Abstract
Retroviruses are associated with a variety of diseases, including immunological and neurological disorders, and various forms of cancer. In humans, the Human T-cell Leukaemia/Lymphotropic virus type 1 (HTLV-1), which belongs to the Oncovirus family, is the aetiological agent of two diverse diseases: Adult T-cell leukaemia/lymphoma (ATLL) (Poiesz et al. 1980; Hinuma et al. 1981; Yoshida et al. 1982), as well as the neurological disorder tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) (Gessain et al. 1985; Rodgers-Johnson et al. 1985; Osame et al. 1986). HTLV-1 is the only human retrovirus known to be the aetiological agent of cancer.
A genetically related virus, HTLV-2, has been identified and isolated (Kalyanaraman et al. 1982). However, there has been no demonstration of a definitive aetiological role for HTLV-2 in human disease to date. Simian T-cell lymphotropic viruses types 1 and 2 (STLV-1 and -2) and bovine leukaemia virus (BLV) have also been classified in same group, Oncoviridae, based upon their similarities in genetic sequence and structure to HTLV-1 and -2 (Burny et al. 1988; Dekaban et al. 1995; Slattery et al. 1999). This article will focus on HTLV-1, reviewing its discovery, molecular biology, and its role in disease pathogenesis.
History
Epidemiological studies had suggested that a transmissible agent was involved in ATLL, as unusual clusters of ATLL were noted in some areas of Japan (Uchiyama et al. 1977). The use of T-cell growth factor, now designated Interleukin 2 (IL-2), enabled the culture of leukaemic T-cells (Morgan et al. 1976). This finding was significant, as it resulted in the detection and isolation of the first human retrovirus HTLV-1 from a T-lymphoblastoid cell line (HUT 102) established from a patient with a cutaneous T-cell lymphoma (Poiesz et al. 1980). Type C retroviral particles were detected in the culture supernatant by electron microscopy. At the same time, an independently isolated cell line (MT-1), derived from a patient with leukaemia, was shown to harbour a retrovirus and to produce antigens reactive against sera from ATLL patients (Hinuma et al. 1981). The viruses from the two different cell lines were shown to be identical and thus, HTLV-1 was identified as the aetiological agent of ATLL (Yoshida et al. 1982).
The neurological disorder tropical spastic paraparesis or HTLV-1 associated myelopathy (TSP/HAM) is the other major disease associated with HTLV-1 infection. In 1985, sera from neurological patients with TSP, which is endemic in the West Indies, were also shown to have immune reactivity to HTLV-1 (Gessain et al. 1985). At the same time in Japan, HTLV-1 serum reactivity was found in non-ATLL patients. This condition was designated HAM (Osame et al. 1986).
HTLV-1 infection has also been associated with a number of other diseases, such as chronic arthropathy, uveitis, infective dermatitis, and polymyositis (Murphy et al. 1989; Blattner 1990; Yamaguchi & Takatsuki 1993). However the role of HTLV-1 in these disorders is still under investigation.
It is estimated that 10–20 million people world-wide are infected with HTLV-1, which is endemic to southern Japan, Africa, the Caribbean, and eastern parts of South America. HTLV and STLV appear to have originated from a common ancestor virus that may have been transmitted to humans by contact with nonhuman primates (Koralnik et al. 1994; Slattery et al. 1999). PCR amplification of HTLV-1 proviral sequences from Andean mummies indicate that HTLV-1 infection has existed in humans for a long period of time (Li et al. 1999).
HTLV-1 has been shown to infect a wide variety of human and nonhuman cells in vitro (Clapham et al. 1984; Krichbaum-Stenger et al. 1987). However, CD4+ T-cells are the predominant target in vivo. The cellular receptor has yet to be identified, although the use of viral interference assays suggests that the receptor localizes to human chromosome 17 (Sommerfelt et al. 1988).
HTLV-1 is transmitted sexually or by blood, blood products, and breast milk and induces a lifelong chronic infection. Unlike HIV, HTLV-1 virions are poorly infectious in vitro and transmission of HTLV-1 occurs mainly through cell-to-cell contact. ATLL occurs in 1–2% of infected carriers generally 20–30 years after infection (Yamaguchi & Takatsuki 1993). Epidemiological studies have shown that ATLL develops mainly in individuals that were infected in infancy. This long latency period of ATLL suggests that the accumulation of genetic mutations, in addition to HTLV-1 infection, may be required for the induction of ATLL.
HTLV-1-associated diseases
ATLL is an aggressive lymphoproliferative disease whose clinical course can be classified into five different stages: asymptomatic, pre-Leukaemic, chronic/smouldering, lymphoma, and acute (Yamaguchi et al. 1983; Yamaguchi & Takatsuki 1993). The majority of HTLV-1 infected individuals are asymptomatic, but capable of transmitting the virus. Morphologically abnormal T-cells with highly lobulated or flower-shaped nuclei are pathognomonic of HTLV-1 infection (Takatsuki et al. 1985) and usually have a mature phenotype (CD2+, CD3+, CD4+, CD8-, CD25+, and HLA-DR +). Clonal populations of T-cells carry HTLV-1 proviral DNA, as detected by southern hybridization and of these, one T-cell clone may become malignant. Approximately one-half of preleukaemic individuals undergo a spontaneous regression, while some progress to chronic/smouldering ATLL. Skin lesions and marrow involvement are typically found in patients with smouldering ATLL, whereas patients with chronic ATLL usually have elevated numbers of circulating leukaemic cells. Patients with smouldering or chronic ATLL can progress into acute ATLL, a very aggressive form of leukaemia, within a period of months. Acute ATLL is characterized clinically by hypercalcemia, elevated lactate dehydrogenase levels (LDH), skin lesions, lymphadenopathy, lymphomatous meningitis, lytic bone lesions, spleen or liver involvement, and immunodeficiency (Kondo et al. 1987; Murphy et al. 1989). There is a dominant clone of malignant cells, as demonstrated by a single rearrangement of the T-cell receptor gene (TCR), as well as one or two (rarely more) proviral copies in the neoplastic T-cells (Shimoyama et al. 1983).
Although HTLV-1 is the cause of ATLL, the various mechanisms by which leukaemogenesis occurs are not fully defined. The virus itself does not carry any host-derived oncogenes (Yamaguchi et al. 1986), nor does it activate a cellular oncogene upon proviral integration at a common site (Seiki et al. 1984). It has been suggested that HTLV-1 infection is only one step in a multistep process that has yet to be entirely elucidated.
TSP/HAM is a chronic demyelinating disease affecting women more often than men, usually beginning in adulthood (Gessain et al. 1985; Rodgers-Johnson et al. 1985; Osame et al. 1986). In contrast to ATLL, TSP/HAM can develop in some patients within years of HTLV-1 infection, which is often a result of a blood transfusion. TSP/HAM is characterized by weakness and spasticity of the extremities, mild peripheral sensory loss, and hyperreflexia. There are lesions in the white matter of the spinal cord with demyelination and axonal changes. The majority of TSP/HAM patients are seropositive for anti-HTLV-1 antibodies (Osame et al. 1987). As with ATLL, morphologically atypical lymphocytes can be seen in the peripheral blood; distinct from ATLL, polyclonal integration of proviral DNA is common. In a few cases however, ATLL and TSP/HAM have been known to occur in the same patient. HTLV-1 DNA can be found in blood and CSF lymphocytes (Yoshida et al. 1987; Bhagavati et al. 1988). Characterization of viral isolates have not demonstrated any obvious differences, biological or genetic, from those isolated from ATLL patients and the disease mechanism underlying TSP/HAM has yet to be fully determined. Some evidence suggests that central nervous system (CNS) damage may be a direct result of infected cells being recognized and lysed by the host's immune system. HTLV-1 can infect neuronal cells in vitro (Lehky et al. 1995), as well as in vivo (Yoshida et al. 1987; Bhagavati et al. 1988) and high levels of HTLV-1 specific cytotoxic T-lymphocytes (CTLs) have been detected in TSP/HAM patients and may contribute to the neurological damage (Greten et al. 1998; Jacobson et al. 1990; Usuku et al. 1991). Alternatively, damage may result indirectly due to autoimmune or cytokine-mediated mechanisms. TNF-α, GM-CSF, IFN-γ, and IL-1 levels found in TSP/HAM patients are increased vs. levels in asymptomatic carriers (Watanabe et al. 1995). Certain HLA haplotypes have been associated with the development of TSP/HAM, which is not the case for ATL (Usuku et al. 1991; Jeffery et al. 1999).
