBNA3A gene has been reported to be important for the initial process of immortalization, whereas EBNA3B seems dispensable (Tomkinson et al. 1993; Kempkes et al. 1995).
LMP1
LMP1 is the best studied EBV latent protein. The LMP1 molecules are integral membrane proteins with 6 transmembrane segments that form multimeric aggregates in the plasma membrane resulting in characteristic patches. Transfection of LMP1 in Burkitt's lymphoma cells lines protects against apoptosis via induction of the Bcl2 and A20 antiapoptotic genes (Henderson et al. 1991; Laherty et al. 1992), and activates the expression of the CD23 cellular gene (Wang et al. 1988). Rodent fibroblastic cells transfected with LMP1 display a transformed phenotype, but LMP1 alone cannot transform human cells (Wang et al. 1985; Baichwal & Sugden 1988).
The introduction of LMP1 in human cells leads to a permanent activation of several signal transduction cascades through its C-terminal intracytoplasmic domains (see below). LMP1 therefore behaves like a constantly activated receptor that is not dependent on extracellular ligands for its activation (Gires et al. 1997). LMP1 shows striking parallels with the CD40 receptor with regard to the signal transduction pathways both molecules utilize (Eliopoulos et al. 1996; Eliopoulos et al. 1997; Floettmann et al. 1998; Kilger et al. 1998). LMP1 expression results in the activation of NFkB and therefore of NFkB cellular targets (Eliopoulos et al. 1996; Eliopoulos et al. 1997; Floettmann et al. 1998; Kilger et al. 1998). The c-jun N-terminal kinase (JNK)/AP-1 pathway is also a major target of LMP1, and this effect is mediated by the CTAR2 domain (Kieser et al. 1997; Eliopoulos & Young 1998). There have been isolated reports of an activation of the JAK-STAT pathway and of the small GTPase cdc42 by LMP1, but their contribution to immortalization is still unknown (Gires et al. 1999; Puls et al. 1999).
Two subdomains of the C-terminal part of LMP1 have been shown to establish protein–protein interactions with cellular proteins (C-terminal activating regions, CTAR1 and 2) (Huen et al. 1995). CTAR1 (amino acids 194–232) has been shown to interact with members of the TRAF (TNF receptor-associated factors) family of proteins (Huen et al. 1995). The binding of TRAF2 to CTAR1 seems to be essential for LMP1 function, but TRAF2 can also indirectly bind to CTAR2 (amino acids 351–386) via TRADD (TNF receptor-associated death domain) that allows interaction between both molecules (Kaye et al. 1996; Izumi & Kieff 1997). The TRAFs are physiologically recruited by membrane receptors like TNF-R, and this binding leads to a rise in NFkB levels.
The genetic analysis of LMP1 has provided important information about the contribution of the different LMP1 subdomains to immortalization. LMP1 was also shown to be necessary for the continued proliferation and maintenance of the immortalized B cell in vitro (Kaye et al. 1993; Zimber-Strobl et al. 1996; Kilger et al. 1998). A further step in the dissection of the function of LMP1 was to generate viral mutants in which only parts of the LMP1 protein were deleted. An EBV which carried a LMP1 mutant allele in which the first 24 aminoacids from the amino-terminal cytoplasmic domain of LMP1 were deleted kept its ability to immortalize B cells (Izumi et al. 1994). In contrast, the first 45 aminoacids of the intracytoplasmic carboxy-terminal part of LMP1 (aa 185–230) were found to be critical for the function of LMP1 whereas deletion of the remaining part of the carboxy-terminus (aa 230–386) could be complemented by cultivating the primary B cells infected with the LMP1 mutant EBV on a feeder cell layer (Kaye et al. 1995). The feeder cells were not absolutely required since a high virus titre could obviate the need for it (Kaye et al. 1999). However, long-term culture of the infected B lymphocytes proved to be more efficient with the wild-type EBV suggesting that the domain between aa 230–386 of LMP1 plays a crucial role for long-term maintenance of B cells in vitro (Kaye et al. 1999). This LMP1 domain is likely to comprise additional subdomains with different functions as a virus carrying an LMP1 gene with a deletion from aa 232–351 does not show any difference to wild-type EBV with regard to B cell immortalization (Izumi et al. 1999). A more refined analysis of the domain comprising the first 45 aminoacids of the intracytoplasmic carboxy-terminal part of LMP1 provided indirect evidence for a critical role of the TRAF binding site. Using overlapping cosmids, Izumi et al. constructed a viral recombinant in which the domain that engages the TRAFs was deleted (aa 185–211) (Izumi & Kieff 1997). After infection of primary B cells with supernatants containing this viral mutant as well as wild-type virus, none out of 412 cell lines contained the mutant virus only, indicating that this particular domain of LMP1 is indispensable for B cell immortalization.
The availability of CD40 -/- mice has allowed a precise evaluation of the respective functions of CD40 and LMP1 (Uchida et al. 1999). In fact, introduction of an LMP1 transgene in CD40 -/- mice can only partly rescue the phenotype observed in these animals, showing that the homology in function is limited (Uchida et al. 1999). The analyis of LMP1 transgenic mice has provided valuable information about the function of this protein and confirmed that this viral protein interacts intimately with the B-cell activation pathways (Uchida et al. 1999). Germinal centre formation is inhibited in these animals, an effect that may be related to the ability of LMP1 and of CD40 to downregulate Bcl6 expression in B-cells. Bcl6 is required for germinal centre formation, as has been shown by the study of Bcl6 knock out mice (Cattoretti et al. 1995). The observation that the LMP1 transgenic CD40 -/- mice can produce high affinity antibodies in the absence of germinal centres is more puzzling. Whether LMP1 can directly interfere with the hypermutation machinery or whether LMP1 unmasks physiological extrafollicular hypermutations is not known.
LMP2A and B
These proteins are encoded by the same viral gene, with the exception of the first exon that is unique to LMP2A. It has been speculated therefore that LMP2B could therefore act as a negative regulator of LMP2A function (Longnecker & Miller 1996). Like LMP1, LMP2A is an integral membrane protein with 12 transmembrane domains that forms patches and interferes with the cell signalling pathways. The intracytoplasmic tail contains an ITAM (immunoreceptor tyrosine based activation motif) domain that can recruit the src and the syk families of kinases (Longnecker & Miller 1996). These protein tyrosine kinases are physiologically involved in signal transduction from the B-cell receptor. Binding of the tyrosine kinases to the phosphorylated LMP2A ITAM therefore suppresses the activating signal normally generated by the B-cell receptor (Longnecker & Miller 1996). Akata, an EBV positive Burkitt's lymphoma cell line, can be induced to produce virions by addition of anti-Ig. This suggests that crosslinking of the B-cell receptor leads to reactivation of the viral lytic cycle. The ability of LMP2A to repress activation signals mediated by the BCR can explain a repressive effect on lytic replication (Longnecker & Miller 1996).
Mice carrying an LMP2A transgene show an increase in bone marrow and peripheral B lymphocytes that did not undergo functional Ig rearrangements (Caldwell et al. 1998). It is tempting to speculate that the ability of LMP2A to generate signals usually transmitted by functional Ig receptors allowed survival of these B-cells. It has been reported that both LMP2A and LMP2B do not contribute to the immortalization process of mature resting B cells in vitro (Kim & Yates 1993; Longnecker & Miller 1993) although a different report exists (Brielmeier et al. 1996) and functional data argue for an essential role in the infection of differentiating B cells (Caldwell et al. 1998; Caldwell et al. 2000). Even more detailed mutations of LMP2A in the context of the complete EBV genome support a role for LMP2A in the interference with immunoglobulin signal transduction (Fruehling et al. 1996; Fruehling & Longnecker 1997; Fruehling et al. 1998; Swart et al. 1999). Recently, it has also been shown that interaction of LMP2A with extracellular matrix proteins may trigger intracellular signalling in epithelial cells involving Csk, a negative regulator of Src kinase (Scholle et al. 1999).
Primary and persistent EBV infection in vivo
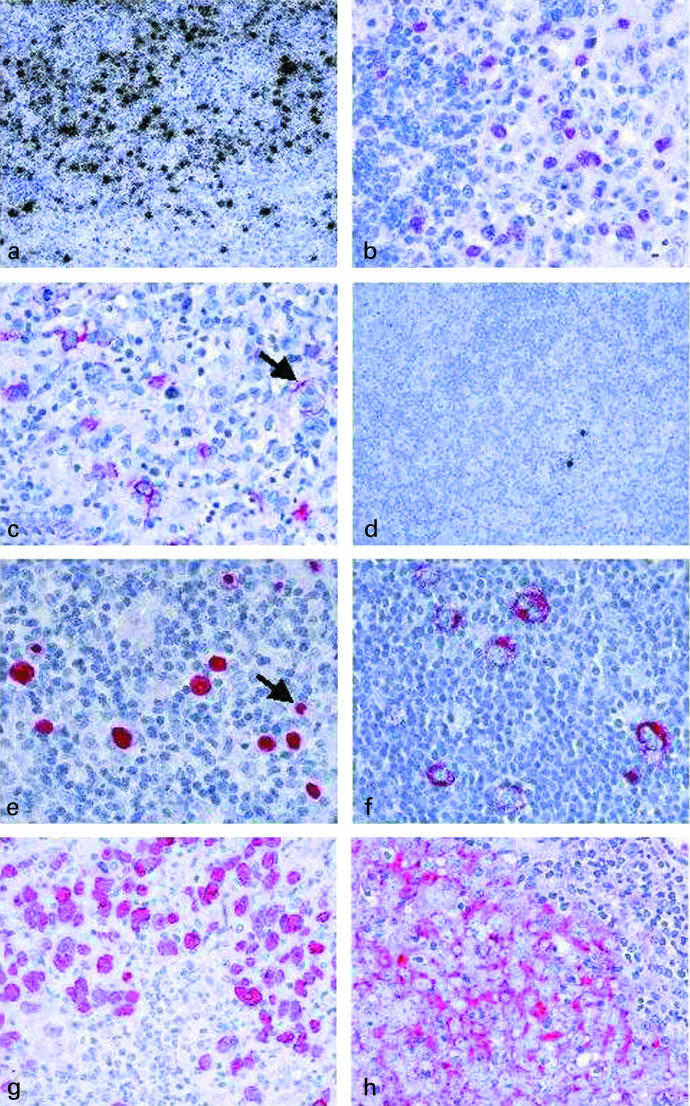
Primary EBV infection occurs usually in childhood and then is asymptomatic in most cases (Evans 1972). In most industrialized countries, primary infection is delayed into adolescence or early adulthood and then may cause a self-limiting lymphoproliferative disorder, infectious mononucleosis (IM) (Evans 1972). Most of what is known of primary EBV infection is derived from studies of IM patients and is based on the assumption that IM is representative also of asymptomatic primary infection. IM is characterized by a proliferation of EBV-infected, activated B-lymphoid blasts in the paracortex of tonsillar lymphoid tissue (Figure 1a) (Niedobitek et al. 1989). In IM, a latency III pattern of viral gene products is detectable in RNA extracts (Figure 1b, c) (Tierney et al. 1994). However, analysis at the single cell level reveals some heterogeneity with cells displaying latencies I, II, and III and cells with an EBNA2+/LMP1− phenotype being detectable (Niedobitek et al. 1997). A proportion of EBV-infected B-cells in IM shows evidence of plasmacytoid differentiation, and these cells may support virus replication (Niedobitek et al. 1997). EBV-infected cells of other lineages, e.g. epithelial cells or T-cells, are rarely if ever detectable in IM (Karajannis et al. 1997; Niedobitek et al. 1997; Niedobitek et al. 2000).
Figure 1.
In situ hybridization with 35S-labelled EBER-specific probes reveals numerous EBV-infected cells in a tonsil from a patient with acute infectious mononucleosis (a, black grains). Immunohistochemical analysis of a tonsil from an infectious mononucleosis patient reveals expression of EBNA2 (b, red labelling) and of LMP1 (c, red labelling; note a LMP1-positive Reed-Sternberg-like cell, arrow) in many cells. Only rare EBV-positive cells are detectable in a tonsil from a chronic virus carrier (d, black grains). EBER-specific in situ hybridization of a case of Hodgkin's disease using nonradioactive probes reveals the presence of the virus in Hodgkin and Reed-Sternberg cells (e, red labelling; note isolated EBV-positive small reactive lymphocytes, arrow). EBV-positive Hodgkin and Reed-Sternberg cells also express LMP1 (f, red labelling). EBV is detected in the neoplastic cells of an undifferentiated nasopharyngeal carcinoma using nonradioactive EBER-specific in situ hybridization (g, red labelling). In the same case, the tumour cells also express LMP1 (h, red labelling).