Animal models
HTLV-1 can infect animals, such as rabbits, rats, and monkeys (Akagi et al. 1985; Nakamura et al. 1987; Oka et al. 1992; Taguchi et al. 1993). Rats infected with HTLV-1 were shown to develop a chronic progressive myeloneuropathy with similarities to HAM (Kushida et al. 1994; Kasai et al. 1999). Several animal models for ATLL have been described in rats and rabbits (Ohashi et al. 1999; Simpson et al. 1996). However, while leukaemic cells can be transplanted in these models, leukaemia of rat or rabbit origin does not develop in any of these animals. Ongoing studies using transgenic animals are currently examining the effect of single or multiple viral genes, particularly the viral transactivator Tax, on leukaemogenesis. Tax has been shown to play an important role in the induction of tumours in transgenic mice (Hinrichs et al. 1987; Grossman et al. 1995; Yamada et al. 1995).
Genetic organization
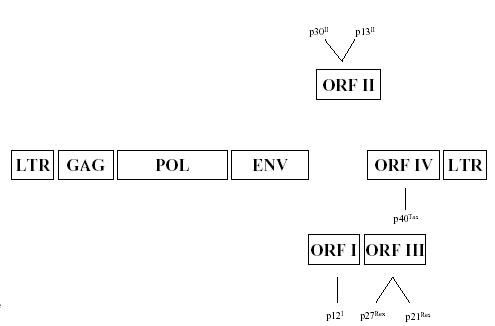
The HTLV-1 carries a single-stranded RNA genome of approximately 9 kb, which encodes the structural and enzymatic proteins, gag, env, and pol, similar to other retroviruses (Figure 1) (Franchini 1995). However, the HTLV-1 genome contains a unique region at the 3′ end, designated the pX region, which encodes regulatory proteins, such as Tax and Rex and additional proteins whose functions are now being investigated (Table 1).
Figure 1.
Schematic representation of the HTLV-1 genome.
Table 1.
Regulatory proteins encoded by the pX region of HTLV-1
| Protein | Size | Localization | Function |
|---|---|---|---|
| p12I | 12 kDa | Endomembranes Golgi and endoplasmic reticulum | Required for viral replication and infectivity of primary lymphocytes in vivo; |
| Binds to β and γc chains of the IL-2 receptor, the 16 kDa subunit of the vacuolar ATPase, and the MHC I heavy chain | |||
| p13II | 13 kDa | Mitochondria | Required for the maintenance of high viral loads in vivo; |
| May interfere with mitochondrial function | |||
| p30II | 30 kDa | Nucleoli | Required for the maintenance of high viral loads in vivo; |
| Modulate the transcription of cellular genes | |||
| Rex, p27III | 27 kDa | Nucleoli | Post-transcriptional regulator of viral gene expression |
| Rex, p21III | 21 kDa | Cytoplasm | Unknown |
| Tax, p40IV | 40 kDa | Nuclei | Transcriptional and post-translational regulator |
HTLV-1 long-terminal repeat (LTR)
The HTLV-1 long-terminal repeat (LTR) located at the 5′ and 3′ ends of the viral genome, contains the viral promoter and other regulatory elements and is divided into U3, R, and U5 regions. The U3 region contains elements that control proviral transcription and mRNA termination and polyadenylation signals. There are three imperfect 21-base pair nucleotide repeats, designated Tax responsive elements (TRE), that are necessary for transcriptional activation by the Tax protein. Similar to other retroviruses, the full length mRNA encodes the gag protein (p55), which is then cleaved by the viral protease to yield the matrix (MA, p19), capsid (CA, p24), and nucleocapsid (NC, p15) proteins. The protease is encoded by a reading frame that spans the 3′ end of gag and the 5′ end of pol and results from ribosomal frameshifting. In addition, the full length mRNA also encodes the pol protein, which is synthesized by ribosomal frameshifting. A single spliced mRNA encodes the env protein and a double spliced mRNA encodes the Tax and Rex regulatory proteins. In addition, several alternatively spliced products are derived from open reading frames (ORFs) I, II, III, IV of the pX region. mRNAs encoding these proteins have been detected in HTLV-1 infected cells in vitro and in ex vivo samples isolated from asymptomatic carriers, ATLL, and TSP/HAM patients (Ciminale et al. 1992; Koralnik et al. 1992a, 1992b).
Tax
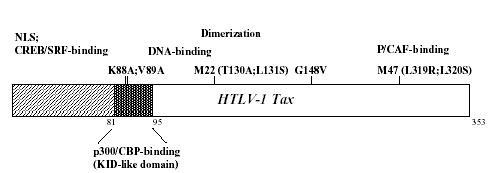
The HTLV-1 trans-activator, Tax, is a 40-kDa protein comprised of 353 amino acid residues that drives viral gene expression from three repetitive 21 bp-enhancer elements located within the U3 region of the long-terminal repeat (LTR) (Beimling & Moelling 1992; Zhao & Giam 1992; Paca-Uccaralertkun et al. 1994). The domain organization of Tax is depicted schematically in Fig. 2. An atypical nuclear localization sequence spans the first 48 amino acids and an amino-terminal domain that interacts with the cellular transcription factors, cyclic AMP-responsive element-binding protein (CREB) and serum-response factor (SRF or p67SRF) overlaps this region (Adya & Giam 1995; Smith & Greene 1992; Suzuki et al. 1993; Goren et al. 1995; Tian et al. 1995). Interactions with these factors are responsible for HTLV-1 LTR-trans-activation, as well as the activation of certain proliferative genes during cellular transformation. The dimerization domain spans the M22 (T130A; L131S) mutation and the transcriptional coactivators, p300/CREB-binding protein (p300/CBP) and the p300/CBP-associated factor (P/CAF), bind two distinct regions in the amino-terminus and carboxyl-terminus of Tax, respectively (Figure 3) (Harrod et al. 1998; Smith & Greene 1990; Tie et al. 1996; Jiang et al. 1999; Harrod et al. 2000). Importantly, a stretch of amino acids located downstream from the p300/CBP-binding domain that interacts with the G/C-rich flanking sequences in the HTLV-1 21 bp-repeats was identified through photo-chemical cross-linking and endopeptidase cleavage experiments (Kimzey & Dynan 1998; Lenzmeier et al. 1998; Kimzey & Dynan 1999).
Figure 2.
Schematic representation of the domain organization of the HTLV-1 trans-activator. The nuclear localization sequence (NLS) as well as the CREB/SRF-binding domains are located at the N-terminus of the protein. A KID-like sequence which recruits p300/CBP spans residues 81–95; a DNA-binding domain that has been shown to contact the minor-groove of the G/C-rich flanking sequences of the U3 21 bp-repeat elements is found immediately adjacent to the co-activator-binding region. The homodimerization domain overlaps the M22 mutation. A trans-activation domain overlaps the M47 mutation and is responsible for the recruitment of the co-adaptor/histone acetyltransferase, P/CAF.
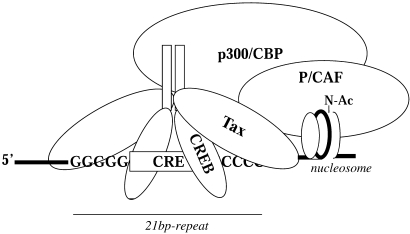
Figure 3.
Diagram of the CREB-dimer bound to the core CRE of the HTLV-1 21 bp-repeat. Tax interacts with CREB as a dimer and independently recruits the cellular coactivators, p300/CBP and P/CAF, which activate transcription by histone-acetylation/chromatin-remodeling.
Tax interacts with CREB and the coactivators p300/CBP on three 21 bp-repeats in the HTLV-1 LTR and is reported to stabilize the formation of CREB/ATF-dimers bound to DNA (Giebler et al. 1997; Zhao & Giam 1992; Perini et al. 1995; Harrod et al. 1998). Each 21 bp-repeat element contains a core cyclic AMP-responsive element (CRE) that is flanked by 5′ and 3′ G/C-rich sequences that contact amino acid residues in Tax (Lenzmeier et al. 1998; Paca-Uccaralertkun et al. 1994). Binding of Tax to p300/CBP occurs independent of CREB Ser-133 phosphorylation and facilitates constitutive viral gene expression in the absence of cellular signalling; however, CREB-phosphorylation enhances the ability of Tax to trans-activate cellular CREs (Kwok et al. 1996). Interestingly, the kinase-inducible domain (KID)-like domain in Tax (spanning residues 81–95) that recognizes the hydrophobic kinase-inducible exchange (KIX) region in p300/CBP was found to bear significant similarity to amino acids comprising the KID surrounding Ser-133 in CREB that undergoes Ca2+-dependent phosphorylation (Parker et al. 1996; Harrod et al. 1998; Yan et al. 1998). This domain in Tax likely mediates many of the interactions through which the viral trans-activator pleiotropically dysregulates the expression of numerous cellular genes. Indeed, competition for utilizing a limiting nuclear pool of p300/CBP may provide a basis for Tax-dependent inactivation/repression of certain transcription factors, including p53 and c-Myb (Ariumi et al. 2000; Colgin & Nyborg 1998; Van Orden et al. 1999). Most recently, the KID-like domain in Tax was shown to mediate Tax-associated cell-death or apoptosis through ‘squelching’ of the nuclear coactivator p300 (Nicot & Harrod, 2000). This observation suggests that aberrant coactivator usage might pose an early barrier to neoplastic transformation that must be selectively overcome for the establishment of malignancy. The carboxyl-terminus of Tax, proximal to the M47 (L319R; L320S) mutation, was shown to interact with P/CAF independent of p300/CBP-binding – providing further complexity to the molecular mechanisms underlying dysregulated gene expression by HTLV-1 (Smith & Greene 1990; Jiang et al. 1999; Harrod et al. 2000). The p300/CBP-binding defective Tax mutants, K88A and V89A, retain their abilities to bind P/CAF and significantly trans-activate an NF-κB-dependent promoter (Nicot & Harrod, 2000). Finally, coexpression of P/CAF increased Tax-dependent trans-activation from the HTLV-1 21 bp-repeats and prevented trans-repression by the adenoviral E1A 12S protein which inhibits the histone acetyl-transferase (HAT) activities of both p300/CBP and P/CAF (Chakravarti et al. 1999; Harrod et al. 2000).
Rex
Rex is a 27-kDa phosphoprotein encoded by ORF III that localizes to the nucleolus of infected cells (Nagashima et al. 1986; Inoue et al. 1991). Rex, p27, plays an essential role in viral replication and the regulation of viral structural genes, by functioning as a post-transcriptional regulator that increases the expression of singly spliced and unspliced viral mRNAs (env, gag, and pol, respectively) (Hidaka et al. 1988). As a result, Rex, whose phosphorylation state appears to be important for its function (Adachi et al. 1992), increases the amount of incompletely spliced viral mRNA in the cytoplasm of infected cells at the expense of Tax/Rex mRNA (Inoue et al. 1986; Hidaka et al. 1988). HIV-1 encodes a protein, Rev, with similar functions.
Rex is a site specific RNA binding protein that binds to a cis-acting Rex responsive element (RxRE), a highly stable stem-loop structure located within the R region of the viral LTR (Ballaun et al. 1991; Yoshida et al. 1987; Seiki et al. 1988; Hanly et al. 1989; Bar-Shira et al. 1991; Unge et al. 1991). Rex-mediated regulation is required to balance the spliced and unspliced mRNAs necessary for the production of infectious virus. The exact mechanism(s) of Rex regulation is not known, but it is suggested that this interaction may promote transport of mRNAs from the nucleus to the cytoplasm (Hanly et al. 1989; Inoue et al. 1991). In addition, Rex may inhibit splicing and degradation of viral mRNAs (Grone et al. 1996).
ORF III also encodes a smaller alternatively spliced protein, p21, whose exact function is unknown and is not discussed (Kiyokawa et al. 1985; Orita et al. 1991).
p13II and p30II
p13II and p30II proteins, encoded by the ORF-II reading frame, are now the subject of intensive studies. Proteins encoded by the ORF-II reading frame are not essential for viral replication in vitro or T-cell immortalization in vitro (Derse et al. 1997; Robek et al. 1998). However, ORF II is important for viral infectivity in vitro, suggesting their importance (Bartoe et al. 2000).
The p30II protein, encoded by a doubly spliced pX-Tax-ORF II mRNA, localizes to the nucleoli (Koralnik et al. 1993). As p30II contains serine-rich domains with distant homologies with several transcriptional activators: Oct-1, Oct-2, Pit-1, Engrailed, and POU-M1, it is suggested that it may modulate transcription of cellular genes (Ciminale et al. 1992).
The p13II protein is encoded by a singly spliced ORF II mRNA and localizes to mitochondria in vitro (Koralnik et al. 1993). As a result of p13II expression, there is an alteration of the mitochondrial structure and a disruption of the inner membrane potential, which could be relevant for HTLV-1 replication or pathogenicity (Ciminale et al. 1999). African swine fever virus has been shown to induce a redistribution of mitochondria to perinuclear viral assembly sites, resulting in mitochondria with enhanced respiratory functions that could provide energy for viral morphogenesis (Rojo et al. 1998).
p12I
ORF I encodes a 12-kDa protein (p12I). While p12I expression has been difficult to demonstrate in HTLV-1-infected cells, indirect evidence suggests its importance. The spliced mRNA encoding p12I has been detected in in vitro and ex vivo HTLV-1-infected T-cells and macrophages (Koralnik et al. 1992a, 1992b). Recently, sera from rabbits experimentally infected with HTLV-1, as well as sera from humans infected with HTLV-1, has been shown to recognize the ORF-1 product (Dekaban et al. 2000). Moroever, a CTL response to ORF-1 products can be detected in HTLV-1-infected individuals (Pique et al. 2000). Importantly, while p12I does not appear necessary for HTLV-1 replication in vitro (Derse et al. 1997; Robek et al. 1998), the ablation of the acceptor splice site for the p12I mRNA results in impairment of viral infectivity in vitro (Collins et al. 1998).
p12I exhibits weak oncogenic activity, shares amino-acid similarities with the bovine papillomavirus (BPV) type 1 E5 oncoprotein (Franchini et al. 1993), and binds to the IL-2-receptor (IL-2R) β and γc chains (Mulloy et al. 1996). Recent work has demonstrated that while IL-2-mediated proliferation and Jak/Stat activation in HTLV- 1 immortalized T-cells appears independent of ORF-1 expression (Collins et al. 1999), p12I is necessary for the infection of primary lymphocytes in vitro (Albrecht et al. 2000). This suggests a role for p12I in the activation of host cells in the early stages of infection where interaction of p12I with components of cell signalling pathways, such as the IL-2-receptor β and γc chains, may contribute to host cell activation, thus resulting in an increased rate of viral infection.
Two natural variants of the p12I protein have been identified: one carries a Lysine at position 88 and is commonly found in HTLV-1 strains from TSP-HAM patients; the second carries an Arginine at position 88 and is found in HTLV-1 strains from all ATLL patients and healthy carriers studied (Trovato et al. 1999). The p12IR88 protein has a much greater stability compared to the p12IK88 protein, which is ubiquitinated and rapidly degraded by the proteasome (Trovato et al. 1999), suggesting that this sequence variation might have a functional relevance.