The proliferation of EBV-infected B-cells in IM elicits a vigorous cytotoxic T-lymphocyte (CTL) response which is directed against latent and lytic viral proteins (Rickinson & Kieff 1996; Steven et al. 1997). CTLs specific for all EBNAs and LMPs with the exception of EBNA1 have been identified (IARC 1997). Recent work has also identified a consistent response of CD4+ T-cells to EBNA1 presented by dendritic cells (Münz et al. 2000). This T-cell response allows the transition from primary infection into asymptomatic life-long persistent infection. In persistent EBV infection, a small number of EBV-carrying B-cells is detectable in peripheral blood and lymphoid as well as other tissues (Figure 1d) (Niedobitek et al. 1992; Hubscher et al. 1994; Khan et al. 1996). The number of these cells varies between individuals but appears to be constant within any given person (Khan et al. 1996). Present evidence suggests that EBV persists in the B-cell system but the exact mechanisms mediating viral persistence are uncertain (Niedobitek & Young 1994; Thorley-Lawson et al. 1996). Thorley-Lawson's group has presented evidence implicating memory B-cells as the major site of EBV persistence and it is possible that EBV utilizes germinal centre reactions to gain access to the memory B-cell pool (Niedobitek et al. 1992; Thorley-Lawson et al. 1996; Babcock et al. 1998). Alternatively, EBV may directly infect memory B-cells as demonstrated for infectious mononucleosis (Kurth et al. 2000). Persistent EBV infection of B-cells is characterized by a restricted pattern of viral latent gene expression, but exactly which genes are expressed is a matter of debate. Using RT-PCR studies, EBNA1 and LMP2A transcripts have been demonstrated in peripheral blood lymphocytes (Tierney et al. 1994). Studying a series of tonsils, Babcock reported the detection of a latency III pattern in naive B-cells and a latency II pattern in germinal centre and memory B-cells (Babcock et al. 2000). Peripheral blood memory B-cells showed an even more restricted expression of latent genes with only LMP2 but remarkably no EBNA1 detectable (Babcock et al. 2000). There are, however, two concerns regarding this study. Firstly, the tonsils studied were apparently poorly characterized. There was no information available regarding the history of the patients or the histopathological changes observed in these specimens. Secondly, the authors used a sensitive RT-PCR method and thus, the relevance of the transcripts detected remains uncertain. Using immunohistochemical techniques, we have detected only rare EBNA2-positive and no LMP1 positive cells in tonsils from chronic virus carriers (Meru et al. in press.).
EBV can clearly infect other cell lineages as illustrated by the detection of the virus in a variety of human tumours derived from, e.g. T-cells, epithelial cells and even smooth muscle cells. Thus, it is possible that, in addition to B-cells, cells of other lineages contribute to EBV persistence and replication. Alternatively, infection of such cell types might be regarded as accidental. In particular, the relevance of oropharyngeal epithelial cells for primary and persistent EBV infection requires re-evaluation, in particular because oral hairy leukoplakia, an epithelial lesion of the tongue, remains the best example for EBV replication in vivo (Niedobitek et al. 1991a).
EBV-associated tumours: a brief survey
EBV-associated lymphomas
Burkitt's lymphoma (BL)
BL is endemic in equatorial regions of Africa and Papua-Guinea where it is a common childhood cancer (Burkitt et al. 1965). In Western countries, BL occurs as a sporadic tumour. BL is a B-cell non-Hodgkin lymphoma (NHL) with an origin from germinal centre B-cells (see below) (Gregory et al. 1987). All BL cases characteristically carry a chromosomal translocation, t(8; 14), t(8; 2), or t(8; 22), placing the c-myc oncogene under the control of an immunoglobulin gene (Klein 1979). This results in an inappropriate overexpression of c-myc. By contrast, endemic and sporadic BL differ with respect to EBV-association: virtually all endemic BLs carry the virus while EBV is detectable only in up to 30% of sporadic cases (Zur Hausen et al. 1970; Hummel et al. 1995). In EBV-associated BL, the viral genome is present as a monoclonal episome and expression of the viral latent genes is restricted to the EBERs and EBNA1 (latency I) (Neri et al. 1991; Raab-Traub & Flynn 1986; Rowe et al. 1987; Niedobitek et al. 1995).
Hodgkin's disease (HD)
HD is characterized histologically by the presence of Hodgkin and Reed-Sternberg (HRS) cells admixed with abundant lymphocytes and other reactive cells. Four histological types of HD have been defined based on the morphology of the HRS cells and the composition of the background infiltrate: lymphocyte predominant (HDlp), nodular sclerosing (HDns), mixed cellularity (HDmc), and lymphocyte depleted (HDld) Hodgkin's disease (Harris et al. 1994). HDlp has been recognized as a separate entity representing a B-cell lymphoma derived from germinal centre B-cells and behaving clinically differently from the other three forms of HD which collectively are called classical HD (Harris et al. 1994). Most cases of classical HD are also derived from B-cells (see below) although a small proportion of T-cell derived HD have been described (Kanzler et al. 1996; Müschen et al. 2000).
In western countries, EBV is detectable in the HRS cells of 20–50% of HD cases while in developing countries up to 100% of cases may be associated with the virus (Ambinder et al. 1993; Herbst et al. 1993). Childhood HD appears to be more frequently associated with EBV than HD occurring in young adults (Armstrong et al. 1998). Moreover, the virus is commonly detected in HDmc cases, while HDns and in particular HDlp are less frequently EBV-associated (Herbst et al. 1993). Analysis of the EBV terminal repeats has revealed monoclonal viral episomes in HD (Anagnostopoulos et al. 1989). In all EBV-associated HD cases, LMP1 and LMP2A are expressed in addition to the EBERs and EBNA1 (Figure 1e,f; latency II) (Herbst et al. 1991; Deacon et al. 1993; Grässer et al. 1994; Niedobitek et al. 1997).
Immunosuppression-related lymphoproliferations
Immunosuppressed transplant patients are at an increased risk of developing lymphoproliferative disorders (post-transplant lymphoproliferative disorders, PTLD). The vast majority of these cases are B-cell-derived and EBV-associated. PTLD comprise a spectrum of diseases ranging from polyclonal polymorphic lesions to frankly malignant monomorphic and monoclonal lymphomas which are morphologically indistinguishable from lymphomas occurring in nonimmunosuppressed individuals (Craig et al. 1993). Polymorphic PTLDs tend to display a type III latency although there is usually a degree of variability at the single cell level (Oudejans et al. 1995). In monoclonal lymphomas, the expression of viral latent genes is generally more restricted, conforming to latency patterns I or II (Niedobitek et al. 1997a). Patients with acquired immunodeficiency syndrome (AIDS) also have an increased risk of developing malignant lymphomas. It has been estimated that at least 1–4% of AIDS patients suffer from this disease (IARC 1997). A large proportion of these lymphomas are primary CNS lymphomas which account for 20–60% of cases (IARC 1997). Morphologically, AIDS-related primary CNS lymphomas represent diffuse large B-cell lymphomas. The vast majority of these cases are EBV-positive with variable expression of LMP1 (MacMahon et al. 1991). Systemic AIDS-related NHL fall into two groups: BL and diffuse large B-cell NHL (Hamilton-Dutoit et al. 1991). The latter are mostly EBV-positive and display latencies II or III (Hamilton-Dutoit et al. 1993). While most of these lymphomas are monoclonal, rare polyclonal cases have been reported suggesting a similar pathogenesis as for PTLD (Gaidano & Dalla-Favera 1995). AIDS-related BL, by contrast, appear to be less frequently EBV-associated and then usually express a type I latency. Thus, these lymphomas may be pathogenetically similar to sporadic BL (Hamilton-Dutoit et al. 1993; IARC 1997).
Other B-cell NHL
In spite of its B-cell tropism, EBV is only infrequently detected in conventional B-cell lymphomas. Moreover, if present, the virus is often detectable only in a proportion of neoplastic cells (Hummel et al. 1995).
T-cell NHL
Since the first reported detection of EBV in T-cell NHL (Jones et al. 1988), it has become clear that EBV is detectable in T-cell NHL more frequently than in B-cell NHL (Pallesen et al. 1994; IARC 1997). Sinonasal angiocentric T-cell NHL (so-called lethal midline granuloma) is the T-cell NHL showing the most consistent association with the virus (Harabuchi et al. 1990). In these tumours the virus is detectable in virtually all tumour cells, the viral episomes are usually clonal and there is a type II pattern of viral latency (IARC 1997). EBV is also frequently detectable in other T-cell NHL. However, in most of these, the virus is present only in variable proportions of tumour cells (Korbjuhn et al. 1993; Pallesen et al. 1994). Moreover, a proliferation of EBV-infected B-cells in lymph nodes affected by EBV-negative T-cell NHL has been reported (Hojo et al. 1995; Ho et al. 1998; Niedobitek et al. 2000a). The significance of these observations for the pathogenesis of T-cell NHL remains uncertain.
EBV-associated carcinomas
Nasopharyngeal carcinoma
NPCs are endemic in South-east Asia, northern Africa, and some other regions, while they occur rarely in western Europe and North America (Niedobitek, 2000). Non-keratinizing undifferentiated NPC, usually associated with a prominent admixture of tumour-infiltrating lymphocytes, represents the most common histological type while conventional squamous cell NPC are less common (Niedobitek et al. 1996). Independently of the geographical origin of the patients, undifferentiated NPCs are always EBV-positive and harbour monoclonal viral episomes (Figure 1g) (Raab-Traub & Flynn 1986; Niedobitek et al. 1996). Expression of LMP1 can be detected at the mRNA or protein level in most undifferentiated NPCs (Figure 1h) (IARC 1997). LMP2A expression in NPC cells is detectable at the transcriptional level but the protein is usually undetectable by immunohistochemical techniques (Niedobitek, 2000). Expression of another EBV latent gene, BARF1, with possible transforming functions has also been detected in NPCs (Decaussin et al. 2000). The association of undifferentiated NPC with EBV has been well documented. By contrast, an association of the virus with squamous cell NPC is controversial (IARC 1997). This issue has been resolved by a study demonstrating that EBV is present in all squamous cell NPCs from an endemic area, while the virus is detectable only in about 30% of cases from regions with a low NPC incidence (Nicholls et al. 1997). This suggests that other factors may be able to replace EBV in the pathogenesis of squamous cell NPC. Smoking and infection with human papillomaviruses are likely to be of importance in this respect (Hording et al. 1994; Vaughan et al. 1996).
Other carcinomas
Carcinomas showing morphological features similar to undifferentiated NPCs, so-called lymphoepithelial carcinomas, can arise at other sites and these tumours have been extensively studied for an association with EBV. These studies have identified three different groups. Lymphoepithelial carcinomas of the stomach are EBV-associated in approximately 80% of cases regardless of the origin of the patients (Osato & Imai 1996). Lymphoepithelial carcinomas of the salivary glands, the lungs and possibly of the thymus are frequently associated with EBV infection in areas where NPC is endemic whereas similar tumours arising, e.g. in Caucasian patients, appear to be EBV-negative (IARC 1997). Finally, there is a large group of EBV-negative lymphoepithelial carcinomas, including carcinomas of the cervix uteri, the urinary bladder, the skin, and the larynx (IARC 1997). It has to be noted, however, that this statement relies largely on single case reports and studies of small series. Systematic comparative studies are lacking, probably owing to the scarcity of these tumours.