Interestingly, the HTLV-1 p12I and Nef proteins of HIV/SIV appear to share common features. Nef is dispensable in vitro, but is required for in vivo replication and pathogenicity (Kestler et al. 1991), as is p12I (Collins et al. 1998). In vitro, p12I has been shown to bind the 16- kDa subunit of the vacuolar ATPase (Franchini et al. 1993) and Nef to bind to the catalytic subunit of the same enzyme (Lu et al. 1998).
Recently, p12I has been shown to interfere with the assembly of the MHC I heavy chain and β2-microglobulin complex and its trafficking to the cell membrane (Johnson et al. Submitted). It may do so by taking advantage of a pathway termed ERAD (ER-associated degradation pathway), whose purpose is to remove misfolded, inappropriately glycosylated or improperly assembled proteins from the ER (Bonifacino & Klausner 1994). Several viruses have evolved mechanisms to escape immune recognition by affecting the expression of MHC I on the cell surface (Ploegh 1998; Tortorella et al. 2000), including Nef, which has been shown to affect MHC I levels at the cell surface (Schwartz et al. 1996). The two natural alleles of p12I, one of which (p12IK88) is ubiquitinated, may differ in their ability to affect antigen presentation and therefore modulate the host-specific immune response. In this regard, the finding that the ubiquitinated form of p12I (p12IK88) is commonly found in TSP-HAM (Trovato et al. 1999), an immune-mediated disease (Osame et al. 1986; Jacobson et al. 1988), indicates that dissecting the functional consequence of the two natural alleles of p12I may further our understanding of HTLV-1 pathogenesis.
It has been suggested that p12I plays an important role early during HTLV-1 infection and in this respect, p12I expression might enable the virus to establish infection in the host. The ability of p12I to target the MHC I heavy chain for degradation may decrease the density of MHC I complexes presenting viral peptides on the cell surface and protect infected cells from lysis by cytotoxic lymphocytes (CTLs). In addition, the down-regulation of MHC I complexes containing Tax peptides would allow Tax-expressing cells to survive; Tax, a protein that plays a major role in HTLV-1 pathogenesis, is extremely immunogenic (Jacobson et al. 1990). Further, the interference of augmentation of the IL-2R-signalling pathway(s) by p12I may contribute to proliferation and activation of HTLV-1 infected T-cells, thus contributing to a persistant viral infection.
Pathogenic mechanisms
HTLV-1 has been shown to induce T-cell activation and proliferation. Activated and dividing T-cells have been shown to have an increased susceptibility to HTLV-1 infection, in comparison with quiescent T-cells (Merl et al. 1984). Thus, T-cell activation may be necessary to enable the virus to establish infection after entry. Spontaneous T-cell proliferation has been observed in cultures (Gazzolo & Duc Dodon 1987; Tendler et al. 1990) or in T-cell colony-forming cells derived from PBMCs of healthy carriers and TSP/HAM patients (Lunardi-Iskandar et al. 1993). T-cells are stimulated by cell contact to divide without the requirement of accessory cells. This activation is mediated by CD2/Lymphocyte function associated molecule-3 (LFA-3) and LFA-1/intracellular adhesion molecule, as well as IL-2/IL-2R. Further, ex vivo derived HTLV-1-infected T-cell clones have been shown to spontaneously proliferate up to two weeks after stimulation without exogenous IL-2 (Wucherpfennig et al. 1992). This proliferative capability is independent of the IL-2/IL-2R pathway. In addition, HTLV-1 virions have been shown to be mitogenic for quiescent human T-cells (Gazzolo & Duc Dodon 1987). As heat-inactivated virions also possess this mitogenic ability, it is suggested that the interaction of the virion with a specific cell surface receptor(s) causes this activation. CD2 has been suggested to be involved in this virion-induced cellular activation (Dodon et al. 1989), although CD2 has been ruled out as a principle receptor for HTLV-1, as CD2- negative cells are susceptible to HTLV-1 infection. However, it is possible that T-cell membrane contamination of the virion preparation could be responsible for this effect (Wucherpfennig et al. 1992; Kimata et al. 1993).
HTLV-1 has been shown to immortalize primary human peripheral blood T-cells in vitro and these cells become IL-2 independent after long-term culture. At this time, the T-cells express high levels of the IL-2 receptor α chain (IL-2Rα), characteristic of ATLL cells, as well as HTLV-1 transformed cells in vitro. In addition, IL-2 independence correlates with the constitutive activation of Jak/Stat pathways (Migone et al. 1995; Xu et al. 1995; Mulloy et al. 1998b), as well as a decreased expression of the src homology 2 (SH2)-containing tyrosine phosphatase 1 (SHP-1) protein that functions to regulate signalling from several haematopoietic surface receptors (Leonard & O'Shea 1998).
The proliferation of ex vivo derived ATLL cells was found to be associated with the constitutive activation of Jak/Stat proteins, which may contribute to neoplastic growth (Takemoto et al. 1997). Indeed, the constitutive activation of Jak and/or Stat proteins has been correlated with cell transformation in other transformation models: Abelson murine leukaemia virus (Danial et al. 1995), Epstein-Barr virus (Weber-Nordt et al. 1996), and spleen focus-forming virus (Ohashi et al. 1995). Jak/Stat proteins were not found to be constitutively activated in T-cell lines infected with HTLV-2 or STLV-2 which suggests that these phylogenetically related viruses may have different mechanisms of pathogenesis from HTLV-1 (Mulloy et al. 1998a).
While HTLV-1 transforms human primary T-cells in vitro and in vivo, leukaemia develops in only a small number of infected individuals after an extended latency period. This suggests that a multistep oncogenic process may take place during this time, resulting in cellular proliferation and the accumulation of genetic mutations (Franchini 1995); several lines of evidence suggests that Tax plays a crucial role in this oncogenic process.
Tax-expressing transgenic mice develop tumours and Tax-transforms rat fibroblasts in combination with a constitutively active, mutant form of the ras oncogene (Nerenberg et al. 1987; Pozzatti et al. 1990; Yamaoka et al. 1996). Most recently, Tax has been demonstrated to cause a CD8+ leukaemia-like condition in transgenic mice when expressed from a construct driven by the T-cell specific, granzyme-B promoter (Grossman et al. 1995). Neoplastic transformation caused by the HTLV-1 Tax requires activation of the NF-κB and SRF transcription pathways (Smith & Greene 1990; Yamaoka et al. 1996; Akagi et al. 1997). Tax activates a number of cellular genes, including IL-2, IL-2Rα, GM-CSF, and PTHRP (Dittmer et al. 1997; Ruben et al. 1988; Wano et al. 1988; Doi et al. 1989; Nimer et al. 1989). However, the expression of certain cellular genes can be inhibited by Tax; the β-polymerase gene is trans-repressed by Tax through interactions with beta-helix-loop-helix (bHLH)-factors (Jeang et al. 1990; Uittenbogaard et al. 1994). The Tax protein interacts with amino acids in the bZip domain of the IκK-γ subunit of the IκB-kinase signalling complex to specifically activate/phosphorylate its IκK-β subunit (Chu et al. 1999; Harhaj & Sun 1999; Jin et al. 1999). Indeed, flat revertants of rat fibroblasts transformed by Tax contained mutations in the NF-κB-essential modulator (NEMO, the rodent homologue of hIκK-γ) and were trans-complemented by a NEMO-expressing cDNA clone (Yamaoka et al. 1998). Human T-cell leukaemia virus, type-1, transformed cells exhibit constitutive NF-κB trans-activation and phosphorylation/degradation of cytoplasmic IκB-α/β (Good et al. 1996). Trans-activation of the adult T-cell leukaemia-derived factor (ADF) by Tax is also believed to play an essential role in activating NF-κB-dependent transcription by stabilizing the redox state of RelA (p65) (Tagaya et al. 1989; Okamoto et al. 1992). Interestingly, Rosin et al. (Rosin et al. 1998) have reported that a S258A Tax-mutant, defective for activation of the NF-κB pathway while retaining its ability to activate CREB-dependent transcription, immortalized primary lymphocytes in vitro when expressed from a herpes saimiri-based vector (Rosin et al. 1998).