The detection of EBV in gastric lymphoepithelial carcinomas has prompted several groups to study conventional gastric adenocarcinomas, revealing the presence of EBV in 2–16% of cases world-wide (Osato & Imai 1996; IARC 1997). Studies of EBV latent gene expression in gastric carcinomas (lymphoepithelial and adenocarcinomas) have revealed the expression of EBNA1 in the absence of EBNA2 and LMP1 (Osato & Imai 1996). Expression of LMP2A and of BARF1 has been detected at the transcriptional level (Osato & Imai 1996; zur Hausen et al. 2000). An association with EBV has also been reported for a proportion of breast carcinomas (Labrecque et al. 1995). This finding has been unexpected since studies of medullary carcinoma of the breast, which shares some morphological features with undifferentiated NPC have consistently failed to identify the virus (Niedobitek et al. 1991; Kumar & Kumar 1994; Lespagnard et al. 1995). Thus, this observation requires confirmation. Finally, EBV has been detected in approximately 35% of hepatocellular carcinomas using Southern blot hybridization (Sugawara et al. 1999). Interestingly, analysis of the terminal repeats of the EBV genome revealed monoclonal viral genomes while immunostaining for the EBNA complex suggested that the virus was present only in a proportion of the tumour cells (Sugawara et al. 1999). The most surprising finding of this study was the absence of detectable expression of the EBERs suggesting a new type of latency (Sugawara et al. 1999).
Other tumours
Unexpectedly, EBV infection has also been detected in leiomyosarcomas, i.e. smooth muscle cell-derived sarcomas, in immunosuppressed patients. Several authors have demonstrated monoclonal or biclonal viral genomes in these tumours. Analysis of viral gene expression has revealed expression of the EBERs and EBNA2 in the absence of LMP1 (Lee et al. 1995; McClain et al. 1995). Clonal EBV infection has also been detected in inflammatory pseudotumours derived from CD21-positive follicular dendritic reticulum cells (Selves et al. 1996).
EBV-associated tumours: specific problems
Role of the immune system
The notion of an antitumour immunosurveillance system is controversial (Prehn 1974). However, there is no doubt regarding the existence of an immunological control of tumour viruses (Prehn 1974). In the case of EBV, this is illustrated by the development of an EBV-specific T-cell immunity in the course of primary infection which permits the establishment of an asymptomatic persistent infection (Rickinson & Kieff 1996). The importance of EBV specific CTLs has been further underlined by the observation of EBV-associated lymphoproliferative disorders in immunosuppressed transplant patients (PTLD) (IARC 1997). PTLD comprise a spectrum of diseases ranging from polyclonal lymphoproliferations to monoclonal monomorphic PTLDs indistinguishable from malignant lymphomas arising in immunocompentent individuals (Craig et al. 1993). The latter notably include not only NHL but also HD cases (Garnier et al. 1996). This diversity is accompanied by diverse patterns of EBV latent gene expression ranging from latency III in many polyclonal PTLDs to latencies II or I in monoclonal monomorphic PTLDs (Oudejans et al. 1995; Niedobitek et al. 1997a). A large body of evidence suggests that the range of lesions encountered in transplant patients may be indicative of a process. According to this model, a relaxation of the EBV-specific immunity allows a polyclonal outgrowth of EBV-infected cells with a latency III pattern of EBV gene expression similar to that seen in infectious mononucleosis. Through the acquisition of additional genetic alterations, e.g. c-myc translocations or p53 mutations, this may eventually progress to frank malignant lymphoma (Delecluse et al. 1995; Knowles et al. 1995). This idea is supported, e.g. by the observation that polyclonal polymorphic PTLD may show progression to monoclonal monomorphic malignant lymphoma, whereas the reverse does not occur (Wu et al. 1996). However, whether those cases which initially present as malignant lymphomas were preceded by a period of subclinical polyclonal proliferation, or represent true de novo development of malignant lymphoma, remains uncertain. The importance of immunosuppression in the pathogenesis of PTLD is also underlined by the frequent regression of PTLD lesions in response to a reduction or cessation of immunosuppressive therapy and by recent successful efforts to prevent and treat PTLD using EBV-specific CTLs generated in vitro (Starzl et al. 1984; Rooney et al. 1995; Rooney et al. 1998).
Apart from the lymphomas arising in immunocompromised individuals, most EBV-associated tumours develop in patients without systemic immunodeficiencies. Specifically, the immune systems of these patients appear to be quite capable of containing EBV infection as illustrated by the absence of lymphoproliferations characteristic for immunodeficient individuals. This raises the question as to why EBV-associated tumours, such as BL, HD or NPC, are not rejected by the immune system. In the case of BL, an important feature appears to be the absence of those viral antigens which provide CTL target epitopes. In particular, EBNA1, the only known latent protein consistently expressed in BL cells, does not elicit a response by CD8+ CTLs. The reason for this appears to be a Gly-Ala repeat sequence within the EBNA1 protein which blocks ubiquitin-dependent intracellular processing and presentation within the context of MHC class I (Levitskaya et al. 1995; Levitskaya et al. 1997). Thus, these tumour cells become invisible for EBV-specific CTL. The role of EBNA1-specific CD4+ T-cells in the pathogenesis of BL remains to be clarified (Münz et al. 2000). Using immunohistochemistry, isolated cells expressing LMP1 or EBNA2 are detectable in some cases of endemic BL (Niedobitek et al. 1995). This illustrates that the phenotypic shift from a type I to a type III latency seen in BL cells in vitro may also occur in vivo. That these cells do not gain a proliferative advantage over the EBNA1+/EBNA2−/LMP1− tumour cells may well be due to an intact EBV-specific CTL response which eliminates such cells. Interestingly, it has been reported recently that EBNA1 induces a response of CD4+ T-cells which predominatly secrete Th2 cytokines. Since many of these cytokines are known growth factors for EBV-infected B-cells this may contribute to the growth of EBV-associated BL (Steigerwald-Mullen et al. 2000).
By contrast, the tumour cells of HD and NPC express LMP1 and LMP2A, antigens known to elicit CTL reponses (Herbst et al. 1991; Niedobitek et al. 1997). Moreover, HD and NPC are characterized by the presence of numerous lymphoid and other inflammatory cells admixed with the tumour cells which, in the case of HD, vastly outnumber the neoplastic cells (Herbst et al. 1993; Niedobitek 2000). In both neoplasms, the tumour cells frequently express CD40, CD70, CD80, and CD95 while the corresponding receptors/ligands (CD40L, CD27, CD28, and CD95L, respectively) are detectable in admixed lymphocytes (Agathanggelou et al. 1995; Poppema et al. 1998; Abdulkarim et al. 2000). This indicates that there may be functional interactions between the neoplastic cells and the tumour-infiltrating lymphocytes in HD and NPC. Moreover, expression of CD40, CD70, and CD80 in nonimmunogenic tumour cells can induce antitumour CTL responses in animal experiments (Nieland et al. 1998). In vitro, the tumour cells of NPC and HD are capable of antigen-processing and are recognized and lysed by HLA class I-restricted virus-specific CTLs (Sing et al. 1997; Khanna et al. 1998; Lee et al. 1998). Moreover, NPCs are susceptible to Fas-mediated lysis although this may be inhibited by CD40 signalling (Sbih-Lammali et al. 1999). Thus, these observations raise the question as to why HD and NPC can grow in vivo in the face of an otherwise functioning EBV-specific immune reponse. Finding an answer to this question is particularly important in view of recent attempts to develop immunotherapeutic approaches to the treatment of HD and NPC which are based on EBV-specific CTLs (Ambinder et al. 1996).
The T-cells surrounding the HRS cells are mainly activated CD4+ cells with a Th2 phenotype (Poppema et al. 1998). Several studies have indicated that HRS cells, through the production of cytokines and chemokines, may contribute to and modulate the accumulation of lymphocytes in affected tissues (Poppema et al. 1998). HRS cells express the thymus and activation regulated chemokine (TARC) which selectively recruits CCR4-positive Th2 cells (Imai et al. 1999; van den Berg et al. 1999). Furthermore, it appears that EBV-specific CTL are absent from lymph nodes affected by EBV-positive but not those affected by EBV-negative HD (Frisan et al. 1995). This effect may be partially mediated by interleukin (IL)-10 which is strongly expressed in EBV-positive HRS cells and to a lesser extent in EBV-negative HRS cells (Herbst et al. 1996; Bejarano & Masucci 1998). Moreover, IL-6 expression has been observed preferentially in EBV-positive HRS cells (Herbst et al. 1997). Thus, although HRS cells are effective antigen presenting cells susceptible to CTL lysis, they seem to be able to modulate actively their microenvironment resulting in an ineffective immune response.
In comparison to HD, the interactions between tumour cells and tumour-infiltrating lymphocytes in NPC are less well understood. Like HRS cells, NPC cells appear to be able to secrete cytokines. Variable expression of IL1α, IL1β, TNFα, and IL-8 has been reported in some NPC cell lines, but a consistent pattern is yet to emerge (Busson et al. 1987; Mahe et al. 1992). Moreover, there is only very limited, and in parts conflicting, information available regarding the cytokine expression patterns of NPCs in vivo. Huang et al. have demonstrated IL-1 expression in NPC tumour cells with a number of other cytokines expressed in the lymphoid stroma (Huang et al. 1999). IL-10 expression has been reported and has even been suggested to be a marker of poor prognosis in NPCs (Yao et al. 1997; Fujieda et al. 1999). However, studying a large series of NPCs by in situ hybridization, we could not confirm this observation (Beck et al. 2001). Using in situ hybridization, IL-10-specific transcripts were only detectable in some tumour-infiltrating lymphocytes but not in the tumour cell population. IL-6 and IL-8 expression was detectable only in exceptional cases and then only in a small fraction of the tumour cells raising questions as to the significance of this finding (Beck et al. 2001). TARC expression was undetectable in NPCs (Beck et al. 2001). Elevated serum levels of TGFβ have been reported in NPC patients but the cellular source of this cytokine is presently uncertain (Xu et al. 1999). Yet another mechanism for immune escape of NPC cells has been suggested by the demonstration that the LMP1 gene in NPCs may be mutated, rendering it less immunogenic in animal experiments (Trivedi et al. 1994; Hu et al. 2000). No such data are available for HD.
In summary, albeit of different lineage, the tumour cells of HD and NPC share some interesting features. In both diseases, the neoplastic cells bear all the hallmarks necessary for antigen presentation and are susceptible to EBV-specific CTL killing in vitro. They share striking phenotypic similarities which allow them to communicate with the tumour-infiltrating lymphocytes. Nevertheless, in both tumours an effective immunological control of the tumour cells is not apparent. In HD, HRS cells are capable of secreting an array of cytokines and chemokines which may modulate the immunological response in the microenvironment of the neoplastic cells and this may allow the tumour cells to escape immune control. In NPC, similar mechanisms may be at work, although consistent patterns of cytokine and chemokine expression have not yet been identified.
Another scenario is encountered in rare cases of high-grade B-cell lymphoma occurring in association with chronic inflammatory processes, e.g. pyothorax or osteomyelitis. In such cases, one may encounter latency forms II or even III suggesting a defect of EBV-specific immunity (Copie-Bergman et al. 1997; Fukayama et al. 1993; Sasajima et al. 1993; Martin et al. 1994). However, the affected patients do not display systemic immunodeficiencies and the lymphomas develop at the site of inflammation. The latter observations suggest that in the context of chronic suppurative inflammation, a local breakdown of EBV-specific immunity may occur. Thus, a loss of immunological control of EBV infection at different levels – systemic, local or in the immediate microenvironment of the tumour cells – may contribute to the development of EBV-associated malignancies.
The timing of EBV infection in the pathogenesis of EBV-associated tumours
In most EBV-associated tumours, the virus is detectable in virtually all tumour cells. Moreover, the viral genomes are usually present as monoclonal episomes, i.e. they are derived from a single infection event (Raab-Traub & Flynn 1986). This has been shown, e.g. for BL, HD, and NPC (Raab-Traub & Flynn 1986; Anagnostopoulos et al. 1989). These findings are taken to imply that EBV infection takes place before expansion of the neoplastic clone. Thus, it appears that EBV infection in these tumours occurs early enough to play a part in the carcinogenic process. Nevertheless, in most virus-associated tumours the question as to when exactly in the carcinogenic process EBV infection occurs remains controversial.