The HTLV-1 trans-activator, Tax, has been demonstrated to aberrantly affect a number of cell-cycle regulatory molecules. The amino-terminus of Tax has also been shown to stabilize and inactivate the tumour suppressor p53 in HTLV-1 transformed cell-lines possibly by inducing hyperphosphorylation of p53 (Cereseto et al. 1996; Mulloy et al. 1998b; Pise-Masison et al. 1998a; Pise-Masison et al. 1998b; Takemoto et al. 2000). Hyperphosphorylation of the p110 retinoblastoma protein (Rb) is associated with Tax expression; Tax also induces phosphorylation of cyclin D1-cdk4/6 and cyclin D3, potentially contributing to G1/S-phase progression in ATLL cells despite elevated levels of p21Waf/Cip1 (Cereseto et al. 1996; Neuveut et al. 1998; Schmitt et al. 1998). Further, the Tax protein is reported to inactivate the cdk-inhibitor, p16Ink4a, as well as the human mitotic arrest-deficiency-1 (MAD-1) protein that regulates the anaphase-promoting complex (APC) and segregation of metaphase chromosomes during mitosis (Suzuki et al. 1996; Jin et al. 1998). Inhibition of MAD-1 by Tax has been proposed to promote the multinucleation that is frequently observed in Reed-Sternberg-like HTLV-1-transformed cells due to the formation of micronuclei, improper chromosomal alignment, or mitotic spindle-damage (Jin et al. 1998; Majone et al. 1993). Thus, Tax acts to promote the acquisition of genetic mutations, inhibits tumour suppressor and cdk-inhibitor functions, and drives viral gene expression and leukaemic proliferation through interactions with the cellular transcriptional machinery.
Conclusion
HTLV-1 infects approximately 10–20 million people worldwide. While only a relatively small percentage of infected individuals develop disease, knowledge of HTLV-1 pathogenic mechanisms has significantly contributed to the general understanding of cellular transformation.
Acknowledgments
The authors would like to thank Drs M.G. Ferrari and R. Fukumoto for the critical reading of this manuscript. We also thank Steven Snodgrass for editorial assistance.
References
- Adachi Y, Copeland TD, Takahashi C, et al. Phosphorylation of the Rex protein of human T-cell leukemia virus type I. J. Biol. Chem. 1992;267:21977–21981. [PubMed] [Google Scholar]
- Adya N, Giam CZ. Distinct regions in human T-cell lymphotropic virus type I tax mediate interactions with activator protein CREB and basal transcription factors. J. Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi T, Ono H, Nyunoya H, Shimotohno K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene. 1997;14:2071–2078. doi: 10.1038/sj.onc.1201045. [DOI] [PubMed] [Google Scholar]
- Akagi T, Takeda I, Oka T, et al. Experimental infection of rabbits with human T-cell leukemia virus type I. Jpn. J. Cancer Res. 1985;76:86–94. [PubMed] [Google Scholar]
- Albrecht B, Collins ND, Burniston MT, et al. Human T-lymphotropic virus type 1 open reading frame I p12 (I) is required for efficient viral infectivity in primary lymphocytes. J. Virol. 2000;74:9828–9835. doi: 10.1128/jvi.74.21.9828-9835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariumi Y, Kaida A, Lin JY, et al. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene. 2000;19:1491–1499. doi: 10.1038/sj.onc.1203450. [DOI] [PubMed] [Google Scholar]
- Ballaun C, Farrington GK, Dobrovnik M, et al. Functional analysis of human T-cell leukemia virus type I rex-response element: direct RNA binding of Rex protein correlates with in vivo activity. J. Virol. 1991;65:4408–4413. doi: 10.1128/jvi.65.8.4408-4413.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shira A, Panet A, Honigman A. An RNA secondary structure juxtaposes two remote genetic signals for human T-cell leukemia virus type I RNA 3′-end processing. J. Virol. 1991;65:5165–5173. doi: 10.1128/jvi.65.10.5165-5173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoe JT, Albrecht B, Collins ND, et al. Functional role of pX open reading frame II of human T-lymphotropic virus type 1 in maintenance of viral loads in vivo. J. Virol. 2000;74:1094–1100. doi: 10.1128/jvi.74.3.1094-1100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beimling P, Moelling K. Direct interaction of CREB protein with 21 bp Tax-response elements of HTLV-ILTR. Oncogene. 1992;7:257–262. [PubMed] [Google Scholar]
- Bhagavati S, Ehrlich G, Kula RW, et al. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in spinal fluid and blood of patients with chronic progressive myelopathy. N. Engl. J. Med. 1988;318:1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- Blattner WA. Epidemiology of HTLV-I and Associated Diseases. New York: Raven; 1990. pp. 251–251. [Google Scholar]
- Bonifacino JS, Klausner RD. Degradation of proteins retained in the endoplasmic reticulum. In: Ciechanover A, Schwartz AL, editors. Cellular Proteolytic Systems. New York: Wiley-Liss; 1994. p. 137. [Google Scholar]
- Burny A, Cleuter Y, Kettmann R, et al. Bovine leukaemia: facts and hypotheses derived from the study of an infectious cancer. Vet. Microbiol. 1988;17:197–218. doi: 10.1016/0378-1135(88)90066-1. [DOI] [PubMed] [Google Scholar]
- Cereseto A, Diella F, Mulloy JC, et al. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T-cells. Blood. 1996;88:1551–1560. [PubMed] [Google Scholar]
- Chakravarti D, Ogryzko V, Kao HY, et al. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell. 1999;96:393–403. doi: 10.1016/s0092-8674(00)80552-8. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Shin YA, Yang JM, Didonato JA, Ballard DW. IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 1999;274:15297–15300. doi: 10.1074/jbc.274.22.15297. 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- Ciminale V, Pavlakis GN, Derse D, Cunningham CP, Felber BK. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV-I. J. Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciminale V, Zotti L, D'Agostino DM, et al. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I) Oncogene. 1999;18:4505–4514. doi: 10.1038/sj.onc.1203047. [DOI] [PubMed] [Google Scholar]
- Clapham P, Nagy K, Weiss RA. Pseudotypes of human T-cell leukemia virus types 1 and 2: neutralization by patients' sera. Proc. Natl. Acad. Sci. USA. 1984;81:2886–2889. doi: 10.1073/pnas.81.9.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin MA, Nyborg JK. The human T-cell leukemia virus type 1 oncoprotein Tax inhibits the transcriptional activity of c-Myb through competition for the CREB binding protein. J. Virol. 1998;72:9396–9399. doi: 10.1128/jvi.72.11.9396-9399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ND, D'Souza C, Albrecht B, et al. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J. Virol. 1999;73:9642–9649. doi: 10.1128/jvi.73.11.9642-9649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ND, Newbound GC, Albrecht B, et al. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- Danial NN, Pernis A, Rothman PB. Jak-STAT signaling induced by the v-abl oncogene. Science. 1995;269:1875–1877. doi: 10.1126/science.7569929. [DOI] [PubMed] [Google Scholar]
- Dekaban GA, Digilio L, Franchini G. The natural history and evolution of human and simian T cell leukemia/lymphotropic viruses. Curr. Opin. Genet. Dev. 1995;5:807–813. doi: 10.1016/0959-437x(95)80015-w. [DOI] [PubMed] [Google Scholar]
- Dekaban GA, Peters AA, Mulloy JC, et al. The HTLV-I orf I protein is recognized by serum antibodies from naturally infected humans and experimentally infected rabbits. Virology. 2000;274:86–93. doi: 10.1006/viro.2000.0406. 10.1006/viro.2000.0406. [DOI] [PubMed] [Google Scholar]
- Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- Dittmer J, Pise-Masison CA, Clemens KE, Choi KS, Brady JN. Interaction of human T-cell lymphotropic virus type I Tax, Ets1, and Sp1 in transactivation of the PTHrP P2 promoter. J. Biol. Chem. 1997;272:4953–4958. doi: 10.1074/jbc.272.8.4953. 10.1074/jbc.272.8.4953. [DOI] [PubMed] [Google Scholar]
- Dodon MD, Bernard A, Gazzolo L. Peripheral T-lymphocyte activation by human T-cell leukemia virus type I interferes with the CD2 but not with the CD3/TCR pathway. J. Virol. 1989;63:5413–5419. doi: 10.1128/jvi.63.12.5413-5419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Hatakeyama M, Itoh S, Taniguchi T. Transient induction of IL-2 receptor in cultured T cell lines by HTLV-1 LTR-linked tax-1 gene. EMBO J. 1989;8:1953–1958. doi: 10.1002/j.1460-2075.1989.tb03600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- Franchini G, Mulloy JC, Koralnik IJ, et al. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J. Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzolo L, Duc Dodon M. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987;326:714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- Gessain A, Barin F, Vernant J-C, et al. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Giebler HA, Loring JE, Van Orden K, et al. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol. Cell. Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good L, Maggirwar SB, Sun S-C. Activation of the IL-2 gene promoter by HTLV-I Tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 1996;15:3744–3750. [PMC free article] [PubMed] [Google Scholar]
- Goren I, Semmes OJ, Jeang KT, Moelling K. The amino terminus of Tax is required for interaction with the cyclic AMP response element binding protein. J. Virol. 1995;69:5806–5811. doi: 10.1128/jvi.69.9.5806-5811.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten TF, Slansky JE, Kubota R, et al. Direct visualization of antigen-specific T cells: HTLV-1 Tax11–19- specific CD8 (+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc. Natl. Acad. Sci. USA. 1998;95:7568–7573. doi: 10.1073/pnas.95.13.7568. 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone M, Koch C, Grassmann R. The HTLV-1 Rex protein induces nuclear accumulation of unspliced viral RNA by avoiding intron excision and degradation. Virology. 1996;218:316–325. doi: 10.1006/viro.1996.0200. 10.1006/viro.1996.0200. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Kimata JT, Wong FH, et al. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly SM, Rimsky LT, Malim MH, et al. Comparative analysis of the HTLV-I Rex and HIV-1 Rev. trans-regulatory proteins and their RNA response elements. Genes Dev. 1989;3:1534–1544. doi: 10.1101/gad.3.10.1534. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Sun SC. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- Harrod R, Kuo YL, Tang Y, et al. p300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J. Biol. Chem. 2000;275:11852–11857. doi: 10.1074/jbc.275.16.11852. 10.1074/jbc.275.16.11852. [DOI] [PubMed] [Google Scholar]
- Harrod R, Tang Y, Nicot C, et al. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell. Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka M, Inoue J, Yoshida M, Seiki M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988;7:519–523. doi: 10.1002/j.1460-2075.1988.tb02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs SH, Nerenberg M, Reynolds RK, Khoury G, Jay G. A transgenic mouse model for human neurofibromatosis. Science. 1987;237:1340–1343. doi: 10.1126/science.2888191. [DOI] [PubMed] [Google Scholar]
- Hinuma Y, Nagata K, Hanaoka M, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J, Itoh M, Akizawa T, Toyoshima H, Yoshida M. HTLV-1 Rex protein accumulates unspliced RNA in the nucleus as well as in cytoplasm. Oncogene. 1991;6:1753–1757. [PubMed] [Google Scholar]
- Inoue J, Seiki M, Yoshida M. The second pX product p27 chi-III of HTLV-1 is required for gag gene expression. FEBS Lett. 1986;209:187–190. doi: 10.1016/0014-5793(86)81108-5. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Raine CS, Mingioli ES, McFarlin DE. Isolation of an HTLV-1-like retrovirus from patients with tropical spastic paraparesis. Nature. 1988;331:540–543. doi: 10.1038/331540a0. [DOI] [PubMed] [Google Scholar]
- Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- Jeang KT, Widen SG, Semmes OJ, Wilson SH. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Usuku K, Hall SE, et al. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc. Natl. Acad. Sci. USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lu H, Schiltz RL, et al. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 1999;19:8136–8145. doi: 10.1128/mcb.19.12.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DY, Giordano V, Kibler KV, Nakano H, Jeang KT. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J. Biol. Chem. 1999;274:17402–17405. doi: 10.1074/jbc.274.25.17402. 10.1074/jbc.274.25.17402. [DOI] [PubMed] [Google Scholar]
- Jin DY, Spencer F, Jeang K-T. Human T-cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:1–20. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman VS, Sarngadharan MG, Robert-Guroff M, et al. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982;218:571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Kasai T, Ikeda H, Tomaru U, et al. A rat model of human T lymphocyte virus type I (HTLV-I) infection: in situ detection of HTLV-I provirus DNA in microglia/macrophages in affected spinal cords of rats with HTLV-I-induced chronic progressive myeloneuropathy. Acta Neuropathol. (Berl) 1999;97:107–112. doi: 10.1007/s004010050962. 10.1007/s004010050962. [DOI] [PubMed] [Google Scholar]
- Kestler HWIII, Ringleer DJ, Mori K, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kimata JT, Palker TJ, Ratner L. The mitogenic activity of human T-cell leukemia virus type I is T-cell associated and requires the CD2/LFA-3 activation pathway. J. Virol. 1993;67:3134–3141. doi: 10.1128/jvi.67.6.3134-3141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimzey AL, Dynan WS. Specific regions of contact between human T-cell leukemia virus type I Tax protein and DNA identified by photocross-linking. J. Biol. Chem. 1998;273:13768–13775. doi: 10.1074/jbc.273.22.13768. 10.1074/jbc.273.22.13768. [DOI] [PubMed] [Google Scholar]
- Kimzey AL, Dynan WS. Identification of a human T-cell leukemia virus type I tax peptide in contact with DNA. J. Biol. Chem. 1999;274:34226–34232. doi: 10.1074/jbc.274.48.34226. 10.1074/jbc.274.48.34226. [DOI] [PubMed] [Google Scholar]
- Kiyokawa T, Seiki M, Iwashita S, et al. p27x-III and p21x-III, proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA. 1985;82:8359–8363. doi: 10.1073/pnas.82.24.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kono H, Nonaka H, et al. Risk of adult T-cell leukaemia/lymphoma in HTLV-I carriers. Lancet. 1987;2:159–159. doi: 10.1016/s0140-6736(87)92359-2. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Boeri E, Saxinger WC, et al. Phylogenetic associations of human and simian T-cell leukemia/lymphotropic virus type I strains: evidence for interspecies transmission. J. Virol. 1994;68:2693–2707. doi: 10.1128/jvi.68.4.2693-2707.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J. Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik I, Gessain A, Klotman ME, et al. Protein isoforms encoded by the pX region of the human T-cell leukemia/lymphotropic virus type I. Proc. Natl. Acad. Sci. USA. 1992a;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik I, Lemp JF, Jr, Gallo RC, Franchini G. In vitro infection of human macrophages by human T-cell leukemia/lymphotropic virus type I (HTLV-I) AIDS Res. Hum. Retroviruses. 1992b;8:1845–1849. doi: 10.1089/aid.1992.8.1845. [DOI] [PubMed] [Google Scholar]
- Krichbaum-Stenger K, Poiesz BJ, Keller P, et al. Specific adsorption of HTLV-I to various target human and animal cells. Blood. 1987;70:1303–1311. [PubMed] [Google Scholar]
- Kushida S, Mizusawa H, Matsumura M, et al. High incidence of HAM/TSP-like symptoms in WKA rats after administration of human T-cell leukemia virus type 1-producing cells. J. Virol. 1994;68:7221–7226. doi: 10.1128/jvi.68.11.7221-7226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok RP, Laurance ME, Lundblad JR, et al. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- Lehky TJ, Fox CH, Koenig S, et al. Detection of human T-lymphotropic virus type I (HTLV-I) tax RNA in the central nervous system of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by in situ hybridization. Ann. Neurol. 1995;37:167–176. doi: 10.1002/ana.410370206. [DOI] [PubMed] [Google Scholar]