EBV infection is detectable in virtually all cases of undifferentiated NPC regardless of the geographical origin of the patients (Niedobitek, 2000). Thus, EBV infection appears to be a rate-limiting step in the pathogenesis of NPC. The viral genomes are present in all tumour cells and are of clonal origin suggesting that infection occurs early in the neoplastic process (Raab-Traub & Flynn 1986; Niedobitek, 2000). This idea is confirmed by the detection of EBV in in situ NPC lesions, which are presumed precursor lesions of invasive NPC (Pathmanathan et al. 1995). Moreover, clonal progression from mild dysplasia to invasive carcinoma has been reported (Jiang & Yao 1996). However, EBV has not been detected in normal nasopharyngeal epithelial cells in a high-risk population nor in normal nasopharyngeal mucosa adjacent to EBV-positive nasopharyngeal carcinoma (Sam et al. 1993). This would seem to suggest that EBV infection of epithelial cells may not be the first step in NPC pathogenesis. This notion is supported by two observations. Chromosome 3p deletions are detectable in 80–100% of nasopharyngeal carcinoma cases and thus appear to be almost as common as EBV infection in these tumours (Chan et al. 2000). Interestingly, 3p deletions are also equally common in EBV-positive dysplatic lesions of the nasopharynx and in EBV-negative normal nasopharyngeal mucosa in individuals from a high-risk population (Chan et al. 2000). This would seem to suggest that 3p deletions precede EBV infection in the pathogenesis of NPC. Moreover, stable EBV infection of CD21-transfected epithelial cells has been shown to require an undifferentiated phenotype (Knox et al. 1996). Thus, it appears that EBV infection is not the first step in the pathogenesis of NPC but occurs before the initiation of invasive growth.
Malignant lymphomas lack well defined preneoplastic stages and thus determination of the timing of EBV infection has to rely on indirect evidence. Endemic BLs are virtually always EBV-positive, while the virus is detectable only in up to 30% of sporadic BLs (IARC 1997). However, all cases harbour a translocation juxtaposing the c-myc gene to one of the immunoglobulin gene loci. In endemic BL, this translocation is reminiscent of a V(D)J recombination event, while it resembles a switch recombination in sporadic BL (Haluska et al. 1986; Vanasse et al. 1999). Early studies have suggested that c-myc translocations occur in EBV-infected B-cells and that a malaria-induced B-cell proliferation increases the chance of such transloactions occurring (Klein 1979). The assumption that c-myc translocations, at least in endemic BL, are the result of an accident during immunoglobulin gene rearrangements in immature B-cells on the other hand has raised the possibility that c-myc translocation may precede EBV infection (Lenoir & Bornkamm 1987). Molecular studies have indicated that EBV infection is a late event in endemic BL occurring after c-myc translocation (Gutierrez et al. 1997). V(D)J recombination of immunoglobulin and T-cell receptor genes physiologically occurs in immature B-and T-cells and is initiated by two recombination activating genes, RAG1 and RAG2 (Oettinger et al. 1990). Interestingly, expression of the RAGs has been reported in EBV-positive endemic but not in EBV-negative sporadic BL cell lines (Kuhn-Hallek et al. 1995; Srinivas & Sixbey 1995). Moreover, RAG expression became detectable in sporadic BL cell lines following EBV infection, an effect apparently mediated by EBNA1 and suppressed by LMP1 (Kuhn-Hallek et al. 1995; Srinivas & Sixbey 1995). These findings suggest that EBV infection in the context of a latency I may be able to induce RAG expression and thus offer a possible link between EBV infection and the occurrence of c-myc translocations in endemic BL. However, using in situ hybridization, we have been unable to detect RAG expression in EBV-associated endemic BLs (Meru et al. 2001). It appears therefore that re-induction of RAG expression by EBV is not an important mechanism for the pathogenesis of EBV-associated endemic BL and in particular is not relevant for the development of c-myc translocations. Taken together, the balance of this evidence suggests that c-myc translocation precedes EBV infection in the pathogenesis of EBV-associated endemic BL. However, that this issue is by no means settled is illustrated by reports suggesting that genetic alterations, including oncogene translocations, may be the result of the somatic hypermutation process in germinal centres (Goossens et al. 1998). All BL cases analysed thus far display somatically mutated V region genes indicating that they have participated in germinal centre reactions. Moreover, two BL cases have been described in which the VκJκ region and the Cκ locus both showed evidence of somatic hypermutation although being separated by c-myc translocations (Goossens et al. 1998). Thus it is possible that the c-myc translocation in BL may occur as a by-product during expansion of EBV-positive B-cells in germinal centre reactions (Niedobitek et al. 1992; Goossens et al. 1998).
In most HD cases the neoplastic cells are derived from B-cells as demonstrated by the detection of clonal immunoglobulin gene rearrangements in the HRS cells, and by sequence analysis of the immunoglobulin V region genes revealing evidence of somatic hypermutation (Kanzler et al. 1996). Moreover, in some cases, stop codons have been detected in these genes rendering them nonfunctional (Kanzler et al. 1996). Thus, it has been argued that HD is a neoplasm derived from ‘crippled’ germinal centre B-cells which have been rescued from apoptotic cell death by a transforming event (Kanzler et al. 1996). Possibly, survival signals in these cells may be delivered by the CD40-like function of LMP1 and/or the B-cell receptor-like signalling activity of LMP2A (Caldwell et al. 1998; Kilger et al. 1998). This scenario would require EBV infection occurring prior to the ‘crippling’ event. Support for this idea has come from reports describing the expression of LMP1 in germinal centre B-cells (Araujo et al. 1999; Babcock et al. 2000).
The observation that in a proportion of T-cell lymphomas EBV is present only in a small proportion of tumour cells has added yet another level of complexity to this discussion (Pallesen et al. 1994). It has to be mentioned in this context, however, that at least some cases of T-cell NHL are characterized by an LCL-like background proliferation of EBV-infected B-cells while the neoplastic T-cells are EBV-negative (Hojo et al. 1995; Ho et al. 1998; Niedobitek et al. 2000a). Nevertheless, in some studies of T-cell NHL, the presence of the virus in a proportion of the neoplastic cells has been confirmed by double labelling experiments (Korbjuhn et al. 1993). Moreover, similar observations have been reported for some cases of B-cell chronic lymphocytic leukaemia and other B-cell lymphomas (Hummel et al. 1995). Thus, EBV infection of a proportion of neoplastic cells appears to occur in some lymphomas while, interestingly, it has not yet been convincingly shown for carcinomas, HD and other virus-associated tumours. This phenomenon is generally believed to be the result of a secondary infection of the neoplastic cells by EBV (Niedobitek 1995). This explanation is supported by a case report demonstrating EBV infection of a metastatic lymph node deposit of an EBV-negative primary intestinal B-cell lymphoma (Ilyas et al. 1995). In this scenario, EBV infection of an already established malignancy may confer a growth advantage to the infected cells leading eventually to the replacement of the EBV-negative population. Thus, it is conceivable that superinfection of a primarily EBV-negative tumour with EBV may give rise to an EBV-positive tumour with monoclonal viral genomes present in all tumour cells. Alternatively, loss of the viral genome from neoplastic cells has to be considered. This explanation is supported by a case report of an EBV-positive HD case relapsing as EBV-negative HD (Delecluse et al. 1997). In addition, viral genomes may be lost from BL and NPC cells cultured in vitro (Lin et al. 1993; Shimizu et al. 1994; Cheung et al. 1999; Ruf et al. 1999). However, at least in the case of BL this appears to coincide with a loss of tumorigenicity (Shimizu et al. 1994). Interestingly, reinfection with EBV, but not transfection with EBNA1, has been demonstrated to reestablish the malignant phenotype (Ruf et al. 1999). Thus, the evidence indicating that loss of viral genomes may occur in EBV-associated tumours is mainly derived from in vitro studies. Moreover, it has been demonstrated only for those tumours in which partial infection of the neoplastic cells in vivo has not been reported as yet.
EBV – a human carcinogen?
EBV is a ubiquitous herpesvirus which establishes a life-long persistent infection in over 90% of adults world-wide. This persistent infection is usually asymptomatic. It has been known for almost 40 years that EBV is associated with two human malignancies, i.e. Burkitt's lymphoma and nasopharyngeal carcinoma (Epstein et al. 1964; Wolf et al. 1973). In addition, EBV has been identified in a large number of other tumours from different cell lineages (IARC 1997). Thus, EBV is one of the more common potential human carcinogens. This makes an assessment of the role of EBV in the pathogenesis of these diverse cancers important. Based on its association with Burkitt's lymphoma, Hodgkin's disease, lymphoproliferations in immunosuppressed individuals, sinonasal angiocentric T-cell lymphoma, and nasopharyngeal carcinoma, EBV has been classified as a group I carcinogen by the IARC (IARC 1997). There is now ample evidence for a transforming function of EBV in vitro. Most importantly, LMP1 has been identified as a viral oncogene with the ability to transform rodent fibroblasts and with functions similar to those of a TNF receptor family member (Wang et al. 1985; Baichwal & Sugden 1988; Gires et al. 1997). Nevertheless, LMP1 alone is not sufficient to transform B-cells in vitro but requires the cooperation of several other viral latent proteins. The effects of EBV infection of B-cells in many ways parallel those of antigen activation and amount to immortalization. While this is an important step in carcinogenesis, it is by no means sufficient.
The role of the virus in the pathogenesis of human neoplasms in vivo remains debated and much of the evidence supporting such a role is circumstantial. In most virus-associated tumours, the EBV genomes appear to be of monoclonal origin (Raab-Traub & Flynn 1986). This would seem to point to an infection before expansion of the malignant cell clone. However, studies of various tumours now suggest that while occurring early, EBV infection may not be the first event in the carcinogenic process. Thus, in some tumours, EBV is detectable only in a proportion of the neoplastic cells. In others, such as BL and NPC, careful genetic studies have demonstrated that characteristic chromosomal alterations occur prior to EBV infection. Whilst this does not exclude a contribution of EBV to the pathogenesis of these tumours it is obvious that further studies are required to define the role of EBV. The expression of viral antigens, notably of LMP1, in many virus-associated tumours has been taken as further evidence to support a role for EBV in the pathogenesis of human tumours. However, the patterns of viral latent protein expression in human tumours are variable, suggesting that the contribution of EBV to different carcinogenic processes may also vary. Moreover, the majority of viral proteins which are required for the immortalization of B-cells in vitro are not expressed in EBV-associated tumours. Also, LMP1 and other viral proteins are clearly detectable in situations not associated with malignancy, notably infectious mononucleosis. The strongest support for an oncogenic role of EBV has come from the observation of EBV-associated lymphoproliferations in immunosuppressed patients (IARC 1997). However, although these lymphoproliferations are frequently fatal, they often do not meet strict criteria of neoplasia. Thus, rather than supporting an oncogenic role of EBV, this observation highlights the importance of the immune system in controlling EBV infection.
In summary, the picture which has emerged after almost 40 years of EBV research is complex. While a role for EBV in the pathogenesis of certain human tumours remains likely, the precise contribution of the virus remains to be defined.