- Lenzmeier BA, Giebler HA, Nyborg JK. Human T-cell leukemia virus type 1 Tax requires direct access to DNA for recruitment of CREB binding protein to the viral promoter. Mol. Cell. Biol. 1998;18:721–731. doi: 10.1128/mcb.18.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, O'Shea JJ. JAKs and STATs: biological implications. Annu. Rev. Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- Li HC, Fujiyoshi T, Lou H, et al. The presence of ancient human T-cell lymphotropic virus type I provirus DNA in an Andean mummy. Nat. Med. 1999;5:1428–1232. doi: 10.1038/71006. 10.1038/71006. [DOI] [PubMed] [Google Scholar]
- Lu X, Yu H, Liu S, Brodsky F, Peterlin B. Interactions between HIV1 Nef and Vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- Lunardi-Iskandar Y, Gessain A, Lam VH, Gallo RC. Abnormal in vitro proliferation and differentiation of T cell colony-forming cells in patients with tropical spastic paraparesis/human T lymphocyte virus type I (HTLV-I) -associated myeloencephalopathy and healthy HTLV-I carriers. J. Exp. Med. 1993;177:741–750. doi: 10.1084/jem.177.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majone F, Semmes OJ, Jeang KT. Induction of micronuclei by HTLV-I Tax: a cellular assay for function. Virology. 1993;193:456–459. doi: 10.1006/viro.1993.1145. 10.1006/viro.1993.1145. [DOI] [PubMed] [Google Scholar]
- Merl S, Kloster B, Moore J, et al. Efficient transformation of previously activated and dividing T lymphocytes by human T cell leukemia-lymphoma virus. Blood. 1984;64:967–974. [PubMed] [Google Scholar]
- Migone TS, Lin JX, Cereseto A, et al. Constitutively activated JAK-STAT pathway in T-cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- Morgan DC, Ruscetti FW, Gallo RC. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mulloy JC, Crowley RW, Fullen J, Leonard WJ, Franchini G. The human T-cell leukemia/lymphotropic virus type I p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J. Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloy JC, Kislyakova T, Cereseto A, et al. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol. 1998b;72:8852–8860. doi: 10.1128/jvi.72.11.8852-8860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloy JC, Migone T-S, Ross TM, et al. Human and simian T-cell leukemia viruses type 2 (HTLV-2 and STLV-2pan-p) transform T-cells independently of Jak/STAT activation. J. Virol. 1998a;72:4408–4412. doi: 10.1128/jvi.72.5.4408-4412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EL, Hanchard B, Figueroa JP, et al. Modeling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int. J. Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Yoshida M, Seiki M. A single species of pX mRNA of human T-cell leukemia virus type I encodes trans-activator p40x and two other phosphoproteins. J. Virol. 1986;60:394–399. doi: 10.1128/jvi.60.2.394-399.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Hayami M, Ohta Y, et al. Protection of cynomolgus monkeys against infection by human T-cell leukemia virus type-I by immunization with viral env gene products produced in Escherichia coli. Int. J. Cancer. 1987;40:403–407. doi: 10.1002/ijc.2910400320. [DOI] [PubMed] [Google Scholar]
- Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Neuveut C, Low KG, Maldarelli F, et al. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin d-cdk and p110Rb. Mol. Cell. Biol. 1998;18:3620–3632. doi: 10.1128/mcb.18.6.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot C, Harrod R. Distinct P300-Responsive Mechanisms Promote Caspase-Dependent Apoptosis by Human T-Cell Lymphotropic Virus Type 1. Mol. Cell. Biol. 2000;20 doi: 10.1128/mcb.20.22.8580-8589.2000. . (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer SD, Gasson JC, Hu K, et al. Activation of the GM-CSF promoter by HTLV-I and -II tax proteins. Oncogene. 1989;4:671–676. [PubMed] [Google Scholar]
- Ohashi T, Hanabuchi S, Kato H, et al. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J. Virol. 1999;73:6031–6040. doi: 10.1128/jvi.73.7.6031-6040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Masuda M, Ruscetti SK. Induction of sequence-specific DNA-binding factors by erythropoietin and the spleen focus-forming virus. Blood. 1995;85:1454–1462. [PubMed] [Google Scholar]
- Oka T, Sonobe H, Iwata J, et al. Phenotypic progression of a rat lymphoid cell line immortalized by human T-lymphotropic virus type I to induce lymphoma/leukemia-like disease in rats. J. Virol. 1992;66:6686–6694. doi: 10.1128/jvi.66.11.6686-6694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Ogiwara H, Hayashi T, et al. Human thioredoxin/adult T cell leukemia-derived factor activates the enhancer binding protein of human immunodeficiency virus type 1 by thiol redox control mechanism. Int. Immunol. 1992;4:811–819. doi: 10.1093/intimm/4.7.811. [DOI] [PubMed] [Google Scholar]
- Orita S, Saiga A, Takagi S, et al. A novel alternatively spliced viral mRNA transcribed in cells infected with human T cell leukemia virus type 1 is mainly responsible for expressing p21X protein. FEBS Lett. 1991;295:127–134. doi: 10.1016/0014-5793(91)81402-t. [DOI] [PubMed] [Google Scholar]
- Osame M, Matsumoto M, Usuku K, et al. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann. Neurol. 1987;21:117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Paca-Uccaralertkun S, Zhao LJ, Adya N, et al. In vitro selection of DNA elements highly responsive to the human T-cell lymphotropic virus type I transcriptional activator. Tax. Mol. Cell. Biol. 1994;14:456–462. doi: 10.1128/mcb.14.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Ferreri K, Nakajima T, et al. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol. Cell. Biol. 1996;16:694–703. doi: 10.1128/mcb.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini G, Wagner S, Green MR. Recognition of bZIP proteins by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- Pique C, Ureta-Vidal A, Gessain A, et al. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J. Exp. Med. 2000;191:567–572. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pise-Masison CA, Choi K-S, Radonovich M, et al. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J. Virol. 1998b;72:1165–1170. doi: 10.1128/jvi.72.2.1165-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pise-Masison CA, Radonovich M, Sakaguchi K, Appella E, Brady JN. Phosphorylation of p53: a novel pathway for p53 inactivation in human T-cell lymphotropic virus type 1-transformed cells. J. Virol. 1998a;72:6348–6355. doi: 10.1128/jvi.72.8.6348-6355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh HL. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol. Cell. Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robek MD, Wong F-H, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J. Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers-Johnson P, Gajdusek DC, Morgan OS, et al. HTLV-I and HTLV-III antibodies and tropical spastic paraparesis. Lancet. 1985;ii:1247–1249. doi: 10.1016/s0140-6736(85)90778-0. [DOI] [PubMed] [Google Scholar]
- Rojo G, Chamorro M, Salas ML, et al. Migration of mitochondria to viral assembly sites in African swine fever virus-infected cells. J. Virol. 1998;72:7583–7588. doi: 10.1128/jvi.72.9.7583-7588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin O, Koch C, Schmitt I, et al. A human T-cell leukemia virus Tax variant incapable of activating NF-kappaB retains its immortalizing potential for primary T-lymphocytes. J. Biol. Chem. 1998;273:6698–6703. doi: 10.1074/jbc.273.12.6698. 10.1074/jbc.273.12.6698. [DOI] [PubMed] [Google Scholar]
- Ruben S, Poteat H, Tan TH, et al. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988;241:89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- Schmitt I, Rosin O, Rohwer P, Gossen M, Grassmann R. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 1998;72:633–640. doi: 10.1128/jvi.72.1.633-640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 nef protein. Nat. Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Seiki M, Eddy R, Shows TB, Yoshida M. Nonspecific integration of the HTLV-I provirus genome into adult T-cell leukaemia cells. Nature. 1984;309:640–642. doi: 10.1038/309640a0. [DOI] [PubMed] [Google Scholar]
- Seiki M, Inoue J, Hidaka M, Yoshida M. Two cis-acting elements responsible for posttranscriptional trans-regulation of gene expression of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA. 1988;85:7124–7128. doi: 10.1073/pnas.85.19.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama M, Minato K, Tobinai K, et al. Atypical adult T-cell leukemia-lymphoma: diverse clinical manifestations of adult T-cell leukemia-lymphoma. Jpn. J. Clin. Oncol. 1983;13:165–187. [PubMed] [Google Scholar]
- Simpson RM, Zhao TM, Hubbard BS, Sawasdikosol S, Kindt TJ. Experimental acute adult T cell leukemia-lymphoma is associated with thymic atrophy in human T cell leukemia virus type I infection. Lab. Invest. 1996;74:696–710. [PubMed] [Google Scholar]
- Slattery JP, Franchini G, Gessain A. Genomic evolution, patterns of global dissemination, and interspecies transmission of human and simian T-cell leukemia/lymphotropic viruses. Genome Res. 1999;9:525–540. [PubMed] [Google Scholar]
- Smith MR, Greene WC. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- Smith MR, Greene WC. Characterization of a novel nuclear localization signal in the HTLV-I tax transactivator protein. Virology. 1992;187:316–320. doi: 10.1016/0042-6822(92)90320-o. [DOI] [PubMed] [Google Scholar]
- Sommerfelt MA, Williams BP, Clapham PR, et al. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988;242:1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hirai H, Fujisawa J, Fujita K, Yoshida M. A trans-activator Tax of human T-cell leukemia virus type 1 binds to NF-kappa B p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-kappa B site and CArG box. Oncogene. 1993;8:2391–2397. [PubMed] [Google Scholar]
- Suzuki T, Kitao S, Matsushime H, Yoshida M. HTLV-I Tax protein interacts with cyclin-dependent kinase inhibitor p16ink4a and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- Tagaya Y, Maeda Y, Mitsui A, et al. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989;8:757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi H, Sawada T, Fukushima A, et al. Bilateral uveitis in a rabbit experimentally infected with human T-lymphotropic virus type I. Lab. Invest. 1993;69:336–339. [PubMed] [Google Scholar]
- Takatsuki K, Yamaguchi K, Kawano F, et al. Clinical diversity in adult T-cell leukemia-lymphoma. Cancer Res. 1985;45:4644s–4645s. [PubMed] [Google Scholar]
- Takemoto S, Mulloy JC, Cereseto A, et al. Proliferation of adult T-cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc. Natl. Acad. Sci. USA. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto S, Trovato R, Cereseto A, et al. p53 stabilization and functional impairment in the absence of genetic mutation or the alteration of the p14 (ARF) -MDM2 loop in ex vivo and cultured adult T-cell leukemia/lymphoma cells. Blood. 2000;95:3939–3944. [PubMed] [Google Scholar]
- Tendler CL, Greenberg SJ, Blattner WA, et al. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogenic implications and a rationale for immunotherapy. Proc. Natl. Acad. Sci. USA. 1990;87:5218–5222. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Adya N, Wagner S, et al. Dissecting protein: protein interactions between transcription factors with an RNA aptamer. RNA. 1995;1:317–326. [PMC free article] [PubMed] [Google Scholar]
- Tie F, Adya N, Greene WC, Giam CZ. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J. Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella D, Gewurz B, Schust D, Furman M, Ploegh H. Down-regulation of MHC class I antigen presentation by HCMV; lessons for tumor immunology. Immunol. Invest. 2000;29:97–100. doi: 10.3109/08820130009062289. [DOI] [PubMed] [Google Scholar]
- Trovato R, Mulloy JC, Johnson JM, et al. A Lysine-to-Arginine change found in natural alleles of the HTLV-I p12I protein greatly influences its stability. J. Virol. 1999;73:6460–6467. doi: 10.1128/jvi.73.8.6460-6467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- Uittenbogaard MN, Armstrong AP, Chiaramello A, Nyborg JK. Human T-cell leukemia virus type I Tax protein represses gene expression through the basic helix-loop-helix family of transcription factors. J. Biol. Chem. 1994;269:22466–22469. [PubMed] [Google Scholar]
- Unge T, Solomin L, Mellini M, et al. The Rex regulatory protein of human T-cell lymphotropic virus type I binds specifically to its target site within the viral RNA. Proc. Natl. Acad. Sci. USA. 1991;88:7145–7149. doi: 10.1073/pnas.88.16.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuku K, Nishizawa M, Osame M, Tabira T. Cytotoxic and suppressor activities in patients with HTLV-I-associated myelopathy. J. Neuroimmunol. 1991;33:199–205. doi: 10.1016/0165-5728(91)90107-i. [DOI] [PubMed] [Google Scholar]
- Van Orden K, Giebler HA, Lemasson I, Gonzales M, Nyborg JK. Binding of p53 to the KIX domain of CREB binding protein. A potential link to human T-cell leukemia virus, type I-associated leukemogenesis. J. Biol. Chem. 1999;274:26321–26328. doi: 10.1074/jbc.274.37.26321. 10.1074/jbc.274.37.26321. [DOI] [PubMed] [Google Scholar]
- Wano Y, Feinberg M, Hosking JB, Bogerd H, Greene WC. Stable expression of the tax gene of type I human T-cell leukemia virus in human T cells activates specific cellular genes involved in growth. Proc. Natl. Acad. Sci. USA. 1988;85:9733–9737. doi: 10.1073/pnas.85.24.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Nakamura T, Nagasato K, et al. Exaggerated messenger RNA expression of inflammatory cytokines in human T-cell lymphotropic virus type I-associated myelopathy. Arch. Neurol. 1995;52:276–280. doi: 10.1001/archneur.1995.00540270068021. [DOI] [PubMed] [Google Scholar]
- Weber-Nordt RM, Egen C, Wehinger J, et al. Constitutive activation of STAT proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV) -related lymphoma cell lines. Blood. 1996;88:809–816. [PubMed] [Google Scholar]
- Wucherpfennig KW, Hollsberg P, Richardson JH, Benjamin D, Hafler DA. T-cell activation by autologous human T-cell leukemia virus type I-infected T-cell clones. Proc. Natl. Acad. Sci. USA. 1992;89:2110–2114. doi: 10.1073/pnas.89.6.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Kang SH, Heidenrich O, et al. Constitutive activation of different Jak tyrosine kinases in human T-cell leukemia virus type 1 (HTLV-1) tax protein or virus-transformed cells. J. Clin. Invest. 1995;96:1548–1555. doi: 10.1172/JCI118193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Ikeda H, Yamazaki H, et al. Cytokine-producing mammary carcinomas in transgenic rats carrying the pX gene of human T-lymphotropic virus type I. Cancer Res. 1995;55:2524–2527. [PubMed] [Google Scholar]
- Yamaguchi K, Nishimura H, Kawano F, et al. A proposal for smoldering adult T-cell leukemia – diversity in clinical pictures of adult T-cell leukemia. Jpn. J. Clin. Oncol. 1983;13:189–199. [PubMed] [Google Scholar]
- Yamaguchi K, Takatsuki K. Adult T cell leukaemia-lymphoma. Baillieres Clin. Haematol. 1993;6:899–915. doi: 10.1016/s0950-3536(05)80183-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Yoshioka R, Kiyokawa T, et al. Lymphoma type adult T-cell leukemia – a clinicopathologic study of HTLV related T-cell type malignant lymphoma. Hematol. Oncol. 1986;4:59–65. doi: 10.1002/hon.2900040108. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Courtois G, Bessia C, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Inoue H, Sakurai M, et al. Constitutive activation of NF-kappa B is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]
- Yan JP, Garrus JE, Giebler HA, Stargell LA, Nyborg JK. Molecular interactions between the coactivator CBP and the human T-cell leukemia virus Tax protein. J. Mol. Biol. 1998;281:395–400. doi: 10.1006/jmbi.1998.1951. 10.1006/jmbi.1998.1951. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Osame M, Usuku K, Matsumoto M, Igata A. Viruses detected in HTLV-I-associated myelopathy and adult T-cell leukaemia are identical on DNA blotting. Lancet. 1987;1:1085–1086. doi: 10.1016/s0140-6736(87)90506-x. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Giam CZ. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein–protein interaction. Proc. Natl. Acad. Sci. USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]