References
- Abbot SD, Rowe M, Cadwallader K, et al. Epstein-Barr virus nuclear antigen 2 (EBNA2) induces expression of the virus-coded latent membrane protein (LMP) J. Virol. 1990;64:2126–2134. doi: 10.1128/jvi.64.5.2126-2134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulkarim B, Sabri S, Deutsch E, et al. Radiation-induced expression of functional Fas ligand in EBV-positive human nasopharyngeal carcinoma cells. Int. J. Cancer. 2000;86:229–237. doi: 10.1002/(sici)1097-0215(20000415)86:2<229::aid-ijc12>3.0.co;2-1. 10.1002/(sici)1097-0215(20000415)86:2<229::aid-ijc12>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- Agathanggelou A, Niedobitek G, Chen R, Nicholls J, Yin W, Young LS. Expression of immune regulatory molecules in Epstein-Barr virus-associated nasopharyngeal carcinomas with prominent lymphoid stroma. Evidence for a functional interaction between epithelial tumor cells and infiltrating lymphoid cells. Am. J. Pathol. 1995;147:1152–1160. [PMC free article] [PubMed] [Google Scholar]
- Aiyar A, Tyree C, Sugden B. The plasmid replicon of EBV consits of multiple cis-acting elements that facilitate DNA synthesis by the cell and a viral maintenance element. EMBO J. 1998;17:6394–6403. doi: 10.1093/emboj/17.21.6394. 10.1093/emboj/17.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambinder R, Browning PJ, Lorenzana I, et al. Epstein-Barr virus and childhood Hodgkin's disease in Honduras and the United States. Blood. 1993;81:462–467. [PubMed] [Google Scholar]
- Ambinder RF, Robertson KD, Moore SM, Yang J. Epstein-Barr virus as a therapeutic target in Hodgkin's disease and nasopharyngeal carcinoma. Sem. Cancer Biol. 1996;7:217–226. doi: 10.1006/scbi.1996.0029. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos I, Herbst H, Niedobitek G, Stein H. Demonstration of monoclonal EBV genomes in Hodgkin's disease and Ki-1 positive anaplastic large cell lymphoma by combined Southern blot and in situ hybridization. Blood. 1989;74:810–816. [PubMed] [Google Scholar]
- Araujo I, Foss H-D, Hummel M, et al. Frequent expansion of Epstein-Barr virus (EBV) infected cells in germinal centres of tonsils from an area with a high incidence of EBV-associated lymphoma. J. Pathol. 1999;187:326–330. doi: 10.1002/(SICI)1096-9896(199902)187:3<326::AID-PATH242>3.0.CO;2-N. 10.1002/(sici)1096-9896(199902)187:3<326::aid-path242>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Armstrong AA, Alexander FE, Cartwright R, et al. Epstein-Barr virus and Hodgkin's disease: further evidence for the three disease hypothesis. Leukemia. 1998;12:1272–1276. doi: 10.1038/sj.leu.2401097. [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- Babcock GJ, Hochberg D, Thorley-Lawson DA. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- Baer R, Bankier AT, Biggin MD, et al. DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Baichwal VR, Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988;2:461–467. [PubMed] [Google Scholar]
- Beck A, Päzolt D, Grabenbauer GG, et al. Expression of cytokine and chemokine genes in Epstein-Barr virus-associated nasopharyngeal carcinoma and Hodgkin's disease. J. Pathol. 2001:867. doi: 10.1002/path.867. Published online, April 2001, DO1 10.1002/path. [DOI] [PubMed] [Google Scholar]
- Bejarano MT, Masucci MG. Interleukin-10 abrogates the inhibition of Epstein-Barr virus-induced B-cell transformation by memory T-cell reponses. Blood. 1998;92:4256–4262. [PubMed] [Google Scholar]
- Birkenbach M, Tong X, Bradbury LE, Tedder TF, Kieff E. Characterization of an Epstein-Barr virus receptor on human epithelial cells. J. Exp. Med. 1992;176:1405–1414. doi: 10.1084/jem.176.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Hutt-Fletcher L. Epstein-Barr virus recombinant lacking expression of glykoprotein gp150 infects B cell normally but is enhanced for infection of epithelial cells. J. Virol. 1998;72:7577–7582. doi: 10.1128/jvi.72.9.7577-7582.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmeier M, Mautner J, Laux G, Hammerschmidt W. The latent membrane protein 2 gene of Epstein-Barr virus is important for efficient B cell immortalization. J. Gen. Virol. 1996;77:2807–2818. doi: 10.1099/0022-1317-77-11-2807. [DOI] [PubMed] [Google Scholar]
- Burkitt D, Hutt MSR, Wright DH. The African lymphoma. Preliminary observations on response to therapy. Cancer. 1965;18:399–410. doi: 10.1002/1097-0142(196504)18:4<399::aid-cncr2820180402>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Busson P, Braham K, Ganem G, et al. EBV containing epithelial cells from nasopharyngeal carcinoma produce IL-1 alpha. Proc. Natl. Acad. Sci. USA. 1987;84:6262–6266. doi: 10.1073/pnas.84.17.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RG, Brown RC, Longnecker R. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J. Virol. 2000;74:1101–1113. doi: 10.1128/jvi.74.3.1101-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Chang CC, Cechova K, et al. BCL-6 protein is expressed in germinal center B cells. Blood. 1995;86:45–53. [PubMed] [Google Scholar]
- Chan ASC, To KF, Lo KW, et al. High frequency of chromosome 3p deletion in histologically normal nasopharyngeal epithelia from Southern Chinese. Cancer Res. 2000;60:5365–5370. [PubMed] [Google Scholar]
- Cheung ST, Huang DP, Hui AB. Nasopharyngeal carcinoma cell line (C666–1) consistently harbouring Epstein-Barr virus. Int. J. Cancer. 1999;83:121–126. doi: 10.1002/(sici)1097-0215(19990924)83:1<121::aid-ijc21>3.0.co;2-f. 10.1002/(sici)1097-0215(19990924)83:1<121::aid-ijc21>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Kieff E. An Epstein-Barr virus nuclear protein 2 domain essential for transformation is a direct transcriptional activator. J. Virol. 1991;65:5880–5885. doi: 10.1128/jvi.65.11.5880-5885.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Picchio GR, Mosier DE. Epstein-Barr virus nuclear protein 2 is a critical determinant for tumor growth in SCID mice and for transformation in vitro. J. Virol. 1992;66:7555–7559. doi: 10.1128/jvi.66.12.7555-7559.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Wang F, Kieff E. Epstein-Barr virus nuclear protein 2 mutations define essential domains for transformation and transactivation. J. Virol. 1991;65:2545–2554. doi: 10.1128/jvi.65.5.2545-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Wang F, Mannick J, Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc. Natl. Acad. Sci. USA. 1989;86:9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copie-Bergman C, Niedobitek G, Mangham DC, et al. Epstein-Barr virus in B-cell lymphomas associated with chronic suppurative inflammation. J. Pathol. 1997;183:287–292. doi: 10.1002/(SICI)1096-9896(199711)183:3<287::AID-PATH932>3.0.CO;2-Q. 10.1002/(sici)1096-9896(199711)183:3<287::aid-path932>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Craig FE, Gulley ML, Banks PM. Posttransplantation lymphoproliferative disorders. Am. J. Clin. Pathol. 1993;99:265–276. doi: 10.1093/ajcp/99.3.265. [DOI] [PubMed] [Google Scholar]
- Deacon EM, Pallesen G, Niedobitek G, et al. Epstein-Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells. J. Exp. Med. 1993;177:339–349. doi: 10.1084/jem.177.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaussin G, Sbih-Lammali F, de Turenne-Tessier M, Bouguermouh A, Ooka T. Expression of BARF1 gene excoded by Epstein-Barr virus in nasopharyngeal carcinoma. Cancer Res. 2000;60:5584–5588. [PubMed] [Google Scholar]
- Delecluse HJ, Hammerschmidt W. The genetic approach to Epstein-Barr virus: from basic virology to gene therapy. J. Clin. Pathol. Mol. Pathol. 2000;53:270–279. doi: 10.1136/mp.53.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delecluse HJ, Rouault JP, Ffrench M, Dureau G, Magaud JP, Berger F. Post-transplant lymphoproliferative disorders with genetic abnormalities commonly found in malignant tumours. Br. J. Haematol. 1995;89:90–97. doi: 10.1111/j.1365-2141.1995.tb08905.x. [DOI] [PubMed] [Google Scholar]
- Delecluse H-J, Marafioti T, Hummel M, Dallenbach F, Anagnostopoulos I, Stein H. Disappearance of the Epstein-Barr virus in a relapse of Hodgkin's disease. J. Pathol. 1997;182:475–479. doi: 10.1002/(SICI)1096-9896(199708)182:4<475::AID-PATH878>3.0.CO;2-6. 10.1002/(sici)1096-9896(199708)182:4<475::aid-path878>3.3.co;2-y. [DOI] [PubMed] [Google Scholar]
- Eliopoulos A, Dawson CW, Mosialos G, et al. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- Eliopoulos A, Young LS. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- Eliopoulos AG, Stack M, Dawson CW, et al. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-kB pathway involving TNF receptor-associated factors. Oncogene. 1997;14:2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;i:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Evans AS. Clinical syndromes associated with EB virus infection. Ann. Intern. Med. 1972;18:77–93. [PubMed] [Google Scholar]
- Fåhraeus R, Jansson A, Ricksten A, Sjöblom A, Rymo L. Epstein-Barr virus-encoded nuclear antigen 2 activates the viral latent membrane protein promotor by modulating the activity of a negative regulatory element. Proc. Natl. Acad. Sci. USA. 1990;87:7390–7394. doi: 10.1073/pnas.87.19.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc. Natl. Acad. Sci. USA. 1984;81:4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floettmann JE, Eliopoulos AG, Jones M, Young LS, Rowe M. Epstein-Barr virus latent membrane protein-1 (LMP1) signalling is distinct from CD40 and involves physical cooperation of its two C-terminus functional regions. Oncogene. 1998;17:2383–2392. doi: 10.1038/sj.onc.1202144. [DOI] [PubMed] [Google Scholar]
- Frisan T, Sjoberg J, Dolcetti R, et al. Local suppression of Epstein-Barr virus (EBV) -specific cytotoxicity in biopsies of EBV-positive Hodgkin's disease. Blood. 1995;86:1493–1501. [PubMed] [Google Scholar]
- Fruehling S, Lee SK, Herrold R, et al. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J. Virol. 1996;70:6216–6226. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- Fruehling S, Swart R, Dolwick KM, Kremmer E, Longnecker R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J. Virol. 1998;72:7796–7806. doi: 10.1128/jvi.72.10.7796-7806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujieda S, Lee K, Sunaga H, et al. Staining of interleukin-10 predicts clinical outcome in patients with nasopharyngeal carcinoma. Cancer. 1999;85:1439–1445. 10.1002/(sici)1097-0142(19990401)85:7<1439::aid-cncr3>3.3.co;2-y. [PubMed] [Google Scholar]
- Fukayama M, Ibuka T, Hayashi Y, Ooba T, Koike M, Mizutani S. Epstein-Barr virus in pyothorax-associated pleural lymphoma. Am. J. Pathol. 1993;143:1–7. [PMC free article] [PubMed] [Google Scholar]
- Gaidano G, Dalla-Favera R. Molecular pathogenesis of AIDS-related lymphomas. Adv. Cancer Res. 1995;67:113–153. doi: 10.1016/s0065-230x(08)60712-5. [DOI] [PubMed] [Google Scholar]
- Garnier J-L, Lebranchu Y, Dantal J, et al. Hodgkin's disease after transplantation. Transplantation. 1996;61:71–76. doi: 10.1097/00007890-199601150-00015. [DOI] [PubMed] [Google Scholar]
- Gires O, Kohlhuber F, Kilger E, et al. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 1999;18:3064–3073. doi: 10.1093/emboj/18.11.3064. 10.1093/emboj/18.11.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gires O, Zimber-Strobl U, Gonnella R, et al. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S. Notch takes a short cut. Nature. 1995;377:288–289. doi: 10.1038/377288a0. [DOI] [PubMed] [Google Scholar]
- Goossens T, Klein U, Küppers R. Frequent occurrence of deletions and duplications during somatic hypermutation: implications for oncogene translocations and heavy chain disease. Proc. Natl. Acad. Sci. USA. 1998;95:2463–2468. doi: 10.1073/pnas.95.5.2463. 10.1073/pnas.95.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grässer FA, Murray PG, Kremmer E, et al. Monoclonal antibodies directed against the Epstein-Barr virus-encoded nuclear antigen 1 (EBNA1): immunohistologic detection of EBNA1 in the malignant cells of Hodgkin's disease. Blood. 1994;84:3792–3798. [PubMed] [Google Scholar]
- Gregory CD, Tursz T, Edwards CF, et al. Identification of a subset of normal B cells with a Burkitt's lymphoma (BL) -like phenotype. J. Immunol. 1987;139:313–318. [PubMed] [Google Scholar]
- Gutierrez MI, Bhatia K, Cherney B, et al. Intraclonal molecular heterogeneity suggests a hierarchy of pathogenetic events in Burkitt's lymphoma. Ann. Oncol. 1997;8:987–994. doi: 10.1023/a:1008265304712. [DOI] [PubMed] [Google Scholar]
- Haluska FG, Finver S, Tsujimoto Y, Croce CM. The t (8; 14) chromosomal translocation occurring during B-cell malignancies results from mistakes in V-D-J joining. Nature. 1986;324:158–161. doi: 10.1038/324158a0. [DOI] [PubMed] [Google Scholar]
- Hamilton-Dutoit SJ, Pallesen G, Franzmann MB, et al. AIDS-related lymphoma. Histopathology, immunophenotype, and association with Epstein-Barr virus as demonstrated by in situ nucleic acid hybridization. Am. J. Pathol. 1991;138:149–163. [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Dutoit SJ, Rea D, Raphael M, et al. Epstein-Barr virus-latent gene expression and tumor cell phenotype in acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Correlation of lymphoma phenotype with three distinct patterns of viral latency. Am. J. Pathol. 1993;143:1072–1085. [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Harabuchi Y, Yamanaka N, Kataura A, et al. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. 1990;335:128–130. doi: 10.1016/0140-6736(90)90002-m. [DOI] [PubMed] [Google Scholar]
- Harada S, Yalamanchili R, Kieff E. Residues 231–280 of the Epstein-Barr virus nuclear protein 2 are not essential for primary B-lymphocyte growth transformation. J. Virol. 1998;72:9948–9954. doi: 10.1128/jvi.72.12.9948-9954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NL, Jaffe E, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- Hedrick JA, Watry D, Speiseries C, O'Donnell P, Lambris JD, Tsoukas CD. Interaction between Epstein-Barr virus and a T cell line (HSB-2) via a receptor phenotypicaly distinct from complement receptor type 2. Eur. J. Immunol. 1992;22:1123–1131. doi: 10.1002/eji.1830220504. [DOI] [PubMed] [Google Scholar]
- Henderson S, Rowe M, Gregory C, et al. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Henkel T, Ling PD, Hayward SD, Peterson MG. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein Jk. Science. 1994;265:92–95. doi: 10.1126/science.8016657. [DOI] [PubMed] [Google Scholar]
- Herbst H, Dallenbach F, Hummel M, et al. Epstein-Barr virus latent membrane protein expression in Hodgkin and Reed-Sternberg cells. Proc. Natl. Acad. Sci. USA. 1991;88:4766–4770. doi: 10.1073/pnas.88.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst H, Foss H-D, Samol J, et al. Frequent expression of interleukin-10 by Epstein-Barr virus-harboring tumor cells of Hodgkin's disease. Blood. 1996;87(87):2918–2929. [PubMed] [Google Scholar]
- Herbst H, Samol J, Foss HD, Raff T, Niedobitek G. Modulation of interleukin-6 expression in Hodgkin and Reed-Sternberg cells by Epstein-Barr virus. J. Pathol. 1997;182:299–306. doi: 10.1002/(SICI)1096-9896(199707)182:3<299::AID-PATH856>3.0.CO;2-8. 10.1002/(sici)1096-9896(199707)182:3<299::aid-path856>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Herbst H, Stein H, Niedobitek G. Epstein-Barr virus in CD30+ malignant lymphomas. Crit. Rev. Oncogenesis. 1993;4:191–239. [PubMed] [Google Scholar]
- Ho JWY, Ho FCS, Chan ACL, et al. Frequent detection of Epstein-Barr virus-infected B cells in peripheral T-cell lymphomas. J. Pathol. 1998;185:79–85. doi: 10.1002/(SICI)1096-9896(199805)185:1<79::AID-PATH52>3.0.CO;2-3. 10.1002/(sici)1096-9896(199805)185:1<79::aid-path52>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hofelmayr H, Strobl LJ, Stein C, et al. Activated mouse Notch1 transactivates Epstein-Barr virus nuclear antigen 2-regulated viral promoters. J. Virol. 1999;73:2770–2780. doi: 10.1128/jvi.73.4.2770-2780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo I, Takanashi M, Hirai K, Mori S. Increased number of Epstein-Barr virus latently infected B-cells in T-cell non-Hodgkin's lymphoma. Arch. Virol. 1995;140:1419–1426. doi: 10.1007/BF01322668. [DOI] [PubMed] [Google Scholar]
- Hording U, Winther Nielsen H, Daugaard S, Albeck H. Human papillomavirus types 11 and 16 detected in nasopharyngeal carcinomas by the polymerase chain reaction. Laryngoscope. 1994;104:99–102. doi: 10.1288/00005537-199401000-00018. [DOI] [PubMed] [Google Scholar]
- Howe JG, Steitz JA. Localisation of Epstein-Barr virus-encoded small RNAs by in situ hybridisation. Proc. Natl. Acad. Sci. USA. 1986;83:9006–9010. doi: 10.1073/pnas.83.23.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Troyanovsky B, Zhang X, Trivedi P, Ernberg I, Klein G. Differences in the immunogenicity of latent membrane protein 1 (LMP1) encoded by Epstein-Barr virus genomes derived from LMP1-positive and -negative nasopharyngeal carcinoma. Cancer Res. 2000;60:5589–5593. [PubMed] [Google Scholar]
- Huang YT, Sheen TS, Chen CL, et al. Profile of cytokine expression in nasopharyngeal carcinomas: a distinct expression of interleukin 1 in tumor and CD4+ T cells. Cancer Res. 1999;59:1599–1605. [PubMed] [Google Scholar]
- Hubscher SG, Williams A, Davison SM, Young LS, Niedobitek G. Epstein-Barr virus in inflammatory diseases of the liver and liver allografts: an in situ hybridization study. Hepatology. 1994;20:899–907. doi: 10.1002/hep.1840200419. [DOI] [PubMed] [Google Scholar]
- Huen DS, Henderson SA, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- Hummel M, Anagnostopoulos I, Korbjuhn P, Stein H. Epstein-Barr virus in B-cell non-Hodgkin's lymphomas: unexpected infection patterns and different infection incidence in low- and high-grade types. J. Pathol. 1995;175:263–271. doi: 10.1002/path.1711750303. [DOI] [PubMed] [Google Scholar]
- IARC. Lyon, France: World Health Organization; 1997. Epstein-Barr Virus and Kaposi's Sarcoma Herpesvirus/Human Herpesvirus 8. [Google Scholar]
- Ilyas M, Niedobitek G, Agathanggelou A, et al. Non-Hodgkin's lymphoma, coeliac disease, and Epstein-Barr virus: a study of 13 cases pf enteropathy-associated T- and B-cell lymphoma. J. Pathol. 1995;177:115–122. doi: 10.1002/path.1711770203. [DOI] [PubMed] [Google Scholar]
- Imai S, Nishikawa J, Takada K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 1998;72:4371–4378. doi: 10.1128/jvi.72.5.4371-4378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Nagira M, Takagi S, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int. Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- Izumi KM, Kaye KM, Kieff ED. Epstein-Barr virus recombinant molecular genetic analysis of the LMP1 amino-terminal cytoplasmic domain reveals a probable structural role, with no component essential for primary B-lymphocyte growth transformation. J. Virol. 1994;68:4369–4376. doi: 10.1128/jvi.68.7.4369-4376.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi KM, Kieff ED. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kB. Proc. Natl. Acad. Sci. USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi KM, McFarland EC, Riley EA, Rizzo D, Chen Y, Kieff E. The residues between the two transformation effector sites of Epstein-Barr virus latent membrane protein 1 are not critical for B-lymphocyte growth. J. Virol. 1999;73:9908–9916. doi: 10.1128/jvi.73.12.9908-9916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz A, Oezel M, Kurzeder C, et al. Infectious Epstein-Barr virus lacking major glykoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 2000;74:10142–10152. doi: 10.1128/jvi.74.21.10142-10152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Yao KT. The clonal progression in the neoplastic process of nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 1996;221:122–128. doi: 10.1006/bbrc.1996.0556. 10.1006/bbrc.1996.0556. [DOI] [PubMed] [Google Scholar]
- Jones JF, Shurin S, Abramowsky C, et al. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N. Engl. J. Med. 1988;318:733–741. doi: 10.1056/NEJM198803243181203. [DOI] [PubMed] [Google Scholar]
- Kaiseries C, Laux G, Eick D, Jochner N, Bornkamm GW, Kempkes B. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J. Virol. 1999;73:4481–4484. doi: 10.1128/jvi.73.5.4481-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzler H, Küppers R, Hansmann M-L, Rajewski K. Hodgkin and Reed-Sternberg cells in Hodgkin's disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J. Exp. Med. 1996;184:1495–1505. doi: 10.1084/jem.184.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karajannis MA, Hummel M, Anagnostopoulos I, Stein H. Strict lymphotropism of Epstein-Barr virus during acute infectious mononucleosis in nonimmunocompromised individuals. Blood. 1997;89:2856–2862. [PubMed] [Google Scholar]
- Kaye KM, Devergne O, Harada JN, et al. Tumor necrosis factor associated factor 2 is a mediator of NF-kB activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc. Natl. Acad. Sci. USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye KM, Izumi KM, Li H, et al. An Epstein-Barr virus that expresses only the first 231 LMP1 amino acids efficiently initiates primary B-lymphocyte growth transformation. J. Virol. 1999;73:10525–10530. doi: 10.1128/jvi.73.12.10525-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye KM, Izumi KM, Mosialos G, Kieff E. The Epstein-Barr virus LMP1 cytoplasmic carboxy terminus is essential for B-lymphocyte transformation; fibroblast cocultivation complements a critical function within the terminal 155 residues. J. Virol. 1995;69:675–683. doi: 10.1128/jvi.69.2.675-683.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher CA, Dreyfus DH, Jones JF, Gelfand EW. EBV infection of T cells: potential role in malignant transformation. Sem. Cancer Biol. 1996;7:197–207. doi: 10.1006/scbi.1996.0027. [DOI] [PubMed] [Google Scholar]
- Kempkes B, Pich D, Zeidler R, Sugden B, Hammerschmidt W. Immortalization of human B lymphocytes by a plasmid containing 71 kilobase pairs of Epstein-Barr virus DNA. J. Virol. 1995;69:231–238. doi: 10.1128/jvi.69.1.231-238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan G, Miyashita EM, Yang B, Babcock GJ, Thorley-Lawson DA. Is EBV persistence in vivo a model for B cell homeostasis? Immunity. 1996;5:173–179. doi: 10.1016/s1074-7613(00)80493-8. [DOI] [PubMed] [Google Scholar]
- Khanna R, Busson P, Burrows SR, et al. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma: evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 1998;58:310–314. [PubMed] [Google Scholar]
- Kieseries A, Kilger E, Gires O, et al. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilger E, Kieseries A, Baumann M, Hammerschmidt W. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 1998;17:1700–1709. doi: 10.1093/emboj/17.6.1700. 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim O-J, Yates JL. Mutants of Epstein-Barr virus with a selective marker disrupting the TP gene transform B cells and replicate normally in culture. J. Virol. 1993;67:7634–7640. doi: 10.1128/jvi.67.12.7634-7640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G. Lymphoma development in mice and humans: diversity of initiation is followed by convergent cytogenetic evolution. Proc. Natl. Acad. Sci. USA. 1979;76:2442–2446. doi: 10.1073/pnas.76.5.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles DM, Cesarman E, Chadburn A, et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood. 1995;85:552–565. [PubMed] [Google Scholar]
- Knox PG, Li Q-X, Rickinson AB, Young LS. In vitro production of stable Epstein-Barr virus-positive epithelial cell clones which resemble the virus: cell interaction observed in nasopharyngeal carcinoma. Virology. 1996;215:40–50. doi: 10.1006/viro.1996.0005. 10.1006/viro.1996.0005. [DOI] [PubMed] [Google Scholar]
- Knutson JC. The level of c-frg RNA is increased by EBNA-2, an Epstein-Barr virus gene required for B-cell immortalization. J. Virol. 1990;64:2530–2536. doi: 10.1128/jvi.64.6.2530-2536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Zhang XK, Imai S, Sugiura M, Usui N, Osato T. Infection of the HTLV-I-harbouring T-lymphoblastoid line MT-2 by Epstein-Barr virus. Virology. 1992;188:859–863. doi: 10.1016/0042-6822(92)90542-w. [DOI] [PubMed] [Google Scholar]
- Korbjuhn P, Anagnostopoulos I, Hummel M, et al. Frequent latent Epstein-Barr virus infection of neoplastic T cells and bystander B cells in human immunodeficiency virus-negative European peripheral pleomorphic T-cell lymphomas. Blood. 1993;82:217–223. [PubMed] [Google Scholar]
- Kuhn-Hallek I, Sage DR, Stein L, Groelle H, Fingeroth JD. Expression of recombination activating genes (RAG-1 and RAG-2) in Epstein-Barr virus-bearing B cells. Blood. 1995;85:1289–1299. [PubMed] [Google Scholar]
- Kumar S, Kumar D. Lymphoepithelioma-like carcinoma of the breast. Mod. Pathol. 1994;7:129–131. [PubMed] [Google Scholar]
- Kurth J, Spieker T, Wustrow J, et al. EBV-infected B cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity. 2000;13:485–495. doi: 10.1016/s1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- Labrecque LG, Barnes DM, Fentiman IS, Griffin BE. Epstein-Barr virus in epithelial cell tumors: a breast cancer study. Cancer Res. 1995;55:39–45. [PubMed] [Google Scholar]
- Laherty CD, Hu HM, Opipari AW, Wang F, Dixit VM. Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor κB. J. Biol. Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- Lee ES, Locker J, Nalesnik M, et al. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N. Engl. J. Med. 1995;332:19–25. doi: 10.1056/NEJM199501053320104. [DOI] [PubMed] [Google Scholar]
- Lee MA, Diamond ME, Yates JL. Genetic evidence that EBNA-1 is needed for efficient, stable latent infection by Epstein-Barr virus. J. Virol. 1999;73:2974–2982. doi: 10.1128/jvi.73.4.2974-2982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, Constandinou CM, Thomas WA, et al. Antigen presenting phenotype of Hodgkin Reed-Sternberg cells: analysis of the HLA class I processing pathway and effects of interleukin-10 on Epstein-Barr virus-specific cytotoxic T-cell recognition. Blood. 1998;92:1020–1030. [PubMed] [Google Scholar]
- Lenoir GM, Bornkamm GW. Burkitt's lymphoma, a human cancer model for the study of the multistep development of cancer: proposal for a new scenario. Adv. Viral. Oncol. 1987;7:173–206. [Google Scholar]
- Lespagnard L, Cochaux P, Larsimont D, et al. Absence of Epstein-Barr virus in medullary carcinoma of the breast as demonstrated by immunophenotyping, in situ hybridization and polymerase chain reaction. Am. J. Clin. Pathol. 1995;103:449–452. doi: 10.1093/ajcp/103.4.449. [DOI] [PubMed] [Google Scholar]
- Levitskaya J, Coram M, Levitsky V, et al. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen-1. Proc. Natl. Acad. Sci. USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QX, Young LS, Niedobitek G, et al. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992;356:347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- Lin CT, Chan WY, Chen W, et al. Characterization of seven newly established nasopharyngeal carcinoma cell lines. Lab. Invest. 1993;68:716–727. [PubMed] [Google Scholar]
- Lindhout E, Lakeman A, Mevissen MLCM, de Groot C. Functionally active Epstein-Barr virus-transformed follicular dendritic cell-like cell lines. J. Exp. Med. 1994;179:1173–1184. doi: 10.1084/jem.179.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R, Miller CL. Regulation of Epstein-Barr virus latency by latent membrane protein 2. Trends Microbiol. 1996;4:38–42. doi: 10.1016/0966-842x(96)81504-6. [DOI] [PubMed] [Google Scholar]
- LonGecker R, Miller CL, Tomkinson B, Miao XQ, Kieff E. Deletion of DNA encoding the first five transmembrane domains of Epstein-Barr virus latent membrane proteins 2A and 2B. J. Virol. 1993;67:5068–5074. doi: 10.1128/jvi.67.8.5068-5074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon EME, Glass JD, Hayward SD, et al. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338:969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- Mahe Y, Hirose K, Clausse B, Chouaib S, Tursz T, Mariame B. Heterogeneity among human nasopharyngeal carcinoma cell lines for inflammatory cytokines mRNA expression levels. Biochem. Biophys. Res. Commun. 1992;187:121–126. doi: 10.1016/s0006-291x(05)81467-6. [DOI] [PubMed] [Google Scholar]
- Mannick JB, Cohen JI, Birkenbach M, Marchini A, Kieff E. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J. Virol. 1991;65:6826–6837. doi: 10.1128/jvi.65.12.6826-6837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal V, Dehee A, Chikhi-Brachet R, et al. Mapping EBNA-1 domains involved in binding to metaphase chromosomes. J. Virol. 1999;73:4385–4393. doi: 10.1128/jvi.73.5.4385-4392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Capron F, Liguory-Brunaud M-D, et al. Epstein-Barr virus-associated primary malignant lymphomas of the pleural cavity occurring in longstanding pleural chronic inflammation. Hum. Pathol. 1994;25:1314–1318. doi: 10.1016/0046-8177(94)90091-4. [DOI] [PubMed] [Google Scholar]
- McClain KL, Leach CT, Jenson HB, et al. Association of Epstein-Barr virus with leiomyosarcomas in young people with AIDS. N. Engl. J. Med. 1995;332:12–18. doi: 10.1056/NEJM199501053320103. [DOI] [PubMed] [Google Scholar]
- Meru N, Jung A, Lisner R, Niedobitek G. Expression of the recombination activating genes (Rag1 and Rag2) is not detectable in Epstein-Barr virus-associated human lymphomas. Int. J. Cancer. 2001;92:75–78. [PubMed] [Google Scholar]
- Meru N, Davison S, Whitehead L, et al. Epstein-Barr virus infection in paediatric liver transplant recipients: detection of the virus in post-transplant tonsillectomy specimens. J. Clin. Pathol. Mol. Pathol. 2001 doi: 10.1136/mp.54.4.264. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- miller G, robinson J, heston L, lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc. Natl. Acad. Sci. USA. 1974;71:4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz C, Bickham KL, Subklewe M, et al. Human CD4+ T lymphocytes consitently respond to the latentn Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müschen M, Rajewsky K, Bräuninger A, et al. Rare occurrence of classical Hodgkin's disease as a T cell lymphoma. J. Exp. Med. 2000;191:387–394. doi: 10.1084/jem.191.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow GR, Wolfert R, McNaughton MW, Cooper NR. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2) J. Virol. 1985;55:347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A, Barriga F, Inghirami G, et al. Epstein-Barr Virus infection precedes clonal expansion in Burkitts and acquired immunodeficiency syndrome-associated lymphoma. Blood. 1991;77:1092–1095. [PubMed] [Google Scholar]
- Nicholls JM, Agathanggelou A, Fung K, Xianggou Z, Niedobitek G. The association of squamous cell carcinomas of the nasopharynx with Epstein-Barr virus shows geographic variation reminiscent of Burkitt's lymphoma. J. Pathol. 1997;183:164–168. doi: 10.1002/(SICI)1096-9896(199710)183:2<164::AID-PATH919>3.0.CO;2-J. 10.1002/(sici)1096-9896(199710)183:2<164::aid-path919>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Niedobitek G. Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. J. Clin. Pathol. Mol. Pathol. 2000;53:248–254. doi: 10.1136/mp.53.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedobitek G. Patterns of Epstein-Barr virus infection in non-Hodgkin's lymphomas. J. Pathol. 1995;175:259–261. doi: 10.1002/path.1711750302. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Agathanggelou A, Herbst H, Whitehead L, Wright DH, Young LS. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J. Pathol. 1997;182:151–159. doi: 10.1002/(SICI)1096-9896(199706)182:2<151::AID-PATH824>3.0.CO;2-3. 10.1002/(sici)1096-9896(199706)182:2<151::aid-path824>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Agathanggelou A, Nicholls JM. Epstein-Barr virus infection and the pathogenesis of nasopharyngeal carcinoma: viral gene expression, tumour cell phenotype, and the role of the lymphoid stroma. Sem. Cancer Biol. 1996;7:165–174. doi: 10.1006/scbi.1996.0023. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Agathanggelou A, Rowe M, et al. Heterogeneous expression of Epstein-Barr virus latent proteins in endemic Burkitt's lymphoma. Blood. 1995;86:659–665. [PubMed] [Google Scholar]
- Niedobitek G, Agathanggelou A, Steven N, Young LS. Epstein-Barr virus (EBV) in infectious mononucleosis: detection of the virus in tonsillar B lymphocytes but not in desquamated oropharyngeal epithelial cells. J. Clin. Pathol. Mol. Pathol. 2000;53:37–42. doi: 10.1136/mp.53.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedobitek G, Baumann I, Brabletz T, et al. Hodgkin's disease and peripheral T-cell lymphoma: composite lymphoma with evidence of Epstein-Barr virus infection. J. Pathol. 2000;191:394–399. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH651>3.0.CO;2-0. 10.1002/1096-9896(2000)9999:9999<::aid-path651>3.3.co;2-s. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Hamilton-Dutoit S, Herbst H, et al. Identification of Epstein-Barr virus-infected cells in tonsils of acute infectious mononucleosis by in situ hybridization. Hum Pathol. 1989;20:796–799. doi: 10.1016/0046-8177(89)90075-0. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Hansmann ML, Herbst H, et al. Epstein-Barr virus and carcinomas: undifferentiated carcinomas but not squamous cell carcinomas of the nasopharynx are regularly associated with the virus. J. Pathol. 1991;165:17–24. doi: 10.1002/path.1711650105. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Herbst H, Young LS, et al. Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood. 1992;79:2520–2526. [PubMed] [Google Scholar]
- Niedobitek G, Kremmer E, Herbst H, et al. Immunohistochemical detection of the Epstein-Barr virus-encoded latent membrane protein 2A (LMP2A) in Hodgkin's disease and infectious mononucleosis. Blood. 1997;90:1664–1672. [PubMed] [Google Scholar]
- Niedobitek G, Mutimer DJ, Williams A, et al. Epstein-Barr virus infection and malignant lymphomas in liver transplant recipients. Int. J. Cancer. 1997;73:514–520. doi: 10.1002/(sici)1097-0215(19971114)73:4<514::aid-ijc10>3.0.co;2-9. 10.1002/(sici)1097-0215(19971114)73:4<514::aid-ijc10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Young LS. Epstein-Barr virus persistence and virus-associated tumours. Lancet. 1994;343:333–335. doi: 10.1016/s0140-6736(94)91167-3. [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Young LS, Lau R, et al. Epstein-Barr virus infection in oral hairy leukoplakia: virus replication in the absence of a detectable latent phase. J. Gen. Virol. 1991;72:3035–3046. doi: 10.1099/0022-1317-72-12-3035. [DOI] [PubMed] [Google Scholar]
- Nieland JD, Graus YF, Dortmans YE, Kremers BL, Kruisbeek AM. CD40 and CD70 co-stimulate a potent in vivo antitumor T cell response. J. Immunother. 1998;21:225–236. doi: 10.1097/00002371-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Nitsche F, Bell A, Rickinson AB. Epstein-Barr virus leader protein enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J. Virol. 1997;71:6619–6628. doi: 10.1128/jvi.71.9.6619-6628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V (D) J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Osato T, Imai S. Epstein-Barr virus and gastric carcinoma. Sem. Cancer Biol. 1996;7:175–182. doi: 10.1006/scbi.1996.0024. [DOI] [PubMed] [Google Scholar]
- Oudejans JJ, Jiwa M, van den Brule AJC, et al. Detection of heterogeneous Epstein-Barr virus gene expression patterns within individual post-transplant lymphoproliferative disorders. Am. J. Pathol. 1995;147:923–933. [PMC free article] [PubMed] [Google Scholar]
- Pallesen G, Hamilton-Dutoit SJ, Zhou X. The association of Epstein-Barr virus (EBV) with T cell lymphoproliferations and Hodgkin's disease: two new developments in the EBV field. Adv. Cancer Res. 1994;62:179–239. doi: 10.1016/s0065-230x(08)60319-x. [DOI] [PubMed] [Google Scholar]
- Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N. Engl. J. Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- Poppema S, Potters M, Visser L, van den Berg AM. Immune escape mechanisms in Hodgkin's disease. Ann. Oncol. 1998;9(Suppl. 5):S21–S24. doi: 10.1093/annonc/9.suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Prehn RT. Immunomodulation of tumor growth. Am. J. Pathol. 1974;77:119–122. [PMC free article] [PubMed] [Google Scholar]
- Puls A, Eliopoulos AG, Nobes CD, Bridges T, Young LS, Hall A. Activation of the small GTPase Cdc42 by the inflammatory cytokines TNF alpha and IL-1, and by the Epstein-Barr virus transforming protein LMP1. J. Cell Sci. 1999;112:2983–2992. doi: 10.1242/jcs.112.17.2983. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Radkov SA, Bain M, Farrell PJ, West M, Rowe M, Allday MJ. Epstein-Barr virus EBNA3C represses Cp, the major promoter for EBNA expression, but has no effect on the promoter of the cell gene CD21. J. Virol. 1997;71:8552–8562. doi: 10.1128/jvi.71.11.8552-8562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radkov SA, Touitou R, Brehm A, et al. Epstein-Barr virus nuclear antigen 3C interacts with histone deacetylase to repress transcription. J. Virol. 1999;73:5688–5697. doi: 10.1128/jvi.73.7.5688-5697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins DR, Milman G, Hayward SD, Hayward GS. Sequence-specific binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985;42:859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Reisman D, Sugden B. Trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol. Cell. Biol. 1986;6:3838–3846. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: Lipincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- Robertson ES, Grossman S, Johannsen E, et al. Epstein-Barr virus nuclear protein 3C modulates transcription through interaction with the sequence-specific DNA-binding protein J kappa. J. Virol. 1995;69:3108–3116. doi: 10.1128/jvi.69.5.3108-3116.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson ES, Lin J, Kieff E. The amino-terminal domains of Epstein-Barr virus nuclear proteins 3A, 3B, and 3C interact with RBPJ (kappa) J. Virol. 1996;70:3068–3074. doi: 10.1128/jvi.70.5.3068-3074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CYC, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- Rooney CM, Smith CA, Ng CYC, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphom in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- Rowe M, Rowe DT, Gregory CD, et al. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma. EMBO J. 1987;6:2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf IK, Rhyne PW, Yang H, et al. Epstein-Barr virus regulates c-myc, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol. Cell. Biol. 1999;19:1651–1660. doi: 10.1128/mcb.19.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam CK, Brooks LA, Niedobitek G, Young LS, Prasad U, Rickinson AB. Analysis of Epstein-Barr virus infection in nasopharyngeal biopsies from a group at high risk of nasopharyngeal carcinoma. Int. J. Cancer. 1993;53:957–962. doi: 10.1002/ijc.2910530616. [DOI] [PubMed] [Google Scholar]
- Sasajima S, Yamabe H, Kobashi Y, Hirai K, Mori S. High expression of the Epstein-Barr virus latent protein EB nuclear antigen-2 on pyothorax-associated lymphomas. Am. J. Pathol. 1993;143:1280–1285. [PMC free article] [PubMed] [Google Scholar]
- Sbih-Lammali F, Clausse B, Ardila-Osorio H, et al. Control of apoptosis in Epstein-Barr virus-positive nasopharyngeal carcinoma cells: opposite effects of CD95 and CD40 stimulation. Cancer Res. 1999;59:924–930. [PubMed] [Google Scholar]
- Schaefer BC, Strominger JL, Speck SH. Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc. Natl. Acad. Sci. USA. 1995;92:10565–10569. doi: 10.1073/pnas.92.23.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholle F, Longnecker R, Raab-Traub N. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2: a role for C-terminal Src kinase. J. Virol. 1999;73:4767–4775. doi: 10.1128/jvi.73.6.4767-4775.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selves J, Meggetto F, Brousset P, et al. Inflammatory pseudotumor of the liver. Evidence for follicular dendritic reticulum cell proliferation associated with clonal Epstein-Barr virus. Am. J. Surg. Pathol. 1996;20:747–753. doi: 10.1097/00000478-199606000-00013. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV) -negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J. Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N, Yoshiyama H, Takada K. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J. Virol. 1996;70:7260–7263. doi: 10.1128/jvi.70.10.7260-7263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silins SL, Sculley TB. Modulation of vimentin, the CD40 activation antigen and Burkitt's lymphoma antigen (CD77) by the Epstein-Barr virus nuclear antigen EBNA-4. Virology. 1994;202:16–24. doi: 10.1006/viro.1994.1317. 10.1006/viro.1994.1317. [DOI] [PubMed] [Google Scholar]
- Sing AP, Ambinder RF, Hong DJ, Jensen M, Batten W, EP, Greenberg PD. Isolation of Epstein-Barr virus (EBV) -specific cytotoxic T lymphocytes that lyse Reed-Sternberg cells: implications for immune-mediated therapy of EBV+ Hodgkin's disease. Blood. 1997;89:1978–1986. [PubMed] [Google Scholar]
- Sixbey JW, Vesterinen E, Nedrud JG, Raab-Traub N, Walton LA, Pagano JS. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature. 1983;306:480–483. doi: 10.1038/306480a0. [DOI] [PubMed] [Google Scholar]
- Sjoblom A, Nerstedt A, Jansson A, Rymo L. Domains of the Epstein-Barr virus nuclear antigen 2 (EBNA2) involved in the transactivation of the latent membrane protein 1 and the EBNA Cp promoters. J. Gen. Virol. 1995;76:2669–2678. doi: 10.1099/0022-1317-76-11-2669. [DOI] [PubMed] [Google Scholar]
- Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- Speck SH, Strominger JL. Transcription of Epstein-Barr virus in latently infected, growth-transformed lymphocytes. Adv. Viral. Oncol. 1989;8:133–150. [Google Scholar]
- Srinivas SK, Sixbey JW. Epstein-Barr virus induction of recombinase-activating genes RAG1 and RAG2. J. Virol. 1995;69:8155–8158. doi: 10.1128/jvi.69.12.8155-8158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;i:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald-Mullen P, Kurilla MG, Braciale TJ. Type 2 cytokines predominate in the human CD4+ T-lymphocyte response to Epstein-Barr virus nuclear antigen 1. J. Virol. 2000;74:6748–6759. doi: 10.1128/jvi.74.15.6748-6759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG, Rickinson AB. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T-cell response. J. Exp. Med. 1997;185:1605–1617. doi: 10.1084/jem.185.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl LJ, Hofelmayr H, Marschall G, Brielmeier M, Bornkamm GW, Zimber-Strobl U. Activated Notch1 modulates gene expression in B cells similarly to Epstein-Barr viral nuclear antigen 2. J. Virol. 2000;74:1727–1735. doi: 10.1128/jvi.74.4.1727-1735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara Y, Mizugaki Y, Uchida T, et al. Detection of Epstein Barr virus (EBV) in hepatocellular carcinoma tissue: a novel EBV latency characterized by the absence of EBV-encoded small RNA expression. Virology. 1999;256:196–202. doi: 10.1006/viro.1999.9619. 10.1006/viro.1999.9619. [DOI] [PubMed] [Google Scholar]
- Swart R, Fruehling S, Longnecker R. Tyrosines 60, 64, and 101 of Epstein-Barr virus LMP2A are not essential for blocking B cell signal transduction. Virology. 1999;263:485–495. doi: 10.1006/viro.1999.9964. 10.1006/viro.1999.9964. [DOI] [PubMed] [Google Scholar]
- Tanner J, Weis J, Fearon D, Whang Y, Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping and endocytosis. Cell. 1987;50:203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Geilinger K. Monoclonal antibodies against the major glycoprotein (gp350/220) of Epstein-Barr virus neutralize infectivity. Proc. Natl. Acad. Sci. USA. 1980;77:5307–5311. doi: 10.1073/pnas.77.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Miyashita EM, Khan G. Epstein-Barr virus and the B cell: that's all it takes. Trends Microbiol. 1996;4:204–208. doi: 10.1016/s0966-842x(96)90020-7. 10.1016/0966-842x(96)10030-5. [DOI] [PubMed] [Google Scholar]
- Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J. Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson B, Robertson E, Kieff E. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J. Virol. 1993;67:2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Yalamanchili R, Harada S, Kieff E. The EBNA-2 arginine-glycine domain is critical but not essential for B-lymphocyte growth transformation; the rest of region 3 lacks essential interactive domains. J. Virol. 1994;68:6188–6197. doi: 10.1128/jvi.68.10.6188-6197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Hu LF, Christensson B, Masucci MG, Klein G, Winberg G. Epstein-Barr virus (EBV) -encoded membrane protein LMP1 from a nasopharyngeal carcinoma is non-immunogenic in a murine model system, in contrast to a B cell-derived homologue. Eur. J. Cancer. 1994;30A:84–88. doi: 10.1016/s0959-8049(05)80024-3. [DOI] [PubMed] [Google Scholar]
- Uchida J, Yasui T, Takaoka SY, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. Am. J. Pathol. 1999;154:1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanasse GJ, Concannon P, Willerford DM. Regulated genomic instability and neoplasia in the lymphoid lineage. Blood. 1999;94:3997–4010. [PubMed] [Google Scholar]
- Vaughan TL, Shapiro JA, Burt RD, et al. Nasopharyngeal cancer in a low-risk population-Defining risk factors by histological type. Cancer Epidemiol. Biomark. Prev. 1996;5:587–593. [PubMed] [Google Scholar]
- Wang D, Leibowitz D, Wang F, et al. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J. Virol. 1988;62:4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wang F, Gregory CD, Rowe M, et al. Epstein-Barr virus nuclear protein 2 specifically induces expression of the B cell activation antigen CD23. Proc. Natl. Acad. Sci. USA. 1987;84:3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gregory CD, Sample C, et al. Epstein-Barr virus latent membrane protein (LMP-1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA2 and LMP-1 cooperatively induce CD23. J. Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Tsang S-F, Kurilla MG, Cohen JI, Kieff E. Epstein-Barr virus nuclear antigen 2 transactivates latent membrane protein LMP1. J. Virol. 1990;64:3407–3416. doi: 10.1128/jvi.64.7.3407-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hutt-Fletcher L. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 1998;72:158–163. doi: 10.1128/jvi.72.1.158-163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watry D, Hedrick JA, Siervo S, et al. Infection of human thymocytes by Epstein-Barr virus. J. Exp. Med. 1991;173:971–980. doi: 10.1084/jem.173.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JB, Bell JL, Levine AJ. Expression of Epstein-Barr virus nuclear antigen-1 induces B cell neoplasia in transgenic mice. EMBO J. 1996;15:3117–3126. [PMC free article] [PubMed] [Google Scholar]
- Wolf H, zur Hausen H, Becker V. EB viral genomes in epithelial nasopharyngeal carcinoma cells. Nature. 1973;244:245–247. doi: 10.1038/newbio244245a0. [DOI] [PubMed] [Google Scholar]
- Wu TT, Swerdlow SH, Locker J, et al. Recurrent Epstein-Barr virus-associated lesions in organ transplant recipients. Hum. Pathol. 1996;27:157–164. doi: 10.1016/s0046-8177(96)90369-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Menezes J, Prasad U, Ahmad A. Elevated serum levels of transforming growth factor beta 1 in Epstein-Barr virus-associated nasopharyngeal carcinoma patients. Int. J. Cancer. 1999;84:396–399. doi: 10.1002/(sici)1097-0215(19990820)84:4<396::aid-ijc11>3.0.co;2-#. 10.1002/(sici)1097-0215(19990820)84:4<396::aid-ijc11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yalamanchili R, Tong X, Grossman S, Johannsen E, Mosialos G, Kieff E. Genetic and biochemical evidence that EBNA 2 interaction with a 63-kDa cellular GTG-binding protein is essential for B lymphocyte growth transformation by EBV. Virology. 1994;294:634–641. doi: 10.1006/viro.1994.1578. [DOI] [PubMed] [Google Scholar]
- Yao M, Ohshima J, Kume T, Shiroshita T, Kikuchi M. Interleukin-10 expression and cytotoxic T-cell response in Epstein-Barr-virus-associated nasopharyngeal carcinoma. Int. J. Cancer. 1997;72:398–402. doi: 10.1002/(sici)1097-0215(19970729)72:3<398::aid-ijc4>3.0.co;2-k. 10.1002/(sici)1097-0215(19970729)72:3<398::aid-ijc4>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- Zimber-Strobl U, Kempkes B, Marschall G, et al. Epstein-Barr virus latent membrane protein (LMP1) is not sufficient to maintain proliferation of B cells but both it and activated CD40 can prolong their survival. EMBO J. 1996;15:7070–7078. [PMC free article] [PubMed] [Google Scholar]
- zur Hausen A, Brink AATP, Craanen ME, et al. Unique transcription pattern of Epstein-Barr virus (EBV) in EBV-carrying gastric adenocarcinomas: expression of the transforming BARF1 gene. Cancer Res. 2000;60:2745–2748. [PubMed] [Google Scholar]
- Zur Hausen H, Schulte-Holthausen H, Klein G, et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228:1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]